Published online Jul 27, 2013. doi: 10.4254/wjh.v5.i7.379
Revised: June 17, 2013
Accepted: June 18, 2013
Published online: July 27, 2013
Processing time: 117 Days and 2 Hours
AIM: To study the effect of dichloromethylene diphosphonate (DMDP), a selective Kupffer cell toxicant in reference to liver damage and postnecrotic liver regeneration in rats induced by sublethal dose thioacetamide (TA).
METHODS: Rats, intravenously (iv) pre-treated with a single dose of DMDP (10 mg/kg), were intraperitoneally (ip) injected with TA 6.6 mmol/kg (per 500 mg/kg body weight). Hepatocytes were isolated from rats at 0, 24, 48 and 72 h following TA intoxication and blood and liver samples were obtained. To evaluate the mechanisms involved in the postnecrotic regenerative state, DNA distribution and ploidy time course were assayed in isolated hepatocytes. Circulating cytokine tumor necrosis factor-alpha (TNF-α) was assayed in serum and determined by reverse transcriptase-polymerase chain reaction in liver extract.
RESULTS: The effect of DMDP induced noticeable changes in postnecrotic regeneration, causing an increased percentage of hepatocytes in the cell cycle S phase. The increase at 24 h in S1 population in rats pretreated with DMDP + TA was significantly (P < 0.05) different compared with that of the TA group (18.07% vs 8.57%). Hepatocytes increased their proliferation as a result of these changes. Also, TNF-α expression and serum level were increased in rats pre-treated with DMDP. Thus, DMDP pre-treatment reduced TA-induced liver injury and accelerated postnecrotic liver regeneration.
CONCLUSION: These results demonstrate that Kupffer cells are involved in TA-induced liver, as well as in postnecrotic proliferative liver states.
Core tip: Over the last 20 years, liposomes, useful models for cell membranes, have become a powerful research tool whose study has resulted in many advances in cell physiology. When encapsulated in liposomes, dichloromethylene diphosphonate, a selective Kupffer cell toxicant, completely eliminates large Kupffer cells from the liver, allowing us to elucidate the role of these macrophages in total damage induced by hepatotoxic compounds such as thioacetamide.
- Citation: Bautista M, del Rio M&G, Benedí J, Sánchez-Reus MI, Morales-González JA, Téllez-López AM, López-Orozco M. Effect of dichloromethylene diphosphonate on liver regeneration following thioacetamide-induced necrosis in rats. World J Hepatol 2013; 5(7): 379-386
- URL: https://www.wjgnet.com/1948-5182/full/v5/i7/379.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i7.379
Dichloromethylene diphosphonate (DMDP) is clinically employed for the treatment of osteolytic bone diseases. When encapsulated in liposomes, DMDP is a selective Kupffer cell toxicant that completely eliminates large Kupffer cells from the liver, resulting in their damage and apoptosis[1]. Degree of depletion depends on the injection route and amount of injected DMDP liposomes. In the majority of studies, not only Kupffer cells, but also splenic macrophages have been depleted by a single intravenous (iv) injection of DMDP. Kupffer cells, due to the macrophages residing in the liver sinusoids, are the first macrophage population to come into contact with drugs. These cells are anchored to the endothelium in the lumen of the sinusoids[2]. Kupffer cells exhibit intra-acinar heterogeneity because those located in the periportal area are larger and exhibit higher phagocytic activity compared with those localized in the perivenous area[3]. It is well known that the function of these cells (cytokine and protease release, superoxide anion production, etc.) plays an important role in the pathogenesis induced by hepatotoxic compounds[4,5]. DMDP is most likely protective because it prevents the release of inflammatory cytokines and toxic oxygen radicals produced by activated Kupffer cells[6,7].
Thioacetamide (TA) is a potent hepatotoxic agent that, when administered at 500 mg/kg body weight doses to rats, gives rise to severe hepatocellular perivenous necrosis[8,9]. The selective destruction of perivenous hepatocytes and the proliferative state of liver cells that immediately follows were employed in the present study as an experimental model by means of which to study the hepatic response against the aggressive attack of a hepatotoxic drug. Thus, this response may be considered from two perspectives: that of hepatocellular necrosis and that of the postnecrotic hepatocellular regeneration linked with restoration of liver function[10,11].
Kupffer cells are also the major source of mitogens such as tumor necrosis factor-alpha (TNF-α) in liver[12,13]. TNF-α is a multifunctional cytokine that in the liver acts as a mediator of the acute phase response and is a cytotoxic agent in many types of hepatic injury. Some authors have suggested that TNF-α may be necessary for hepatocyte proliferation[14]. The observation that TNF-α is required for liver regeneration is surprising because TNF-α is a proinflammatory cytokine and an acute phase response mediator[15]. The proliferative and anti-apoptotic effect of this cytokine appears to take place only under special conditions, such as those existing after partial hepatectomy. Although TNF-α appears to be beneficial and required for liver regeneration after partial hepatectomy, the need for this factor has not been as clearly established after liver injury, a more common regenerative stimulus. In fact, a number of studies have suggested that TNF increases liver injury after toxic damage[16,17]. Moreover, Fujita et al[18] demonstrated that the absence of TNF-α does not impair liver regeneration.
The purpose of the present study was to elucidate the role of Kupffer cells in regeneration after liver injury, specifically blocking Kupffer cell function by DMDP. The proliferative postnecrotic response was assayed by evaluating ploidy and DNA distribution in the cell cycle phases in isolated hepatocytes by flow cytometry.
DMDP (dichloromethylene diphosphonate) was provided by Roche Diagnostics (Mannheim, Germany), phosphatidylcholine by Lipoid EPC, LIPOID (Ludwigshafen, Germany) and monoclonal ED-1 (MCA1018G) and monoclonal ED-2 antibodies were provided by Serotec, Hilversum (The Netherlands). Enzymes were obtained from Boehringer Mannheim (Germany). Substrates and coenzymes were from Sigma Chemical Co. (St. Louis, MO, United States). Standard analytical grade laboratory reagents were obtained from Merck (Darmstadt, Germany).
Liposomal clodronate was prepared as previously described[19]. Briefly, 86 mg phosphatidylcholine and 8 mg cholesterol were dissolved in chloroform in a round-bottom flask. The thin film that formed on the interior of the flask after high vacuum rotary evaporation was dispersed by gentle rotation under low vacuum conditions for 10 min in 10 mL phosphate buffered saline (PBS) (control liposomes) or in 10 mL of a 0.6 mol/L DMDP (2.5 g DMDP in 10 mL distilled water and clodronate-containing liposomes). After swelling, sonication and washing in PBS, the liposomes were resuspended in 4 mL PBS. The resulting liposomal formulation contained clodronate at a concentration of 0.7 mol/L.
Two month old male Wistar rats (weighing 200-220 g) were obtained from the Bioterio, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo (UAEH), Mexico, and acclimated to our animal room for 2 wk, during which time the rats were supplied with food (Purina de México, S.A.) and water ad libitum, exposed to a 12 h light-dark cycle, and administered intraperitoneally (ip) with a single necrogenic dose of thioacetamide (TA) 6.6 mmol (500 mg/kg body weight) (TA) freshly dissolved in 0.9% NaCl. The TA dose was chosen as the highest dose with survival of > 90%[20,21]. Experiments were performed on two different groups. Rats were treated with a single dose of TA and rats pre-treated with DMDP 24 h prior to TA (DMDP + TA). DMDP encapsulated in liposomes was injected into tail vein (10 mg/kg). Untreated animals received 0.5 mL of 0.9% NaCl. Rats were cervically dislocated and blood and liver samples were obtained and processed as previously described[21]. Blood was collected from hearts and maintained at 4 °C for 24 h, centrifuged at 3000 g for 15 min, and serum was obtained as the supernatant. Hepatocytes were isolated from rats by the classic perfusion method[22] at 0, 24, 48 and 72 h following TA (24 h). The viability of isolated hepatocytes (> 90%) was assessed by trypan blue exclusion as previously described[10].
Each experiment was performed in duplicate on four different animals and following the International Criteria of Experimental Animals outlined in Care and Use of Laboratory Animals, DHEW Publication No. (NIH) 85-23, 1985, and all procedures involving experimental animals were conducted according to our Federal Regulations for Animal Experimentation and Care (Ministry of Agriculture; SAGAR, Mexico) and The Guiding Principles in the Use of Animals in Toxicology adopted by the Society of Toxicology in 1989.
Enzymatic determinations were carried out in serum under optimal conditions of pH, temperature, substrate and co-factor concentrations. Aspartate aminotransferase (AST) and isocitrate dehydrogenase (ICDH) were determined in serum as a biochemical indicator of hepatocellular necrosis according to the manufacturer’s protocol. AST (EC 2.6.2.1) activity was assayed following the method of Rej and Horder[25]. ICDH (E.C 1.1.1.39) was determined as described previously[26]. Concentrations of immunoreactive TNF-α was determined by the enzyme-linked immunosorbent assay (ELISA) system (Amersham Pharmacia Biotech) according to the manufacturer’s protocol. In brief, the extracted plasma was reacted with the assay reagents in the TNF-α kit and analyzed spectrophotometrically at 450 nm absorbance. TNF-α levels were calculated from kit standards and expressed as pg/mL of plasma.
Total RNA was isolated from rat liver following the guanidinium thiocyanate/phenol reagent method[27]. For reverse transcriptase-polymerase chain reaction (RT-PCR), total RNA (1 μg) was subjected to random primer first-strand complementary DNA (cDNA) synthesis in 40 μL reactions composed of 50 mmol/L Tris-HCl, 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L DTT, 1 mmol/L dNTP (each), 50 ng of random hexamer and 0.5 IU/μL Mo-Mu-LV reverse transcriptase (Super-Script Pre-Amplification System; Gibco-BRL, Life Technologies). The reactions were incubated for 60 min at 42 °Cand terminated at 65 °C for 15 min. First-strand cDNA were subsequently amplified by PCR; β-actin cDNA was utilized as an internal control. Sequences of the primers were as follows: TNF-α sense: 5’-TGG CCC AGA CCC TCA CAC TC-3’; TN-α antisense: 5’-CTC CTG GTA TGA AAT GGC AAA TC-3’; β-actin sense: 5’-TAC AAC CTC CTT GCA GCT CC-3’; and β-actin antisense: 5’-GGA TCT TCA TGA GGT AGT CAG TC-3’. The PCR reaction mixture contained PCR buffer [20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl], 1.5 mmol/L MgCl2, 100 mmol/L dNTP (each), 0.4 mmol/L primers and 0.0025 U/μL of Taq polymerase in a final volume of 50 μL. Number of PCR cycles was adjusted to avoid saturation of the amplification system [at 94 °C for 1 min, 59 °C for 1 min and 72ºC for 1 min (35 cycles) for TNF-α, and at 94 °C for 30 s, 58 °C for 45 s and 72 °C for 30 s (24 cycles) for β-actin], with a final elongation at 72 °C for 10 min. Amplification products were visualized on 1.8% agarose gels containing ethidium bromide (1 µg/mL), TNF-α product, 281 bp, and β-actin product, 630 bp. A 100 bp DNA ladder was used as a marker. The products were quantified by laser densitometry.
DNA content was obtained from 106 isolated viable hepatocytes stained with propidium iodide following the multistep procedure of Vindeløv et al[28]. The fluorescence emitted from the DNA-propidium iodide complex was assayed in a FACScan flow cytometer (Becton-Dickinson) in the FL2-A channel. A double discriminator module was employed to distinguish between signals deriving from a single nucleus and nuclear aggregation products. Data analysis was carried out by evaluation of single inputs (104 nuclei/assay) and was expressed as the percentage of DNA distribution in cell cycle phases G0/G1 (2N), S1, G2 + M (4N), S2, (G2 + M)2 (8N) and hypodiploid peak (< 2N).
The results were calculated as the mean ± SD of four experimental observations in duplicate (four animals). Differences between groups were analyzed by analysis of variance (ANOVA) following Snedecor F (α = 0.05). The Student’s t test (statistical significance P < 0.05) was performed for statistical evaluation as follows: (1) all values against their control; and (2) differences between two groups: DMDP + TA vs TA.
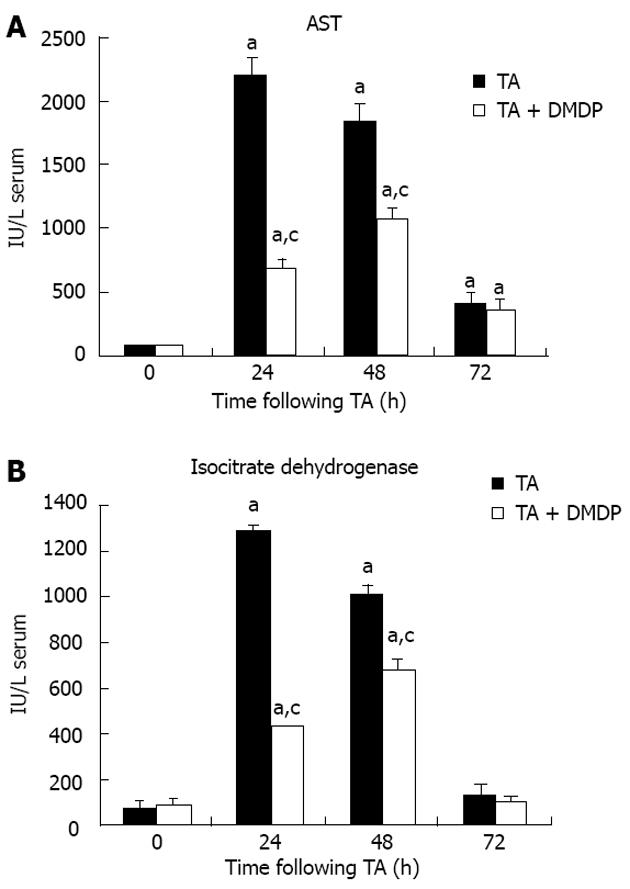
Liver damage induced by xenobiotics is characterized by the release in serum of hepatic enzymes due to the necrosis of hepatocytes. AST is randomly distributed in the hepatic acinus and is the enzyme activity utilized as the marker of necrosis. The increase in AST and ICDH in serum reached the maximum at 24 h (Figure 1). The extent of TA-induced necrosis was detected by a peak of 30 and 15 times baseline values for AST and ICDH activity, respectively. When rats were pre-treated with DMDP, the 24 h peaks were reduced to 30% and 40%, respectively. However, at 48 h of intoxication, the DMDP-associated difference was 58% for AST activity, which indicates that DMDP delays TA-induced liver injury because maximal necrosis appeared at 48 h of intoxication. No effects were detected on serum activities when empty liposomes were administered (data not shown).
Table 1 shows the percentages of cell cycle populations related with ploidy and DNA content, as associated with histograms determined on the basis of fluorescence emission at 623 nm by the DNA propidium iodide complex. Following TA, liver cells exhibit marked variations in the pattern of DNA distribution, which can be summarized as a sharp decrease at 48 h in tetraploid population parallel to an increase in diploid population, followed by restoration to nearly normal values at 72 h. It can also be observed how the S1 population is increased from 24 h, reaching maximal increase at 48 h. When rats were pre-treated with DMDP, variations in the pattern of DNA distribution is very similar to that observed in the TA group. However, we are able to detect an important difference: the highest increase in S1 population is reached at 24 h (18.07% vs 8.57%) instead of at 48 h; thus, the proliferative state in hepatocytes is reached 24 h prior to that obtained in single dose TA-treated rats. No changes were detected in DNA ploidy when empty liposomes were administered (data not shown).
| Group | Hypodiploid | Diploid | S1 Phase | Tetraploid | S2 Phase | Octoploid |
| (< 2N) | (2N) | (2N→4N) | (4N) | (4N→8N) | (8N) | |
| Control | 0.98 | 12.3 | 2 | 75.31 | 2.48 | 3.97 |
| Control DMDP | 0.74 | 18.86 | 0.98 | 71.09 | 5.6 | 2.67 |
| TA 24 h | 1.61 | 41.74a | 8.57a | 39.0ª | 7.82ª | 0.9 |
| TA-DMDP 24 h | 2.01 | 25.45ac | 18.07ac | 49.20ac | 3.92 | 0.76 |
| TA 48 h | 1.59 | 52.87a | 11.95a | 22.86a | 10.18a | 0.2 |
| TA-DMDP 48 h | 2.35 | 42.77a | 14.92a | 28.0a | 10.25ª | 1.7 |
| TA 72 h | 3.78a | 47.61a | 7.21a | 35.6ª | 4.29ª | 1.12 |
| TA-DMDP 72 h | 2.65ac | 45.99a | 1.44c | 41.71ª | 5.98 | 1.83 |
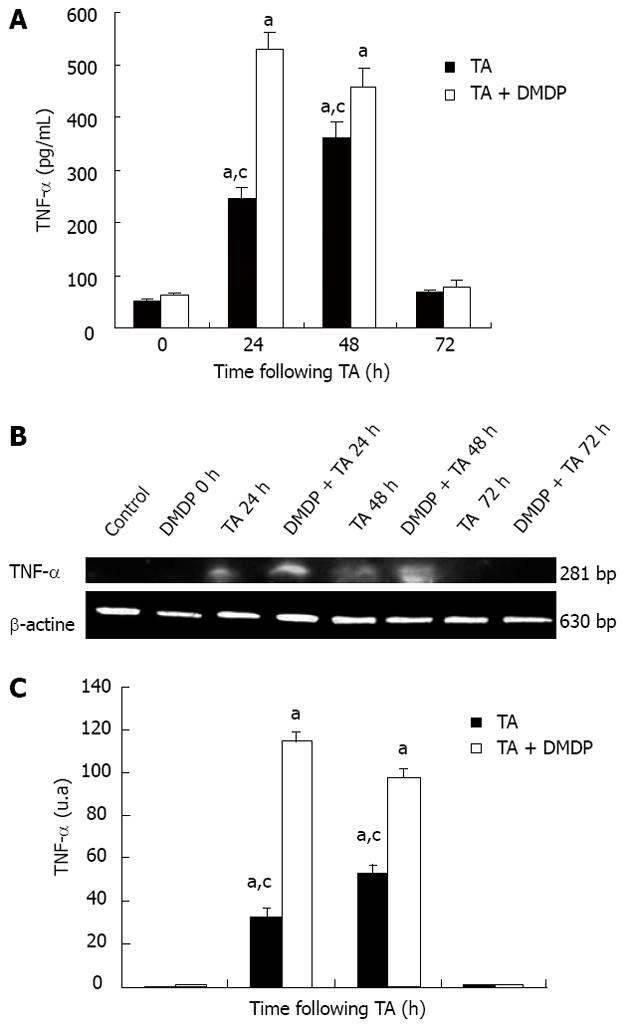
TNF-α is a multifunctional cytokine that, in the liver, acts as a mediator of the acute phase response and is a cytotoxic agent in many types of hepatic injury. TNF-α determination was performed in serum and liver. In TA-intoxicated rat serum, the level of this cytokine increased at 24 h of intoxication and when DMDP was pre-administered; this increase was significant (Figure 2).
Figure 2B and C depict the levels of TNF-α messenger RNA (mRNA) assayed by RT-PCR. As observed in serum levels of TNF-α , mRNA follow the same pattern, which corroborates with the results obtained by ELISA.
Macrophages such as Kupffer cells in liver are multifunctional cells. They are involved in host defense mechanisms and possess a regulatory role in many biomedical processes. Their selective depletion[6], employing liposome-encapsulated drugs, forms a widely accepted approach to studying their functional aspects in vivo. There is evidence that liposome-mediated DMDP delivery actually depleted Kupffer cell in rat liver. We and others[7,29] found this “suicide” approach highly effective in depletion of Kupffer cell in liver tissue.
On the other hand, TA-induced liver injury is a well-established area of considerable pharmacological interest because reactive oxygen species (ROS) and free radicals, generated in microsomal drug oxidation, participate in the mechanisms of cell death[20,21,30]. Xenobiotics may act directly on hepatocytes, causing toxicity by interacting with target molecules, and may also act indirectly by means of activating phagocytic cells. The active phagocytes participate in the pathogenesis of tissue injury by releasing, among others, inflammatory cytokines that upregulate the expression of adhesion molecules. Tissue damage initiates an inflammatory response characterized by an accumulation of neutrophils at the site of injury[31].
Kupffer cells and infiltrating neutrophils contribute to liver injury in different experimental models of hepatotoxicity[32-34]. In our experiments, DMDP significantly attenuates TA-induced liver damage. Blockade of Kupffer cell function by DMDP appears to result in a disruption of a part of the sequence of events leading to hepatotoxicity.
In addition, the role of DMDP in TNF-α expression by Kupffer cells has been widely debated. Depletion of Kupffer cells, the major source of TNF-α production in liver, should give rise to a decrease in serum and in the mRNA TNF-α level in liver, a fact that has been described and corroborated by several authors[5,35,36]. However, other authors have reported opposite data[37,38] after partial hepatectomy in rats pre-treated with gadolinium, another inhibitor of Kupffer cells. Additionally, depletion of Kupffer cells with DMDP appears to increase hepatocyte proliferation and liver regeneration following partial hepatectomy; however, the responsible mechanism remains unknown.
On the other hand, it has already been reported that DMDP protects the liver from a number of toxicants that require biotransformation to elicit toxicity[39,40]. Badger et al[41] demonstrated in hepatocytes isolated from DMDP pre-treated rats that CYP-450 activity was reduced and the susceptibility of hepatocytes was altered. It has also been shown that hepatic injury induced by ischemia/reperfusion is modulated by the Kupffer cells[42].
In previous reports, we described that when TA was administered to rats, necrosis developed and peaked at 24 h of intoxication and that a synchronous proliferative response was immediately initiated, reaching a peak of DNA synthesis at 48 h. Postnecrotic proliferative response after experimental liver cell death constitutes an interesting area in which to study the factors involved in the regulation of hepatocyte proliferation.
Regarding postnecrotic regeneration, the peak of DNA synthesis was similar in both groups, although it is noteworthy that initial DNA synthesis levels were significantly higher due to the effect of DMDP, indicating that in our experiments, this compound also exerts mitogenic action, which can lead to liver hyperplasia.
After depleting Kupffer cells with DMDP, we explain that elevation of serum TNF-α levels and enhanced mRNA levels in the liver by hepatic cells other than Kupffer cells may contribute to cytokine synthesis or that TA-inducible cells residing in the liver contribute to cytokine levels in plasma. Endothelial cells may be a hepatic source of cytokines because this cell type readily responds to TA stimulation[35,43] and may not be affected directly by clodronate.
Following TA, liver cells exhibit marked variations in the DNA distribution pattern, which can be summarized as a sharp decrease at 48 h in tetraploid population parallel to an increase in diploid population, followed by restoration to nearly normal values at 72 h. It can also be observed how the S1 population increases from 24 h, reaching the maximum at 48 h. When rats were pre-treated with DMDP, variations in the pattern of DNA distribution are very similar to those observed in the TA group. However, we can detect an important difference: the highest increase in S1 population is reached at 24 h (17.17% vs 10.01%) instead of at 48 h; thus, the proliferative state in hepatocytes is reached 24 h prior to that obtained in rats treated with the single dose of TA.
Our results clearly indicate that administration of DMDP + TA in rats results in stimulated tissue repair. From these results, we are able to speculate that Kupffer cells may play a crucial role in inducing DNA synthesis by secreting the priming factors (TNF-α) in the early phase of oval cell-mediated liver regeneration[44].
We conclude that DMDP pre-treatment significantly attenuates TA-induced hepatotoxicity. These results demonstrate that Kupffer cells are involved in TA-induced liver, as well as in postnecrotic proliferative liver states.
Modulation of Kupffer cell function by DMDP may serve as a potential target for therapeutics and could be useful for preventing drug-induced liver damage.
Thioacetamide (TA)-induced liver injury is a well-established area of considerable pharmacological interest because reactive oxygen species (ROS) and free radicals generated in microsomal drug oxidation participate in the mechanisms of cell death. In the present study, TA-induced hepatotoxicity was used to investigate the effect of a single dose of dichloromethylene diphosphonate (DMDP) (clinically employed for the treatment of osteolytic bone diseases); but in the present study, when encapsulated in liposomes, DMDP is a selective Kupffer cell toxicant.
The aim of this study was to elucidate the role of Kupffer cells in regeneration after liver injury, specifically blocking Kupffer cell function by DMDP. The effect was assayed on an experimental model of liver injury induced by a single sublethal dose of TA.
Macrophages such as Kupffer cells in the liver are multifunctional cells. They are involved in host defense mechanisms and possess a regulatory role in many biomedical processes. Their selective depletion, utilizing liposome-encapsulated drugs, forms a widely accepted approach of studying their functional aspects in vivo.
As it is generally accepted that Kupffer cell function is involved in the severity of drug-induced liver damage and that DMDP induces a selective blockade of Kupffer cell function when administered intravenously, the purpose of the present study was to elucidate the role of Kupffer cells in regeneration after liver injury, opening a window to novel therapeutic strategies.
Liposomes can be used for intracellular drug delivery into macrophages. In the present study, the authors utilized a liposome-mediated macrophage “suicide” technique based on intraphagocytic accumulation of the liposomes delivered.
This is a nice experimental study showing that Kupffer cells play an important role in experimental hepatotoxicity by TA and in the regenerating process. The paper is well written.
P- Reviewers Tang N, Teschke R S- Editor Wen LL L- Editor Roemmele A E- Editor Li JY
| 1. | Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N, Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Laskin DL. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990;10:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Bautista AP, Skrepnik N, Niesman MR, Bagby GJ. Elimination of macrophages by liposome-encapsulated dichloromethylene diphosphonate suppresses the endotoxin-induced priming of Kupffer cells. J Leukoc Biol. 1994;55:321-327. [PubMed] |
| 4. | Ishiyama H, Sato M, Matsumura K, Sento M, Ogino K, Hobara T. Proliferation of hepatocytes and attenuation from carbon tetrachloride hepatotoxicity by gadolinium chloride in rats. Pharmacol Toxicol. 1995;77:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Iimuro Y, Yamamoto M, Kohno H, Itakura J, Fujii H, Matsumoto Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats--analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol. 1994;55:723-728. [PubMed] |
| 6. | Andrés D, Sánchez-Reus I, Bautista M, Cascales M. Depletion of Kupffer cell function by gadolinium chloride attenuates thioacetamide-induced hepatotoxicity. Expression of metallothionein and HSP70. Biochem Pharmacol. 2003;66:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Landon EJ, Naukam RJ, Rama Sastry BV. Effects of calcium channel blocking agents on calcium and centrilobular necrosis in the liver of rats treated with hepatotoxic agents. Biochem Pharmacol. 1986;35:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Cascales M, Martin-Sanz P, Alvarez A, Sanchez-Pérez M, Diez Fernández C, Boscá L. Isoenzymes of carbohydrate metabolism in primary cultures of hepatocytes from thioacetamide-induced rat liver necrosis: responses to growth factors. Hepatology. 1992;16:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Díez-Fernández C, Boscá L, Fernández-Simón L, Alvarez A, Cascales M. Relationship between genomic DNA ploidy and parameters of liver damage during necrosis and regeneration induced by thioacetamide. Hepatology. 1993;18:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Sanz N, Díez-Fernández C, Alvarez AM, Fernández-Simón L, Cascales M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol Appl Pharmacol. 1999;154:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Harstad EB, Klaassen CD. Gadolinium chloride pretreatment prevents cadmium chloride-induced liver damage in both wild-type and MT-null mice. Toxicol Appl Pharmacol. 2002;180:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Iszard MB, Liu J, Klaassen CD. Effect of several metallothionein inducers on oxidative stress defense mechanisms in rats. Toxicology. 1995;104:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Theocharis SE, Kanelli H, Margeli AP, Spiliopoulou CA, Koutselinis AS. Metallothionein and heat shock protein expression during acute liver injury and regeneration in rats. Clin Chem Lab Med. 2000;38:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Theocharis SE, Margeli AP, Karandrea DN, Tsarpalis KS, Agapitos EV, Spiliopoulou CA, Koutselinis AS. Liver metallothionein expression in thioacetamide-intoxicated rats. Pathol Res Pract. 2000;196:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Fujita J, Marino MW, Wada H, Jungbluth AA, Mackrell PJ, Rivadeneira DE, Stapleton PP, Daly JM. Effect of TNF gene depletion on liver regeneration after partial hepatectomy in mice. Surgery. 2001;129:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1430] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 20. | Cascales M, Martín-Sanz P, Craciunescu DG, Mayo I, Aguilar A, Robles-Chillida EM, Cascales C. Alterations in hepatic peroxidation mechanisms in thioacetamide-induced tumors in rats. Effect of a rhodium(III) complex. Carcinogenesis. 1991;12:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Sanz N, Díez-Fernández C, Fernández-Simón L, Alvarez A, Cascales M. Necrogenic and regenerative responses of liver of newly weaned rats against a sublethal dose of thioacetamide. Biochim Biophys Acta. 1998;1384:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Seglen PO. Isolation of hepatocytes by collagenase perfusion. Methods in Toxicology. In vitro biological systems. New York, London: Academic Press 1993; 231-243. |
| 23. | Barbé E, Damoiseaux JG, Döpp EA, Dijkstra CD. Characterization and expression of the antigen present on resident rat macrophages recognized by monoclonal antibody ED2. Immunobiology. 1990;182:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Rej R, Horder M. Aspartate aminotransferase. L-aspartate: 2-oxoglutarate aminotranferase, EC 2.6.2.1. Routine U.V. method. Methods of Enzymatic Analysis. 3rd ed, vol III. Weinheim: Verlag Chemie 1984; 416-424. |
| 26. | Goldberg DM, Ellis G. Isocitrate dehydrogenase. Methods of Enzymatic Analysis. Vol 3, 3rd ed. Weinheim: Verlag Chemie 1986; 183-189. |
| 27. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39086] [Article Influence: 1028.6] [Reference Citation Analysis (0)] |
| 28. | Vindeløv LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1220] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 29. | Gregory SH, Wing EJ, Danowski KL, van Rooijen N, Dyer KF, Tweardy DJ. IL-6 produced by Kupffer cells induces STAT protein activation in hepatocytes early during the course of systemic listerial infections. J Immunol. 1998;160:6056-6061. [PubMed] |
| 30. | Bautista M, Andres D, Cascales M, Morales-González JA, Sánchez-Reus MI, Madrigal-Santillán E, Valadez-Vega C, Fregoso-Aguilar T, Mendoza-Pérez JA, Gutiérrez-Salinas J. Role of Kupffer cells in thioacetamide-induced cell cycle dysfunction. Molecules. 2011;16:8319-8331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 1995;133:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 249] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990;192:245-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 628] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 33. | Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647-653. [PubMed] |
| 34. | Essani NA, Fisher MA, Farhood A, Manning AM, Smith CW, Jaeschke H. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995;21:1632-1639. [PubMed] |
| 35. | Prins HA, Meijer C, Boelens PG, Nijveldt RJ, Siroen MP, Masson S, Daveau M, Scotté M, Diks J, van Leeuwen PA. The role of Kupffer cells after major liver surgery. JPEN J Parenter Enteral Nutr. 2005;29:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Lázár G, Lázár G, Kaszaki J, Oláh J, Kiss I, Husztik E. Inhibition of anaphylactic shock by gadolinium chloride-induced Kupffer cell blockade. Agents Actions. 1994;41 Spec No:C97-C98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996;270:G909-G918. [PubMed] |
| 38. | Rose ML, Bradford BU, Germolec DR, Lin M, Tsukamoto H, Thurman RG. Gadolinium chloride-induced hepatocyte proliferation is prevented by antibodies to tumor necrosis factor alpha. Toxicol Appl Pharmacol. 2001;170:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 1986;86:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Hardonk MJ, Dijkhius FWJ, Jonker AM. Selective depletion of Kupffer cells by gadolinium Chloride attenuates both acute galactosemine-induced hepatitis and carbon tetrachloride toxicity in rats. Cells of the hepatic sinusoid. Rijswijk, The Netherlands: The Kupffer Cell Foundation 1995; 29-32. |
| 41. | Badger DA, Kuester RK, Sauer JM, Sipes IG. Gadolinium chloride reduces cytochrome P450: relevance to chemical-induced hepatotoxicity. Toxicology. 1997;121:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Mehendale HM, Roth RA, Gandolfi AJ, Klaunig JE, Lemasters JJ, Curtis LR. Novel mechanisms in chemically induced hepatotoxicity. FASEB J. 1994;8:1285-1295. [PubMed] |
| 44. | Zhang W, Chen XP, Zhang WG, Zhang F, Xiang S, Dong HH, Zhang L. Hepatic non-parenchymal cells and extracellular matrix participate in oval cell-mediated liver regeneration. World J Gastroenterol. 2009;15:552-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |