Published online Apr 27, 2013. doi: 10.4254/wjh.v5.i4.170
Revised: November 24, 2012
Accepted: December 22, 2012
Published online: April 27, 2013
AIM: To investigate the effect of hypothermia on the function of the liver remnant (LR) after extended hepatectomy.
METHODS: We performed a 75% partial hepatectomy (PH) in male C57BL/6J mice. Body temperature was measured with a rectal probe. The study mice were prospectively grouped as hypothermic (HT) or normothermic (NT) if their body temperature was < 34 °C vs≥ 34 °C, respectively. Blood and liver samples were obtained at 24 and 48 h after 75% PH. Various factors during and after 75% PH were compared at each time point and the most important factor for a good outcome after 75% PH was determined.
RESULTS: At 24 and 48 h after 75% PH, LR weight was decreased in HT mice compared with that in NT mice and the assay results in the HT mice were consistent with liver failure. NT mice had normal liver regeneration. Each intra- and post-operative factor which showed statistical significance in univariate analysis was evaluated by multivariate analysis. The most important factor for a good outcome after 75% PH was body temperature at both 24 and 48 h after surgery.
CONCLUSION: Hypothermia after an extensive hepatectomy predicts impending liver failure and may be a useful clinical marker for early detection of liver failure after extended hepatectomy.
- Citation: Ohashi N, Hori T, Uemoto S, Jermanus S, Chen F, Nakao A, Nguyen JH. Hypothermia predicts hepatic failure after extensive hepatectomy in mice. World J Hepatol 2013; 5(4): 170-181
- URL: https://www.wjgnet.com/1948-5182/full/v5/i4/170.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i4.170
Liver failure (LF) occurs in various conditions, including acute LF, LF of an insufficient liver remnant (LR) after extended hepatectomy (EH) and LF of transplanted whole or split liver grafts[1-6]. Without a suitable liver replacement, death imminently ensues. When LF occurs in an insufficient LR, it becomes clinically apparent by postoperative days 3-7 or later[2,7,8]. Biochemically, transaminases and bilirubin levels are markedly elevated, with prolonged coagulopathy. Liver histology shows destruction of sinusoids and hepatocellular parenchyma. Clinically, ascites and possibly encephalopathy can develop in patients[6,8]. Hepatic failure can have lethal consequences but the mechanisms responsible for LF in an insufficient LR are unknown.
In early studies, terminal LF was associated with hypothermia[9]. Hypothermia is also common in advanced stages of acute LF[10,11]. Similarly, earlier studies that conducted EH in rodent models demonstrated that the animals that failed to survive after EH had significant hypothermia, whereas those that survived maintained normothermia[12,13]. In a recent outcome analysis of living-donor liver transplantation, hypothermia < 34 °C was one of the significant predictors of hospital death in adult recipients[14]. In our laboratory, preliminary results showed that mice which failed to survive after 75% partial hepatectomy (PH) had significant hypothermia and the surviving animals remained normothermic. Therefore, we hypothesized that hypothermia predicts imminent LF after EH. In this study, we investigated body temperature (BT) after 75% PH and prospectively evaluated the correlation of hypothermia to biochemical, histological and molecular factors of LF in mice after 75% PH. Our results suggest that hypothermia predicts LF after EH.
Male C57BL/6J mice (10-14 wk old), purchased from Jackson Laboratory (Bar Harbor, ME), were housed in a conventional mouse room with a 12 h light/dark cycle and were given food and water. The study was institutionally approved in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Hepatectomy models in mice have been well documented[15-17] and our surgical procedures have been described in detail elsewhere[17]. Left posterior, left and right anterior and right posterior lobes were resected[15-17]. After the mice underwent isoflurane general anesthesia, the liver lobes were mobilized. A hemostatic clip (Hemoclip, Edward Weck and Co., Research Triangle Park, NC) was applied across the pedicle at the base of the liver lobes, which were cut distally to the applied clip. For the sham operation performed on control subjects, laparotomy without liver resection was performed. Before closing the abdominal incision, 2 mL of warm saline was administered intraperitoneally. Cefalexin (30 mg/kg) and buprenorphine (0.1 mg/kg) were given subcutaneously. Postoperatively, the mice were housed under controlled temperature, humidity and light, with free access to food and water. No dextrose was provided. At the end of the procedure, all LRs were pink. Control mice had a normal BT and no postoperative mortality.
No heating pad or lamp was used after surgery. BT was measured with a rectal probe (RET-3, Physitemp Instrument Inc, Clifton, NJ) at 24 h after 75% PH.
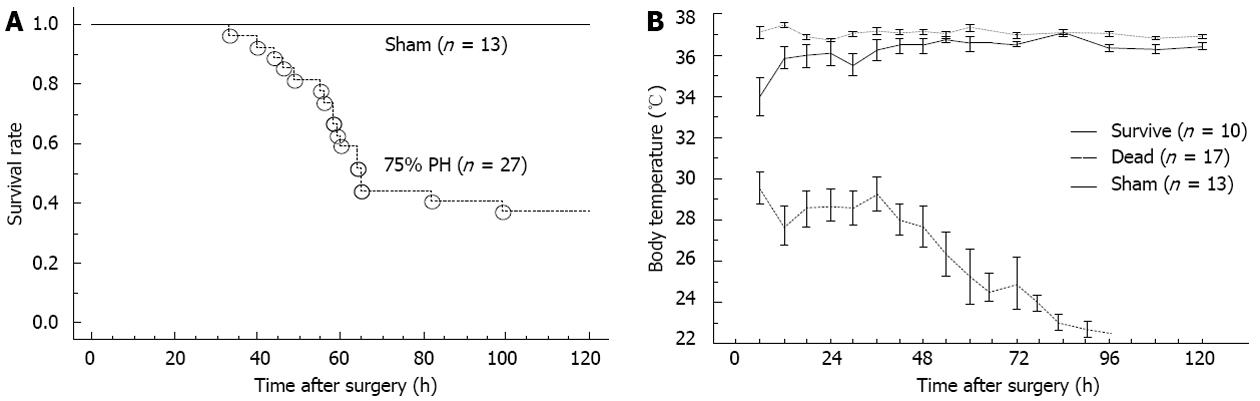
In a preliminary study, the mice that failed to survive had hypothermia of < 34 °C after 75% PH (0 of 20 mice after 75% PH). None of the mice that had a BT ≤ 30 °C survived (0 of 6 mice after 75% PH). In contrast, all but one mouse that had a BT over 34 °C survived beyond 5 d, consistent with previous reports[18,19].
In a separate preliminary study, after 75% PH in hypothermic mice, we achieved normothermia with a heating pad, as previously described[20]. However, a heating pad did not offer any benefits for survival (P > 0.05), consistent with a previous report[12]. Therefore, we did not employ a heating pad after surgery in this study.
To simulate clinical conditions[3,4], 75% PH was reproduced according to a previous description[15] in 27 mice (n = 27) and sham operations were performed in 13 control mice (n = 13).
Based on the preliminary studies and other reports[8], we conducted the following experiments prospectively to test the hypothesis that hypothermia precedes LF after 75% PH in mice. Since LF occurs between 3 and 7 d postoperatively, we sacrificed the study mice at 24 and 48 h after 75% PH. Rectal temperature was measured at 24 h after 75% PH and we divided the mice into the following groups based on their BT: normothermic (NT) with a BT ≥ 34 °C or hypothermic (HT) with a BT < 34 °C.
When mice were sacrificed at 24 and 48 h after 75% PH, liver and blood samples were collected (n = 10 in each group). Excised LR was weighed and snap-frozen at -80 °C. The sera were used for biochemical analysis.
Serum levels of aspartate aminotransferase (AST) and alanine aminotransaminase (ALT) were determined by a colorimetric kit (BioTron Diagnostics Co., Hemet, CA). Total bilirubin (T-Bil) levels were determined by the QuantiChrom™ Bilirubin Assay Kit (BioAssay Systems, Heyward, CA). The prothrombin time-international normalized ratio (PT-INR) was measured by i-STAT analysis (Abbott Laboratories, North Chicago, IL).
LR samples were homogenized in a buffer containing 10 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% Triton-X, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L ethylene diamine tetra-acetic acid, 1 mmol/L ethylene glycol tetra-acetic acid, 1 mmol/L phenyl-methyl-sulfonyl fluoride and protease and phosphatase inhibitors. Homogenates were centrifuged at 105000 g for 1 h at 4 °C. Supernatants were collected. Protein concentration was determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Samples were stored at -80 °C until use. Forty micrograms of protein was separated via SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA). Membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBS-T) [20 mmol/L Tris (pH 7.4), 500 mmol/L NaCl, and 0.05% Tween-20] and probed using the following antibodies: phospho-STAT3, STAT3, phospho-Akt, Akt, phospho-p44/42MAPK, p44 42 MAPK, phospho-SAPK/JNK, SAPK/JNK, phospho-p38 MAPK, p38 MAPK, cyclin D1, Met, caspase 3 (Cell Signaling, Danvers, MA) and vascular endothelial growth factor (VEGF) (Abcam, Cambridge, MA). Immunoblots were incubated with peroxidase-conjugated secondary antibodies (Southern Biotech, Birmingham, AL) followed by enhanced chemi-luminescence or ECL-plus reagent (Amersham Biosciences, Piscataway, NJ). Blots were reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase primary antibody (IMGENEX, San Diego, CA) followed by mouse secondary-HRP antibody for confirmation of equal loading. Signals were quantified using an ImageQuant program (Molecular Dynamics, Sunnyvale, CA).
Serum samples were analyzed using an anti-rat hepatic growth factor (HGF) enzyme-linked immunosorbent assay kit (B-Bridge International, Inc. Mountain View, CA) according to the manufacturer’s instructions.
LR tissues were homogenized in 0.6 mol/L trichloroacetic acid and centrifuged at 9000 g for 5 min at 4 °C. The supernatant was neutralized with 5 mol/L KOH mixed with 0.4 mol/L imidazole using color pHast (EMD, Gibbstown, NJ). Adenosine triphosphate (ATP) was measured using an ATP Determination Kit (Invitrogen, Carlsbad, CA).
Formalin-fixed liver specimens were embedded in the paraffin and cut into 5-μm sections and stained with hematoxylin and eosin. Immunohistochemical proliferation analyses for Ki-67 and proliferating cell nuclear antigen (PCNA) were performed after antigen retrieval with citric acid (pH 6.0) and 3% H2O2. Rabbit anti-Ki-67 and PCNA antibodies (Abcam, Cambridge, MA) were incubated at 4 °C overnight. An Elite ABC kit (Vector Laboratories Inc, Burlingame, CA) was used for immunostaining detection according to the manufacturer’s instructions. The number of Ki-67-positive and PCNA-positive cells was counted in 10 random fields at ×200. Immunohistochemical analysis for c-Met was performed using mouse anti-c-Met antibody (Cell Signaling). Blocking and detection were performed with the Vectastain MOM Kit (Vector Laboratories, Burlingame, CA).
Activation of caspase-3 was assayed by western blot, as described above. TdT-mediated DUTP-biotin nick end labeling (TUNEL) staining was performed using the ApopTag Apoptosis Detection kit (Chemicon, Billerica, MA) on formalin-fixed liver sections. DNA laddering detection was performed with extracted DNA samples using the TACS DNA Laddering Kit (R and D, Minneapolis, MN).
Surgery time (minutes), anesthesia time (minutes), estimated % hepatectomy (%), LR weight/body weight (%), BT (°C), AST (U/L), ALT (U/L), T-Bil (mg/dL), Hb (g/dL), hematocrit (%), glucose (mg/dL) and PT-INR were assessed at 24 and 48 h after 75% PH. A total of 152 mice at 24 h and 108 mice at 48 h after 75% PH were evaluated.
Some differences in the behavior between survivors and mice that eventually died were observed from the early postoperative period after 75% PH. We divided postoperative mice into two groups, based on the postoperative behavior (i.e., asymptomatic and sick mice at 24 and 48 h after 75% PH). Postoperative behavior was consistent with outcomes. A total of 152 mice at 24 h and 108 mice at 48 h after 75% PH were evaluated. Each factor, which showed statistical significance in univariate analysis, was evaluated by multivariate analysis and the most important factor for a good outcome after 75% PH was determined.
Data are presented as the mean ± SD. Statistical comparisons were performed using analysis of variance followed by t tests with a Bonferroni adjustment for continuous unpaired variables, the Kaplan-Meier method (the log-rank) for survival rates and logistic regression analysis for important factors for survival. Statistical calculations were performed using SPSS Software Version 16.0 (SPSS Inc., Chicago, IL). A P value less than 0.05 was considered statistically significant.
No deaths were observed at 24 h among the mice that had 75% PH. However, after 48 h, the survival rate was decreased (Figure 1A). The overall 5 d mortality rate was 60%, consistent with previous reports[18,19]. The survival rate of HT mice (BT < 34 °C) was significantly lower than that of NT mice after 75% PH (Fisher’s exact test, P = 0.003) (Figure 1B). This finding suggested that BT might be a useful marker for impending hepatic failure after EH.
After 75% PH, the animals were monitored prospectively. HT and NT mice were sacrificed at 24 and 48 h. Although HT mice had decreased physical activity, they were still active and not moribund. There were no differences in surgery time, anesthesia time or the ratio of resected liver weight to body weight between HT and NT mice (Table 1).
| 24 h after PH | 48 h after PH | |||||
| HT | NT | P value | HT | NT | P value | |
| Surgery time (min) | 12.5 ± 0.6 | 11.9 ± 0.4 | NS | 12.0 ± 0.4 | 12.0 ± 0.4 | NS |
| Anesthesia time (min) | 16.0 ± 0.6 | 15.2 ± 0.6 | NS | 15.3 ± 0.5 | 15.4 ± 0.3 | NS |
| Estimated % of hepatectomy (%) | 79.5 ± 1.8 | 77.1 ± 2.4 | NS | 76.1 ± 1.2 | 78.0 ± 0.1 | NS |
| Remnant liver weight/body weight (%) | 1.50 ± 0.03 | 1.73 ± 0.05 | 0.0002 | 1.74 ± 0.05 | 2.02 ± 0.05 | < 0.0001 |
| Last measured body temperature (°C) | 24.4 ± 0.6 | 36.3 ± 0.2 | < 0.0001 | 25.7 ± 0.7 | 36.1 ± 0.6 | < 0.0001 |
| AST (U/L) | 1627 ± 722 | 342 ± 241 | 0.0124 | 1354 ± 514 | 51 ± 34 | < 0.0001 |
| ALT (U/L) | 1389 ± 681 | 182 ± 34 | 0.0567 | 1465 ± 497 | 22 ± 7 | 0.0252 |
| T-Bil (mg/dL) | 12.9 ± 3.0 | 3.0 ± 0.5 | 0.0041 | 23.9 ± 3.1 | 3.1 ± 0.2 | < 0.0001 |
| Hb (g/dL) | 9.5 ± 0.7 | 12.4 ± 0.5 | 0.0031 | 13.5 ± 0.8 | 12.5 ± 0.4 | NS |
| Hematocrit (%) | 28.0 ± 2.1 | 36.5 ± 1.4 | 0.0028 | 39.7 ± 2.3 | 36.7 ± 1.4 | NS |
| Glucose (mg/dL) | 133.9 ± 18.9 | 136.4 ± 5.1 | NS | 92.9 ± 11.7 | 128.9 ± 5.6 | 0.0117 |
| PT- INR | 0.9 ± 0.0 | 0.9 ± 0.0 | NS | 1.37 ± 0.24 | 1.84 ± 0.17 | NS |
At the time of surgery there was no difference in BT between the two groups. However, at 24 and 48 h, we found that some mice were HT compared with others that were clearly NT. The mice that were HT at 24 h were sacrificed, as well as some of the NT mice. Similarly, at 48 h, the mice were divided into groups according to BT. The mean BT of HT mice was significantly lower than that of NT mice (24.7 ± 0.8 °C vs 36.4 ± 0.2 °C, P < 0.0001) at 24 h and at 48 h (24.9 ± 1.1 °C vs 36.2 ± 0.4 °C, P < 0.0001).
Perioperatively, there was no difference in the amount of liver resected (Table 1), but at the time of sacrifice, the LR weight was significantly less in HT mice compared with that in NT mice (24 h, 0.36 ± 0.00 mg vs 0.44 ± 0.07 mg, P = 0.0100; and 48 h, 0.45 ± 0.04 mg vs 0.51 ± 0.09 mg, P = 0.0270). Similarly, the liver remnant weight/body weight ratio was significantly lower in HT mice than that in NT mice (24 h, 1.50 ± 0.03 vs 1.73 ± 0.05, P = 0.0002; and 48 h, 1.74 ± 0.05 vs 2.02 ± 0.05, P < 0.0001). These results indicate that liver growth was decreased in HT mice.
Paradoxically, after 75% PH, some differences in behavior between survivors and mice that finally died were observed from the early postoperative period. To determine the most important factor, multivariate analyses at each time point were performed for the significant factors in univariate analysis (Table 1). BT had a significant effect at each time point on murine symptoms after 75% PH and the LR weight/body weight also had a significant effect at 48 h after 75% PH (Table 2). In this preliminary study, we speculated that BT is a critical factor for the postoperative course and/or survival and may be a reliable predictor from the early postoperative period.
| 24 h after 75% PH (n = 152) | P value |
| Remnant liver weight/body weight | 0.0829 |
| Body temperature (°C) | 0.0372 |
| AST (U/L) | 0.1263 |
| ALT (U/L) | 0.3826 |
| T-Bil (mg/dL) | 0.8753 |
| Hb (g/dL) | 0.6974 |
| Hematocrit (%) | 0.7365 |
| 48 h after 75% PH (n = 108) | P value |
| Remnant liver weight/body weight | 0.0012 |
| Body temperature (°C) | 0.0161 |
| AST (U/L) | 0.9182 |
| ALT (U/L) | 0.8827 |
| T-Bil (mg/dL) | 0.7322 |
| Glucose (mg/dL) | 0.9913 |
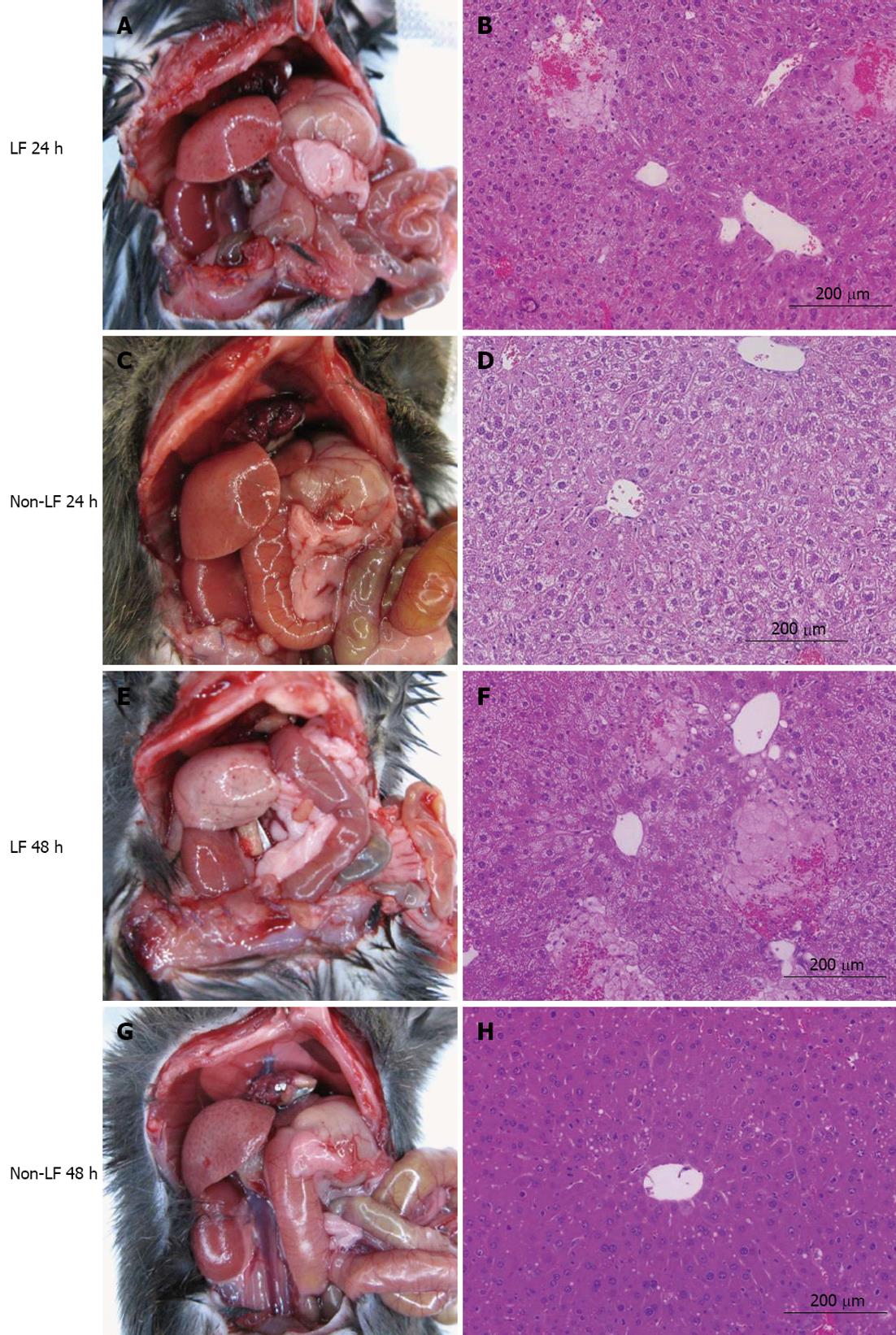
On gross inspection at 24 h, the LR of HT mice appeared relatively normal (Figure 2A) but it was pale and severely abnormal at 48 h (Figure 2E). There was no intraperitoneal bleeding. Histologically, there was a significant number of hemorrhagic nodules throughout the liver parenchyma at 24 h. Significant microvesicular steatosis was observed at 24 h (Figure 2B) and it was worse at 48 h (Figure 2F). These findings are consistent with previous reports of LF in insufficient LRs[12,19,21]. In contrast, in NT mice, the LR appeared normal at both 24 and 48 h (Figure 2C and G). Although there was significant microvesicular steatosis in livers of NT mice at 24 h (Figure 2D), it had largely resolved by 48 h (Figure 2H). The parenchymal architecture was essentially preserved in NT mice, as reported elsewhere[12,19,21].
HT mice had markedly elevated T-Bil levels at 24 h (12.9 ± 3.0 mg/dL vs 3.0 ± 0.5 mg/dL, P = 0.0008) and at 48 h (23.9 ± 3.1 mg/dL vs 3.1 ± 0.2 mg/dL, P < 0.0001) compared with those in NT mice. Serum AST levels were significantly higher in HT mice at 24 h (1627 ± 722 U/L vs 342 ± 241 U/L, P = 0.0124) and 48 h (1354 ± 514 U/L vs 51 ± 34 U/L, P < 0.0001) compared with those in NT mice. ALT levels were similar in both groups at 24 h but became significantly elevated at 48 h in HT mice compared with those in NT mice (1465 ± 497 U/L vs 22 ± 7 U/L, P = 0.0016). Hemoglobin (Hb) and hematocrit were significantly lower at 24 h in HT mice compared with those in NT mice (P < 0.0001). The anemia in HT mice was probably related to intrahepatic hemorrhage, consistent with a previous report[22]. At 48 h, Hb was the same in both groups (Table 1).
Serum glucose levels were similar in HT and NT mice at 24 h. However, serum glucose levels in HT mice were significantly lower than those in NT mice at 48 h (92.9 ± 11.7 mg/dL vs 128.9 ± 5.6 mg/dL, P = 0.0117), which is consistent with early hepatic insufficiency[18]. The PT-INR was measured in a separate study. There was no significant difference in the PT-INR between HT and NT mice at either 24 or 48 h. However, HT mice had a trend for an increased PT-INR at 48 h (1.84 ± 0.17 vs 1.37 ± 0.24, P = 0.06) (Table 1).
These results collectively showed that at 24 and 48 h, HT mice had gross, histological and biochemical evidence consistent with early stages of LF after 75% PH. In contrast, NT mice showed preserved liver function.
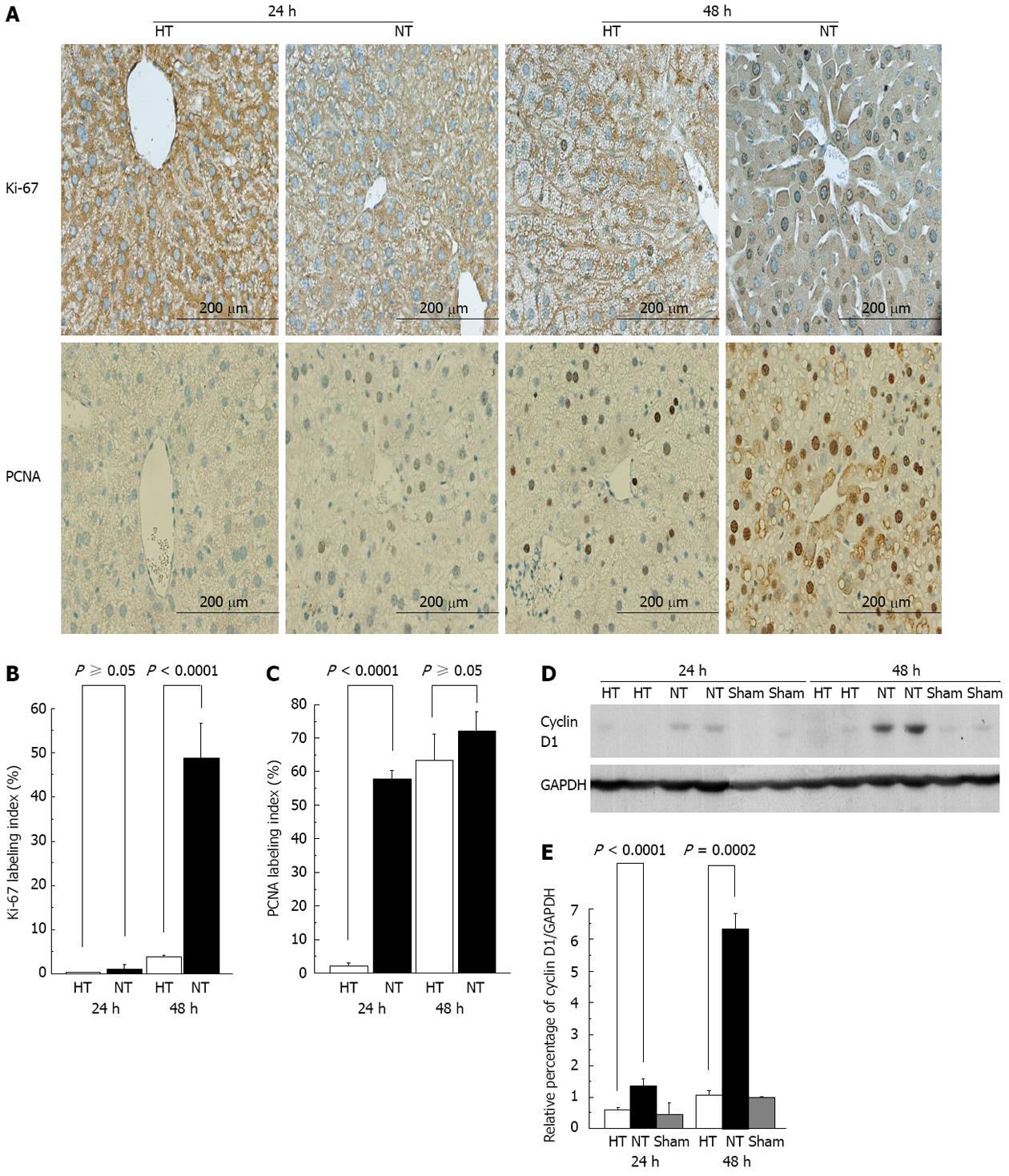
In mice, DNA synthesis starts at approximately 24 h and peaks at approximately 36 to 48 h after standard 2/3 PH[23]. The proliferative capability was assessed using Ki-67 and PCNA[18,24,25] (Figure 3A). Ki-67 staining showed minimal Ki-67 activity in HT and NT mice at 24 h. HT mice continued to have a minimal Ki-67 index at 48 h. In contrast, NT mice showed significantly more Ki-67 immunostaining at 48 h compared with HT mice (P < 0.0001) (Figure 3B). Using PCNA, HT mice showed no proliferative activity at 24 h but proliferative activity was observed at 48 h. There were significant differences between HT and NT mice at 24 h (P < 0.0001) (Figure 3C). In contrast, NT mice had elevated proliferative activity at 24 and 48 h compared with HT mice, indicating normal progression and proliferation of hepatocytes. The initial lack of proliferative activity in HT mice is consistent with LF[18,19,26].
Hepatocytes progress through the cell cycle to proliferate. Cyclin D1 is a marker for when hepatocytes enter the G1 phase and continue to proliferate[27,28]. In NT mice, we observed a significant increase in cyclin D1 expression at 24 h (P < 0.0001) and an even more pronounced increase in cyclin D1 at 48 h (P = 0.0002) compared with HT mice (Figure 3E). These results suggested that proliferation was normal in NT mice. In contrast, HT mice had no detectable cyclin D1 at 24 h and minimal activity at 48 h (Figure 3D). These results are consistent with LF in HT mice[26,29].
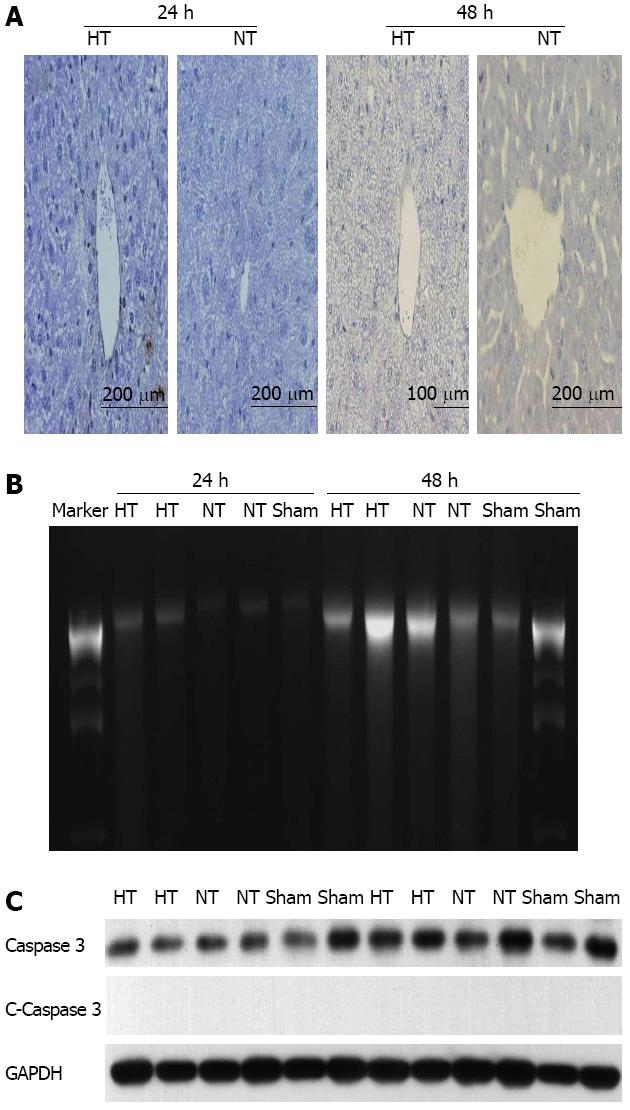
Apoptosis is prevalent in LF after extensive PH[30,31]. However, necrosis is a major mechanism of liver failure in certain models of PH[12,19,21,32]. To determine the contribution of apoptosis, we performed TUNEL, DNA laddering and caspase-3 assays. The TUNEL assay showed no differences between HT and NT mice at 24 and 48 h (Figure 4A). No DNA laddering patterns were detected at each time point (Figure 4B). No cleaved caspase-3 expression was found (Figure 4C).
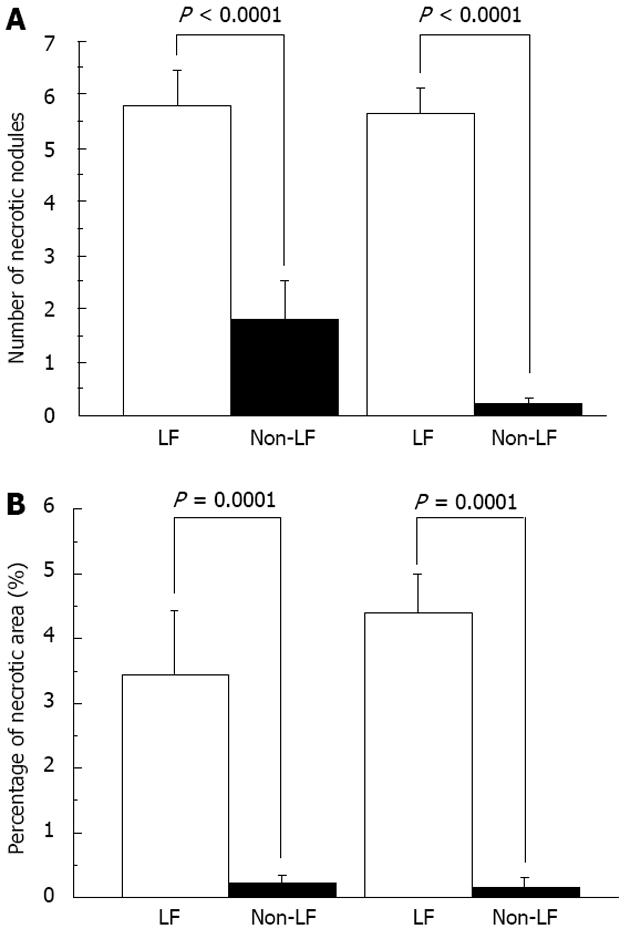
There was a large amount of hemorrhagic/necrotic nodules and their total area was large throughout the liver in HT mice at 24 and 48 h. In contrast, minimal or no hemorrhage and necrosis were found in NT mice (Figure 5). These results suggested that necrosis but not apoptosis was the main pathway for cell death in LR, consistent with previous reports[12,19,21,32].
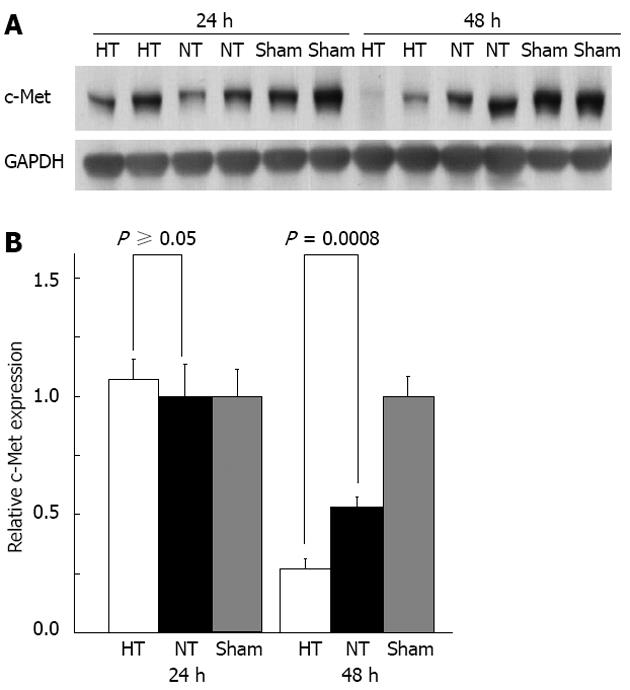
By Western blot, c-Met expression was the same in both study groups at 24 h but was significantly lower in HT mice compared with that in NT mice at 48 h (P = 0.0008) (Figure 6). By immunohistochemistry, there was no significant difference in c-Met expression per cell between the groups, suggesting that the decrease in c-Met expression in HT mice was due to a loss of hepatocytes in the hemorrhagic and necrotic nodules.
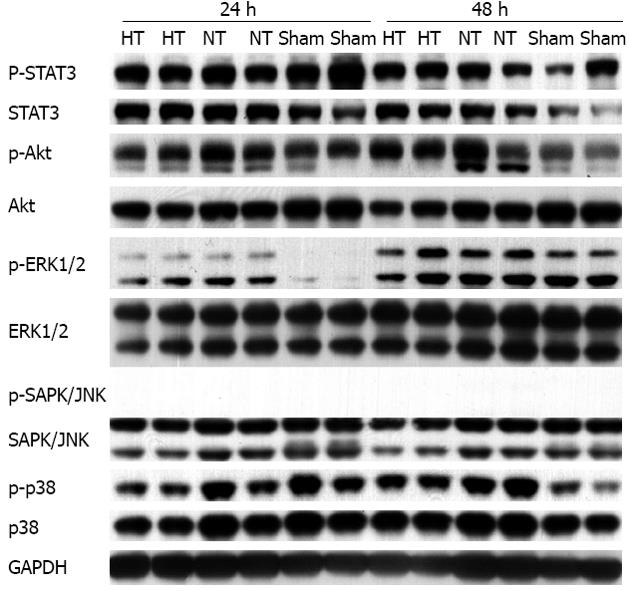
We found no significant differences in phosphorylated levels of STAT3, Akt and MAP kinases, including ERK1/2, SAPK/JNK and p38 MAPK, between HT and NT mice at 24 and 48 h, although the levels were higher than baseline levels found in the sham animals (Figure 7). These results are consistent with a previous report demonstrating that Akt and STAT3 are elevated in LR[19]. However, an increase in signal transduction in an insufficient LR might not be adequate for liver regeneration, as previously reported[33].
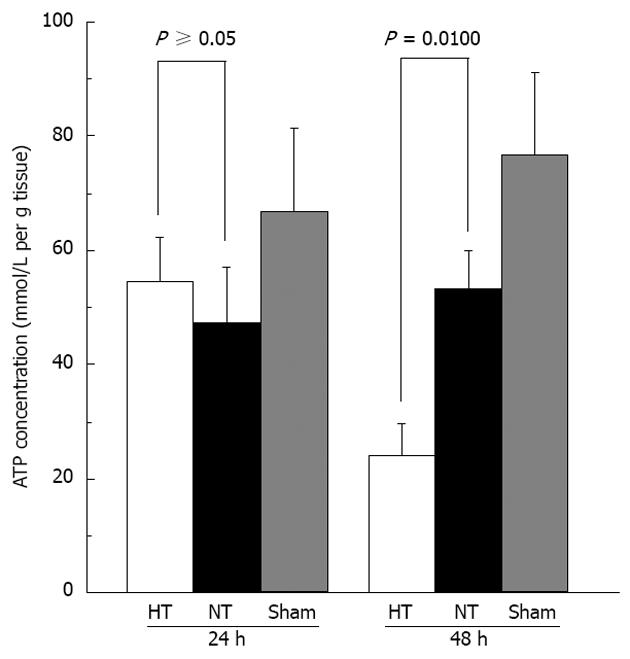
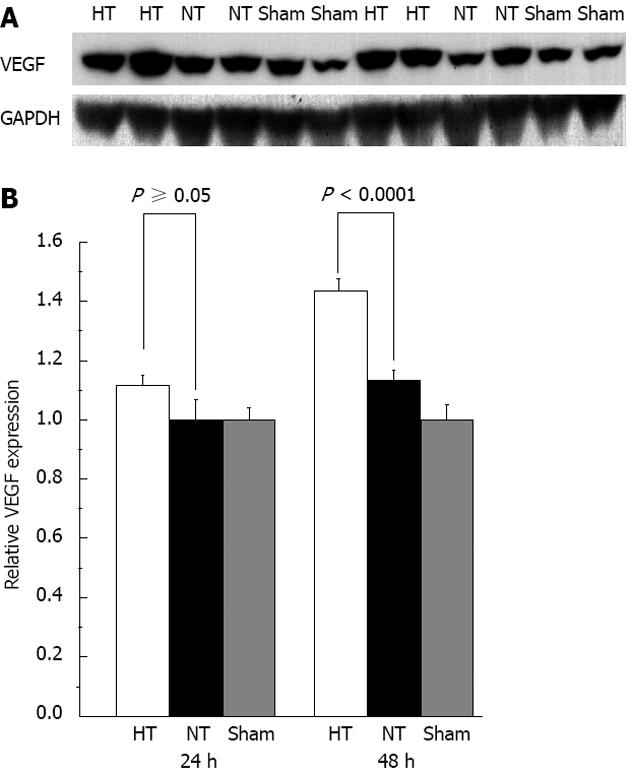
We also found that ATP concentrations were significantly lower at 48 h in HT mice compared with those in NT mice (P = 0.0100)[19,29] but we did not observe any difference in ATP levels at 24 h (P = 0.0758) (Figure 8). We did not find a difference in cytochrome c among the groups. We also did not observe a difference in VEGF levels between the groups at 24 h but HT mice had significantly higher VEGF levels than those in NT mice at 48 h (P < 0.0001) (Figure 9). We also found no differences in HGF in the sera of the study mice.
The clinical indicators for early detection of LF in an insufficient LR after EH are unknown. Most current studies on the mechanisms of LF have focused only on surviving animals because there have been no reliable indicators of the onset of LF in an insufficient LR[19].
In this study, we prospectively demonstrated that hypothermia is directly correlated with biochemical, histological and molecular factors of LR after 75% PH in mice. Consistent with previous studies[12-14], HT mice in this study had ongoing LF. Their LRs appeared abnormal, showed hemorrhage and necrosis, had a severely disrupted liver function profile, and demonstrated markedly suppressed proliferative indices and cyclin D1. The lack of cyclin D1 in HT mice is consistent with the absence of liver regeneration and inadequate liver regeneration results in high mortality rates[29]. In addition, in our model, LF was due to hepatocellular necrosis rather than apoptosis[19,21].
Two-thirds PH (2/3 PH) in rodents is the standard model for investigating LF and hepatic regeneration[34,35]. With 2/3 PH, all animals survive. A > 70% PH is routinely performed as a model of LF. Longo et al[18] showed an increased mortality after 24 h with ≥ 75% PH in mice. Emond et al[12] used ≥ 90% PH in rats, resulting in 90% mortality. The rats that died after 90% PH failed to achieve a BT above 34 °C. Moreover, maintaining normothermia by external warming does not improve survival[12]. Similarly, Eguchi et al[13] found suppressed LR growth and an altered biochemical liver profile in 92% PH rats and those rats had hypothermia of 29 °C at 30 h. Moreover, in those animals, maintaining normothermia by external warming led to increased mortality[13]. These data are consistent with our results, which collectively support the hypothesis that hypothermia is predictive of LF after EH.
In a retrospective analysis, a prothrombin time < 50% and T-Bil level > 50 μmol/L at day 5 after hepatectomy predicted a mortality rate of over 50%[36]. Similarly, a low platelet count (< 100000/μL), PT-INR >2.0 and T-Bil level > 6.6 mg/dL at 48 h were correlated with LF and death unless a liver transplant was performed[37]. Therefore, LF is lethal and usually has reached an advanced stage when it becomes clinically and biochemically evident. Moreover, these retrospectively derived prognostic factors may not be applicable universally and remain to be validated[7].
Our findings of hemorrhagic and necrotic nodules in the liver of HT mice suggest mechanisms of injury and potential therapy similar to those previously reported[12,18,19,21,32]. For example, specific blockade of tumor necrosis factor prevents LF after EH[32]. Interleukin-6 (IL-6) might protect against oxidative injury and necrosis by promoting STAT3 and Akt pathways[19]. Mitochondrial injury and dysfunction with increased free radical production could be a focus of therapy[21,29]. In addition, portal hyperperfusion is implicated in an insufficient LR and small-for-size graft (SFSG)[38,39]. Attenuating portal hyperperfusion has been shown to ameliorate liver dysfunction in residual extreme small LRs[40], as well as in liver transplantation with SFSGs[41-44]. However, the exact mechanisms for LR in an insufficient LR remain incompletely understood. The role of free radical scavengers, tumor necrosis factor blockage, IL-6 and portal diversion in clinical practice require further evaluation.
After 75% PH, MAPK cascades were activated above baseline levels in the sham-operated mice in this study but we did not observe any difference in the MAPK cascades, including MAPK p38, ERK1/2, JNK, Akt and STAT3, in HT and NT mice. The lack of liver regeneration in HT mice in the presence of activated MAPK cascades indicates severe hepatic dysfunction, as previously suggested[33]. These results suggest that understanding the mechanisms responsible for hemorrhage and necrosis, which might be independent of MAPKs, is essential for preventing and treating LF.
Our study is limited by a potentially confounding factor, namely, whether hypothermia was the cause rather than the result of the observed LF. It has been suggested that hypothermia impedes liver regeneration[45,46]. However, hypothermia is a well-known sequel of acute LF[10]. Mild hypothermia can ameliorate brain edema as well as liver injury in acute LF[46-48]. In our study, we monitored BT prospectively and separated the study animals by BT before the animals became moribund[19]. Although we could not completely rule out the potential contribution of hypothermia as a cause of LF after EH, the prospective characterizations of the mice in this study indicate that hypothermia has an important role as an early indicator of LF.
In conclusion, hypothermia after EH portends impending LF. Hypothermia might be a clinically useful marker for investigators to focus on the molecular pathways that are important during the early development of LF in an insufficient LR. Further study to validate the role of hypothermia as a predictor of LF in an insufficient LR is warranted.
The authors wish to acknowledge Lisa Maroski and Kathleen E Norton for their editorial assistance. Hori T paid the fee for English editing of the final draft (edanz editing, ID: J1112-40831-Hori1).
Liver failure occurs in various conditions, including acute liver failure and an insufficient liver remnant after extended hepatectomy. Without a suitable liver replacement, death imminently ensues. Hepatic failure can have lethal consequences but the mechanisms responsible for liver failure in an insufficient liver remnant are unknown.
In early studies, terminal liver failure was associated with hypothermia. Hypothermia is also common in advanced stages of acute liver failure. Similarly, earlier studies that conducted extended hepatectomy in rodent models demonstrated that the animals that failed to survive after extended hepatectomy had significant hypothermia, whereas those that survived maintained normothermia.
In the laboratory, preliminary results showed that mice which failed to survive after extended hepatectomy had significant hypothermia and the surviving animals remained normothermic. Therefore, the authors hypothesized that hypothermia predicts imminent liver failure after extended hepatectomy. In this study, the authors investigated body temperature after extended hepatectomy and evaluated the hypothermia as a predictor of liver failure after extended hepatectomy.
Hypothermia might be a clinically useful predictor for the early development of liver failure in an insufficient liver remnant after extended hepatectomy.
Here, the authors focused only on the liver surgery field. In the near future, the authors will investigate the impact of hypothermia on the liver failure in the split liver transplantation model with portal hypertension and cold ischemia/warm reperfusion injury.
There is merit in describing the differences between hypothermic and normothermic mice in terms of the pathological, biochemical and immunological findings, which are novel. The manuscript is well-written.
P- Reviewer Yong BH S- Editor Song XX L- Editor Roemmele A E- Editor Li JY
| 1. | Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [PubMed] |
| 3. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio > or = 0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [PubMed] |
| 7. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 8. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 10. | Traber P, DalCanto M, Ganger D, Blei AT. Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology. 1989;96:885-891. [PubMed] |
| 11. | Vaquero J, Belanger M, Blei AT, Butterworth RF. Lack of assessment of body temperature in mice with acetaminophen toxicity. Hepatology. 2006;44:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Emond J, Capron-Laudereau M, Meriggi F, Bernuau J, Reynes M, Houssin D. Extent of hepatectomy in the rat. Evaluation of basal conditions and effect of therapy. Eur Surg Res. 1989;21:251-259. [PubMed] |
| 13. | Eguchi S, Kamlot A, Ljubimova J, Hewitt WR, Lebow LT, Demetriou AA, Rozga J. Fulminant hepatic failure in rats: survival and effect on blood chemistry and liver regeneration. Hepatology. 1996;24:1452-1459. [PubMed] |
| 14. | Fan ST, Lo CM, Liu CL, Yong BH, Wong J. Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg. 2003;238:864-869; discussion 869-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 449] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 16. | Nikfarjam M, Malcontenti-Wilson C, Fanartzis M, Daruwalla J, Christophi C. A model of partial hepatectomy in mice. J Invest Surg. 2004;17:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hori T, Ohashi N, Chen F, Baine AM, Gardner LB, Hata T, Uemoto S, Nguyen JH. Simple and reproducible hepatectomy in the mouse using the clip technique. World J Gastroenterol. 2012;18:2767-2774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Longo CR, Patel VI, Shrikhande GV, Scali ST, Csizmadia E, Daniel S, Sun DW, Grey ST, Arvelo MB, Ferran C. A20 protects mice from lethal radical hepatectomy by promoting hepatocyte proliferation via a p21waf1-dependent mechanism. Hepatology. 2005;42:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Nguyen JH, Yamamoto S, Steers J, Sevlever D, Lin W, Shimojima N, Castanedes-Casey M, Genco P, Golde T, Richelson E. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, Lehnert M, Mason RP, Thurman RG, Lemasters JJ. Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology. 2005;129:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Miller DJ, Pichanick GG, Fiskerstrand C, Saunders SJ. Experimental liver necrosis: hepatic erythrocyte sequestration as a cause of acute anemia. Am J Dig Dis. 1977;22:1055-1059. [PubMed] |
| 24. | Assy N, Gong Y, Zhang M, Pettigrew NM, Pashniak D, Minuk GY. Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats. J Lab Clin Med. 1998;131:251-256. [PubMed] |
| 25. | Assy N, Minuk GY. Liver regeneration: methods for monitoring and their applications. J Hepatol. 1997;26:945-952. [PubMed] |
| 26. | Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, Tanaka K, Shimizu T, Matsuo K, Nagashima Y. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564-8568. [PubMed] |
| 28. | Rickheim DG, Nelsen CJ, Fassett JT, Timchenko NA, Hansen LK, Albrecht JH. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology. 2002;36:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Zhong Z, Schwabe RF, Kai Y, He L, Yang L, Bunzendahl H, Brenner DA, Lemasters JJ. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of c-Jun N-terminal kinase, cyclin D1, and defective energy supply. Transplantation. 2006;82:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Morita T, Togo S, Kubota T, Kamimukai N, Nishizuka I, Kobayashi T, Ichikawa Y, Ishikawa T, Takahashi S, Matsuo K. Mechanism of postoperative liver failure after excessive hepatectomy investigated using a cDNA microarray. J Hepatobiliary Pancreat Surg. 2002;9:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Yoshida N, Iwata H, Yamada T, Sekino T, Matsuo H, Shirahashi K, Miyahara T, Kiyama S, Takemura H. Improvement of the survival rate after rat massive hepatectomy due to the reduction of apoptosis by caspase inhibitor. J Gastroenterol Hepatol. 2007;22:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ogata T, Yamashita K, Horiuchi H, Okuda K, Todo S. A novel tumor necrosis factor-alpha suppressant, ONO-SM362, prevents liver failure and promotes liver regeneration after extensive hepatectomy. Surgery. 2008;143:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Debonera F, Wang G, Xie J, Que X, Gelman A, Leclair C, Xin D, Shaked A, Olthoff KM. Severe preservation injury induces Il-6/STAT3 activation with lack of cell cycle progression after partial liver graft transplantation. Am J Transplant. 2004;4:1964-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1205] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 35. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 36. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 37. | Otsuka Y, Duffy JP, Saab S, Farmer DG, Ghobrial RM, Hiatt JR, Busuttil RW. Postresection hepatic failure: successful treatment with liver transplantation. Liver Transpl. 2007;13:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Ueno S, Kobayashi Y, Kurita K, Tanabe G, Aikou T. Effect of prior portosystemic shunt on early hepatic hemodynamics and sinusoids following 84% hepatectomy in dogs. Res Exp Med (Berl). 1995;195:1-8. [PubMed] |
| 39. | Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986-993. [PubMed] |
| 40. | Wang H, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, Sekiguchi S, Kawagishi N, Fujimori K, Sato A. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:2014-2022; discussion 2023-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Ku Y, Fukumoto T, Nishida T, Tominaga M, Maeda I, Kitagawa T, Takao S, Shiotani M, Tseng A, Kuroda Y. Evidence that portal vein decompression improves survival of canine quarter orthotopic liver transplantation. Transplantation. 1995;59:1388-1392. [PubMed] |
| 42. | Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Asakura T, Ohkohchi N, Orii T, Koyamada N, Tsukamoto S, Sato M, Enomoto Y, Usuda M, Satomi S. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl Int. 2003;16:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Wang HS, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Asakura T, Masuoka H, Aiso T, Fukushima K, Narita T. Excessive portal flow causes graft failure in extremely small-for-size liver transplantation in pigs. World J Gastroenterol. 2005;11:6954-6959. [PubMed] |
| 45. | Munoz SJ. Hypothermia may impair hepatic regeneration in acute liver failure. Gastroenterology. 2005;128:1143-1144; author reply 1144-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Vaquero J, Rose C, Butterworth RF. Keeping cool in acute liver failure: rationale for the use of mild hypothermia. J Hepatol. 2005;43:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Vaquero J, Bélanger M, James L, Herrero R, Desjardins P, Côté J, Blei AT, Butterworth RF. Mild hypothermia attenuates liver injury and improves survival in mice with acetaminophen toxicity. Gastroenterology. 2007;132:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |