Revised: September 19, 2011
Accepted: January 15, 2012
Published online: January 27, 2012
AIM: To analyze the relationship between the glycated albumin (GA) to glycated hemoglobin (HbA1c) ratio and the histological grading of liver fibrosis.
METHODS: The study retrospectively included consecutive hepatitis C virus positive chronic liver disease patients (n = 142) who had undergone percutaneous liver biopsy between January 2008 and March 2010 at our institution. The ratios of GA/HbA1c were calculated in all patients to investigate the relationship with the degree of the liver fibrosis. The values of the aspartate aminotransferase-to-platelet ratio index (APRI), an excellent marker for the evaluation of liver fibrosis, were also calculated. In addition, we combined the ratio of GA/HbA1c and the APRI in order to improve our ability to detect the presence of significant liver fibrosis.
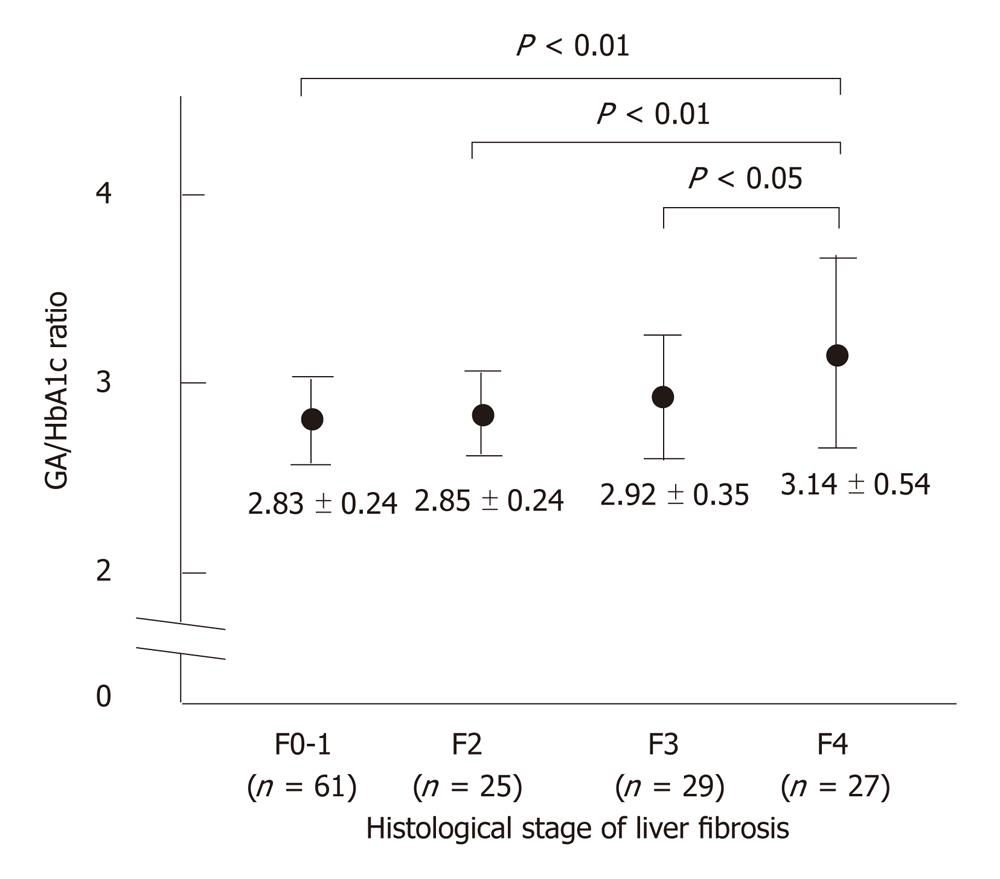
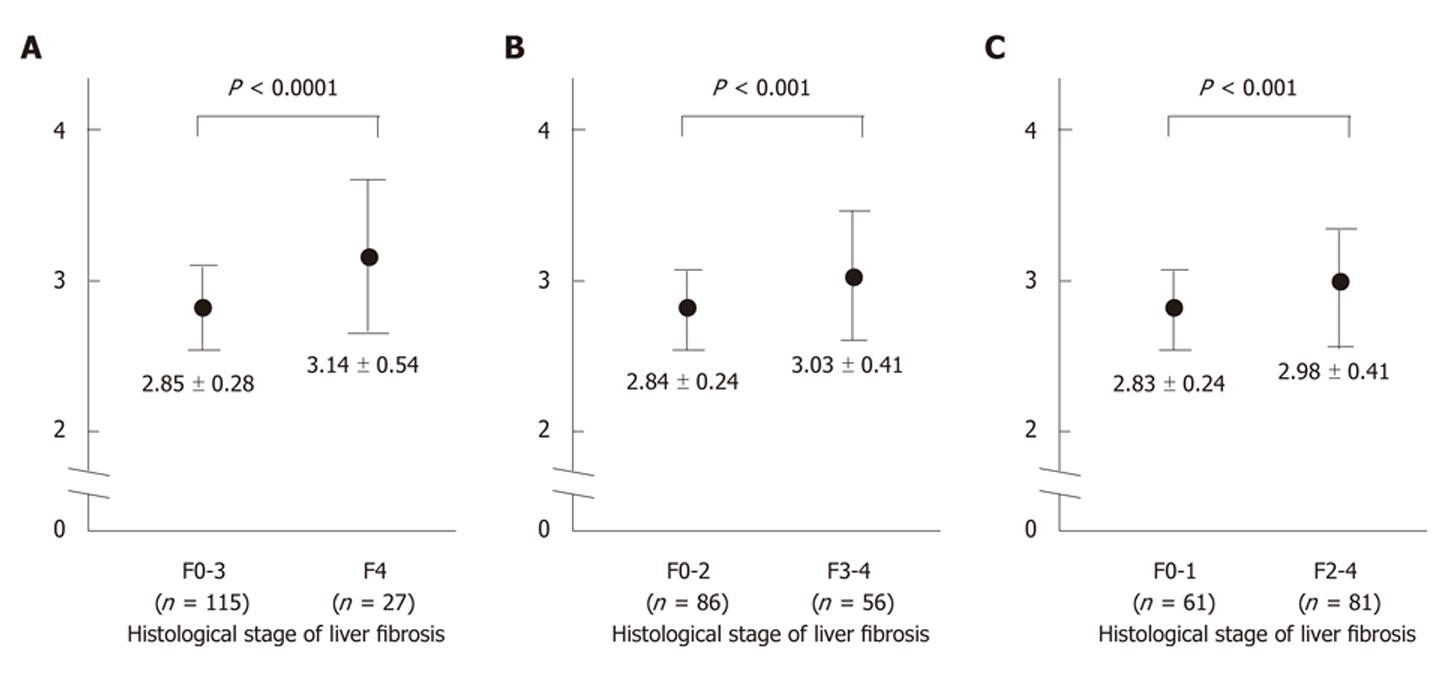
RESULTS: Sixty-one (43%) patients had either no fibrosis or minimal fibrosis (METAVIR score: F0-F1), while 25 (17%) had intermediate fibrosis (F2). Fifty-six (39%) patients had severe fibrosis (F3-F4) and 27 of them had cirrhosis (F4). The mean values of the GA/HbA1c increased with the progression of the fibrosis (F0-1: 2.83 ± 0.24, F2: 2.85 ± 0.24, F3: 2.92 ± 0.35, F4: 3.14 ± 0.54). There was a significant difference between the F0-F1 vs F4, F2 vs F4, and F3 vs F4 groups (P < 0.01, P < 0.01, P < 0.01 and P < 0.05, respectively). The GA/HbA1c ratio was significantly higher in the patients with cirrhosis (F4) than in those without cirrhosis (F0-F3) (3.14 ± 0.54 vs 2.85 ± 0.28, P < 0.0001). The GA/HbA1c ratio was also significantly higher in the patients with severe fibrosis (F3-F4) than in those without severe liver fibrosis (F0-F2) (3.03 ± 0.41 vs 2.84 ± 0.24, P < 0.001). Furthermore, the GA/HbA1c ratio was also significantly higher in the patients with significant fibrosis (F2-F4) than in those without significant liver fibrosis (F0-F1) (2.98 ± 0.41 vs 2.83 ± 0.24, P < 0.001). The diagnostic performance of the increased GA/HbA1c ratio (> 3.0) was as follows: its sensitivity and specificity for the detection of liver cirrhosis (F4) were 59.3% and 70.4%, respectively and its sensitivity and specificity for the detection of severe liver fibrosis (F3-F4) were 50.0% and 74.4%, respectively. With regard to the detection of significant fibrosis (F2-F4), its sensitivity was 44.4% and its specificity was 77.0%. Although even the excellent marker APRI shows low sensitivity (25.9%) for distinguishing patients with or without significant fibrosis, the combination of the APRI and GA/HbA1c ratio increased the sensitivity up to 42.0%, with only a modest decrease in the specificity (from 90.2% to 83.6%).
CONCLUSION: The GA/HbA1c ratio increased in line with the histological severity of liver fibrosis, thus suggesting that this ratio is useful as a supportive index of liver fibrosis.
- Citation: Aizawa N, Enomoto H, Imanishi H, Saito M, Iwata Y, Tanaka H, Ikeda N, Sakai Y, Takashima T, Iwai T, Moriwaki EI, Shimomura S, Iijima H, Nakamura H, Nishiguchi S. Elevation of the glycated albumin to glycated hemoglobin ratio during the progression of hepatitis C virus related liver fibrosis. World J Hepatol 2012; 4(1): 11-17
- URL: https://www.wjgnet.com/1948-5182/full/v4/i1/11.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i1.11
Glycated proteins are known to reflect the plasma glucose level and glycated hemoglobin (HbA1c) is used as a standard index of glycemic control in patients with diabetes mellitus[1,2]. Since the lifespan of erythrocytes is about 120 d, HbA1c reflects the glycemia for the recent few months[3]. Glycated albumin (GA) is another index of glycemic control which correlates with the plasma glucose levels during the past few weeks because the turnover of albumin is about 20 d[4,5]. Although the ratio of GA/HbA1c is usually close to 3, the value changes based on the patient’s condition[6]. In patients with chronic liver disease (CLD), hypersplenism causes a shortened lifespan of erythrocytes, leading to lower HbA1c levels relative to the plasma glucose level. In contrast, the turnover periods of serum albumin in CLD patients is prolonged in order to compensate for the reduced production of albumin. Therefore, the GA levels in CLD patients are higher relative to the degree of glycemia[6].
Since HbA1c shows lower and GA shows higher values in CLD patients, the GA/HbA1c ratio is thought to be high in patients with liver cirrhosis. Indeed, the GA/HbA1c ratio in patients with CLD has been reported to show an inverse correlation with some indicators of hepatic function (including the hepaplastin test, cholinesterase and bilirubin) independent of the mean plasma glucose levels, thus suggesting that the GA/HbA1c ratio increases as the liver cirrhosis progresses[7]. However, it has not been examined whether the GA/HbA1c ratio correlates with the histological fibrotic stage in CLD patients.
Hepatitis C virus (HCV) is one of the main causes of liver cirrhosis and hepatocellular carcinoma and knowledge about the progression of liver fibrosis is important. In the present study, we analyzed the relationship between the histological grading of liver fibrosis and the GA/HbA1c ratio in 142 patients with HCV-related CLD. Our findings suggest that the GA/HbA1c ratio is associated with the progression of liver fibrosis and cirrhosis in HCV-positive patients.
We retrospectively studied HCV-positive CLD patients (n = 142) who had undergone percutaneous liver biopsy between January 2008 and March 2010 at our institution who met the following conditions: (1) HCV infection diagnosed by detectable HCV antibodies and HCV RNA in serum; and (2) blood samples were obtained on the same day of the liver biopsies. Patients with the following conditions were excluded from the study: the presence of other liver diseases, hepatocellular carcinoma, immunosuppressive therapy, hepatitis B virus co-infection and those with insufficient liver tissue for staging of fibrosis. The present study did not include patients whose GA/HbA1c ratios could have been influenced by poorly controlled diabetes.
The routine studies, including platelet counts, prothrombin time international normalized ratio (PT-INR), liver functional tests [alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and total bilirubin] were performed. Since the index calculated by the combination of GA and HbA1c (CLD-HbA1c: defined as the average of the measured HbA1c and GA/3) was reported to be a good indicator for the evaluation of the mean plasma glucose level in patients with CLD[8], HbA1c and GA were also routinely measured in all patients. The values of GA and HbA1c were determined in the same sample and on the same day as the liver biopsies were performed. The AST-to-platelet ratio index (APRI), an excellent marker for the evaluation of liver fibrosis, was also calculated based on the formula proposed by Wai et al[9]: APRI = [(AST level/ upper limit of normal)/platelet counts (109/L)] × 100. Written informed consent regarding the liver biopsy and retrospective use of clinical data was obtained from all patients on admission. This study was approved by the ethics committees of the institutional review board.
Liver biopsy examinations were performed using the standard procedures and all liver specimens were evaluated by well-trained pathologists at our institute, with evaluation of the fibrosis stage and activity grade according to the METAVIR scoring system[10]. Fibrosis was staged on a scale of 0-4 (F0: no fibrosis, F1: portal fibrosis without septa, F2: portal fibrosis with rare septa, F3: numerous septa without cirrhosis, F4: liver cirrhosis). The histological evaluation of the biopsy samples was also routinely performed in our department. All authors participated in the conference about the histological evaluation and the final results were confirmed by two authors (Enomoto H and Imanishi H) who received training for histological studies.
In the present study, we attempted to clarify whether the GA/HbA1c ratio was associated with liver fibrosis and cirrhosis. The data for the comparisons among the groups “F0-1 vs F2 vs F3 vs F4” was analyzed by non-repeated measurements ANOVA and statistical significance was further examined by the Student-Newman-Keuls test. We compared the “F0-F3 (no cirrhosis) vs F4 (cirrhosis)”, “F0-F2 (no - intermediate fibrosis) vs F3-F4 (severe fibrosis)” and “F0-F1 (no approximately minimal fibrosis) vs F2-F4 (significant fibrosis)” groups. The differences in the baseline characteristics and GA/HbA1c ratios of the groups were evaluated. Quantitative variables were expressed as the mean ± SD and those with an abnormal distribution were expressed as the median values (range). Statistical analysis was performed using Student’s t test or the Mann-Whitney U test, as appropriate.
From January 2008 to March 2010, a total of 142 patients with HCV were consecutively included in the present study, based on the inclusion and exclusion criteria as described in the “Patients and Methods” section. The characteristics of the study population are summarized in Table 1. The population consisted of 60 (42%) males and 82 (58%) females, and the age of patients ranged from 19 to 78 years old (median 60). According to the METAVIR liver fibrosis staging[10], 56 (39%) patients had significant fibrosis (F3-F4) and 27 (19%) had cirrhosis (F4).
| Age (yr) | 60 (19-78) |
| Gender (male/female) | 60/82 |
| AST (IU/L) | 37.5 (14-328) |
| ALT (IU/L) | 36 (10-388) |
| γ-GTP (IU/L) | 29 ( 7-259) |
| ALP (IU/L) | 217 ( 97-556) |
| Total bilirubin (mg/dL) | 0.7 (0.1-2.1) |
| Albumin (g/dL) | 3.96 ± 0.36 |
| Hemoglobin (g/dL) | 13.4 ± 1.8 |
| Platelet (× 104/mm3) | 15.9 ± 5.5 |
| PT-INR | 1.04 ± 0.07 |
The GA/HbA1c ratio in patients with CLD has been reported to show an inverse correlation with certain indicators of hepatic function. As shown in Figure 1, the mean values of the GA/HbA1c increased with the progression of the fibrosis stage, suggesting that the GA/HbA1c ratio was associated with the histological severity of liver fibrosis.
Comparing the F0-F3 (no cirrhosis) and F4 (cirrhosis) groups, we found that there was a significant difference in several parameters which correlated with hepatic function; that is, higher AST, ALT, γ-GTP alkaline phosphatase (ALP) and PT-INR levels and also a lower platelet count, and albumin values in the presence of cirrhosis (Table 2; left). However, no significant difference was observed in other parameters such as age and gender, which were not related to the hepatic function. Between the two groups, the GA/HbA1c ratio was significantly higher in patients with cirrhosis (Figure 2A), thus suggesting that the GA/HbA1c ratio is associated with the cirrhotic changes in the liver.
| F0-F3(n = 115) | F4(n = 27) | P value | F0-F2(n = 86) | F3-F4(n = 56) | P value | F0-F1(n = 61) | F2-F4(n = 81) | P value | |
| Age (yr) | 60 (19-78) | 62 (23-78) | NS | 60 (19-78) | 62 (23-78) | NS | 60 (19-78) | 62 (23-78) | NS |
| Gender (male/female) | 48/67 | 12/15 | NS | 31/55 | 29/37 | NS | 25/36 | 35/46 | NS |
| AST (IU/L) | 35 (14-195) | 50 (20-328) | < 0.001 | 32 (14-175) | 46 (20-328) | < 0.001 | 32 (14-104) | 42 (18-328) | < 0.001 |
| ALT (IU/L) | 38 (10-388) | 47 (10-310) | < 0.05 | 31.5 (10-388) | 48 (10-310) | < 0.01 | 31 (11-388) | 46 (10-310) | < 0.01 |
| γ-GTP (IU/L) | 25 ( 7-183) | 50 (12-259) | < 0.001 | 22 ( 7-183) | 42.5 (12-259) | < 0.0001 | 22 ( 8-183) | 36 ( 7-259) | < 0.01 |
| ALP (IU/L) | 207 ( 97-490) | 267 (133-556) | < 0.001 | 186 (97-465) | 275 (133-556) | < 0.0001 | 207 ( 97-465) | 258 (101-556) | < 0.001 |
| Total bilirubin (mg/dL) | 0.7 (0.1-1.6) | 0.7 (0.3-2.1) | NS | 0.7 (0.1-1.6) | 0.8 (0.3-2.1) | NS | 0.7 (0.1-1.6) | 0.7 (0.3-2.1) | NS |
| Albumin (g/dL) | 4.02 ± 0.31 | 3.70 ± 0.43 | < 0.001 | 4.03 ± 0.32 | 3.84 ± 0.37 | < 0.01 | 4.05 ± 0.31 | 3.89 ± 0.38 | < 0.01 |
| Hemoglobin (g/dL) | 13.5 ± 1.7 | 12.8 ± 2.0 | NS | 13.5 ± 1.8 | 13.3 ± 1.7 | NS | 13.7 ± 1.7 | 13.2 ± 1.8 | NS |
| Platelet (× 104/mm3) | 16.5 ± 5.3 | 13.2 ± 5.9 | < 0.001 | 17.2 ± 5.2 | 13.8 ± 5.5 | < 0.001 | 17.2 ± 4.8 | 14.9 ± 5.9 | < 0.05 |
| PT-INR | 1.03 ± 0.05 | 1.08 ± 0.06 | < 0.001 | 1.02 ± 0.05 | 1.07 ± 0.06 | < 0.001 | 1.02 ± 0.05 | 1.05 ± 0.08 | < 0.05 |
Next, we examined whether the GA/HbA1c ratio differed in patients with or without severe liver fibrosis. Comparing the F0-F2 (without severe fibrosis) and F3-F4 (with severe fibrosis) groups, we found significant differences, with higher AST, ALT, γ-GTP, ALP and PT-INR values and a lower platelet count, and albumin values in the presence of severe fibrosis (Table 2; middle). In patients with severe liver fibrosis, the GA/HbA1c ratio was significantly higher (Figure 2B) than that in patients without severe fibrosis, suggesting that the GA/HbA1c ratio also correlates with the progression of liver fibrosis.
We also examined whether the GA/HbA1c ratio differed in patients with or without significant liver fibrosis. When we compared the F0-F1 (no or minimal fibrosis: without significant fibrosis) and F2-F4 (with significant fibrosis) groups, we also found significant differences, with higher AST, ALT, γ-GTP ALP and PT-INR values and a lower platelet count and albumin values in the presence of significant fibrosis (Table 2; right). In patients with significant liver fibrosis, the GA/HbA1c ratio was significantly higher than that in patients without significant fibrosis (Figure 2C).
Although the GA/HbA1c ratio is usually about 3, we found that the ratio increased in line with the progression of liver fibrosis (Figure 2). We therefore evaluated the diagnostic performance of the increased GA/HbA1c ratio (> 3.0) for the detection of patients with cirrhosis (F4), severe fibrosis (F3-F4) and significant fibrosis (F2-F4) (Table 3). Its sensitivity for the detection of liver cirrhosis was 16/27 (59.3%) and the specificity was 81/115 (70.4%). With regard to the detection of severe fibrosis, the sensitivity of the increased GA/HbA1c ratio (> 3.0) was 28/56 (50.0%) and its specificity was 64/86 (74.4%). With regard to the detection of significant fibrosis, the sensitivity of the increased GA/HbA1c ratio (> 3.0) was 36/81 (44.4%) and its specificity was 47/61 (77.0%).
| F4 | F0-F3 | F3-F4 | F0-F2 | F2-F4 | F0-F1 | |
| GA/HbA1c > 3.0 | 16/27 (59.3) | 34/115 (29.6) | 28/56 (50.0) | 22/86 (25.6) | 36/81 (44.4) | 14/61 (23.0) |
| GA/HbA1c ≤ 3.0 | 11/27 (40.7) | 81/115 (70.4) | 28/56 (50.0) | 64/86 (74.4) | 45/81 (55.6) | 47/61 (77.0) |
As described above, the GA/HbA1c ratio in patients with significant liver fibrosis was higher than that in patients without significant fibrosis. However, the differences were small and the GA/HbA1c ratio had difficulty in distinguishing between F1 and F2.
Several biomarkers for the evaluation of fibrosis have been reported previously and the APRI is a simple and useful marker for the prediction of significant fibrosis. We combined the GA/HbA1c ratio and the APRI in order to examine their utility for the detection of patients with significant liver fibrosis. At first, based on prior studies[9,11,12], we assessed two cut-off points (0.50 and 1.50) of the APRI to predict the absence or presence of significant fibrosis (Table 4). When we used the cut-off point as 0.5 (Table 4; left), the sensitivity was 68/81 (84.0%) and the specificity was 29/61 (47.5%). When we used the cut-off value of 1.5 (Table 4; right), the sensitivity was 21/81 (25.9%) and the specificity was 55/61 (90.2%). Therefore, as previously reported, the cut-off point of 1.50 had a high specificity but a low sensitivity to detect significant fibrosis.
| F2-F4 (%) | F0-F1 (%) | F2-F4 (%) | F0-F1 (%) | ||
| APRI > 0.5 | 68/81 (84.0) | 32/61 (52.5) | APRI > 1.5 | 21/81 (25.9) | 6/61 (9.8) |
| APRI ≤ 0.5 | 13/81 (16.0) | 29/61 (47.5) | APRI ≤ 1.5 | 60/81 (74.1) | 55/61 (90.2) |
We next asked whether a combination of the GA/HbA1c and the APRI could improve the sensitivity to detect the presence of significant fibrosis and help distinguish between the two groups (F0-F1 and F2-F4). When we examined the criteria “APRI >1.5 or GA/HbA1c ratio > 3.0”, the sensitivity and the specificity for the detection of significant liver fibrosis was 43/81 (53.1%) and 43/61 (70.5%), respectively (Table 5; left). In addition, when we used the criteria “APRI >1.5 or GA/HbA1c ratio > 3.2”, the sensitivity was 34/81 (42.0%) and the specificity was 51/61 (83.6%) (Table 5; right). Therefore, compared with the detection of significant liver fibrosis by using the APRI alone, the combination of GA/HbA1c and the APRI (APRI >1.5 or GA/HbA1c ratio > 3.2) improved the sensitivity from 25.9% to 42.0% without a major decrease in the specificity (only a modest reduction from 90.2% to 83.6% was observed).
| F2-F4 (%) | F0-F1 (%) | F2-F4 (%) | F0-F1 (%) | ||
| APRI > 1.5 or GA/HbA1c > 3.0 | 43/81 (53.1) | 18/61 (29.5) | APRI > 1.5 or GA/HbA1c > 3.2 | 34/81 (42.0) | 10/61 (16.4) |
| Others | 38/81 (46.9) | 43/61 (70.5) | Others | 47/81 (58.0) | 51/61 (83.6) |
Liver biopsy is the gold standard method for histological evaluation of liver fibrosis[13]. Although a liver biopsy is generally a safe procedure, it is costly, invasive and has a small risk of complications. In addition, only 1/50 000 of the organ is removed and there can be sampling errors[13]. Furthermore, it has also been reported that there are inter- and intra-observer discrepancies of 10% to 20%[14,15]. Therefore, many noninvasive biomarkers readily available via laboratory tests have been proposed to predict the presence of significant fibrosis or cirrhosis in patients with HCV.
The Fibro-Test score is computed using the patient’s age, sex and results of the analyses of serum haptoglobin, α2-macroglobulin, apolipoprotein A1, γ-GTP and bilirubin levels[16]. Forns et al[17] developed the Forns score, which is an algorithm including the platelet count, γ-GTP, age and cholesterol level. Wai et al[8] reported the APRI for fibrosis and cirrhosis prediction. In addition, some models such as the Hepascore[18], FibroMeter[19], FibroIndex[20] and FIB-4[21] have also been proposed for the evaluation of liver fibrosis. In addition, there are several noninvasive methods for the evaluation of liver fibrosis using ultrasound waves[22-26] such as Transient Elastography (FibroScan)[22,26]; SonoElastography (Real-Time Tissue Elastography)[23] and Acoustic Radiation Force Impulse[24-26]. Although each noninvasive tool has an excellent positive predictive value for the diagnosis of moderate or significant fibrosis, none of the available methods completely meets the criteria of an ideal (simple, inexpensive and easily reproducible) method.
The Fibro-Test[16] is a combination of 6 markers and the Forns score[17] contains a complicated formula, indicating that while these markers are excellent, they lack simplicity. Recently introduced markers including APRI, FIB-4 and the FibroIndex are well-established, simple and inexpensive tools to assess liver fibrosis[9,20,21]. However, the values of these markers in one patient can vary within a short period, since the levels of AST or ALT or platelet count in the same patient often change daily. In addition, regarding APRI and FIB-4, the appropriate definition of the upper limit of normal (ULN) of the AST level remains uncertain, since each laboratory uses a different value for the ULN. With regard to the methods using special ultrasound tools, they are costly and cannot be routinely evaluated in all medical institutes.
In the present study, we have shown that the GA/HbA1c ratio of HCV-positive patients increases with the progression of liver fibrosis. Unlike the other previously established methods, the GA/HbA1c ratio is a simple and unique tool which is calculated based on the two glycated proteins and correlates with the degree of liver fibrosis. Since GA and HbA1c are stable over several weeks, the GA/HbA1c ratio does not change in a short period, resulting in a high reproducibility of its value. The stability of the two glycated proteins over weeks is a unique point, different from other biomarkers.
Bando et al[7] previously reported that the GA/HbA1c ratio in patients with CLD have an inverse correlation with the some indicators of hepatic function, regardless of the mean plasma glucose levels, thus suggesting that the increase of GA/HbA1c ratio indicates a reduction in the liver function caused by the progression of liver cirrhosis. Consistent with that report, our current histological evaluation revealed that the GA/HbA1c ratios of the cirrhotic patients were significantly higher than those of the patients without cirrhosis (Figure 2A). Furthermore, as shown in Figure 2B, the GA/HbA1c ratios increased in patients with severe fibrosis (F3-F4) compared to those in patients without severe fibrosis (F0-F2), thus suggesting that the GA/HbA1c ratio increased in correlation with the progression of fibrosis.
Since the GA/HbA1c ratio is usually about 3, we examined the diagnostic performance of the elevated GA/HbA1c ratio (GA/HbA1c > 3.0) and determined the sensitivity and specificity (Table 3). As described in the “Results” section, its solo diagnostic performance did not achieve satisfactory levels. However, when we combined the GA/HbA1c ratio with the APRI, the sensitivity to distinguish patients with significant fibrosis (F2-F4) from those without significant fibrosis was improved, with only a modest reduction in the specificity (Table 5). These findings suggest that the GA/HbA1c ratio can be used as a supportive index for the evaluation of liver fibrosis. Since only a small number of patients were investigated in the present study, we will therefore need to rigorously investigate the ratios in both larger and different populations.
In summary, we have shown that the GA/HbA1c ratio increases with the progression of the histological findings of liver fibrosis. However, its rate of change is relatively small. Although we have shown that the GA/HbA1c ratio improves the diagnostic performance of the APRI for the detection of significant fibrosis, it will be necessary to establish a new and better biomarker using a combination of the GA/HbA1c ratio and other parameter(s).
Hepatitis C virus (HCV) is one of the main causes of liver cirrhosis and hepatocellular carcinoma, and knowledge about the progression of liver fibrosis is important. Many noninvasive biomarkers readily available via laboratory tests have been proposed to predict the presence of significant fibrosis or cirrhosis in patients with HCV. The glycated albumin (GA)/glycated hemoglobin (HbA1c) ratio in patients with chronic liver disease (CLD) has been reported to show an inverse correlation with some indicators of hepatic function independent of the mean plasma glucose levels, thus suggesting that the GA/HbA1c ratio increases as the liver cirrhosis progresses. However, it has not been examined whether the GA/HbA1c ratio correlates with the histological fibrotic stage in CLD patients.
Liver biopsy is the gold standard method for histological evaluation of liver fibrosis. Although a liver biopsy is generally a safe procedure, it is costly, invasive and has a small risk of complications. It is very important to establish a simple, inexpensive and easily reproducible method for the evaluation of liver fibrosis.
In the previous studies, many excellent noninvasive methods for the evaluation of liver fibrosis have been proposed. However, none of the available methods completely meets the criteria of an ideal (simple, inexpensive and easily reproducible) method. The present study has shown that the GA/HbA1c ratio of HCV-positive patients increases with the progression of liver fibrosis. Unlike the other previously established methods, the GA/HbA1c ratio is a simple and unique tool which is calculated based on the two glycated proteins and correlates with the degree of liver fibrosis.
The study showed that the GA/HbA1c ratio increased in line with the histological severity of liver fibrosis, thus suggesting that this ratio is useful as a supportive index of liver fibrosis.
HbA1c is used as a standard index of glycemic control in patients with diabetes mellitus. Since the lifespan of erythrocytes is about 120 d, HbA1c reflects the glycemia for the recent few months; GA is another index of glycemic control which correlates with the plasma glucose levels during the past few weeks because the turnover of albumin is about 20 d.
The study focuses on the power of the GA/HbA1c ratio in estimation of liver fibrosis in people with HCV infection. Previously defined noninvasive fibrosis markers exist but none of them have proved to be equal to liver biopsy. Therefore, research on defining new but more effective fibrosis markers should be encouraged. People with HCV are always a good research base in this context. Therefore, the present study may be interesting for the readers.
Peer reviewers: Ilker Tasci, Professor, Department of Internal Medicine, Gulhane School of Medicine, GATA Ic Hastaliklari Bilim Dali, Ankara 06018, Turkey; Lang Zhuo, Dr., Department of Cell and Tissue Engineering, Institute of Bioengineering and Nanotechnology, Singapore 138669, Singapore
S- Editor Wu X L- Editor Roemmele A E- Editor Li JY
| 1. | Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 834] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978;200:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 655] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Dolhofer R, Wieland OH. Glycosylation of serum albumin: elevated glycosyl-albumin in diabetic patients. FEBS Lett. 1979;103:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Guthrow CE, Morris MA, Day JF, Thorpe SR, Baynes JW. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci USA. 1979;76:4258-4261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Bando Y, Kanehara H, Toya D, Tanaka N, Kasayama S, Koga M. Association of serum glycated albumin to haemoglobin A1C ratio with hepatic function tests in patients with chronic liver disease. Ann Clin Biochem. 2009;46:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Koga M, Kasayama S, Kanehara H, Bando Y. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3245] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 10. | The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1415] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 11. | Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, Lecomte L, Castellani P, Rosenthal-Allieri MA, Gerolami R. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 15. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 16. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1037] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 17. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 382] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 20. | Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 22. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1935] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 23. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] |
| 26. | Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |