Published online Jul 27, 2010. doi: 10.4254/wjh.v2.i7.289
Revised: June 29, 2010
Accepted: July 6, 2010
Published online: July 27, 2010
Transarterial chemoembolization (TACE) is an effective modality for the treatment of Hepatocellular Carcinoma. It is used to treat small tumors and to downstage large tumors to meet liver transplant criteria. TACE can be associated with multiple side effects, including fever, right upper quadrant pain, nausea, vomiting, hepatic failure, hepatic encephalopathy, cholecystitis and pancreatitis. Neurological complications after TACE are rare, usually caused by cerebral embolism, and confirmed by means of imaging studies. Spinal cord ischemia secondary to TACE is extremely rare and can lead to significant morbidity. We report a case of paraparesis caused by TACE with normal imaging and nerve conduction studies, suggestive of localized vasculitis.
- Citation: Tufail K, Araya V, Azhar A, Hertzog D, Khanmoradi K, Ortiz J. Paraparesis caused by transarterial chemoembolization: A case report. World J Hepatol 2010; 2(7): 289-291
- URL: https://www.wjgnet.com/1948-5182/full/v2/i7/289.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i7.289
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and is the third most common cause of cancer-related death[1]. It is the fastest growing cause of cancer-related deaths in males in the United States[2]. Only 30%-40% of patients with HCC are diagnosed at a stage where curative therapy can be offered[3]. In appropriate candidates, liver resection and transplantation are associated with long-term response and improved survival. The remaining 60%-70% already have advanced HCC and are eligible only for palliative interventions, which include transarterial chemoembolization (TACE), ablation, radiation, and systemic chemotherapy. TACE is also used to treat small tumors, and to downstage large tumors to meet liver transplant criteria[4]. Although considered a relatively safe procedure, TACE may be associated with fever, right upper quadrant pain, nausea and transaminitis, known as post-embolization syndrome. The complication rate after TACE is reported to be 2.9%[5]. Examples include hepatic failure, cholecystitis, pancreatitis, hepatic encephalopathy, common bile duct stricture, biloma, tumor lysis syndrome, pulmonary and cerebral embolism. Neurological deficits after TACE are very rare, usually caused by cerebral embolism of iodized oil (lipoidal), and confirmed by abnormal findings on imaging. We report a case of paraparesis caused by TACE and normal imaging of brain, thoracic and lumbo-sacral spine, and normal electromyography and nerve conduction studies.
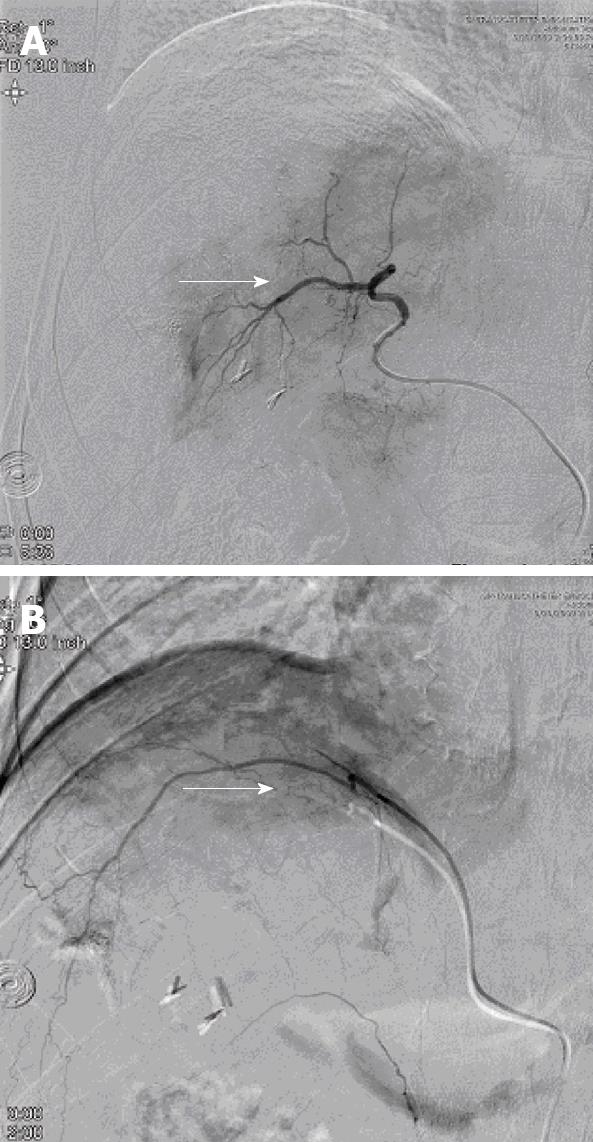
A 45 year-old African American male with hepatitis C-related cirrhosis underwent open microwave ablation of a single 5.6 cm right hepatic lobe HCC. Follow-up MRI revealed a residual tumor, which was treated by TACE of the right hepatic artery, but follow up imaging revealed a persistent residual tumor. Hepatic angiogram confirmed that part of the tumor was supplied by the right inferior phrenic artery. The patient underwent repeat TACE, in which the right hepatic (Figure 1A) and inferior phrenic arteries (Figure 1B) were successfully chemoembolized with doxorubicin, cisplatin and mitomycin.
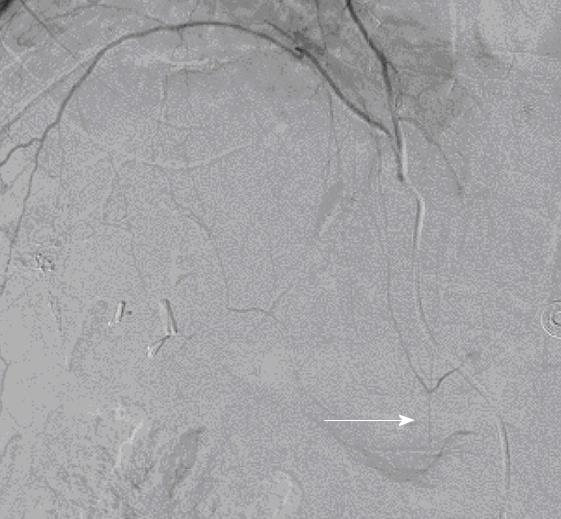
The patient developed bilateral lower extremity weakness eight hours after the procedure. His strength was 3/5 in the right and 4/5 in the left lower extremity. This was associated with numbness in the left anterior thigh and scrotum, together with urinary retention. Reflexes were 2+ bilaterally and there was no loss of rectal tone. MRI of the brain, thoracic, lumbar and sacral spine did not reveal stroke, mass or cord compression. Electromyography and nerve conduction studies were all within normal limits. The patient was diagnosed with localized vasculitis of the branches of the anterior spinal artery, caused by the chemotherapy. An angiogram revealed the anastomoses between the branches of the right inferior phrenic artery and intercostal arteries, confirmed by the filling of the anterior spinal artery, which was believed to be the route of the embolism causing the injury to the spinal cord (Figure 2). The patient was started on high dose steroids, which were then systematically reduced over a period of one mo. Aggressive physical therapy was also instituted. His symptoms markedly improved and he was able to walk without assistance. The patient underwent liver transplantation two months later and remained free of neurological complications.
Neurological complications occur rarely in association with TACE, and are usually due to lipoidal embolism in the central nervous system. Focal symptoms develop in the area of distribution of the affected vessel, which can be confirmed by imaging studies. Spinal cord injury is an extremely rare complication after TACE. Our patient developed bilateral lower extremity weakness following the procedure, associated with numbness in the left anterior thigh, together with urinary retention. All these symptoms can be explained by ischemia to the lumbar spinal cord in the region of L1-L3. Since the MRI/MRA of the brain and spinal cord did not show any infarction or cord compression, we surmise that the symptoms occurred due to localized vasculitis caused by one of the chemotherapeutic agents used during TACE.
Neurological complications after TACE usually occur after the second or third session, which can be explained by hepatic artery injury or decreased flow at the previous TACE site, leading to collateral recruitment[6]. Our patient had previously undergone one initial TACE procedure of the right hepatic artery without any complications. The second session of TACE involved the right inferior phrenic artery, which supplied part of the tumor and resulted in this complication.
The inferior phrenic artery is the most common source of extra-hepatic collateral blood supply for HCC[7]. The right and left inferior phrenic arteries usually arise from the aorta above the celiac artery, and each divides into medial and lateral branches. They are the main source of blood supply to the diaphragm, and also send a few branches to adrenal glands, and, occasionally, to the liver and spleen. The medial branch joins with its fellow of the opposite side, and with the musculophrenic and pericardiophrenic arteries, whereas the lateral branch joins with the lower intercostal arteries and musculophrenic arteries. We believe that the anastomosis between the lateral branches of the right inferior phrenic artery and intercostal arteries which supply the spinal cord through the anterior spinal artery, was the route of the embolism which caused the injury to the spinal cord in our patient. Few cases of spinal cord injury after TACE causing paraplegia or paraparesis, sensory loss, and urinary retention, confirmed by imaging studies and usually caused by communication through intercostal arteries have been reported[8]. Although the spinal cord injury in these case reports was seen on MRI, it was not classic for ischemia, and was found to be steroid-responsive. In our patient, since the extensive workup for paraparesis had been essentially unremarkable, with normal imaging, electromyography, and nerve conduction studies, the diagnosis of localized vasculitis was made, and treatment with steroids resulted in the resolution of symptoms.
In conclusion, we have reported a case of paraparesis following TACE with normal imaging, and its improvement with steroids, suggesting localized vasculitis. This emphasizes the fact that TACE and other loco-regional therapies can be associated with serious side effects which may lead to significant morbidity, and could jeopardize liver transplant candidacy. Physicians should be aware of these risks and appropriate consent should be obtained.
Peer reviewers: Giuseppe R Nigri, MD, PhD, FACS, Assistant Professor, Department of Surgery, University of Rome La Sapienza, II School of Medicine, St. Andrea Hospital, Rome, Italy; Martin Loss, MD, Associate Professor, Klinik und Poliklinik für Chirurgie, Universitätsklinikum Regensburg, Franz-Josef-Strauss-Allee 11, Regensburg 93053, Germany
| 1. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. |
| 2. | Cancer Facts & Figures. American Cancer Society. Atlanta: Georgia 2008; . |
| 3. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. |
| 4. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. |
| 5. | Pietrosi G, Miraglia R, Luca A, Vizzini GB, Fili’ D, Riccardo V, D’Antoni A, Petridis I, Maruzzelli L, Biondo D. Arterial chemoembolization/embolization and early complications after hepatocellular carcinoma treatment: a safe standardized protocol in selected patients with Child class A and B cirrhosis. J Vasc Interv Radiol. 2009;20:896-902. |
| 6. | Lee KH, Sung KB, Lee DY, Park SJ, Kim KW, Yu JS. Transcatheter arterial chemoembolization for hepatocellular carcinoma: anatomic and hemodynamic considerations in the hepatic artery and portal vein. Radiographics. 2002;22:1077-1091. |
| 7. | Gwon DI, Ko GY, Yoon HK, Sung KB, Lee JM, Ryu SJ, Seo MH, Shim JC, Lee GJ, Kim HK. Inferior phrenic artery: anatomy, variations, pathologic conditions, and interventional management. Radiographics. 2007;27:687-705. |
| 8. | Kim JH, Yeon JE, Jong YK, Seo WK, Cha IH, Seo TS, Park JJ, Kim JS, Bak YT, Byun KS. Spinal cord injury subsequent to transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Dig Liver Dis. 2010;42:67-70. |