Published online Mar 27, 2013. doi: 10.4254/wjh.v5.i3.145
Revised: December 7, 2012
Accepted: January 23, 2013
Published online: March 27, 2013
Processing time: 204 Days and 12.9 Hours
Hepatic adenoma (HA) is a rare indication for liver transplantation (LTx). So far 20 cases of LTx for HA are reported in PubMed. In rare cases HA presents as multiple hepatic adenomas or recurrent adenoma after initial liver resection and in such cases LTx is the only potential cure and prevents the risk of bleeding or cancer transformation into hepatocellular carcinoma. We report the case of a 56 years old lady who underwent a left hepatectomy for giant adenoma in 2005 and resection of segment V-VI for recurrence of liver adenoma in 2007. She developed a second recurrence of HA with 3 new lesions in the right liver in 2008. The patient underwent LTx. After 3 years the patient is alive with no evidence of disease. LTx is indicated in patients with HA in which resection is not technically feasible.
- Citation: Vennarecci G, Santoro R, Antonini M, Ceribelli C, Laurenzi A, Moroni E, Burocchi M, Lepiane P, Ettorre GM. Liver transplantation for recurrent hepatic adenoma. World J Hepatol 2013; 5(3): 145-148
- URL: https://www.wjgnet.com/1948-5182/full/v5/i3/145.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i3.145
Hepatic adenoma (HA) is a benign tumor of the liver, which is mainly related to the estrogen-based oral contraception in women. It is often diagnosed, in asymptomatic patients, as an incidental finding during radiological procedures. Symptomatic patients usually present vague abdominal pain with normal hepatic function. HA greater than 5 cm in diameter are suitable of liver resection to prevent the risks of spontaneous rupture, bleeding and malignant transformation into hepatocellular carcinoma (HCC). Liver cell adenomatosis is a different clinical entity, that can occur in 10%-24% of the patients with HA. The peculiarity of liver cell adenomatosis is the presence of multiple HA, the association with oral contraceptive use is not as high as in solitary liver cell adenomas. These lesions can recur even after a complete surgical resection. HA and liver cell adenomatosis are a rare indication for liver transplantation (LTx). So far 20 cases of LTx for HA are reported in PubMed[1-6]. Here we report a case of recurrent HA, that underwent LTx after two previous hepatic resections.
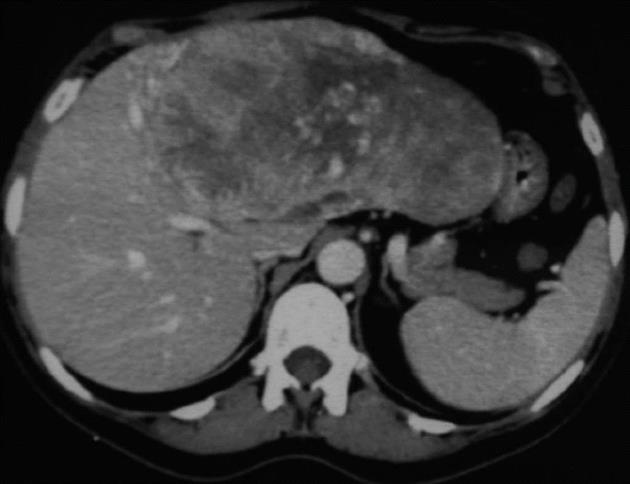
A 56 years old lady presented in 2005 with a huge palpable mass in the upper abdomen. A computed tomography (CT) scan showed a 20 cm mass occupying the all left hemi-liver, this was compatible with a giant HCC, the right lobe was normal without signs of dysmorphism (Figure 1). Her past medical history was relevant for arterial hypertension, meningioma surgically removed in 2001 and Danazol assumption for 1 year. Laboratory tests did not show hepatitis C virus and hepatitis B virus infection, but a slight elevation of gamma-glutamyltransferase. Alpha-fetoprotein was normal. There was no evidence of alcohol or drug abuse. She underwent a left hepatectomy and the pathological examination revealed a giant adenoma.
During follow-up, 18 mo later, an ultrasound of the liver showed a small nodule 3 cm in diameter located in the sixth segment and a CT scan confirmed this nodule as a recurrent adenoma. The nodule was followed-up but imaging radiology showed an increase of its dimension. Therefore it was decided to remove it by an atypical resection of segment V and VI. Pathological examination revealed again a liver adenoma.
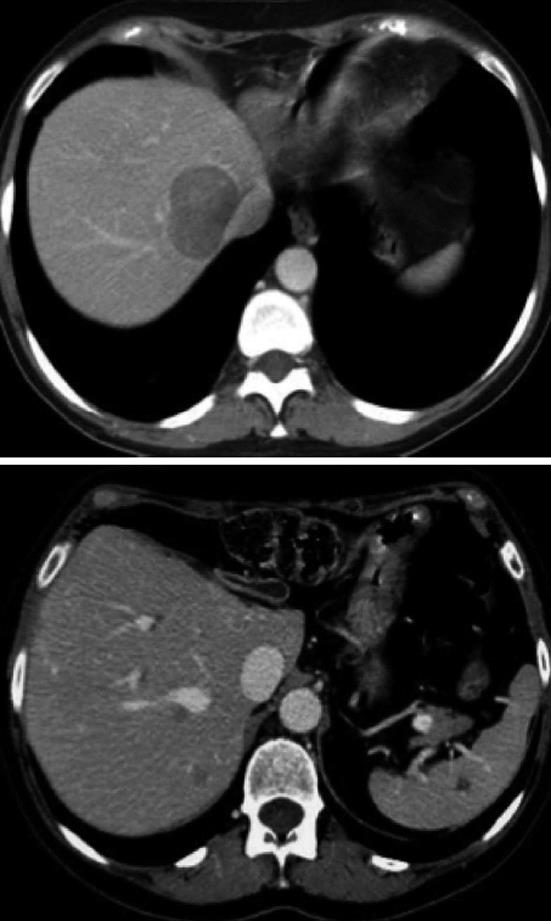
The patient underwent close postoperative follow-up. Nine months later, 3 new lesions were discovered in the liver: the largest, measuring 5-6 cm in diameter, was located close to the inferior vena cava and the right hepatic vein; the other two lesions of 2.5 cm and 1.8 cm in diameter were deeply located in the right lobe of liver (Figure 2). Considering this second recurrence after two hepatic resections and the size and location of nodules a surgical resection and less invasive treatment such as radio frequency ablation (RFA) and transarterial embolization (TAE) were judged not feasible and the option of LTx was recommended. After a deep medical examination the patient was listed. One month later a CT scan of the liver showed an increase in size of the three lesions. Two months later she underwent a LTx. The donor was 16-year old boy died in a car accident. The transplant procedure took 5 h and was performed with the so called “piggy-back” technique and caval flow preservation without veno-venous by-pass, cold ischaemic time was 5 h and 45 min. During the procedure, several technical problems were met and they were related to the two previous hepatic resections: division of adhesions, mobilization of the right lobe adherent to the right diaphragm, exposure of the supra hepatic vein and dissection of the hepatic pedicle with left branch of hepatic artery and portal vein absent. Immunosuppression was based on a triple association of steroids, prograf and mycophenolic acid. The postoperative course was uneventful and the patient was discharged after 10 d. Pathological examination of the explanted right lobe showed an healthy liver with 3 HA measuring 6, 3.5 and 2.2 cm respectively. Three years after LTx the patient is carrying on a normal life.
HA is a rare benign epithelial monoclonal tumor of the liver with an incidence of 3/1000.000 per year in Europe and North America[1]. It is frequently seen in clinical practice due to diffuse use of imaging radiology performed for unrelated reasons and consequently it is often discovered incidentally[1]. Its natural history has been better defined recently. HA are more frequent in women and are usually solitary lesions. Oral contraception is a classical cause of HA in women while the metabolic syndrome appears as an emerging condition associated with malignant transformation of HA in men[7,8]. The risk of bleeding varies from 21% to 50% and the risk of malignancy is around 8%-10%[9,10]. It seems that the risk of complication is unrelated to the number of HA but associated with a size > 5 cm particularly in telangiectatic and unclassified subtypes[8]. HA have been recently classified into four different groups due to the expression of three gene mutations: hepatocyte nuclear factor 1-alpha (35%-50%), β-catenin (15%-18%), serum amyloid A and C reactive protein (40%-55%) and unclassified type (< 10%)[11,12]. HA with aberrant nuclear β-catenin expression have higher risk of HCC development[13]. The risk of cancer degeneration is superior in men (47%) and the prevalence of malignancy is ten times more frequent in men than in women[9] and in lesions larger than 4-5 cm.
Although several strategies are available for HA management, there is still a debate on the best treatment. Clinical observation is indicated in small adenomas less than 4 cm in diameter and for lesions that show a regression after stopping oral contraceptives (OC). Surgical resection should be taken into consideration in cases in which the risk of complication (bleeding and malignant transformation) is increased, in female patients with a wish for pregnancy with HA ≥ 5 cm, in post-menopausal women with HA > 5 cm after 6 mo of stop OC and in males regardless of HA size due to the high risk of β-catenin mutation[9,14]. It is more controversial the management of patients with multiple HA. Surveillance, hepatic resection or non-surgical treatments such as RFA and TAE are all valid options[15,16].
After resection a close monitoring is mandatory even if HA are benign tumors. HA have the tendency to recur (8%) as was highlighted even by our case[8]. The peculiar aspect of our case is that despite a complete resection of the nodule the patient had two local recurrences over a period of three years and at the time of the second recurrence the lesions were ill located and the patient was no more suitable for hepatic resection. LT appeared to be the best treatment of choice.
In such situation of recurrent HA the management is initially non-operative and based on strict radiological follow-up. Hepatic liver resection is again indicated when HA reaches 5 cm with an increased risk for complications. In patients who are not eligible for surgical resection or RFA and TAE, liver transplantation may be a valid option in highly selected cases.
Hepatic resection for HA should be always attempted when technically feasible. The postoperative results are excellent even in cases of extended liver resections. Depending on center experience, the morbidity associated to liver resections for benign tumors ranges from 10% to 25% and mortality from 0% to 3%[17]. In such situations surgery is often curative, avoids the risk of a liver transplant procedure, delays the exposition to immunosuppressor. Moreover the pathological examination excludes the possibility of malignant transformation, that in case of giant adenoma is often not possible through radiology or core needle biopsy.
Indications for LTx in patients with HA are very limited to cases of HA (giant, multiple or recurrent and progressive adenoma) in which hepatic resection is not technically feasible. In PubMed literature only 20 cases of LTx for HA are reported[1-6]. However up to 2008, United Network for Organ Sharing and European Liver Transplant Registry count 103 cases of LTx for HA which represent about 6%-11% all LTx performed for benign liver conditions and 0.085%-0.094% of all indications[18]. Literature highlights the risk of HCC development in the transplanted liver or lung metastasis[1]. This review of HA may rise some concerns particularly with regard to patient’s follow-up and management of immunosuppression after transplantation.
LTx for recurrent HA is a technically demanding procedure as showed by our case and shows some different technical aspects if compared to a LTx performed for chronic liver disease. First, surgeons must take into consideration that in such situation a previous hepatectomy is responsible for the presence of adhesions that need to be divided before reaching the liver and the hepatic pedicle. Furthermore, after a previous major hepatic resection at least one of major hepatic veins is missing and the hepatic pedicle at bifurcation misses one major branch of portal vein and hepatic artery. These anatomical changes may render the liver implantation more difficult. On the contrary, the absence of portal hypertension could be an advantage when the adhesions are divided since they do not show an intrinsic vascular pattern.
HA and liver cell adenomatosis are benign tumors of the liver and they are often diagnosed incidentally. Nevertheless these clinical conditions can be complicated by bleeding and a malignant transformation can occur in about 10% of the cases with a prevalence in men with an associated metabolic syndrome. Liver resection is considered to be the treatment of choice in case of single lesions that can be completely resected. In case of recurrence liver transplantation represents a definitive and curative treatment but it should be taken into consideration as the last therapeutic option in patients in whom hepatic resection is indicated but not technically feasible. Moreover, the donor grafts shortage and the high risk of morbidity and mortality related to liver transplantation, let this treatment be not a standard option but an extraordinary choice in a few particular cases.
P- Reviewers Detry O, Herrero JI, Schemmer P S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford). 2005;7:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Di Sandro S, Slim AO, Lauterio A, Giacomoni A, Mangoni I, Aseni P, Pirotta V, Aldumour A, Mihaylov P, De Carlis L. Liver adenomatosis: a rare indication for living donor liver transplantation. Transplant Proc. 2009;41:1375-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tepetes K, Selby R, Webb M, Madariaga JR, Iwatsuki S, Starzl TE. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Yunta PJ, Moya A, San-Juan F, López-Andújar R, De Juan M, Orbis F, Mir J. A new case of hepatic adenomatosis treated with orthotopic liver transplantation. Ann Chir. 2001;126:672-674. [PubMed] |
| 5. | Santambrogio R, Marconi AM, Ceretti AP, Costa M, Rossi G, Opocher E. Liver transplantation for spontaneous intrapartum rupture of a hepatic adenoma. Obstet Gynecol. 2009;113:508-510. [PubMed] |
| 6. | Carreiro G, Villela-Nogueira CA, Coelho Hu, Basto S, Pannain VL, Caroli-Bottino A, Ribeiro Filho J. Orthotopic liver transplantation in glucose-6-phosphatase deficiency--Von Gierke disease--with multiple hepatic adenomas and concomitant focal nodular hyperplasia. J Pediatr Endocrinol Metab. 2007;20:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, Osio C, Vertemati M, Forti D. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Cho SW, Marsh JW, Steel J, Holloway SE, Heckman JT, Ochoa ER, Geller DA, Gamblin TC. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol. 2008;15:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 11. | Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg. 2010;27:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 14. | van Aalten SM, Terkivatan T, de Man RA, van der Windt DJ, Kok NF, Dwarkasing R, Ijzermans JN. Diagnosis and treatment of hepatocellular adenoma in the Netherlands: similarities and differences. Dig Surg. 2010;27:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Atwell TD, Brandhagen DJ, Charboneau JW, Nagorney DM, Callstrom MR, Farrell MA. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol. 2005;184:828-831. [PubMed] |
| 16. | Erdogan D, van Delden OM, Busch OR, Gouma DJ, van Gulik TM. Selective transcatheter arterial embolization for treatment of bleeding complications or reduction of tumor mass of hepatocellular adenomas. Cardiovasc Intervent Radiol. 2007;30:1252-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Belghiti J, Pateron D, Panis Y, Vilgrain V, Fléjou JF, Benhamou JP, Fékété F. Resection of presumed benign liver tumours. Br J Surg. 1993;80:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ercolani G, Grazi GL, Pinna AD. Liver transplantation for benign hepatic tumors: a systematic review. Dig Surg. 2010;27:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |