Published online Dec 27, 2013. doi: 10.4254/wjh.v5.i12.696
Revised: November 5, 2013
Accepted: November 18, 2013
Published online: December 27, 2013
Processing time: 114 Days and 22 Hours
A 68-year-old Caucasian man with hepatitis C virus-related cirrhosis was admitted to our Unit in February 2010 for a diagnostic evaluation of three centimetric hypoechoic focal liver lesions detected by regular surveillance ultrasound. The subsequent computer tomography (CT) led to a diagnosis of unifocal hepatocellular carcinoma (HCC) in VI hepatic segment, defined the other two nodules in the VI and VII segment as suspected metastases, and showed a luminal narrowing with marked segmental circumferential thickening of the hepatic flexure of the colon. Colonoscopy detected an ulcerated, bleeding and stricturing lesion at the hepatic flexure, which was subsequently defined as adenocarcinoma with a moderate degree of differentiation at histological examination. Finally, ultrasound-guided liver biopsy of the three focal liver lesions confirmed the diagnosis of HCC for the nodule in the VI segment, and characterized the other two lesions as metastases from colorectal cancer. The patient underwent laparotomic right hemicolectomy with removal of thirty-nine regional lymph nodes (three of them tested positive for metastasis at histological examination), and simultaneous laparotomic radio-frequency ablation of both nodule of HCC and metastases. The option of adjuvant chemotherapy was excluded because of the post-surgical onset of ascites. Abdomen CT and positron emission tomography/CT scans performed after 1, 6 and 12 mo highlighted a complete response to treatments without any radiotracer accumulation. After 18 mo, the patient died due to progressive liver failure. Our experience emphasizes the potential coexistence of two different neoplasms in a cirrhotic liver and the complexity in the proper diagnosis and management of the two tumours.
Core tip: A 68-year-old man with hepatitis C virus-related cirrhosis was admitted to our Unit for a diagnostic evaluation of three focal liver lesions detected by regular surveillance ultrasound. Computer tomography scans of abdomen allowed a diagnosis of single nodule hepatocellular carcinoma (HCC) and showed two centimetric liver nodules suspected for metastases and a luminal narrowing with thickening of the colon. The subsequent colonoscopy and ultrasound-guided biopsy of the three focal liver lesions confirmed a diagnosis of colorectal cancer with liver metastases together with a single nodule HCC. Our experience highlights the potential coexistence of two different neoplasms in a cirrhotic liver and the complexity in the proper diagnosis and management of the two tumours.
- Citation: Maida M, Macaluso FS, Galia M, Cabibbo G. Hepatocellular carcinoma and synchronous liver metastases from colorectal cancer in cirrhosis: A case report. World J Hepatol 2013; 5(12): 696-700
- URL: https://www.wjgnet.com/1948-5182/full/v5/i12/696.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i12.696
The incidence of hepatocellular carcinoma (HCC) is currently increasing worldwide, being the leading cause of death in patients with cirrhosis[1,2]. Despite intensive surveillance programs, considerable recent therapeutic advances and the use of potentially radical treatments, prognosis and life expectancy remain poor in this setting[3]. Curative treatments are applicable for early stage tumors only, and include resection, liver transplantation and percutaneous ablation, whereas transarterial chemoembolization and sorafenib are regarded as non-curative treatments able to improve survival in intermediate and advanced stages, respectively[4].
The burden of colorectal cancer (CRC) is huge, since it is the third most common diagnosed cancer in men and the second in women and it is the third leading cause of cancer death in the United States[5]. The liver is the most frequent metastatic site for patients with CRC: up to 50% of patients develop hepatic metastases during the course of the disease[6]. However, few data can be retrieved from the literature about the incidence and management of liver metastases from CRC in patients with cirrhosis.
We describe the coexistence of HCC and liver metastases from CRC in a patient with hepatitis C virus (HCV)-related cirrhosis.
A 68-year-old Caucasian man with HCV-related cirrhosis was admitted to our Unit in February 2010 for a diagnostic evaluation of three centimetric hypoechoic focal liver lesions in VI and VII segment, detected by surveillance ultrasound. His medical history included type 2 diabetes mellitus requiring insulin treatment and chronic obstructive pulmonary disease diagnosed few years before.
Upon admission the patient was asymptomatic and there was no evidence of hepatic encephalopathy, ascites or peripheral edema. Physical examination was unremarkable, except for hepatosplenomegaly and palmar erythema. Laboratory tests showed microcytic anemia (haemoglobin 9.1 g/dL, haematocrit 30%, mean corpuscular volume 70 fL) and mild hypoalbuminemia (3.2 g/dL), while all other liver function tests were normal. The Child-Pugh score was A6 showing a good residual liver function.
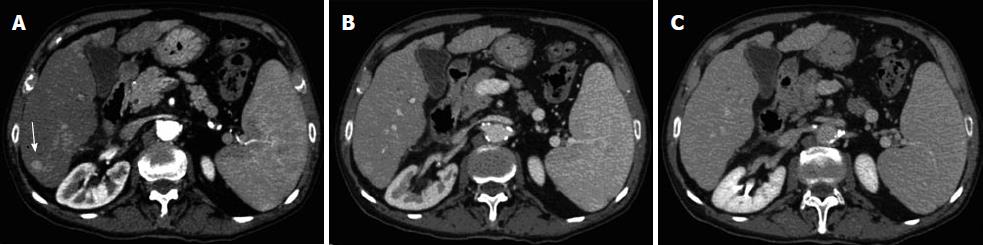
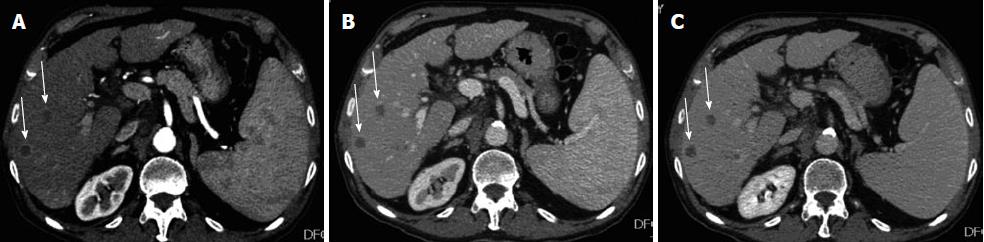
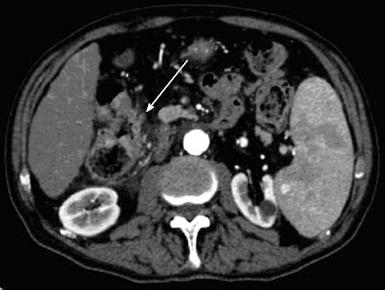
Esophagogastroduodenoscopy detected the presence of medium-size (F2) oesophageal varices and portal hypertensive gastropathy without signs of active bleeding. The subsequent dynamic computer tomography (CT) led to a diagnosis of unifocal HCC in VI hepatic segment (Figure 1), defined the other two nodules in VI and VII segment as suspected metastases (Figure 2), and showed a luminal narrowing with marked segmental circumferential thickening of the hepatic flexure of the colon (Figure 3). Colonoscopy revealed an ulcerated, bleeding and stricturing lesion at the hepatic flexure, which was defined as adenocarcinoma with a moderate degree of differentiation at histological examination. The levels of carcinoembryonic antigen (CEA) and α-fetoprotein were 21.5 ng/mL (NV < 5) and 3.2 ng/mL (NV < 5), respectively.
Ultrasound-guided liver biopsy of the three focal liver lesions confirmed the diagnosis of HCC (grading G2 according to Edmondson-Steiner System) for the nodule in the VI segment and characterized the other two lesions as metastases from CRC. Finally, CT scans of the chest did not show metastasis. On one hand, considering the good performance status (PS 0), the preserved liver function (Child-Pugh score A6), and the tumour stage (single nodule < 3 cm, absence of vascular invasion), our patient could be allocated in stage A according to Barcelona Clinical Liver Cancer staging system; on the other hand, tumour stage of colon cancer was IVa (T3, Nx, M1) according to Tumor-Node-Metastasis Classification of Malignant Tumours.
The patient underwent right hemicolectomy with removal of thirty-nine regional lymph nodes (three of them tested positive for metastasis at histological examination), and simultaneous laparotomic radio-frequency ablation (RFA) of either nodule of HCC and metastasis. No operative complications occurred. As a probable consequence of surgery, the patient developed hepatic decompensation after about 2 wk, with onset of moderate degree ascites. Consequently, the option of adjuvant chemotherapy was excluded and the patient began oral diuretic treatment with furosemide and canrenone with complete clinical response. Abdomen CT and positron emission tomography/computerized tomography (PET/CT) scans performed after 1, 6 and 12 mo showed a complete response to treatments according to mRECIST[7,8] for HCC, and RECIST 1.1[9] criteria for measurable target lesion of colon cancer, without any radiotracer accumulation. Finally, determinations of CEA were repeatedly negative.
After 18 mo, the patient died due to hepatic decompensation and progressive liver failure.
Multiple primary cancers are quite rare. Their incidence ranges from 0.73% to 11.7%[10], even if it is steadily rising as a result of the continuous improvement of treatments and the consequent increased cancer survival. Coexistence of multiple primary tumors is defined by Warren and Gates Criteria, i.e., demonstrating that (1) each tumor is distinct, (2) each tumor is clearly malignant on histological examination, and (3) one of the two tumors is not a metastasis of the other one. In addition, multiple primary tumors are classified into synchronous and metachronous according to Moertel classification[11] if they occur within or after 6 mo since the diagnosis of the first tumor, respectively.
We described a case of synchronous HCC and CRC with liver metastases. As already mentioned, the presence of multiple primary tumors is rare, and even rarer the presence of synchronous neoplasms. In addition to the rarity of the case, the complexity in distinguishing the simultaneous presence of HCC nodules and metastases in a cirrhotic liver must be emphasized. Indeed, the baseline probability of a new focal liver lesion to be a HCC in cirrhosis increases according to nodule size, ranging from 66% for nodules between 1 and 2 cm, to 80% for nodules between 2 and 3 cm in size and up to 95% for nodules larger than 3 cm[12]. Nevertheless, it is possible to find metastases from tumors aroused from extra-hepatic sites in a cirrhotic liver, although unlikely; consequently, an accurate and complete characterization of the focal liver lesions is mandatory in this setting. In our case, we reached the diagnosis through dynamic CT scans and subsequent histological confirmation.
The presence of liver cirrhosis, if the disease is compensated, does not change per se the therapeutic indications for non-hepatic tumors[13]. In view of the good residual function of the liver disease, and after screening for portal hypertension and fluid retention, our patient was surgically treated with laparotomic right colectomy, without operative complications.
The standard of care for patients with colorectal hepatic metastases (CRHM) is the systemic chemotherapy with or without biologic agents. When metastasis are confined to liver and there is no vascular invasion or regional lymph node involvement, surgical resection is the standard of care. In patients with cirrhosis, as in non surgical candidates, loco-regional therapies as RFA may be regarded as one of the best options for both curative and palliative approaches[6]. In our patient, the single nodule of HCC, as well as the two CRHM, were simultaneously treated during the laparotomic session with RFA.
Finally, adjuvant chemotherapy was excluded because of the post-surgical onset of ascites, whereas CT and PET scans performed after six and twelve months showed a complete response to treatments.
Our experience highlights the possible coexistence of two different neoplasms in a cirrhotic liver, although rare, and the complexity in the proper diagnosis and management of the two tumors. Even if dynamic CT or MR play a key role in the diagnosis of HCC, the identification of synchronous metastasis may be difficult, due to the inherent morphological characteristics of the cirrhotic liver and to the high pre-test probability of a new lesion to be a HCC. Therefore, despite the potential risk of tumour seeding in this setting, ranging from 10% to 19% according to several reports[14], liver biopsy remains a critical diagnostic tool recommended in doubtful cases and/or when hepatic metastases are suspected, in order to confirm the histological origin of the primary neoplasm and to provide the best therapeutic algorithm based on the correct diagnosis.
In absence of recommendations regarding the best combined therapeutic approach, it is desirable to carry out the best available therapy for each tumor, when feasible. In this line, RFA can be regarded as one of the best options for both curative and palliative approaches of CRHM in compensated cirrhosis[6].
Upon admission the patient was asymptomatic, and physical examination was unremarkable, except for hepatosplenomegaly and palmar erythema.
The patient presented with microcytic anemia and imaging evidence of three hepatic focal lesions on a background of liver cirrhosis.
Differential diagnosis was performed between hepatocellular carcinoma and hepatic metastases from colorectal cancer, using dynamic computer tomography (CT) and histology of the primary lesion and the three hepatic nodules.
Laboratory tests showed microcytic anemia (haemoglobin 9.1 g/dL, haematocrit 30%, mean corpuscular volume 70 fL) and mild hypoalbuminemia (3.2 g/dL); all other liver function tests were normal, whereas levels of carcinoembryonic antigen andα-fetoprotein were 21.5 ng/mL (NV < 5) and 3.2 ng/mL (NV < 5), respectively.
Dynamic CT showed a unifocal hepatocellular carcinoma in VI hepatic segment and other two nodules suspected for metastases, and it pointed out a luminal narrowing with marked thickening of the hepatic flexure of the colon. This latter was further detected by colonoscopy as an ulcerated, bleeding and stricturing lesion at the hepatic flexure.
Histological examination defined the stricturing lesion of the colon as adenocarcinoma with a moderate degree of differentiation, whereas biopsy of the three focal liver lesions confirmed a diagnosis of single nodule hepatocellular carcinoma (HCC) together with two liver metastases from colorectal cancer.
The patient underwent laparotomic right hemicolectomy with removal of thirty-nine regional lymph nodes, and simultaneous laparotomic radio-frequency ablation of both nodule of HCC and metastases.
This case report emphasizes the possible coexistence of two different neoplasms in a cirrhotic liver, although rare, and the complexity in the proper diagnosis and management of the two tumors.
Even if dynamic CT or colorectal cancer play a key role in the diagnosis of HCC, the identification of synchronous metastasis may be difficult, due to the inherent characteristics of the cirrhotic liver and to the high pre-test probability of a new lesion to be a HCC. Therefore, liver biopsy remains a critical diagnostic tool recommended in doubtful cases and it is mandatory when a liver metastasis from another primary tumour is suspected.
This manuscript is interesting and presents a careful observation and discussion regarding diagnosis and management of primary HCC and liver metastasis from colorectal cancer.
P- Reviewers: Charatcharoenwitthaya P, Nojiri S, Sugimoto K, Verhelst X S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 2. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 3. | Cabibbo G, Craxì A. Hepatocellular cancer: optimal strategies for screening and surveillance. Dig Dis. 2009;27:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] |
| 5. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 6. | Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 8. | Maida M, Cabibbo G, Brancatelli G, Genco C, Alessi N, Genova C, Romano P, Raineri M, Giarratano A, Midiri M. Assessment of treatment response in hepatocellular carcinoma: a review of the literature. Future Oncol. 2013;9:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21625] [Article Influence: 1351.6] [Reference Citation Analysis (1)] |
| 10. | Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162-167. [PubMed] |
| 11. | Moertel CG. Multiple primary malignant neoplasms: historical perspectives. Cancer. 1977;40:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 13. | Cabibbo G, Palmeri L, Palmeri S, Craxì A. Should cirrhosis change our attitude towards treating non-hepatic cancer? Liver Int. 2012;32:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Cresswell AB, Welsh FK, Rees M. A diagnostic paradigm for resectable liver lesions: to biopsy or not to biopsy? HPB (Oxford). 2009;11:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |