Published online Dec 27, 2013. doi: 10.4254/wjh.v5.i12.685
Revised: September 26, 2013
Accepted: November 15, 2013
Published online: December 27, 2013
Processing time: 130 Days and 22.3 Hours
AIM: To investigate clinical and biochemical features of hepatorenal syndrome (HRS), to assess short and long-term survival evaluating potential predictors of early mortality.
METHODS: Sixty-two patients with liver cirrhosis and renal failure, defined as a serum creatinine value > 1.5 mg/dL on at least two measurements within 48 h, admitted to our tertiary referral Unit from 2001 to 201, were retrospectively reviewed. Among them, 33 patients (53.2%) fulfilled the revised criteria of the International Ascites Club for the diagnosis of HRS. Twenty-eight patients were treated with combinations of terlipressin and albumin, two with dopamine and albumin, and three with albumin alone. No patients were suitable for liver transplantation. Complete response was defined as normalization of creatinine levels to less than 1.5 mg/dL, partial response as a decrease of at least 50% but not to less than 1.5 mg/dL, no response as no reduction in creatinine or a decrease of less 50% compared to pre-treatment values. All of the patients were followed up for at least 1 year until January 2013.
RESULTS: HRS type 1 was diagnosed in 15 patients (45.5%). Hepatitis C virus infection was the primary etiology (69.6%), followed by alcohol (15.2%), and cryptogenesis (15.2%). Complete response to therapy was obtained in only 3 cases (9.1%) and partial response in 7 patients (21.2%). Median survival was 30 d (range: 10-274) without significant differences between type 1 and type 2 HRS. By univariate analysis, Child-Pugh class C (P = 0.009), presence of hepatocellular carcinoma (P = 0.04), low serum sodium (P = 0.02), high bilirubin values (P = 0.009) and high Model for End-stage Liver Disease (MELD) score (P = 0.03) were predictive factors of 30-d mortality. By multivariate analysis, only serum sodium < 132 mEq/L (OR = 31.39; P = 0.02) and MELD score > 27 (OR = 18.72; P = 0.01) were independently associated with a survival of less than one month.
CONCLUSION: HRS still has a poor prognosis, even when vasoactive drug therapies are extensively used.
Core tip: Hepatorenal syndrome (HRS) is a life-threatening complication of advanced liver disease. The aims of this study were to investigate the clinical and biochemical features of HRS, to assess short- and long-term survival, and to evaluate the presence of potential predictors of early mortality. Thirty-three patients with liver cirrhosis and HRS were retrospectively reviewed. Median survival was 30 d. By univariate analysis Child-Pugh class C, hepatocellular carcinoma, low serum sodium, high bilirubin and high Model for End-stage Liver Disease (MELD) score were predictive factors of 30-d mortality. By multivariate analysis, only serum sodium < 132 mEq/L and MELD score > 27 were independently associated with survival of less than one month.
- Citation: Licata A, Maida M, Bonaccorso A, Macaluso FS, Cappello M, Craxì A, Almasio PL. Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J Hepatol 2013; 5(12): 685-691
- URL: https://www.wjgnet.com/1948-5182/full/v5/i12/685.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i12.685
Hepatorenal syndrome (HRS) is a life-threatening complication of advanced liver cirrhosis. It is characterized by functional renal failure, which develops as a result of portal hypertension, splanchnic vasodilatation and consequential deterioration of all systemic circulatory function[1]. Its incidence is approximately 8% per year in cirrhotic patients with ascites, but its incidence is greater in patients with advanced Child-Pugh scores[2]. The development of portal hypertension is associated with vascular arterial vasodilation in the splanchnic district, as a result of increased local production of nitric oxide and other vasodilators[3]. In the early stages of liver cirrhosis, the reduction of vascular resistance is compensated for by the development of hyperdynamic circulation, characterized by increased cardiac output and heart rate[4]. HRS usually develops during the terminal stages of liver disease, as soon as the hyperdynamic circulation is no longer able to compensate for the relative hypovolemia caused by splanchnic storage of blood[5].
Some recent studies have shown a marked reduction of cardiac output in patients with cirrhosis and HRS, in comparison to patients with cirrhosis without HRS, thus reappraising reduction in cardiac function as an important cofactor in the pathogenesis of HRS[6,7]. Several lines of evidence have finally proved that the results of systemic vasoconstriction can also cause significant hypoperfusion in other organs, such as the skin, muscles, brain and the liver itself. HRS can therefore be better considered a complex syndrome with systemic involvement[8].
There are two well-recognized types of HRS[9]. Type-1 HRS is characterized by rapid progression of renal failure, with doubling of serum creatinine values to greater than 2.5 mg/dL in less than 2 wk. It is often triggered by a precipitating event, mainly bacterial infections, and it is associated with rapid deterioration of circulatory status, with arterial hypotension and multiorgan failure. Type-1 HRS has a poor prognosis, with median survival of only 2 wk, and its main consequence is represented by hepato-renal failure and death. In contrast, type-2 HRS is characterized by gradual renal failure, with a moderate increase in serum creatinine to between 1.5 and 2.5 mg/dL. It represents the natural functional renal failure that develops in patients with End-stage Liver Disease, as a result of the natural history of portal hypertension, and it often does not recognize a specific trigger. Type-2 HRS has a better prognosis compared to type-1, with a median survival of 6 mo, and its principal consequence is represented by refractory ascites[10].
According to the European Association for the Study of the Liver (EASL) Clinical Practice Guidelines, pharmacological therapy with terlipressin plus albumin should be considered as the first-line therapy[11]. However, the prognosis remains poor even when pharmacological therapy is extensively used, and orthotopic liver transplant (OLT) remains the best treatment for HRS.
The aims of our study were to investigate the clinical and biochemical features of HRS, to assess short-term and long-term survival, and to evaluate potential predictors of early mortality.
All patients with liver cirrhosis and renal failure, defined as a serum creatinine value > 1.5 mg/dL on at least two measurements within 48 h, admitted to our tertiary referral unit from 2001 to 2012, were retrospectively reviewed. Among them, we selected those who fulfilled the revised criteria of the International Ascites Club for the diagnosis of HRS[12]. The criteria were: (1) cirrhosis with ascites; (2) serum creatinine > 1.5 mg/dL; (3) no improvement in serum creatinine (decrease to a level < 1.5 mg/dL) after two days of diuretics withdrawal and volume expansion with albumin (1 g/kg of body weight up to a maximum of 100 g/d); (4) absence of shock; (5) no current or recent treatment with nephrotoxic drugs; and (6) absence of parenchymal kidney disease, as indicated by a urinary protein concentration < 500 mg/d, a urinary red blood cell count < 50 cells per high power field, and normal renal ultrasonography. Type-1 HRS was defined by a large and rapid increase in serum creatinine to final values greater than 2.5 within 2 wk, while type-2 HRS was defined by a slower and more moderate increase in serum creatinine. The following were considered precipitating factors for type-1 HRS: spontaneous bacterial peritonitis and other infections diagnosed by standard methods; gastrointestinal bleeding; acute hepatitis superimposed on cirrhosis; and major surgical procedures.
Terlipressin was administered at a starting dose of between 0.5 and 1 mg every 4 h, increased to a maximum dose of 2 mg every 4 h if there was no reduction in serum creatinine compared to the baseline value by day 3 of therapy. Albumin was administered at an average dose of 40 g/d.
All of the patients were followed up for at least 1 year until January 2013. No patients were suitable for liver transplantation. Complete response was defined as normalization of creatinine levels to less than 1.5 mg/dL, partial response as a decrease of at least 50% but not to less than 1.5 mg/dL, and no response as no reduction in creatinine or a decrease of less 50% compared to pre-treatment values.
The results are reported as frequencies, medians or means and standard deviations. Student’s t-test, the Mann-Whitney and the (χ2 test were used to compare continuous or categorical variables. Multivariate analysis, including all of the significant baseline variables (P < 0.05), was also performed, using binary logistic regression to identify independent predictors of outcomes. For this analysis, continuous variables were categorized using receiver operating characteristic curves. Survival analysis was conducted using the Kaplan-Meier method. The log-rank test was used to compare survival between type-1 and type-2 HRS. All of the statistical analysis was performed using SPSS software, version 20.0 for Macintosh (SPSS Inc., Chicago, IL, United States).
Among 62 patients with liver cirrhosis and acute renal failure, 33 patients (53.2%) fulfilled the revised criteria of the International Ascites Club for the diagnosis of HRS[12]. The main clinical and laboratory characteristics are summarized in Tables 1 and 2. The mean age of the patients was 65.9 years old (range: 39-83), with a comparable sex distribution. Chronic hepatitis C virus infection was the main etiology, and approximately one third of patients were affected by hepatocellular carcinoma (HCC). Type-1 HRS was diagnosed in 15 patients (45.5%).
| Age (yr), (mean ± SD) | 65.9 ± 9.6 |
| Sex | |
| Male | 17 (51.5) |
| Female | 16 (48.5) |
| Etiology | |
| HCV | 23 (69.6) |
| Alcohol | 5 (15.2) |
| Cryptogenic | 5 (15.2) |
| Child-Pugh | |
| B | 18 (54.5) |
| C | 15 (45.5) |
| Esophageal varices | |
| Absent | 4 (14.3) |
| F1 | 17 (60.7) |
| F2 | 7 (25.0) |
| Diabetes mellitus | 13 (39.4) |
| Hepatocellular carcinoma | 12 (36.4) |
| Portal Vein Thrombosis | 7 (21.2) |
| HRS | |
| Type-1 | 15 (45.5) |
| Type-2 | 18 (54.5) |
| Hemoglobin (g/dL) | 10.3 ± 2.2 |
| White blood cells (× 103/mmc) | 8.5 ± 5.7 |
| Platelets (× 103/mmc) | 104.8 ± 85.2 |
| BUN (mg/dL) | 145.17 ± 61.82 |
| Creatinine (mg/dL) | 3.29 ± 1.09 |
| Creatinine clearance | 19 (8-25.25) |
| Sodium (mEq/L) | 130.7 ± 5.5 |
| Potassium (mEq/L) | 4.94 ± 0.85 |
| Urinary sodium (mEq/L) | 6.5 (2-14) |
| Urinary potassium (mEq/L) | 17.5 (11-29) |
| Total bilirubin (mg/dL) | 3.63 (1.63-13.2) |
| INR | 1.47 (1.2-1.62) |
| MELD score | 26 (22-32) |
Twenty-eight patients (84.8%) were treated with terlipressin plus albumin, 2 patients (6.1%) with dopamine and albumin, and 3 patients (9.1%) with albumin alone. The median duration of therapy was 7 d (range: 3-14). All of the patients with partial or no response discontinued treatment within 14 d. No significant adverse events occurred during vasoactive therapy. At the end of treatment, a complete response was obtained in 3 cases (9.1%), one with type-1 and two with type-2 HRS, and partial response was obtained in 7 patients (21.2%), four with type-1 and three with type-2 HRS.
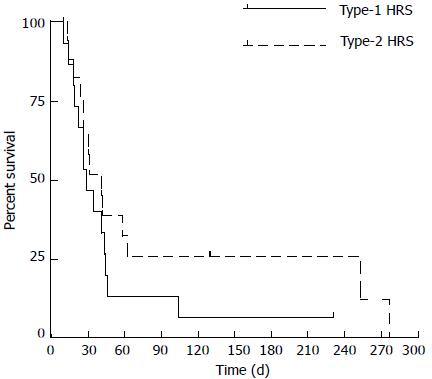
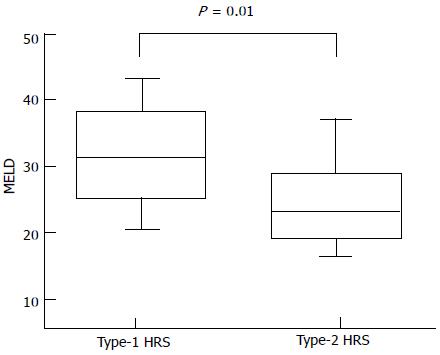
The overall median survival was 30 d (range: 10-274) (Figure 1) without significant differences between type-1 and type-2 HRS (P = 0.2 by log-rank test) (Figure 2). Between the two groups of type-1 and type-2 HRS patients, there were no statistically significant differences except for serum creatinine, creatinine clearance, international normalized ratio (INR) values and End-stage Liver Disease (MELD) score (Table 3) (Figure 3).
| Type-1 HRS(n = 15) | Type-2 HRS(n = 18) | P value | |
| Age (yr) | 63.3 ± 8.5 | 68.2 ± 10.2 | 0.150 |
| Sex | |||
| Male | 8 (53.3) | 9 (50.0) | 0.800 |
| Female | 7 (46.7) | 9 (50.0) | |
| Etiology | |||
| HCV | 9 (60.0) | 14 (77.8) | 0.500 |
| Alcohol | 3 (20.0) | 2 (11.1) | |
| Cryptogenic | 3 (20.0) | 2 (11.1) | |
| Child-Pugh | |||
| B | 8 (53.3) | 10 (55.6) | 0.900 |
| C | 7 (46.7) | 8 (44.4) | |
| Diabetes mellitus | 5 (33.3) | 8 (44.4) | 0.500 |
| Hepatocellular carcinoma | 6 (40.0) | 6 (33.3) | 0.700 |
| Portal vein thrombosis | 4 (26.7) | 3 (16.7) | 0.500 |
| Platelets (× 103/mmc) | 108.7 ± 96.6 | 101.6 ± 7711 | 0.800 |
| BUN (mg/dL) | 152 ± 73.4 | 139.5 ± 51.7 | 0.500 |
| Creatinine (mg/dL) | 3.95 ± 1.13 | 2.75 ± 0.71 | 0.001 |
| Creatinine clearance | 8 (5.2-17) | 24.5 (15-26) | 0.030 |
| Sodium (mEq/L) | 130.8 ± 4.24 | 130.7 ± 6.47 | 0.900 |
| Potassium (mEq/L) | 4.9 ± 0.96 | 4.97 ± 0.78 | 0.800 |
| Urinary sodium (mEq/L) | 11 (2.0-17.7) | 6 (2-11) | 0.700 |
| Urinary potassium (mEq/L) | 19 (7.2-38.2) | 16 (11-27.75) | 0.900 |
| Total bilirubin (mg/dL) | 6.37 (1.6-27.1) | 2.62 (1.3-13.2) | 0.200 |
| INR | 1.51 (1.3-2.2) | 1.29 (1.18-1.6) | 0.040 |
| MELD score | 31 (26-33) | 23 (20-26) | 0.010 |
Comparing the two groups of patients according to 30-d mortality, the variables associated with poorer prognosis were Child-Pugh class C (P = 0.009), presence of HCC (P = 0.04), low serum sodium (P = 0.02), high bilirubin values (P = 0.009) and high MELD score (P = 0.03) (Table 4). By multivariate analysis, only serum sodium < 132 mEq/L (OR = 31.39; P = 0.02) and MELD score > 27 (OR = 18.72; P = 0.01) were independently associated with survival of less than one month (Table 5).
| < 30-d mortality(n = 16) | ≥30-d mortality(n = 17) | P value | |
| Age (yr) | 62.9 ± 11.4 | 68.7 ± 6.6 | 0.080 |
| Sex | |||
| Male | 8 (50.0) | 9 (52.9) | 0.800 |
| Female | 8 (50.0) | 8 (47.1) | |
| Etiology | |||
| HCV | 10 (62.5) | 13 (76.5) | 0.600 |
| Alcohol | 3 (18.8) | 2 (11.8) | |
| Cryptogenic | 3 (18.8) | 2 (11.8) | |
| Child-Pugh | |||
| B | 5 (31.3) | 13 (76.5) | 0.009 |
| C | 11 (68.8) | 4 (23.5) | |
| Esophageal varices | |||
| Absent | 2 (14.3) | 2 (14.3) | 0.900 |
| F1 | 8 (57.1) | 9 (64.3) | |
| F2 | 4 (28.6) | 3 (21.4) | |
| Diabetes mellitus | 6 (37.5) | 7 (41.2) | 0.800 |
| Hepatocellular carcinoma | 3 (18.8) | 9 (52.9) | 0.040 |
| Portal vein thrombosis | 2 (12.5) | 5 (29.4) | 0.200 |
| Platelets (× 103/mmc) | 103.4 ± 87.9 | 106.1 ± 85.2 | 0.900 |
| BUN (mg/dL) | 134.8 ± 40.5 | 154.8 ± 76.8 | 0.300 |
| Creatinine (mg/dL) | 3.14 ± 0.8 | 3.44 ± 1.32 | 0.400 |
| Creatinine clearance | 26 (20-37) | 13 (8-24) | 0.050 |
| Sodium (mEq/L) | 128.5 ± 4.6 | 132.8 ± 5.5 | 0.020 |
| Potassium (mEq/L) | 4.77 ± 0.87 | 5.1 ± 0.82 | 0.200 |
| Urinary sodium (mEq/L) | 11 (7-18) | 4 (2-13) | 0.200 |
| Urinary potassium (mEq/L) | 25 (19-29) | 15 (10-27) | 0.200 |
| Total bilirubin (mg/dL) | 10.8 (3.2-21.2) | 1.8 (1.5 - 3.6) | 0.009 |
| INR | 1.6 (1.3-1.7) | 1.2 (1.2-1.5) | 0.560 |
| MELD score | 31 (26-33) | 23 (20-26) | 0.030 |
| HRS | |||
| Type-1 | 8 (50) | 7 (41.2) | 0.600 |
| Type-2 | 8 (50) | 10 (58.8) | |
| Variable | < 30-d mortalityn (%) | ≥30-d mortalityn (%) | Crude OR(95%CI) | Adjusted OR(95%CI) | P value |
| Age, (yr) | |||||
| < 65 | 7 (43.8) | 4 (23.5) | 1 | - | |
| ≥ 65 | 9 (56.2) | 13 (76.5) | 2.53 (0.57-11.26) | ||
| Child-Pugh class | |||||
| B | 5 (31.2) | 13 (76.5) | 1 | 11.98 (0.97-148.23) | 0.053 |
| C | 11 (68.8) | 4 (23.5) | 7.15 (1.53-33.37) | ||
| Sodium | |||||
| ≥ 132 mEq/L | 3 (18.8) | 10 (58.8) | 1 | ||
| < 132 mEq/L | 13 (81.2) | 7 (41.2) | 6.19 (1.27-30.17) | 31.39 (1.54-641.83) | 0.020 |
| Total bilirubin | |||||
| ≤ 3.6 mg/dL | 4 (25.0) | 12 (70.6) | 1 | ||
| > 3.6 mg/dL | 12 (75.0) | 5 (29.4) | 7.20 (1.55-33.56) | 0.69 (0.17-28.06) | 0.800 |
| Hepatocellular carcinoma | |||||
| Absent | |||||
| Present | 13 (81.2) | 8 (47.1) | 1 | ||
| 3 (18.8) | 9 (52.9) | 4.88 (1.01-23.57) | 1.37 (0.13-14.91) | 0.700 | |
| MELD score | |||||
| ≤ 27 | 4 (25.0) | 14 (82.4) | 1 | ||
| > 27 | 12 (75.0) | 3 (17.6) | 14.0 (2.60-75.41) | 18.72 (1.63-214.56) | 0.010 |
HRS is a life-threatening complication in patients with advanced liver cirrhosis, and it should always be distinguished from other causes of renal failure. It is not always easy to recognize because there are no specific clinical or laboratory parameters that clearly allow for its diagnosis, so diagnosis is mainly based on the exclusion of other causes of renal failure. Currently, the new criteria of the International Ascites Club are regarded as the gold standard for HRS diagnosis[12]. Nonetheless, a recent study by Salerno et al[13] showed that these criteria allow for a correct diagnosis in only two thirds of cases.
According to the EASL Clinical Practice Guidelines, pharmacological therapy with terlipressin plus albumin should be considered the first-line therapy[11], even if terlipressin is not available in some countries, such as in the United States, and where it is available, there is not a standardized dose, due to the absence of dose-finding studies. However, prognosis remains poor even when pharmacological therapy is extensively used, and OLT remains the best treatment for both type-1 and type-2 HRS.
In our cohort, the prevalence of HRS among patients with liver cirrhosis and renal failure was 53.2%, similar to the incidences found by Salerno et al[13] (45.8%) and Martín-Llahí et al[14] (43%). However, in contrast to above-cited studies, we found an equal prevalence of type-1 and type-2 HRS (45.5% vs 54.5%, respectively). This difference might have been due to the small sample size and to the elevated number of patients with end-stage cirrhosis who are followed in our Unit, who tend to develop mainly type-2 HRS.
Comparing type-1 and type-2 HRS patients, there were no statistically significant differences between the two groups, except for serum creatinine, creatinine clearance, INR values and MELD score. Similarly, we found no differences in survival at 30 d between the groups. A difference in favor of patients with type-2 HRS was observed over 30 d, but the number of patients with survival longer than 30 d was very low, and the difference was not significant. Interestingly, we observed a poor rate of response to vasoactive therapy, although this drug was administered at effective doses and for an adequate period of time. In particular, a response was observed in only 30.3% of cases (complete response: 9.1%; partial response: 21.2%). These results were lower compared to those observed in the literature[15-21]. In a recent study, Salerno et al[13] achieved a response in 50% of cases, with a higher percentage of complete than partial response (30% vs 20%, respectively). This finding might be secondary to the differences between the patients in our cohort and those recruited for prospective studies, particularly for randomized controlled trials. In addition, the numbers of patients with hepatocellular carcinoma and with portal vein thrombosis were not specified in Salerno and colleagues’ paper; however, patients with advanced HCC and advanced cirrhosis did not receive any treatment. In our cohort, 36.4% of patients had HCC, most of whom were in advanced stages, and 21.2% had a portal vein thrombosis, none of whom were suitable for liver transplantation. Consequently, we likely obtained a worse response to therapy because of the worse pre-treatment prognosis of our patients.
Comparing the two groups of patients with survival shorter and longer than 30 d, Child-Pugh class C, presence of HCC, low serum sodium, high bilirubin values and high MELD scores were associated with lower survival (Table 4). By multivariate analysis, only serum sodium less than 132 mEq/L, and MELD score greater than 27 were independently associated with 30-d mortality.
Overall, these results confirm that prognosis was negatively influenced by the severe impairment of liver function, highlighting the role of MELD score as a prognostic factor in patients with cirrhosis and renal failure, as already described in other studies[22-24].
This study had several limitations. First, the sample size was small. Second, there were a relatively large number of patients with co-morbidities that adversely affected the prognosis, such as hepatocellular carcinoma, as confirmed by the ineligibility of any patients for OLT. Third, none of the patients included were treated with a combination of midodrine and octreotide. This combination would have been useful to compare the outcomes of patients treated with terlipressin to those treated with midodrine and octreotide. Finally, none of the patients underwent liver transplantation, so we were not able to evaluate the impact of vasoactive therapy as a bridge to OLT in our cohort.
HRS still has a poor prognosis, even when drug therapy is extensively used. The impact of vasoactive drugs is poor, and the true effectiveness of these drugs is in prolonging short-term survival, as a bridge to transplantation, in patients suitable for OLT. In this setting, the use of TIPS has been limited by the absence of large, prospective studies and by restricted use in patients with end-stage cirrhosis[25,26]. Prognostic factors for short-term mortality (low serum sodium and high MELD score) could be used to choose candidate patients for OLT as soon as possible after the onset of HRS.
Hepatorenal syndrome (HRS) is a life-threatening complication of advanced liver cirrhosis. It is characterized by functional renal failure, which develops as a result of portal hypertension, splanchnic vasodilatation and consequential deterioration of all systemic circulatory function.
HRS still has a poor prognosis, even when vasoactive drug therapies are extensively used. The aims of the study were to investigate the clinical and biochemical features of HRS, to assess short-term and long-term survival, and to evaluate potential predictors of early mortality.
Comparing the two groups of patients with survival shorter and longer than 30 d, Child-Pugh class C, presence of hepatocellular carcinoma, low serum sodium, high bilirubin values and high Model for End-stage Liver Disease (MELD) scores were associated with lower survival. By multivariate analysis, only serum sodium less than 132 mEq/L, and MELD score greater than 27 were independently associated with 30-d mortality.
The impact of vasoactive drugs is poor, and the true effectiveness of these drugs is in prolonging short-term survival, as a bridge to transplantation, in patients suitable for orthotopic liver transplant (OLT). Prognostic factors for short-term mortality (low serum sodium and high MELD score) could be used to choose candidate patients for OLT as soon as possible after the onset of HRS.
HRS is a life-threatening complication in patients with advanced liver cirrhosis, and it should always be distinguished from other causes of renal failure. It is not always easy to recognize because there are no specific clinical or laboratory parameters that clearly allow for its diagnosis, so diagnosis is mainly based on the exclusion of other causes of renal failure. Currently, the new criteria of the International Ascites Club are regarded as the gold standard for HRS diagnosis.
In this manuscript, the authors investigated the clinical and biochemical features of HRS, the short and long-term survival of HRS patients, and the potential predictors for early mortality. This manuscript may provide useful information for the clinicians. The data analysis and presentation were appropriate, and the manuscript was well prepared.
P- Reviewers: Chuang WL, Kietzmann T S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol. 2012;57:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [PubMed] |
| 3. | Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106-1115. [PubMed] |
| 4. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. [PubMed] |
| 5. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 6. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [PubMed] |
| 10. | Guevara M, Arroyo V. Hepatorenal syndrome. Expert Opin Pharmacother. 2011;12:1405-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | European Association For The Study Of The Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 12. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 13. | Salerno F, Cazzaniga M, Merli M, Spinzi G, Saibeni S, Salmi A, Fagiuoli S, Spadaccini A, Trotta E, Laffi G. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488-496.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 401] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 17. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 542] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 18. | Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, Siringo S, Castellino P. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830-835. [PubMed] |
| 19. | Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152-156. [PubMed] |
| 20. | Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012;9:CD005162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229. [PubMed] |
| 23. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [PubMed] |
| 24. | Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282-1289. [PubMed] |
| 25. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |