Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.107541
Revised: May 5, 2025
Accepted: June 27, 2025
Published online: July 27, 2025
Processing time: 122 Days and 3.6 Hours
Liver diseases are of growing interest to clinicians and researchers due to their high prevalence, difficulty in early diagnosis, and limited treatment options. The liver is an important organ at the intersection of many metabolic and immune pathways. To this end, it contains a large number of immune cells of both the innate and adaptive immune system that perform multiple functions, detecting and destroying pathogens that enter the body through the intestine, as well as recognizing endogenous antigens. Immune cells in the liver have a complex regulation that can be impaired in various diseases such as metabolic dysfunction-associated steatotic liver disease (MASLD), liver cancer, and biliary diseases. A growing body of evidence reinforces the realization that not only impaired metabolism but also many immune mechanisms underlie MASLD. The liver has complex bilateral immune and metabolic links with the gut microbiota, and disruptions of these links underlie the development and progression of both gas
Core Tip: The liver is an important organ at the intersection of many metabolic and immune pathways. Immune cells in the liver have a complex regulation that can be impaired in various diseases such as metabolic dysfunction-associated steatotic liver disease, liver cancer, and biliary disorders. Interactions with the gut microbiota play an important role in liver immunology. Effects on immune mechanisms are considered a promising therapeutic target for liver diseases.
- Citation: Kotlyarov SN. Liver immunology: Biological role and clinical significance. World J Hepatol 2025; 17(7): 107541
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/107541.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.107541
Liver diseases are an urgent medical problem due to their high prevalence, as well as significant underdiagnosis and underestimation in real clinical practice. The problem of liver diseases is also reinforced by the difficulty and expense of diagnosing and monitoring the disease course, which contribute to many patients going for long periods of time without a confirmed diagnosis[1,2]. In addition, patients may not seek medical attention due to mild or no symptoms for long periods of time. Metabolic dysfunction-associated steatotic liver disease (MASLD), for example, is often not diagnosed in a timely manner, although it is one of the most common metabolic diseases, occurring in 17%-46% of the adult popu
The liver is the largest gland in the body, representing about 3% of total body weight, and performs many different functions, the full consideration of which is beyond the scope of this review[5,6]. According to current thinking, the liver is a central regulator of many metabolic and immune processes. Indeed, the liver acts as an important barrier to various toxic substances and pathogens that may enter the bloodstream from the intestine. At the same time, on the one hand the liver must detoxify and neutralize the harmful substances that enter the body through the bloodstream; on the other hand the liver needs to maintain immune tolerance in order to ensure adequate immune responses to commensal microorganisms and food antigens[7-10]. Thus, the liver is an important defense organ, and impaired immune function is associated with various diseases. It is important to note that this complex combination of immune and metabolic functions characteristic of the liver is not random, but rather is a product of evolutionary pressures that have sculpted the liver into a vital immune organ[11].
Therefore, the aim of the current review is to discuss the immune function of the liver and how these functions are impaired in various noninfectious liver diseases.
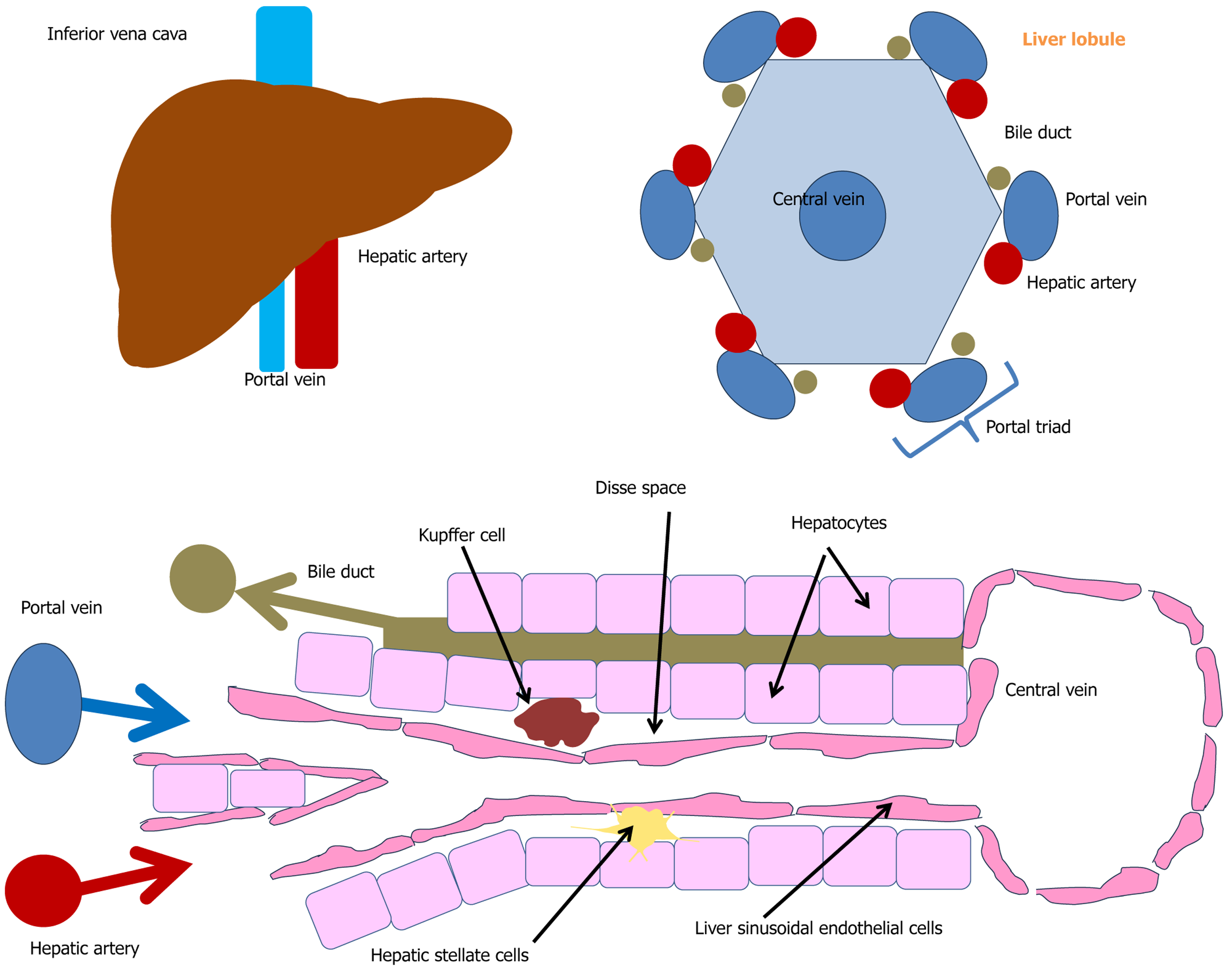
The liver is located at the crossroads of blood flow pathways, and consequently, has a very intensive blood supply. It receives about one-quarter of the cardiac output, and the blood volume in the liver is about 14% of the total blood volume in the body. The liver has a dual blood supply: Approximately 20%-30% comes from the hepatic artery, primarily supplying oxygen, while the remaining 70%-80% arrives via the portal vein, which is mainly responsible delivering nutrients[12,13].
The structural and functional units of the liver are hepatic lobules (Figure 1), which are hexagonal structures with a central vein[14-16]. Each lobule consists of hepatocytes arranged as plates diverging from the central vein; sinusoids, i.e. capillary-like vessels between the plates of hepatocytes through which blood flows from the portal triads into the central vein; and portal triads, which are located in the corners of the lobules and consist of a branch of the hepatic artery, a branch of the portal vein, and the bile duct[14-16]. The intra-lobular blood capillaries are lined with liver sinusoidal endothelial cells (LSECs). Numerous Kupffer cells are located between the endothelial cells. The space between hepatocytes and LSECs is known as the space of Disse, where hepatocytes exchange substances with the blood[13].
The liver is composed of parenchymatous cells (hepatocytes) and non-parenchymatous cells, which include LSECs, Kupffer cells, hepatic stellate cells (HSCs) (Ito cells), lymphocytes, and a number of other cells. Parenchymatous cells account for about 70% of its mass, and non-parenchymatous cells make up the remaining 30%. The liver contains many cells of innate and adaptive immunity, including Kupffer cells, dendritic cells, natural killer (NK) cells and T cells, which are essential for detecting and responding to pathogens.
Hepatocytes constitute about 70% of all liver cells. They have an irregular polygonal shape and are about 20-25 μm in diameter[14,15]. Many of these cells (up to 20%) contain two or more nuclei. The number of these cells depends on their functional state. Hepatocytes form a cellular barrier separating the sinusoidal blood from the bile ducts. Hepatocytes are characterized by tissue polarity in which their basal membrane faces the endothelial cells of liver sinusoids, and the apex of hepatocytes forms numerous bile tubules directly adjacent to neighboring hepatocytes[13].
The main function of hepatocytes is to participate in the metabolism of substances, protein synthesis, and the neutralization of toxins. Hepatocytes also produce fibrinogen, a key factor in the blood coagulation system[17-19]. It should be noted that the blood coagulation system is evolutionarily linked to immunity, as it is designed to ensure that the bloodstream is sealed in the event of damage. In addition, hepatocytes are directly involved in immunity by synthesizing acute phase proteins and complement, as well as producing opsonins that promote phagocytosis of foreign pathogens including C-reactive protein, serum amyloid A, and serum amyloid P[19]. Hepatocytes also express a number of immune receptors such as Toll-like receptors (TLRs), retinoic acid-inducible gene 1 and nucleotide-binding and oligomerization domain (NOD)-like receptors. In addition, they are a major source of lipopolysaccharide (LPS) binding protein, soluble cluster of differentiation 14 (sCD14), and soluble myeloid differentiation factor 2 required for LPS signaling[19-22]. In response to cytokine action during liver injury or in response to bacterial infection, hepatocytes can produce acute phase proteins and also produce several chemokines, such as monocyte chemoattractant protein 1 (MCP-1) and C-X-C motif chemokine ligand 1, involved in the recruitment of immune cells (e.g., macrophages and neutrophils)[19].
Hepatocytes are also involved in efferocytosis, the engulfment of other cells, including dead immune cells such as apoptotic T lymphocytes[23,24]. In addition to engulfment by hepatocytes, autoreactive CD8+ T lymphocytes can themselves actively infiltrate hepatocytes where they undergo lysosomal degradation in a process known as suicidal emperipolesis[23,25,26]. In addition to being involved in inflammasome activation, hepatocytes also exert important anti-inflammatory functions, for example through the production of peptidoglycan recognition protein 2. This immunomodulatory protein promotes the hydrolysis of bacterial peptidoglycan to smaller fragments, which impairs its recognition by TLR of immune cells[19].
In addition to mechanisms of the innate immune system, hepatocytes may participate in adaptive immunity by expressing major histocompatibility complex (MHC) I molecules and molecules associated with antigen presentation. During inflammation, some hepatocytes may express MHC II molecules and interact with lymphocytes and induce their activation. MHC II expression on hepatocytes, for example, has been found in alcoholic and non-alcoholic hepatitis[13,27]. Hepatocytes do not express costimulatory factors, cluster of differentiation 80 (CD80) and CD86, so they cannot induce sustained activation and survival of T cells.
Interestingly, the function of hepatocytes depends on their anatomical location. Analysis of hepatocyte populations by single-cell RNA sequencing, identified several clusters of hepatocytes that differ in function, including hepatocyte clusters found to express genes involved in immunity[28].
Thus, hepatocytes, as the main parenchymatous cells of the liver, perform a number of immune functions, which are of importance in various diseases.
LSECs are highly specialized cells that play a critical role in maintaining liver homeostasis and are involved in a variety of physiological and pathological processes. LSECs have a unique fenestrated structure that is essential for the exchange of nutrients and waste products between the blood and liver parenchyma[29,30]. To perform this function, LSECs do not have a dense basal membrane (unlike normal endothelium), which facilitates the exchange of molecules. Fenestrae are small pores 50-300 nm in diameter, and their total area is approximately 10% of the entire surface area of the LSEC[31-34]. Fenestrae are absent in other types of endothelium, and in the liver provide direct filtration of substances between blood and hepatocytes. Fenestrae allow large lipoproteins (e.g., chylomicrons) to penetrate into the space of Disse for subsequent metabolism. In addition, LSECs, like vascular endothelial cells, are involved in the control of vascular tone through the production of vasoactive substances (e.g., nitric oxide [NO]), regulating sinusoid diameter and pressure in the portal system. In addition, NO produced by LSECs is involved in the regulation of LSECs differentiation and hepatic lipid homeostasis[35,36].
In addition to transport and vasoregulatory functions, LSECs play an important role in liver immunology. They act as scavenger cells, removing potentially harmful macromolecules from the blood via receptor-mediated endocytosis[30,37-40]. LSECs also remove soluble colloidal or macromolecular wastes via clathrin-mediated endocytosis[32,39,40]. Thus, LSECs act together with Kupffer cells to remove bacteria as well as dead and dying cells and insoluble waste by phagocytosis. LSECs have, for example, been shown to promote the uptake and destruction of enterovirus bacteria[41]. A peculiarity of LSECs is that they contain about 15% of the total volume of lysosomes and about 45% of the total volume of pinocytosed vesicles in the liver, although they represent only 2.8% of the total liver volume[42,43].
To exert immune function, LSECs express a wide range of pattern recognition receptors (PRRs) including TLR-3, TLR-4, TLR-7, TLR-9, and MHC and costimulatory molecules[44]. LSECs are also involved in immune mechanisms through cytokine secretion. By secreting interleukin 10 (IL-10), transforming growth factor beta (TGF-β), and other mediators, LSECs modulate the activity of macrophages (Kupffer cells) and liver stellate cells. LSECs interact with other cells, including Kupffer cells, hepatocytes, and other immune cells, playing a central role in the liver's response to injury and disease[45-47]. In liver injury, such as in MASLD and alcoholic liver disease (ALD), LSECs alter the expression of chem
In addition, LSECs participate in liver regeneration by secreting growth factors (hepatocyte growth factor [HGF] and vascular endothelial growth factor [VEGF]) that support hepatocyte proliferation after injury. LSECs secrete angiogenic signals that are crucial for liver regeneration after injury[51]. On the other hand, LSECs are involved in the development and progression of liver fibrosis. They interact with HSCs and also contribute to the fibrotic process through capillarization, i.e. loss of their fenestrated structure[52]. In chronic damage (alcohol, viruses), LSECs activate HSCs via TGF-β, promoting collagen deposition and fibrosis.
Disruption of the fenestrated structure and function of LSECs plays an important role in the pathogenesis of liver disease. The appearance of the basal membrane and loss of fenestra transform sinusoids into capillaries (a process known as capillarization). Capillarization of LSECs impairs the ability of LSECs to absorb and transport various substances, which impairs many liver processes and function. In cirrhosis, alcoholic disease or MASLD, defenestration impairs metabolism and contributes to portal hypertension.
Thus LSECs, although they are endothelial cells, have a number of structural and functional features, which are associated with their diverse functions in the liver.
Kupffer cells are the population of resident macrophages in the liver. Kupffer cells account for 80%-90% of the total number of fixed macrophages in mammals. They have an amoeboid shape and are usually found in the lumen of the liver sinusoids, attaching to the endothelial lining, and are also capable of migrating along the sinusoid walls[43]. Kupffer cells and macrophages differentiated from monocytes (monocyte-derived macrophages) are distinct subgroups of mac
Kupffer cells serve as the first line of defense against pathogens, effectively recognizing and phagocytosing pathogens[55,56]. Liver macrophages play differential roles in inflammation depending on their immunometabolic polarization: Pro-inflammatory M1 phenotype and anti-inflammatory M2 phenotype. The phenotypes are switched due to changes in cell metabolism. The M1 phenotype is characterized by increased glycolysis, reduced oxidative phosphorylation, and a disrupted tricarboxylic acid cycle; by contrast, the M2 phenotype is characterized by normal oxidative phosphorylation and the tricarboxylic acid cycle and moderate glycolysis. M1 polarization is induced by interferon gamma (IFN-γ) and LPS and characterized by the production of pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α). In addition, macrophages with M1 polarization also produce reactive oxygen and nitrogen species and are involved in phagocytosis and antigen presentation. M2 polarizing macrophages are induced by IL-4 and IL-13 and are characterized by the production of anti-inflammatory cytokines such as IL-10. M2 macrophages are involved in tissue repair, angiogenesis, and immune regulation. This is due to the switch of arginine metabolic pathways from NO production (in M1 macrophages) to ornithine and proline production (in M2 macrophages) required for tissue repair after inflammation[57-60].
Disruption of macrophage polarization has been reported in a variety of diseases including cancer, metabolic and inflammatory diseases. In cancer, tumor-associated macrophages promote tumor growth by stimulating angiogenesis and suppressing the immune response. In metabolic disorders such as obesity and type 2 diabetes, macrophages in adipose tissue undergo M1 polarization and contribute to chronic inflammation, insulin resistance, and glucose intolerance.
Kupffer cells are major effector cells that damage hepatocytes by producing pro-inflammatory cytokines, lysosomal enzymes, phospholipase, reactive oxygen species (ROS) and NO. In addition to participating in inflammation activation, Kupffer cells may play an important role in immunological tolerance by suppressing the activity of effector T cells and activating regulatory T cells (Tregs)[54,61-63]. Kupffer cells are also the major antigen-presenting cells (APCs) in the liver.
Kupffer cells play an important role in cholesterol metabolism. They take up and transport low-density lipoprotein (LDL)-derived cholesterol into hepatocytes and are involved in the regulation of high-density lipoprotein (HDL) and LDL cholesterol levels by the cholesterol ester transfer protein (CETP) surface receptor[53,64]. Kupffer cells produce CETP in plasma[64]. CETP is a plasma protein that transfers cholesterol from HDL to the atherogenic LDL fraction[65]. CETP plays an important role in reverse cholesterol transport, a homeostatic pathway to maintain cholesterol balance. During reverse cholesterol transport, excess cholesterol is transported from macrophages to HDL and then to the liver for utilization. It is important to note that reverse cholesterol transport can be used not only for cholesterol transport but also as a pathway for utilization of LPS in the liver. In this regard, the pathways of cholesterol metabolism are closely related to the immune function of macrophages. Thus, Kupffer cells are important participants in the innate immune system of the liver.
HSC are non-parenchymatous cells located in the Disse space between hepatocytes and endothelial cells of liver sinusoids. The most characteristic feature of these cells is the presence of cytoplasmic perinuclear droplets containing retinol (vitamin A) esters, as HSCs carry out storage of this vitamin[66].
In the quiescent state, HSCs can produce cytokines and growth factors such as HGF[67]. Liver stellate cells participate in liver regeneration by producing growth factors necessary for liver development and repair, and they also maintain the balance of the extracellular matrix (ECM), which is essential for maintaining liver structure and function. In addition, HSCs can interact with LSECs to regulate hepatic blood flow[66].
Upon liver injury, HSCs switch from a quiescent state to an activated myofibroblast-like phenotype. This activation includes proliferation, increased ECM production, and secretion of pro-inflammatory and profibrogenic cytokines, all of which contribute to the development of hepatic fibrosis. HSC activation involves loss of lipid droplets and expression of contractile fibers, increased proliferation and chemotactic activity of HSCs, and signaling for leukocyte recruitment[67]. Upon activation, human HSCs express smooth muscle α-actin and begin to produce an ECM composed of collagen (e.g., collagen type I alpha 1 chain, collagen type I alpha 2 chain). Alpha smooth muscle actin production during HSC activation contributes to vascular deformation, increased vascular resistance, and the development of portal hypertension[66]. Activated HSCs are a major source of collagen in liver fibrosis, leading to excessive ECM deposition and tissue scarring. HSCs contribute to the inflammatory response and participate in the development of HCC by interacting with cancer cells and promoting tumor growth and angiogenesis. NK cells can induce remission of liver fibrosis by destroying HSCs and producing IFN-γ in a mouse model. At the same time, activated hepatic star cells reduce the ability of NK cells to fight fibrosis through TGF-β-dependent emperipolesis, which has been shown in patients with hepatitis B virus (HBV)-induced cirrhosis[68].
Lymphocytes are a major cell population in the liver and are represented by many different subtypes. Both resident and migratory T-cell subtypes are present in the liver and play a critical role in regulating inflammation and antimicrobial defense of the liver. Given the peculiarities of the liver structure, circulating T cells in the blood may interact with antigen-presenting liver cells in the sinusoids. As already mentioned, APCs in the liver are dendritic cells, LSECs, and Kupffer cells, which are readily accessible to circulating naive T cells in the blood. In addition, hepatocytes can also perform antigen-presenting functions in inflammation[69,70].
CD4+ T cells in the liver coordinate the immune response by secreting cytokines and are also involved in chronic liver inflammation and fibrosis[70,71]. Cytotoxic CD8+ T cells directly destroy infected or cancerous cells and play an important role in viral hepatitis (e.g., HBV, hepatitis C virus [HCV])[72,73]. Recognition and destruction of APCs is performed by CD8+ T cells through binding of MHC class I molecules and the T-cell receptor (TCR) on the cell surface[74]. Upon activation, CD8+ T cells produce IFN-γ and other cytokines and exhibit cytotoxic activity through the release of perforin and granzyme B[72,75,76].
CD8+ T cells play an important role in liver inflammation, affecting the liver in a variety of ways and contributing to both direct and indirect liver damage. CD8+ T cells can exert a direct cytotoxic effect on hepatocytes through the production of perforin, leading to the release of signaling molecules such as IL-33, which can activate other immune cells and promote further liver inflammation[75,77,78]. CD8+ T cells can cause secondary liver injury by stimulating Kupffer cells to produce TNF-α, which enhances the inflammatory response and promotes liver damage[79]. CD8+ T cells can travel to the liver sinusoids where they interact with platelets and detect antigens in hepatocytes, leading to their activation and subsequent inflammatory responses[72,80,81].
Another important subgroup of liver lymphocytes is represented by NK cells. NK cells account for 25%-50% of the total number of liver lymphocytes and play a central role in innate immunity, including early antiviral and antitumor properties[82-84]. The number of NK cells in the liver is altered in various diseases due to an increase in the influx of circulating NK cells into the liver[85,86]. These cells include conventional NK cells (circulating subset), which mediate antiviral and antitumor responses and resident liver NK cells (tissue-specific), which exhibit adaptive properties and regulate fibrosis[87,88]. NK cells can increase liver inflammation by secreting pro-inflammatory cytokines and cytotoxic action on hepatocytes and cholangiocytes. At the same time, NK cells can also attenuate liver inflammation by killing activated T cells and pathogenic cells located in the liver. Thus, the effect of NK cells on liver disease is mediated by their direct cytotoxic function against hepatocytes, cholangiocytes or immune cells in the liver, including T cells and APCs, as well as by the secretion of cytokines, including IFN-γ[84].
NK T cells (NKT cells) are another unique subset of lymphocytes that link innate and adaptive immunity and play a critical role in liver homeostasis, inflammation, and disease. In the liver, NKT cells are particularly abundant compared to other organs, making them key players in liver immunology. NKT cells are divided into two major subsets according to their TCR diversity and antigen specificity: (1) Type I invariant NKT cells recognize lipid antigens via CD1d and express a semi-invariant TCR[89,90] and play diverse roles in liver diseases including viral, autoimmune, and toxic liver diseases[82,91,92]; and (2) The second group consists of type II NKT cells, which have a variety of TCRs. Thus, two different subtypes of NKT cells recognize antigens differently and may play opposite roles in inflammatory liver diseases[91,93,94]. Type I NKT cells, when activated, secrete T helper 1 (Th1) cytokines (IFN-γ, TNF-α) and Th2 cytokines (IL-4, IL-13) and destroy infected or stressed cells with cytotoxic granules (perforin, granzyme). These cells modulate immune responses in viral hepatitis (HBV, HCV), fibrosis, and HCC, which contribute to liver protection against viral infections and tumors. On the other hand, overactivation of these cells leads to liver damage[95-97]. Type II NKT cells often counteract the effects of type I NKT cells by promoting tolerance or suppressing inflammation[90]. Type II NKT cells are associated with autoimmune liver diseases[98]. When activated, type II NKT cells can produce cytokines such as IL-13 and IFN-γ, although their cytokine profile may vary depending on the stimulus[99].
Mucosal-associated invariant T (MAIT) cells comprise about 10%-40% of all T lymphocytes in the human liver[100]. These cells express TCR-α and TCR-β, but differ from conventional T cells in that this receptor has a limited TCR diversity, which consists of a semi-invariant TCR-α chain linked to a limited repertoire of TCR-β chains. In addition, MAIT cells are restricted not by MHC but by the nonpolymorphic antigen-presenting molecule MHC class I-related protein 1[101,102]. MAIT cells exert protective effects on chronic viral hepatitis, alcoholic hepatitis, MASLD, primary sclerosing cholangitis (PSC), and decompensated cirrhosis[103]. A decrease in the number of MAIT cells in the blood and liver of patients with primary biliary cholangitis (PBC) compared with the control group has been shown. Moreover, MAIT cells in patients with PBC are activated, depleted, and persistently deficient, including after ursodeoxycholic acid treatment[104].
γδ T cells are another important cell population that accounts for about 3%-5% of the total number of lymphocytes in the liver[105]. γδ T cells continuously populate the liver, especially in the sinusoids and periportal regions. γδ T cells can function as an innate immune system because their TCRs function as PRRs and can also produce cytokines such as TNF-α and IFN-γ in response to infection or tumor antigens and are capable of phagocytosis[106-108]. At the same time, γδ T cells can also participate in the adaptive immune response due to the ability to rearrange TCR genes, providing greater diversity potential in putative ligand binding sites, and also exhibit the development of a memory phenotype[109-112]. Because of this dual function, γδ T cells are considered an evolutionarily early lymphocyte population that is functionally intermediate between innate and adaptive immunity[113,114]. The immune functions of γδ T cells are also ensured by the production of various biologically active substances, including representatives of the C-type lectin family (antimicrobial peptide RegIIIγ, which kills Gram-positive bacteria, and its related lectin RegIIIβ), granzymes and perforins, which have cytotoxic effects against infected and tumor cells, as well as immunosuppressive cytokines such as TGF-β or IL-10 that regulate other cells involved in innate immunity mechanisms[115-119].
γδ T cells show different activities against tumors. On the one hand, they are characterized by antitumor activity, as they recognize stress ligands (e.g., MHC class I polypeptide-related sequence A and B) on tumor cells and mediate cytotoxicity, as well as produce IFN-γ to enhance antitumor immunity and inhibit angiogenesis[120-122]. On the other hand, tumor-infiltrating γδ T cells can acquire immunosuppressive phenotypes (e.g., programmed cell death 1 [PD-1], IL-17), contributing to impaired immunity. IL-17 produced by γδ T cells[120,123] can stimulate angiogenesis and tumor growth[124-128]. It was also shown that HCC-infiltrating γδ T cells were cytotoxic but depleted. HCC-infiltrating γδ T cells show reduced proliferation and cell cycle arrest at the G2/M stage[129].
In general, γδ T cells may play important roles in a variety of diseases and conditions, including liver infections, MASLD, autoimmune hepatitis, fibrosis and cirrhosis, cholangitis, and in the development of liver cancer and regen
Thus, liver lymphocytes are represented by a vast group of functionally heterogeneous cells that maintain immune balance by linking innate and adaptive immunity.
Chronic liver diseases involve a number of common links regardless of etiology. Repetitive liver injury leads to fibrosis, cirrhosis, and eventually terminal disease leading to liver failure. All these processes involve closely overlapping immune links, a better understanding of which will improve approaches to the diagnosis and treatment of liver disease.
MASLD is a widespread disease that is closely associated with other metabolic disorders including metabolic syndrome, obesity and diabetes mellitus. MASLD ranges from simple steatosis, to MASH with progressive hepatic fibrosis and possible outcome to cirrhosis[136]. The term MASLD was recently proposed and it replaced the previously used term non-alcoholic fatty liver disease, as MASLD more accurately reflects the contribution of risk factors. The key path
The current understanding of the pathogenesis of MASLD corresponds to the concept of “multiple parallel-hit”, i.e. it is believed that the disease develops as a result of a complex chain of events, many links of which are cross-linked, including insulin resistance, de novo lipogenesis, local and systemic inflammation, disturbances in the structure of gut microbiota, and oxidative stress. The gut microbiota has a close bilateral relationship with the liver, and disruption of the microbiota may contribute to the development and maintenance of inflammation in the liver. The liver-microbiota relationship on the one hand involves the production of primary bile acids (BAs) by the liver, which can regulate the composition of the microbiota; on the other hand, the microbiota can metabolize primary BAs into secondary BAs, which after reabsorption in the intestine, can re-enter the liver and partly the general bloodstream, where they have a regulatory effect on some immune and metabolic processes[140-142]. Secondary BAs are ligands for many receptors, such as the nuclear farnesoid X receptor (FXR), which regulates bile acid synthesis and metabolism[143]. In addition, disruption of the intestinal epithelial immune barrier may lead to an increase in its permeability to bacterial LPS, which contribute to the activation of hepatic immune cells, which is relevant for the development of MASLD. The gut microbiota under normal conditions is also a source of various substances that are involved in various immune and metabolic processes in the human body. For example, short-chain FAs (SCFAs) produced by the gut microbiota are transported to the portal vein, liver and partly to the general bloodstream, where they have various immunomodulatory effects. Disturbances in the composition of the gut microbiota may lead to decreased production of SCFAs, which has important clinical implications, including for liver function[144-147]. In addition, changes in the intestinal microbiota may lead to other metabolic processes, such as excessive ethanol production. Thus, in recent years, there has been increasing interest in the gut-liver axis, in which the gut microbiota plays an important role. Disruption of the function of this axis is considered to be an important link in the development of liver diseases such as MASLD[148-150].
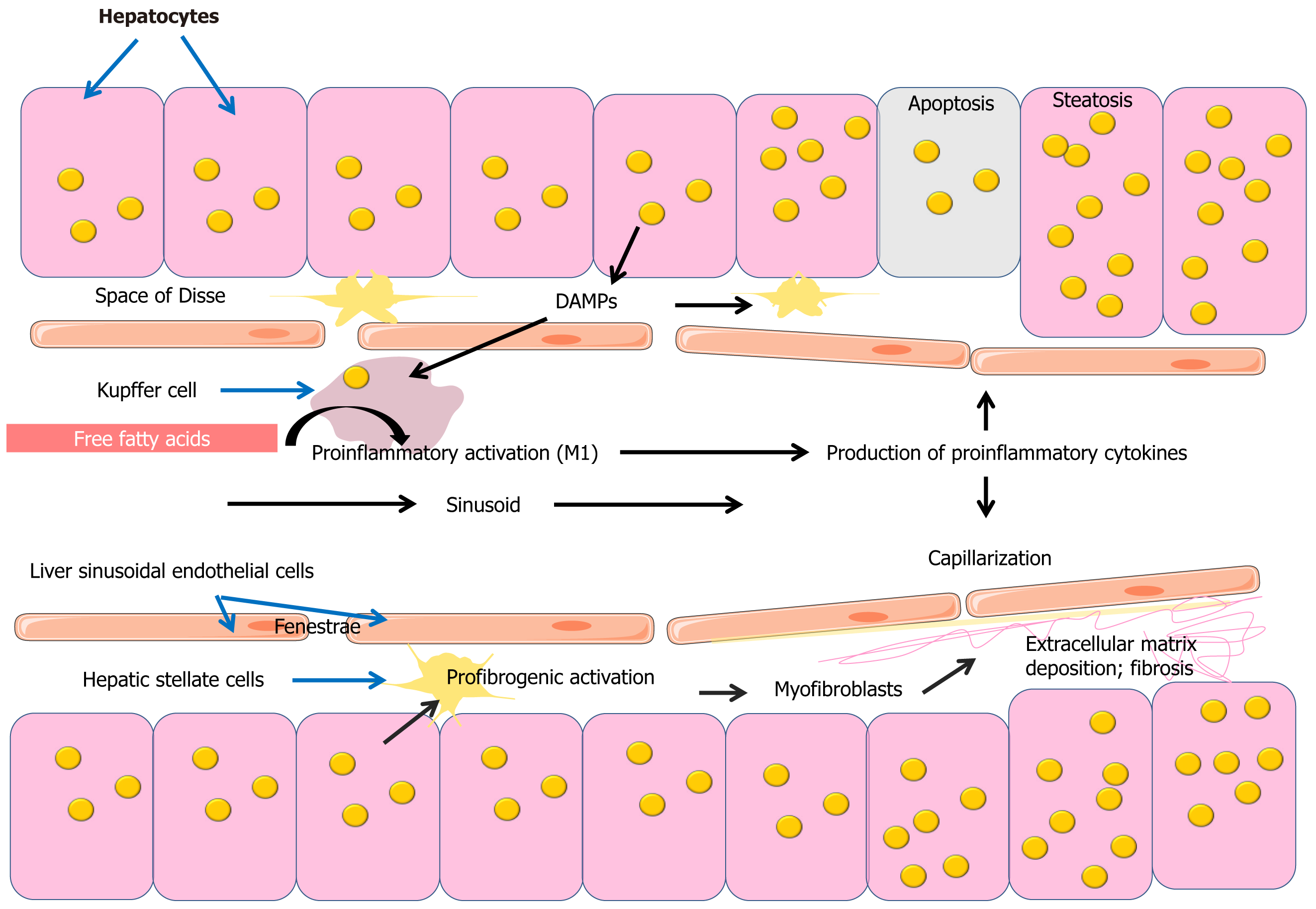
A complex of mechanisms results in the accumulation of excessive amounts of lipid droplets containing triglycerides (triacylglycerols [TAGs]) in hepatocytes in MASLD (Figure 2)[151-153]. Lipid accumulation results from an imbalance between lipid influx, de novo lipogenesis, and lipid export. The most widely accepted model of lipid droplet formation to date suggests that excess TAG accumulates on endoplasmic reticulum membranes, forming vesicles of a lipid bilayer of TAG and cholesterol esters in the center and a monolayer of phospholipids and sphingomyelin on the outside[154]. TAG biosynthesis utilizes FAs, which may enter hepatocytes from the blood or be formed by de novo lipogenesis and endocytotic recycling of lipoprotein residues. Moreover, the main source of FAs used for TAG synthesis is FAs from blood[152,155,156]. Insulin resistance contributes to increased delivery of FFAs to the liver, leading to triglyceride accumulation in hepatocytes. It should be noted that data suggest that TAG accumulation is not toxic to liver cells and is even considered s a certain protective mechanism against lipotoxicity induced by FFAs[157,158].
Excess lipids in liver cells leads to organelle dysfunction, which contributes to mitochondrial dysfunction and endoplasmic reticulum stress[159]. The endoplasmic reticulum performs an important cellular function, namely, it is involved in protein and lipid synthesis. Therefore, endoplasmic reticulum stress against the background of excess lipid accumulation is characterized by the accumulation of misfolded or unfolded proteins and leads to the activation of an adaptive cellular response called the unfolded protein response (UPR). This mechanism restores endoplasmic reticulum homeostasis and promotes cell survival. However, if recovery fails, prolonged endoplasmic reticulum stress and UPR activation promote cell death[160]. In addition, endoplasmic reticulum stress is also associated with activation of inflammatory mechanisms through excessive production of ROS and activation of nuclear factor kappa B and c-Jun N-terminal kinase pathways[157,161].
Mitochondrial dysfunction is another important part of the pathogenesis of MASLD, as these organelles have an increased ability to oxidize FAs, producing ROS. The resulting imbalance between ROS and defense mechanisms, contributes to oxidative stress[159]. Oxidative stress can lead to hepatocyte death and is another link in the mechanisms of the development of MASLD[162].
A growing body of evidence is increasing the understanding of the importance of the innate immune system in the development of MASLD. Innate immune cells such as Kupffer cells, neutrophils, dendritic cells, and NK cells play an important role in the pathogenesis of MASLD. In MASLD, the liver is affected by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) such as bacterial LPS and some metabolites such as FFAs and microparticles including mitochondrial DNA derived from steatotic hepatocytes. PAMPs and DAMPs are recognized by PRRs such as TLRs and NOD-like receptors, leading to activation of proinflammatory signaling pathways[163-166].
The transition from steatosis to MASH is associated with the development of liver inflammation (Figure 2). In steatohepatitis, there is an increase and aggregation of Kupffer cells in perivenular areas. Scattered large fat vacuoles are found within the Kupffer cells[167]. Kupffer cells take up large amounts of FFAs, which contributes to their proinflammatory activation, in which Kupffer cells are involved in the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, which contribute to the progression of inflammation and development of MASH[168]. TLRs are important contributors to inflammation in MASH. Saturated FAs can activate proinflammatory TLR-4 pathways, mediating the link between lipid metabolism and the innate immune system[169-171].
LSECs are another important player in the pathogenesis of MASLD. Disruption of the normal LSEC phenotype promotes capillarization of sinusoidal spaces, a critical step in the fibrotic process in the liver. LSECs are also involved in the regulation of HSC activation and play an important role in liver immunology and the development of MASLD[172].
Liver-derived CD4+ T cells may be involved in the pathogenesis of MASH. Naive CD4+ T cells can be activated in the liver by various APCs and then transform into effector T cells with several polarization variants, such as Th1, Th2, and Th17. The different profile of cytokines produced by these effector cells is associated with the development of MASH. Th1 cells are involved in the production of IFN-γ and predominate in the liver of patients with MASH, while Th17 cells are present in the liver and peripheral blood of mice with MASH and are characterized by the production of IL-17A, IL-17F and IL-22. The IL-17A and IL-17F axis has been shown to play an important role in the development and progression of MASH, whereas the use of monoclonal antibodies against IL-17 in the experiment improved liver function, reduced hepatic lipid accumulation, decreased Kupffer cell activation, and decreased proinflammatory cytokine levels in a model of MASH induced by a high-fat diet[70,173-176]. In addition, effector T cells can form effector memory T cells (TEMs) and central TEMs, whose cytokines are also associated with the development of MASH[70].
CD8+ T cells in the liver may also be involved in the development of MASH. The number of CD8+ T cells is increased in MASH and they directly activate HSCs, promoting inflammation and fibrosis[77]. CD8+ T cells produce pro-inflammatory cytokines such as IFN-γ and TNF-α, which play a key role in the development of inflammation and liver damage[77,79,177,178].
Inflammation and fibrosis are important steps in the progression of MASH (Figure 2). Inflammatory mediators such as cytokines and chemokines enhance the inflammatory response. This cascade of events triggers the activation of HSCs and deposition of ECM, leading to hepatic fibrosis. Importantly, increasing the stage of fibrosis significantly increases the risk of liver-related mortality, emphasizing the importance of fibrosis[179]. Liver stellate cells, which differentiate into a myofibroblast phenotype, play an important role in the development of liver fibrosis and are involved in the synthesis and deposition of ECM proteins[180,181]. Accumulation of free cholesterol in HSC also exacerbates hepatic fibrosis[182,183].
Thus, the development and progression of MASLD is a complex process in which immune and metabolic mechanisms are intertwined and occur not only in the liver but also at the systemic level, which affects the course of comorbidities.
The role of immunotherapy in the treatment of MASLD and its complications is an emerging area of research, although it is still in its early stages. Immune checkpoint inhibitors (ICIs), including anti-PD-1/programmed death-ligand 1 (PD-L1) therapy, are promising avenues for immunotherapy. Immunotherapy using antibodies to PD-1 and PD-L1 has shown efficacy in slowing the progression of MASLD and treating HCC associated with MASLD. However, the efficacy of these therapies is still under investigation, and results regarding patient prognosis are inconsistent[184].
Therapies targeting immune cells, such as macrophage polarization, are another promising strategy. Understanding the role of immune cells in MASLD is crucial for the development of targeted immunotherapy[185].
Mesenchymal stem cells (MSCs) have shown promising results in preclinical and early clinical studies in liver diseases. It has been shown that MSCs can improve liver histology, reduce fibrosis, and restore liver functional capacity. Extracellular vesicles (EVs) and exosomes derived from MSCs may find applications in treating liver failure and mitigating post-transplant complications. Autologous MSC-derived hepatocytes are of interest for the treatment of cirrhosis, and allogeneic MSCs may have applications in liver failure and liver transplantation. However, a number of issues, including long-term safety, need to be addressed for the use of MSCs[186,187].
Immunotherapy is a promising treatment for liver fibrosis by modulating the immune response and affecting the immune microenvironment of the liver. Regulation of the immune microenvironment is considered a promising thera
FXR agonists are a promising therapeutic approach for the treatment of MASLD, including its more severe form, MASH. FXR is a nuclear receptor that plays a key role in the regulation of bile acid synthesis, lipid metabolism, glucose homeostasis and inflammation, making it an important target for the treatment of MASLD[190].
Activation of FXR lowers hepatic lipid levels by reducing FA synthesis and absorption. This is achieved by suppressing genes involved in lipogenesis, such as sCD1, diacylglycerol O-acyltransferase 2, and lipin-1, and by reducing bile acid absorption[191,192]. FXR regulates bile acid synthesis and transport, which is critical for maintaining lipid and glucose homeostasis. This regulation helps prevent bile acid-induced cytotoxicity. FXR agonists also inhibit inflammation and fibrosis, which is particularly important in preventing the progression of MASLD[193,194]. In this regard, drugs targeting FXR are of clinical interest[191].
The clinical use of fibroblast growth factor 21 (FGF21) analogs is also of interest. FGF21 is a hormone-like growth factor that is produced by several organs, including the liver, and plays an important role in regulating glucose and lipid metabolism, insulin sensitivity, and energy expenditure[195,196]. FGF21 increases hepatic insulin sensitivity, reduces lipogenesis, triggers β-oxidation of FAs, reduces endoplasmic reticulum stress of hepatic cells, and reduces LDL entry into the liver[195,197]. FGF21 analogs have shown promising results in reducing fasting insulin levels, body weight and total cholesterol levels in patients with metabolic syndrome[198]. They also improve insulin sensitivity and promote weight loss in animal models[199]. Clinical studies have shown that FGF21 analogs can improve conditions in dyslipidemia, especially hypertriglyceridemia, and in hepatic steatosis. They also reduce inflammation, levels of biomarkers of fibrosis and liver damage, and eliminate steatohepatitis associated metabolic dysfunction[200-202].
Thus, MASLD is an immunometabolic disease in which various cells are involved in the development and progression, and immunotherapy is considered as a promising strategy for the treatment of MASLD (Table 1)[78,157,184,190-228].
| Therapeutic approach | Mechanism | Current state | Ref. |
| Anti-programmed cell death 1/programmed death-ligand 1 therapy | Inhibition of immune checkpoint | Early phase, mixed results | Lombardi et al[74], Jeong et al[184], Zhang et al[203], Pinto et al[204] |
| MSCs | Immunomodulatory, anti-inflammatory agent | Promising preclinical results. MSC heterogeneity and long-term safety issues remain serious obstacles | Moayedfard et al[157], Niu et al[205], Yang et al[206], Hu et al[207] |
| Extracellular vesicles | Modulation of the immune response | Promising preclinical results | Ipsen et al[208], Abreo Medina et al[209], Niu et al[205], Yang et al[206] |
| Probiotics (Lactobacillus plantarum, Bifidobacterium bifidum) | Modulation of intestinal microbiota, anti-inflammatory agent | Lu et al[210], Zhang et al[211], Wen et al[212], Tang et al[213] | |
| Targeting cytokines (e.g., IL-17), IL-1β inhibitors tumor necrosis factor-alpha inhibitors nucleotide-binding and oligomerization domain-like receptor protein 3 inflammasome inhibition | Reducing inflammation | Preclinical studies have shown mixed results | Rafaqat et al[214], Paquissi et al[215], Kucsera et al[216], Duan et al[217], Bakhshi et al[218], Mridha et al[219] |
| Natural anti-inflammatory agents, astaxanthin. Apigenin, ginsenoside Rg1 | Reducing inflammation | Preclinical studies have shown promising results | Lv et al[220], Yang et al[221], Xu et al[222] |
| Inhibition of apoptosis signal-regulating kinase 1 (selonsertib [GS-4997]) | Reduction of inflammation and fibrosis | Patients with MASH may develop liver scarring (fibrosis), including cirrhosis, which increases the risk of liver failure and liver cancer | Harrison et al[223], Loomba et al[224] |
| Caspase inhibition (Emricasan [IDN-6556]) | Reduced hepatocyte apoptosis in a mouse model of MASH liver injury and fibrosis are suppressed by inhibiting hepatocyte apoptosis. | Emricasan treatment did not improve liver histologic pattern in patients with MASH fibrosis. Emricasan administration was not associated with improved hepatic venous pressure gradient or clinical outcomes in patients with MASH-related cirrhosis and severe portal hypertension | Harrison et al[225], Barreyro et al[226], Frenette et al[227], Garcia-Tsao et al[228] |
| FXR agonists | Regulation of bile acid synthesis, lipid metabolism, glucose homeostasis and inflammation | FXR agonists have shown promising results | Gandhe et al[190], Tang et al[191], Clifford et al[192], Adorini et al[193], Xu et al[194] |
| FGF21 analogs | Regulation of glucose and lipid metabolism, insulin sensitivity | FGF21 analogs have shown promising results | Su et al[195], Harrison et al[196], Falamarzi et al[197], Carbonetti et al[198], Corbee et al[199], Raptis et al[200], Chui et al[201], Theofilis et al[202] |
Alcoholic liver damage is an important medical and social problem. Ethanol is metabolized in the liver mainly by alcohol dehydrogenase and the microsomal ethanol–oxidizing system, which includes cytochrome P450 2E1. The resulting acetaldehyde is a toxic metabolite that contributes to liver damage. In addition, ethanol metabolism leads to the formation of ROS, which causes oxidative stress. ROS can damage hepatocytes, Kupffer cells, and HSCs, causing inflammation and fibrosis[229,230].
In alcoholic fatty liver disease, hepatocytes are damaged by chronic alcohol consumption and may activate Kupffer cells through the production of EVs. Alcohol can also damage the intestinal barrier by increasing its permeability to Gram-negative bacteria. LPS from these bacteria is involved in the activation of Kupffer cells and promotes their production of proinflammatory cytokines, including IL-9, IL-1β, TNF-α, and MCP-1. In addition, a population of macrophages differentiated from recruited blood monocytes increases in the liver. These macrophages may play various roles in inflammation, which may influence the course of ALD[231]. Chronic ethanol exposure enhances the ability of LPS to increase ROS production in Kupffer cells[232].
A significant accumulation of CD4+ T cells, exceeding the number of CD8+ T cells, was found in the liver of patients with ALD, in contrast to patients with MASLD and healthy subjects, whereas the number of NK cells and γδ T cells in the intrahepatic infiltration was reduced[233]. Patients with alcoholic hepatitis showed marked immunosuppression, increased production of proinflammatory cytokines/chemokines, lower levels of anti-inflammatory cytokines, and more activated but dysfunctional immune cells[234]. In addition, alcoholic fatty liver disease, alcoholic cirrhosis, and mixed cirrhosis (alcoholic + viral) have significantly reduced the levels of MAIT cells. This is due to the tendency to apoptosis of these cells in patients with alcoholic cirrhosis. Moreover, the decreased number of circulating MAIT cells correlated with liver function in patients with cirrhosis may play a role as an indicator of disease severity[235,236]. The reduced number of MAIT cells in the blood in severe alcoholic hepatitis, corresponded to their hyperactivation and impaired antibacterial cytokine/cytotoxic responses[237]. Reduced MAIT cells in patients with ALD contribute to fibrogenesis and bacterial translocation due to impaired antibacterial activity[237,238]. Alcohol withdrawal does not completely eliminate MAIT cell impairment in the blood of patients with alcoholic hepatitis[239]. In addition, MAIT cells in alcoholic liver cirrhosis patients produce more IL-17 and express more early and late activation markers CD69, PD-1, and lymphocyte-activation gene 3 compared to healthy individuals. In addition to MAIT cell deficiency, patients with alcoholic liver cirrhosis exhibit a deficiency of circulating NKT cells and NK cells, as well as altered cytokine production and activation status. NKT cells in alcoholic liver cirrhosis patients showed a decreased ability to produce IFN-γ and IL-4[235]. Alcohol consumption suppresses the antifibrotic function of NK cells, thereby inducing liver fibrosis[240].
Chronic alcohol consumption also affects the composition of the intestinal microbiota. Alcohol-induced dysbiosis affects the production of microbiome metabolites, including SCFAs[241].
Thus, alcoholic liver damage is characterized by a complex combination of impaired metabolic and immune mechanisms.
HCC arises from hepatocytes and is one of the leading causes of cancer mortality worldwide[242-244]. The main risk factors for HCC are chronic infection with HBV or HCV, ALD, and MASH. Moreover, 70%-90% of patients with HCC have chronic liver disease and cirrhosis[245]. Thus, HCC is usually associated with chronic inflammation that disrupts the immune homeostasis of the liver, including leading to immunosuppression[246]. The immune system attempts to control tumor progression through tumor-infiltrating lymphocytes, which does not always prevent tumor growth, due to failure in immune control mechanisms[247].
Several immune cells are involved in the mechanism of hepatocarcinogenesis: (1) Macrophages; (2) Myeloid suppressor cells; (3) Kupffer cells; (4) Neutrophils; (5) Dendritic cells; and (6) NK cells[248]. The immune microenvironment of the liver is a multicomponent system that plays an important role in tumor development and progression. The tumor microenvironment (TME) can contribute to both protumor and antitumor activity[249,250]. Macrophages play an important role in oncogenesis. Tumor-associated macrophages (TAMs) are one of the most common tumor-infiltrating immune cell types and contain two polarized phenotypes: (1) Tumor-suppressive M1; and (2) Oncogenic M2. The function of M1 macrophages promotes tumor cell apoptosis, reduces drug resistance, and suppresses invasion, migration, and tumor development. By contrast, M2 macrophages promote tumor development, progression, and metastasis through various mechanisms[251-254]. Various cytokines such as ILs, TNFs, and chemokines are involved in the immune response to HCC. They can have both stimulatory and suppressive effects on the tumor depending on their specific role[255].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of early myeloid precursors and immature granulocytes at different stages of differentiation and are known for their immunosuppressive properties. These cells play an important role in the TME in HCC, promoting tumor progression, angiogenesis, metastasis, and resistance to therapy[256,257]. They accomplish this by creating an immunosuppressive TME that promotes cancer cell survival and proliferation. These cells can suppress the innate immunity of NK cells and the adaptive immune response of CD4+ and CD8+ T cells, which are critical for antitumor immunity[252,258]. MDSCs also induce an increase in Treg and TAM, further suppressing the immune response against HCC[259,260].
Tumor-associated neutrophils (TAN) infiltrate the TME and may exert antitumor N1 and protumor N2 functions. The accumulation of infiltrating TANs in the peritumoral region is negatively correlated with the number of T cells, and the ratio of these cells correlates with the patients' life expectancy after surgical treatment, which may have prognostic sig
HCC utilizes multiple mechanisms to circumvent the immune system, including the suppression of effector T cells by Tregs and alteration of immune cell functions through metabolic reprogramming and cytokine production[262]. Tregs play an important role in immune evasion by suppressing the functions of other immune cells and thereby supporting tumor growth[247,262]. Interestingly, HCC cells are able to engulf neighboring cells through entosis, a pathological process involving E-cadherin and β-catenin[23,263]. Internalized cells primarily undergo non-apoptotic death and undergo lysosomal degradation; however, they can survive and be released. The significance of entosis is a subject of research, but it may support tumor cells with energy substrates[23].
Immunotherapy has emerged as a promising treatment for HCC. ICIs involve the use of cytotoxic T-lymphocyte-associated protein 4 and PD1/PD-L1 inhibitors. These drugs block inhibitory pathways in T cells, enhancing the immune response against tumor cells. Clinical trials have shown that immune response checkpoint inhibitors can improve survival in patients with HCC[264,265]. The PD-1 receptor is a negative regulator of T-cell activity. Binding of PD-1 to PD-L1 and PD-L2 ligands, which are capable of being expressed by tumor cells or other cells in the TME, results in inhibition of T cell proliferation and cytokine secretion. In studies in mouse models, blocking PD-1 activity resulted in reduced tumor growth. Therefore, studies of drugs that block the PD-1 system are of clinical interest[184,266-269].
Combining ICIs with other therapies such as anti-VEGF drugs such as bevacizumab has demonstrated significant improvement in patient outcomes compared to monotherapy[270,271]. The combination of the PD-L1 inhibitor atezolizumab and the monoclonal anti-VEGF antibody bevacizumab intravenously is considered as first-line systemic therapy for HCC to improve tumor growth control and patient survival[272]. In patients with initial signs of decompensated cirrhosis or contraindications to the use of protein kinase inhibitors, nivolumab immunotherapy is recommended as an alternative to improve tumor growth control and patient survival in one of the recommended treatment regimens[273]. Nivolumab potentiates the immune response through blockade of PD-1 binding to PD-L1 and PD-L2 ligands.
Adaptive cell therapy involves the use of chimeric antigen receptor (CAR) T cells. These T cells with a CAR are designed to target specific antigens in HCC cells and are showing promising results in clinical trials[271,274,275]. Vaccines designed to elicit an immune response against HCC-specific antigens are under investigation and have shown efficacy in enhancing antitumor immunity[276,277]. Immunotherapy is being studied as a preoperative and postoperative treatment to reduce the risk of recurrence and improve long-term outcomes. This includes shrinking tumors to make them resectable and controlling tumor growth after surgery[278,279].
Thus, HCC is characterized by complex immune abnormalities involving various cells in the TME, which is a promising target for therapy.
The biliary immune system is a complex and specialized system that plays a critical role in maintaining homeostasis and defending against infection. Biliary epithelial cells (BECs) have TLRs that recognize PAMPs. Disruption of TLR regulation, especially TLR-3 and TLR-4, leads to hypersensitivity to PAMPs, contributing to chronic inflammation and cholangitis in PBC[280-282]. BECs produce MCP-1/C-C motif ligand 2, fractalkine (chemokine [C-X3-C motif] ligand 1), and macrophage inflammatory protein-3α (MIP-3α), which attract inflammatory cells and promote liver fibrosis[281]. The bile ducts are also home to various immune cells, including macrophages, T cells and neutrophils. Kupffer cells and NK cells interact with BECs to modulate the immune response. NK cells stimulated by TLR ligands can destroy BECs in the presence of IFN-α produced by monocytes[280,283]. The innate immune responses in the bile ducts trigger the production of two chemokines, fractalkine and MIP-3α, which induce migration of inflammatory cells and APC populations and lead to chronic cholangitis specific to the bile duct epithelium in PBC[281]. Secretory immunoglobulin A and BAs exert local antimicrobial effects and protect the biliary tract from damage by signaling through receptors, mainly the Takeda G protein-coupled receptor 5 receptor on cholangiocytes[284].
The biliary system, traditionally considered sterile, contains a complex microbiota even in non-pathologic conditions. Dysbiosis, or microbial imbalance, in the biliary or intestinal microbiota may influence the development of biliary diseases[285]. Bacterial translocation and molecular mimicry are mechanisms by which the gut microbiota can lead to liver inflammation and immune system activation, contributing to AILD such as PBC and PSC[286,287]. BAs synthesized in the liver are modified by the intestinal microbiota, which may affect their reabsorption and enterohepatic circulation. This interaction is critical in the development of biliary tract diseases, including cholelithiasis and cholestasis[286].
Biliary diseases such as PBC and PSC involve complex immune mechanisms that contribute to their pathogenesis. PBC, also known as PBC, is a chronic autoimmune disease characterized by progressive non-purulent destructive inflammation of the small intrahepatic bile ducts, their further destruction followed by fibrosis formation up to cirrho
The etiology and pathogenesis of PBC, as well as other AILD, are not fully understood. An important part of the pathogenesis of PBC is the formation of anti-mitochondrial antibodies (AMA), which target the pyruvate dehydrogenase complex-E2 (PDC-E2) on the inner mitochondrial membrane of BECs[293,294]. These antibodies play a key role in the autoimmune response against BECs[295].
CD4+ T cells also play an important role in the pathogenesis of PBC, producing cytokines that create an inflammatory environment that activates other immune cells, including autoreactive CD8+ T cells that directly cause BEC damage [282,296,297]. B cells are responsible for the production of AMA and other autoantibodies, contributing to the autoimmune response[294,296,298]. Macrophages, NK cells and dendritic cells are also involved in immune-mediated BEC damage. In particular, macrophages contribute to the initiation and progression of hepatic fibrosis and cirrhosis.
In PBC, changes in the gut microbiome may influence the development and progression of the disease. Patients with PBC show significant changes in the composition of the gut microbiota compared to healthy controls. These changes include decreased bacterial diversity and specific shifts in microbial populations[299,300]. Multivariate analysis has shown that overgrowth of Sphingomonadaceae and Pseudomonas is an independent risk factor for PBC[301]. In addition, the common alpha-proteobacterium Novosphingobium aromaticivorans, a common alpha-proteobacterium, was previously found to be a potential factor causing PBC. N. aromaticivorans shares epitopes with the human PDC-E2, which is a major target of AMA in PBC. This molecular mimicry may lead to impaired immune tolerance and autoantibody production[302,303].
As already mentioned, PSC is characterized by inflammation and fibrosis of the bile ducts, leading to cholestasis, cirrhosis and an increased risk of cholangiocarcinoma. The key immune mechanisms involved in PSC is genetic susceptibility. PSC is associated with certain haplotypes of human leukocyte antigen, indicating a genetic predisposition[304]. The pathogenesis of PSC involves both innate and adaptive immune responses. Cells of innate immunity are activated by molecular patterns associated with bacterial pathogens, leading to the production of pro-inflammatory cytokines[305]. Adaptive immune responses include recruitment of gut-activated TEMs to the liver, which contribute to peribiliary inflammation and fibrosis[306]. Cholangiocytes lining the bile ducts play a critical role in PSC. They can be activated by infection, inflammation and cholestasis, leading to increased expression of adhesion molecules and antigen-presenting molecules. Activated cholangiocytes release inflammatory and fibrogenic mediators, promoting immune cell recruitment and fibrosis progression[305]. Adaptive immune responses include recruitment of gut-activated TEMs to the liver, which contribute to peribiliary inflammation and fibrosis. Cholangiocytes lining the bile ducts play a critical role in PSC. They can be activated by infection, inflammation and cholestasis, leading to increased expression of adhesion molecules and antigen-presenting molecules. Activated cholangiocytes release inflammatory and fibrogenic mediators, promoting immune cell recruitment and fibrosis progression[305]. An important feature of PSC is the association with inflammatory bowel disease. Translocation of bacterial products from the inflamed gut to the liver and migration of TEM activated in the gut to the bile ducts are key processes in the pathogenesis of PSC[307].
Thus, the biliary immune system is a complex system of interactions between cholangiocytes, Kupffer cells, T cells and the biliary microbiota. Understanding these interactions is critical for the development of effective therapies for biliary tract diseases and cancer.
Liver disease is a clinical problem whose awareness of its importance has been increasing in the last decade. This has been matched by an increasing number of studies focusing on liver immunology and the cross-talk between metabolism and immunity. The results of these studies indicate that most liver cells have diverse immune functions, increasing the understanding of the complexity of maintaining normal liver immune homeostasis. Importantly, many of the liver diseases share common pathogenetic immune mechanisms or may overlap in the same patient. Therefore, the immune mechanisms of liver diseases are of clinical interest to improve the diagnosis and treatment of these diseases.
Of interest are the data on the role of the gut-liver axis, in which microbial metabolites and components from the gut can influence liver function and immune responses[308]. On one hand gut microbiota components such as LPS and lipoteichoic acid activate TLR on liver immune cells, which triggers the production of pro-inflammatory cytokines, contributing to liver inflammation and immune regulation[150]. On the other hand, the gut microbiota and its metabolites, such as SCFAs and secondary BAs, play an important role in regulating liver immune responses and influence the development of liver diseases such as MASLD, MASH, and liver cancer[148,308]. It has been suggested, for example, that secondary BAs such as deoxycholic acid, produced by gut bacteria as a result of 7α-dehydroxylation of primary BAs, cause DNA damage and contribute to alterations in the TME[150]. The gut microbiota may influence the efficacy of ICIs used in the treatment of liver cancer, emphasizing their role in the therapeutic effects[309]. In this regard, dietary adjustments aimed at maintaining a normal microbiota are a promising therapeutic strategy. Dietary fibers, especially prebiotic fibers such as inulin, promote the growth of beneficial intestinal bacteria such as bifidobacteria and fecal bacteria that produce SCFAs[310,311].
Fecal microbiota transplantation (FMT) is another promising therapeutic approach for the treatment of various liver diseases, utilizing the gut-liver axis to restore microbial balance and improve liver function. FMT has been shown to be effective in the treatment of chronic liver diseases, including cirrhosis and MASLD, by improving microbial diversity and reducing disease severity[312,313].
The role of lipid mediators of inflammation is also a promising area of future research for the clinical application of liver immunology knowledge. Pro-resolution mediators (SPMs) are involved in the resolution of inflammation. These mediators are synthesized from ω-3 and ω-6 polyunsaturated FAs by various cells. SPMs are currently classified into four classes: (1) Lipoxins; (2) Resolvins (D-series and E-series); (3) Maresins; and (4) Protectins. They inhibit neutrophil infiltration, reduce the production of pro-inflammatory mediators (both lipid mediators and cytokines), stimulate the capture of apoptotic neutrophils by macrophages as well as phagocytosis and removal of bacteria, and promote phenotypic switching of macrophages[314-318]. SPMs can modulate insulin signaling pathways, increase insulin sensitivity and glucose uptake, as a consequence of which they are a promising agent for the correction of insulin resistance and in the treatment of obesity as well[319,320]. In this regard, SPMs and their synthetic analogs are attractive therapeutic targets in many inflammatory diseases, including liver diseases[321-323].
Participation of ATP-binding cassette (ABC) transporters in immune mechanisms, which is realized through regulation of lipid transport, transport of LPS, xenobiotics, and interaction with gut microbiota, is also of clinical interest[324]. ATP subfamily A member 1 (ABCA1) gene polymorphisms are considered as a predictor of the development of MASLD[325]. ABCA1 may also be a therapeutic target in fatty liver disease. It has been shown, for example, that the glucagon-like peptide-1 agonist exendin-4 stimulates ABCA1 expression in the liver and reduces lipid accumulation through the Ca2+/calmodulin (CaM)-dependent protein kinase kinase/CaM-dependent protein kinase IV pathway[326].
Targeting immune cells such as macrophages and Kupffer cells is another promising therapeutic strategy. Various natural components and drugs such as CCR2/CCR5 antagonists and galectin-3 inhibitors are being investigated in animal experiments[327,328]. The use of glucagon-like peptide-1 receptor agonists has shown promise as a potential therapeutic strategy for MASLD, which is achieved through several known mechanisms[329]. Liraglutide has been shown to protect against inflammation in MASLD by modulating Kupffer M2 cell polarization through the cyclic adenosine mono
In this regard, studies that identify novel diagnostic biomarkers and targets for immunotherapy are of interest. A better understanding of the links between immune and non-immune functions of liver cells may improve diagnostic and treatment approaches.
| 1. | Soresi M, Giannitrapani L, Cervello M, Licata A, Montalto G. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol. 2014;20:18131-18150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (3)] |
| 2. | Gao E, Hercun J, Heller T, Vilarinho S. Undiagnosed liver diseases. Transl Gastroenterol Hepatol. 2021;6:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1444] [Article Influence: 722.0] [Reference Citation Analysis (2)] |
| 4. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 5. | Mitra V, Metcalf J. Metabolic functions of the liver. Anaesth Intensive Care Med. 2009;10:334-335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Pritchard MT, Apte U. Models to Study Liver Regeneration. In: Apte U, editor. Liver Regeneration. Netherlands: Elsevier, 2015. [DOI] [Full Text] |
| 7. | Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Grant DM. Detoxification pathways in the liver. J Inherit Metab Dis. 1991;14:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 775] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 10. | Zheng M, Tian Z. Liver-Mediated Adaptive Immune Tolerance. Front Immunol. 2019;10:2525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 11. | Qu B, Zhang S, Ma Z, Gao Z. Hepatic cecum: a key integrator of immunity in amphioxus. Mar Life Sci Technol. 2021;3:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Juza RM, Pauli EM. Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat. 2014;27:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Zhang X, Li Y, Yu J, Chen Z, Niu Y, Ran S, Wang S, Ye W, Luo Z, Li X, Hao Y, Zong J, Xia C, Xia J, Wu J. Interorgan communication with the liver: novel mechanisms and therapeutic targets. Front Immunol. 2023;14:1314123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Zhang S, Chen W, Zhu C. Liver Structure. In: Li L, editor. Artificial Liver. Singapore: Springer, 2021. [DOI] [Full Text] |
| 15. | Devi SS. Structure and Function of Hepatic Parenchymal Cells. In: McQueen CA, editor. Comprehensive Toxicology. Netherlands: Elsevier, 2018. [DOI] [Full Text] |
| 16. | Ou Q, Mu H, Zhou C, Zheng Z, Geng J. Liver Diseases. In: Pan S, Tang J, editors. Clinical Molecular Diagnostics. Singapore: Springer, 2021. [DOI] [Full Text] |
| 17. | Tennent GA, Brennan SO, Stangou AJ, O'Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109:1971-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13:301-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 20. | Grube BJ, Cochane CG, Ye RD, Green CE, McPhail ME, Ulevitch RJ, Tobias PS. Lipopolysaccharide binding protein expression in primary human hepatocytes and HepG2 hepatoma cells. J Biol Chem. 1994;269:8477-8482. [PubMed] |
| 21. | Zhang WJ, Li KY, Huang BH, Wang H, Wan SG, Zhou SC. The hepatocyte in the innate immunity. Virology. 2022;576:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Scott MJ, Chen C, Sun Q, Billiar TR. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J Hepatol. 2010;53:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Davies SP, Terry LV, Wilkinson AL, Stamataki Z. Cell-in-Cell Structures in the Liver: A Tale of Four E's. Front Immunol. 2020;11:650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Sierro F, Tay SS, Warren A, Le Couteur DG, McCaughan GW, Bowen DG, Bertolino P. Suicidal emperipolesis: a process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr Mol Med. 2015;15:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, Breen E, Allison AC, van Rooijen N, McGuffog C, Schlitt HJ, Bowen DG, McCaughan GW, Bertolino P. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A. 2011;108:16735-16740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Lu JG, Iyasu A, French B, Tillman B, French SW. Overexpression of MHCII by hepatocytes in alcoholic hepatitis (AH) compared to non-alcoholic steatohepatitis (NASH) and normal controls. Alcohol. 2020;84:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD, McGilvray ID. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 1017] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 29. | Gracia-Sancho J, Caparrós E, Fernández-Iglesias A, Francés R. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18:411-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 30. | Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver Sinusoidal Endothelial Cells. Compr Physiol. 2015;5:1751-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 31. | Wisse E, De Zanger RB, Jacobs R, McCuskey RS. Scanning electron microscope observations on the structure of portal veins, sinusoids and central veins in rat liver. Scan Electron Microsc. 1983;1441-1452. [PubMed] |
| 32. | Sørensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrød B. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1217-R1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 427] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 490] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Dai Q, Ain Q, Seth N, Rooney M, Zipprich A. Liver sinusoidal endothelial cells: Friend or foe in metabolic dysfunction- associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis. Dig Liver Dis. 2025;57:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal. 2013;6:rs1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Smedsrød B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. 1997;322 (Pt 2):567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Enomoto K, Nishikawa Y, Omori Y, Tokairin T, Yoshida M, Ohi N, Nishimura T, Yamamoto Y, Li Q. Cell biology and pathology of liver sinusoidal endothelial cells. Med Electron Microsc. 2004;37:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Seternes T, Sørensen K, Smedsrød B. Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules. Proc Natl Acad Sci U S A. 2002;99:7594-7597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Smedsrød B, Pertoft H, Gustafson S, Laurent TC. Scavenger functions of the liver endothelial cell. Biochem J. 1990;266:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 224] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Øie CI, Wolfson DL, Yasunori T, Dumitriu G, Sørensen KK, McCourt PA, Ahluwalia BS, Smedsrød B. Liver sinusoidal endothelial cells contribute to the uptake and degradation of entero bacterial viruses. Sci Rep. 2020;10:898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 638] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Seternes T, Bøgwald J, Dalmo RA. Scavenger endothelial cells of fish, a review. J Fish Dis. 2021;44:1385-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Wu J, Meng Z, Jiang M, Zhang E, Trippler M, Broering R, Bucchi A, Krux F, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology. 2010;129:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 45. | Gardner C, Laskin J, Laskin D. Hepatic Sinusoidal Cells and Liver-Associated Lymphocytes. In: McQueen CA, editor. Comprehensive Toxicology. Netherlands: Elsevier, 2018. [DOI] [Full Text] |
| 46. | Jiang T, Hu Y, Cao J. [The role of sinusoidal endothelial cells in liver injury: a review]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2023;35:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Sørensen KK, Smedsrød B. The Liver Sinusoidal Endothelial Cell. In: Arias IM, Alter HJ, Boyer JH, Cohen DE, Shafritz DA, Thorgeirsson SS, Wolkoff AW, editors. The Liver: Biology and Pathobiology, Sixth Edition. United States: Wiley, 2020. [DOI] [Full Text] |
| 48. | Papaioannou S, See JX, Jeong M, De La Torre C, Ast V, Reiners-Koch PS, Sati A, Mogler C, Platten M, Cerwenka A, Stojanovic A. Liver sinusoidal endothelial cells orchestrate NK cell recruitment and activation in acute inflammatory liver injury. Cell Rep. 2023;42:112836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 49. | Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol. 2018;15:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 50. | Wilkinson AL, Qurashi M, Shetty S. The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer Within the Liver. Front Physiol. 2020;11:990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 51. | Cooper SA, Kostallari E, Shah VH. Angiocrine Signaling in Sinusoidal Health and Disease. Semin Liver Dis. 2023;43:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Chen W, Yang A, Zhang N, You H. Liver fibrosis. In: Gracia-Sancho J, editor. Sinusoidal Cells in Liver Diseases. Netherlands: Elsevier, 2024. [DOI] [Full Text] |
| 53. | Kulle A, Thanabalasuriar A, Cohen TS, Szydlowska M. Resident macrophages of the lung and liver: The guardians of our tissues. Front Immunol. 2022;13:1029085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 55. | Elchaninov AV, Fatkhudinov TK, Vishnyakova PA, Lokhonina AV, Sukhikh GT. Phenotypical and Functional Polymorphism of Liver Resident Macrophages. Cells. 2019;8:1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Zeng Z, Surewaard BG, Wong CH, Geoghegan JA, Jenne CN, Kubes P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe. 2016;20:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 57. | Van den Bossche J, O'Neill LA, Menon D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017;38:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 817] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 58. | Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 876] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 59. | Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 837] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 60. | Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, Striz I. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). 2023;137:1067-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 227] [Reference Citation Analysis (0)] |
| 61. | You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 62. | Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, Luedde T, Ginhoux F, Kurts C, Trautwein C, Tacke F. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 63. | Huang H, Lu Y, Zhou T, Gu G, Xia Q. Innate Immune Cells in Immune Tolerance After Liver Transplantation. Front Immunol. 2018;9:2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Wang Y, van der Tuin S, Tjeerdema N, van Dam AD, Rensen SS, Hendrikx T, Berbée JF, Atanasovska B, Fu J, Hoekstra M, Bekkering S, Riksen NP, Buurman WA, Greve JW, Hofker MH, Shiri-Sverdlov R, Meijer OC, Smit JW, Havekes LM, van Dijk KW, Rensen PC. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology. 2015;62:1710-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 639] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 66. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 570] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 67. | Kamm DR, McCommis KS. Hepatic stellate cells in physiology and pathology. J Physiol. 2022;600:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 68. | Shi J, Zhao J, Zhang X, Cheng Y, Hu J, Li Y, Zhao X, Shang Q, Sun Y, Tu B, Shi L, Gao B, Wang FS, Zhang Z. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-β-dependent emperipolesis in HBV cirrhotic patients. Sci Rep. 2017;7:44544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 70. | Muscate F, Woestemeier A, Gagliani N. Functional heterogeneity of CD4(+) T cells in liver inflammation. Semin Immunopathol. 2021;43:549-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Benechet AP, Iannacone M. Determinants of hepatic effector CD8(+) T cell dynamics. J Hepatol. 2017;66:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | O'Connor JH, McNamara HA, Cai Y, Coupland LA, Gardiner EE, Parish CR, McMorran BJ, Ganusov VV, Cockburn IA. Interactions with Asialo-Glycoprotein Receptors and Platelets Are Dispensable for CD8(+) T Cell Localization in the Murine Liver. J Immunol. 2022;208:2738-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Lombardi R, Piciotti R, Dongiovanni P, Meroni M, Fargion S, Fracanzani AL. PD-1/PD-L1 Immuno-Mediated Therapy in NAFLD: Advantages and Obstacles in the Treatment of Advanced Disease. Int J Mol Sci. 2022;23:2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Welz M, Eickhoff S, Abdullah Z, Trebicka J, Gartlan KH, Spicer JA, Demetris AJ, Akhlaghi H, Anton M, Manske K, Zehn D, Nieswandt B, Kurts C, Trapani JA, Knolle P, Wohlleber D, Kastenmüller W. Perforin inhibition protects from lethal endothelial damage during fulminant viral hepatitis. Nat Commun. 2018;9:4805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Yang L, Shao X, Jia S, Zhang Q, Jin Z. Interleukin-35 Dampens CD8(+) T Cells Activity in Patients With Non-viral Hepatitis-Related Hepatocellular Carcinoma. Front Immunol. 2019;10:1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Breuer DA, Pacheco MC, Washington MK, Montgomery SA, Hasty AH, Kennedy AJ. CD8(+) T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G211-G224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 78. | Zhang Y, Qi C, Li L, Hua S, Zheng F, Gong F, Fang M. CD8(+) T cell/IL-33/ILC2 axis exacerbates the liver injury in Con A-induced hepatitis in T cell-transferred Rag2-deficient mice. Inflamm Res. 2019;68:75-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Giannandrea M, Pierce RH, Crispe IN. Indirect action of tumor necrosis factor-alpha in liver injury during the CD8+ T cell response to an adeno-associated virus vector in mice. Hepatology. 2009;49:2010-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Guidotti LG, Iannacone M. Effector CD8 T cell trafficking within the liver. Mol Immunol. 2013;55:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Inverso D, Iannacone M. Spatiotemporal dynamics of effector CD8+ T cell responses within the liver. J Leukoc Biol. 2016;99:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis. 2010;28:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 84. | Highton AJ, Schuster IS, Degli-Esposti MA, Altfeld M. The role of natural killer cells in liver inflammation. Semin Immunopathol. 2021;43:519-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 85. | Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front Immunol. 2012;3:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 86. | Fahrner R, Gröger M, Settmacher U, Mosig AS. Functional integration of natural killer cells in a microfluidically perfused liver on-a-chip model. BMC Res Notes. 2023;16:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 87. | Zecca A, Barili V, Boni C, Fisicaro P, Vecchi A, Rossi M, Reverberi V, Montali A, Pedrazzi G, Ferrari C, Cariani E, Missale G. High CD49a+ NK cell infiltrate is associated with poor clinical outcomes in Hepatocellular Carcinoma. Heliyon. 2023;9:e22680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 88. | Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S, Invernizzi P, Lugli E, Torzilli G, Gershwin ME, Mavilio D. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2016;66:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 89. | Almeida CF, Sundararaj S, Le Nours J, Praveena T, Cao B, Burugupalli S, Smith DGM, Patel O, Brigl M, Pellicci DG, Williams SJ, Uldrich AP, Godfrey DI, Rossjohn J. Distinct CD1d docking strategies exhibited by diverse Type II NKT cell receptors. Nat Commun. 2019;10:5242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | Dasgupta S, Kumar V. Type II NKT cells: a distinct CD1d-restricted immune regulatory NKT cell subset. Immunogenetics. 2016;68:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Gu X, Chu Q, Ma X, Wang J, Chen C, Guan J, Ren Y, Wu S, Zhu H. New insights into iNKT cells and their roles in liver diseases. Front Immunol. 2022;13:1035950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 92. | Ajuebor MN. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol. 2007;293:G651-G656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Ware R, Kumar V. Complexity and function of natural killer T cells with potential application to hepatic transplant survival. Liver Transpl. 2017;23:1589-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol. 2016;13:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 95. | Mattner J. Natural killer T (NKT) cells in autoimmune hepatitis. Curr Opin Immunol. 2013;25:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Zhu S, Zhang H, Bai L. NKT cells in liver diseases. Front Med. 2018;12:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Wang H, Yin S. Natural killer T cells in liver injury, inflammation and cancer. Expert Rev Gastroenterol Hepatol. 2015;9:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Sebode M, Wigger J, Filpe P, Fischer L, Weidemann S, Krech T, Weiler-Normann C, Peiseler M, Hartl J, Tolosa E, Herkel J, Schramm C, Lohse AW, Arrenberg P. Inflammatory Phenotype of Intrahepatic Sulfatide-Reactive Type II NKT Cells in Humans With Autoimmune Hepatitis. Front Immunol. 2019;10:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Zhao J, Weng X, Bagchi S, Wang CR. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci U S A. 2014;111:2674-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 100. | Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 795] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 101. | Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 893] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 102. | Hinks TS. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology. 2016;148:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 103. | Czaja AJ. Incorporating mucosal-associated invariant T cells into the pathogenesis of chronic liver disease. World J Gastroenterol. 2021;27:3705-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Setsu T, Yamagiwa S, Tominaga K, Kimura N, Honda H, Kamimura H, Tsuchiya A, Takamura M, Terai S. Persistent reduction of mucosal-associated invariant T cells in primary biliary cholangitis. J Gastroenterol Hepatol. 2018;33:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. γδT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front Immunol. 2019;10:477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183:5622-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 107. | Hu Y, Hu Q, Li Y, Lu L, Xiang Z, Yin Z, Kabelitz D, Wu Y. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther. 2023;8:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 131] [Reference Citation Analysis (0)] |
| 108. | Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 110. | Mohamed AA, Al-Ramadi BK, Fernandez-Cabezudo MJ. Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions. Int J Mol Sci. 2024;25:1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 111. | Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 969] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 112. | Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F 3rd, Schubert WD, Freitag NE, Lefrançois L. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 113. | Wan F, Hu CB, Ma JX, Gao K, Xiang LX, Shao JZ. Characterization of γδ T Cells from Zebrafish Provides Insights into Their Important Role in Adaptive Humoral Immunity. Front Immunol. 2016;7:675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 114. | Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Kühl AA, Pawlowski NN, Grollich K, Blessenohl M, Westermann J, Zeitz M, Loddenkemper C, Hoffmann JC. Human peripheral gammadelta T cells possess regulatory potential. Immunology. 2009;128:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 116. | O'Neill K, Pastar I, Tomic-Canic M, Strbo N. Perforins Expression by Cutaneous Gamma Delta T Cells. Front Immunol. 2020;11:1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity. 2001;15:419-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 223] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 118. | Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, Hooper LV. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743-8748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 119. | Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, Lam KT, Peiris JS, Lau YL, Tu W. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 120. | Patil R, Sureshbabu SK, Chiplunkar SV. Immunosuppressive role of γδ T cells in cancer: the other side of the coin. Transl Cancer Res. 2017;6:S22-S25. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Liu J, Wu M, Yang Y, Wang Z, He S, Tian X, Wang H. γδ T cells and the PD-1/PD-L1 axis: a love-hate relationship in the tumor microenvironment. J Transl Med. 2024;22:553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 122. | Yin KL, Chu KJ, Li M, Duan YX, Yu YX, Kang MQ, Fu D, Liao R. Immune Regulatory Networks and Therapy of γδ T Cells in Liver Cancer: Recent Trends and Advancements. J Clin Transl Hepatol. 2024;12:287-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 123. | Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, de Visser KE. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1232] [Cited by in RCA: 1335] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 124. | Lopes N, Silva-Santos B. Functional and metabolic dichotomy of murine γδ T cell subsets in cancer immunity. Eur J Immunol. 2021;51:17-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 126. | Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, Chen Z, Wang K, Zhang T, Xu J, Han Y, Zhang T, Wu X, Wang J, Gong W, Zheng S, Qiu F, Yan J, Huang J. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 127. | Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med. 2018;16:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 128. | Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 129. | He W, Hu Y, Chen D, Li Y, Ye D, Zhao Q, Lin L, Shi X, Lu L, Yin Z, He X, Gao Y, Wu Y. Hepatocellular carcinoma-infiltrating γδ T cells are functionally defected and allogenic Vδ2(+) γδ T cell can be a promising complement. Clin Transl Med. 2022;12:e800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 130. | Ichiki Y, Aoki CA, Bowlus CL, Shimoda S, Ishibashi H, Gershwin ME. T cell immunity in autoimmune hepatitis. Autoimmun Rev. 2005;4:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 131. | Rajoriya N, Fergusson JR, Leithead JA, Klenerman P. Gamma Delta T-lymphocytes in Hepatitis C and Chronic Liver Disease. Front Immunol. 2014;5:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Wang X, Tian Z. γδ T cells in liver diseases. Front Med. 2018;12:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Chen S, Lv T, Sun G, Li S, Duan W, Zhang C, Jin H, Tian D, Li M, Shan S, Ma H, Ou X, You H, Zhang D, Jia J. Reciprocal alterations in circulating and hepatic gamma-delta T cells in patients with primary biliary cholangitis. Hepatol Int. 2022;16:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 134. | Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, Gumber S, Speck P, Elrod EJ, Burd EM, Kitchens WH, Magliocca JF, Adams AB, Weiss DS, Mohamadzadeh M, Grakoui A. Alterations in Intestinal Microbiota Lead to Production of Interleukin 17 by Intrahepatic γδ T-Cell Receptor-Positive Cells and Pathogenesis of Cholestatic Liver Disease. Gastroenterology. 2018;154:2178-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 135. | Marinović S, Lenartić M, Mladenić K, Šestan M, Kavazović I, Benić A, Krapić M, Rindlisbacher L, Cokarić Brdovčak M, Sparano C, Litscher G, Turk Wensveen T, Mikolašević I, Fučkar Čupić D, Bilić-Zulle L, Steinle A, Waisman A, Hayday A, Tugues S, Becher B, Polić B, Wensveen FM. NKG2D-mediated detection of metabolically stressed hepatocytes by innate-like T cells is essential for initiation of NASH and fibrosis. Sci Immunol. 2023;8:eadd1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 136. | Monteiro JM, Monteiro GM, Caroli-Bottino A, Pannain VL. Nonalcoholic fatty liver disease: different classifications concordance and relationship between degrees of morphological features and spectrum of the disease. Anal Cell Pathol (Amst). 2014;2014:526979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 138. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 139. | Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 140. | Tang J, Xu W, Yu Y, Yin S, Ye BC, Zhou Y. The role of the gut microbial metabolism of sterols and bile acids in human health. Biochimie. 2025;230:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 141. | Larabi AB, Masson HLP, Bäumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes. 2023;15:2172671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 154] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 142. | Hild B, Heinzow HS, Schmidt HH, Maschmeier M. Bile Acids in Control of the Gut-Liver-Axis. Z Gastroenterol. 2021;59:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 143. | Mpountouridis A, Tsigalou C, Bezirtzoglou I, Bezirtzoglou E, Stavropoulou E. Gut microbiome in non-alcoholic fatty liver disease. Front Gastroenterol. 2025;3. [DOI] [Full Text] |
| 144. | Houtman TA, Eckermann HA, Smidt H, de Weerth C. Gut microbiota and BMI throughout childhood: the role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci Rep. 2022;12:3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 145. | Xiong RG, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, Shang A, Zhao CN, Gan RY, Li HB. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods. 2022;11:2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 181] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 146. | Luu M, Visekruna A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 147. | Zhang W, Mackay CR, Gershwin ME. Immunomodulatory Effects of Microbiota-Derived Short-Chain Fatty Acids in Autoimmune Liver Diseases. J Immunol. 2023;210:1629-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 148. | Ohtani N, Kamiya T, Kawada N. Recent updates on the role of the gut-liver axis in the pathogenesis of NAFLD/NASH, HCC, and beyond. Hepatol Commun. 2023;7:e0241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 149. | Quesada-Vázquez S, Aragonès G, Del Bas JM, Escoté X. Diet, Gut Microbiota and Non-Alcoholic Fatty Liver Disease: Three Parts of the Same Axis. Cells. 2020;9:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 150. | Ohtani N, Hara E. Gut-liver axis-mediated mechanism of liver cancer: A special focus on the role of gut microbiota. Cancer Sci. 2021;112:4433-4443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 151. | Mashek DG, Khan SA, Sathyanarayan A, Ploeger JM, Franklin MP. Hepatic lipid droplet biology: Getting to the root of fatty liver. Hepatology. 2015;62:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 152. | Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 153. | Poss AM, Summers SA. Too Much of a Good Thing? An Evolutionary Theory to Explain the Role of Ceramides in NAFLD. Front Endocrinol (Lausanne). 2020;11:505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 154. | Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 155. | Mashek DG. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol Metab. 2021;50:101115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 156. | Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 157. | Moayedfard Z, Sani F, Alizadeh A, Bagheri Lankarani K, Zarei M, Azarpira N. The role of the immune system in the pathogenesis of NAFLD and potential therapeutic impacts of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2022;13:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 158. | Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1538] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 159. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1511] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 160. | Liu X, Green RM. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019;3:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 161. | Win S, Than TA, Le BH, García-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. 2015;62:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 162. | Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 163. | Federico A, Dallio M, Caprio GG, Ormando VM, Loguercio C. Gut microbiota and the liver. Minerva Gastroenterol Dietol. 2017;63:385-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 164. | Zhao GN, Zhang P, Gong J, Zhang XJ, Wang PX, Yin M, Jiang Z, Shen LJ, Ji YX, Tong J, Wang Y, Wei QF, Wang Y, Zhu XY, Zhang X, Fang J, Xie Q, She ZG, Wang Z, Huang Z, Li H. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med. 2017;23:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 165. | Yan ZZ, Huang YP, Wang X, Wang HP, Ren F, Tian RF, Cheng X, Cai J, Zhang Y, Zhu XY, She ZG, Zhang XJ, Huang Z, Li H. Integrated Omics Reveals Tollip as an Regulator and Therapeutic Target for Hepatic Ischemia-Reperfusion Injury in Mice. Hepatology. 2019;70:1750-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 166. | Cai J, Xu M, Zhang X, Li H. Innate Immune Signaling in Nonalcoholic Fatty Liver Disease and Cardiovascular Diseases. Annu Rev Pathol. 2019;14:153-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 167. | Lefkowitch JH, Haythe JH, Regent N. Kupffer cell aggregation and perivenular distribution in steatohepatitis. Mod Pathol. 2002;15:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 168. | Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 383] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 169. | Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635-646, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 624] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 170. | Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G, Conway JRW, Lee MKS, Timpson P, Murphy AJ, Masters SL, Gerondakis S, Bartonicek N, Kaczorowski DC, Dinger ME, Meikle PJ, Bond PJ, Febbraio MA. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018;27:1096-1110.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 171. | Rogero MM, Calder PC. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients. 2018;10:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 172. | Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918-927.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 173. | Inzaugarat ME, Ferreyra Solari NE, Billordo LA, Abecasis R, Gadano AC, Cherñavsky AC. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol. 2011;31:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 174. | Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, Mombello C, Follenzi A, Novelli F, Carini R. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond). 2016;130:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 175. | Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 589] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 176. | Xu R, Tao A, Zhang S, Zhang M. Neutralization of interleukin-17 attenuates high fat diet-induced non-alcoholic fatty liver disease in mice. Acta Biochim Biophys Sin (Shanghai). 2013;45:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 177. | Gao Y, Li L, Hu X, Zhang W, Li Y. Interleukin-35 has a Protective Role in Infectious Mononucleosis-Induced Liver Inflammation Probably by Inhibiting CD8(+) T Cell Function. Arch Immunol Ther Exp (Warsz). 2022;70:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 178. | Rood JE, Canna SW, Weaver LK, Tobias JW, Behrens EM. IL-10 distinguishes a unique population of activated, effector-like CD8(+) T cells in murine acute liver inflammation. J Leukoc Biol. 2017;101:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 179. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1432] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 180. | Finney AC, Das S, Kumar D, McKinney MP, Cai B, Yurdagul A Jr, Rom O. The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease. Front Cardiovasc Med. 2023;10:1116861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 181. | Zhu C, Tabas I, Schwabe RF, Pajvani UB. Maladaptive regeneration - the reawakening of developmental pathways in NASH and fibrosis. Nat Rev Gastroenterol Hepatol. 2021;18:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 182. | Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, Okada Y, Kurihara C, Irie R, Yokoyama H, Shimamura K, Usui S, Ebinuma H, Saito H, Watanabe C, Komoto S, Kawaguchi A, Nagao S, Sugiyama K, Hokari R, Kanai T, Miura S, Hibi T. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 183. | Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, Hiroi S, Irie R, Okada Y, Kurihara C, Ebinuma H, Saito H, Hokari R, Sugiyama K, Kanai T, Miura S, Hibi T. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152-164.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 184. | Jeong S, Shin WY, Oh YH. Immunotherapy for NAFLD and NAFLD-related hepatocellular carcinoma. Front Endocrinol (Lausanne). 2023;14:1150360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 185. | Guo Z, Wu Q, Xie P, Wang J, Lv W. Immunomodulation in non-alcoholic fatty liver disease: exploring mechanisms and applications. Front Immunol. 2024;15:1336493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 186. | Cheng CH, Hao WR, Cheng TH. Mesenchymal stem cells: A promising therapeutic avenue for non-alcoholic fatty liver disease. World J Stem Cells. 2024;16:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 187. | Huang WC, Li YC, Chen PX, Ma KS, Wang LT. Mesenchymal stem cell therapy as a game-changer in liver diseases: review of current clinical trials. Stem Cell Res Ther. 2025;16:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 188. | Anstee QM, Lucas KJ, Francque S, Abdelmalek MF, Sanyal AJ, Ratziu V, Gadano AC, Rinella M, Charlton M, Loomba R, Mena E, Schattenberg JM, Noureddin M, Lazas D, Goh GBB, Sarin SK, Yilmaz Y, Martic M, Stringer R, Kochuparampil J, Chen L, Rodriguez-Araujo G, Chng E, Naoumov NV, Brass C, Pedrosa MC. Tropifexor plus cenicriviroc combination versus monotherapy in nonalcoholic steatohepatitis: Results from the phase 2b TANDEM study. Hepatology. 2023;78:1223-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 189. | Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S, Fischer L, Loomba R, Abdelmalek MF, Tacke F. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology. 2020;72:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 190. | Gandhe A, Kumari S, Elizabeth Sobhia M. Rational design of FXR agonists: a computational approach for NASH therapy. Mol Divers. 2024;28:3363-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 191. | Tang Y, Fan Y, Wang Y, Wang D, Huang Q, Chen T, Cao X, Wen C, Shen X, Li J, You Y. A Current Understanding of FXR in NAFLD: The multifaceted regulatory role of FXR and novel lead discovery for drug development. Biomed Pharmacother. 2024;175:116658. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 192. | Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, Barshop W, Wohlschlegel J, Calkin AC, Liu Y, Thorell A, Meikle PJ, Drew BG, Mack JJ, Marschall HU, Tarling EJ, Edwards PA, de Aguiar Vallim TQ. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671-1684.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 193. | Adorini L, Trauner M. FXR agonists in NASH treatment. J Hepatol. 2023;79:1317-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 194. | Xu JY, Li ZP, Zhang L, Ji G. Recent insights into farnesoid X receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:13493-13500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 195. | Su X, Kong Y, Peng D. Fibroblast growth factor 21 in lipid metabolism and non-alcoholic fatty liver disease. Clin Chim Acta. 2019;498:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 196. | Harrison SA, Rolph T, Knott M, Dubourg J. FGF21 agonists: An emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J Hepatol. 2024;81:562-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 197. | Falamarzi K, Malekpour M, Tafti MF, Azarpira N, Behboodi M, Zarei M. The role of FGF21 and its analogs on liver associated diseases. Front Med (Lausanne). 2022;9:967375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 198. | Carbonetti MP, Almeida-Oliveira F, Majerowicz D. Use of FGF21 analogs for the treatment of metabolic disorders: a systematic review and meta-analysis. Arch Endocrinol Metab. 2023;68:e220493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 199. | Corbee RJ, van Everdingen DL, Kooistra HS, Penning LC. Fibroblast growth factor-21 (FGF21) analogs as possible treatment options for diabetes mellitus in veterinary patients. Front Vet Sci. 2022;9:1086987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 200. | Raptis DD, Mantzoros CS, Polyzos SA. Fibroblast Growth Factor-21 as a Potential Therapeutic Target of Nonalcoholic Fatty Liver Disease. Ther Clin Risk Manag. 2023;19:77-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 201. | Chui ZSW, Shen Q, Xu A. Current status and future perspectives of FGF21 analogues in clinical trials. Trends Endocrinol Metab. 2024;35:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 202. | Theofilis P, Oikonomou E, Karakasis P, Pamporis K, Dimitriadis K, Kokkou E, Lambadiari V, Siasos G, Tsioufis K, Tousoulis D. FGF21 Analogues in Patients With Metabolic Diseases: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Liver Int. 2025;45:e70016. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 203. | Zhang D, Hart J, Ding X, Zhang X, Feely M, Yassan L, Alpert L, Soldevila-Pico C, Zhang X, Liu X, Lai J. Histologic patterns of liver injury induced by anti-PD-1 therapy. Gastroenterol Rep (Oxf). 2020;8:50-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 204. | Pinto E, Meneghel P, Farinati F, Russo FP, Pelizzaro F, Gambato M. Efficacy of immunotherapy in hepatocellular carcinoma: Does liver disease etiology have a role? Dig Liver Dis. 2024;56:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 205. | Niu Q, Wang T, Wang Z, Wang F, Huang D, Sun H, Liu H. Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte. 2022;11:572-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 206. | Yang F, Wu Y, Chen Y, Xi J, Chu Y, Jin J, Yan Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023;5:100746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 207. | Hu J, Li S, Zhong X, Wei Y, Sun Q, Zhong L. Human umbilical cord mesenchymal stem cells attenuate diet-induced obesity and NASH-related fibrosis in mice. Heliyon. 2024;10:e25460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 208. | Ipsen DH, Tveden-Nyborg P. Extracellular Vesicles as Drivers of Non-Alcoholic Fatty Liver Disease: Small Particles with Big Impact. Biomedicines. 2021;9:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 209. | Abreo Medina ADP, Shi M, Wang Y, Wang Z, Huang K, Liu Y. Exploring Extracellular Vesicles: A Novel Approach in Nonalcoholic Fatty Liver Disease. J Agric Food Chem. 2025;73:2717-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 210. | Lu J, Shataer D, Yan H, Dong X, Zhang M, Qin Y, Cui J, Wang L. Probiotics and Non-Alcoholic Fatty Liver Disease: Unveiling the Mechanisms of Lactobacillus plantarum and Bifidobacterium bifidum in Modulating Lipid Metabolism, Inflammation, and Intestinal Barrier Integrity. Foods. 2024;13:2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 211. | Zhang J, Zhou J, He Z, Li H. Bacteroides and NAFLD: pathophysiology and therapy. Front Microbiol. 2024;15:1288856. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 212. | Wen X, Liu H, Luo X, Lui L, Fan J, Xing Y, Wang J, Qiao X, Li N, Wang G. Supplementation of Lactobacillus plantarum ATCC14917 mitigates non-alcoholic fatty liver disease in high-fat-diet-fed rats. Front Microbiol. 2023;14:1146672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 213. | Tang C, Zhou W, Shan M, Lu Z, Lu Y. Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice. Food Sci Hum Wellness. 2022;11:1428-1439. [RCA] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 214. | Rafaqat S, Gluscevic S, Mercantepe F, Rafaqat S, Klisic A. Interleukins: Pathogenesis in Non-Alcoholic Fatty Liver Disease. Metabolites. 2024;14:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 215. | Paquissi FC. Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front Immunol. 2016;7:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 216. | Kucsera D, Tóth VE, Sayour NV, Kovács T, Gergely TG, Ruppert M, Radovits T, Fábián A, Kovács A, Merkely B, Ferdinandy P, Varga ZV. IL-1β neutralization prevents diastolic dysfunction development, but lacks hepatoprotective effect in an aged mouse model of NASH. Sci Rep. 2023;13:356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 217. | Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL, Luo M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front Immunol. 2022;13:880298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 185] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 218. | Bakhshi S, Shamsi S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: Latest evidence and therapeutic outcomes. Int Immunopharmacol. 2022;106:108595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 219. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 829] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 220. | Lv Y, Gao X, Luo Y, Fan W, Shen T, Ding C, Yao M, Song S, Yan L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J Nutr Biochem. 2019;71:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 221. | Yang M, Kimchi ET, Staveley-O'Carroll KF, Li G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int J Mol Sci. 2021;22:11037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 222. | Xu Y, Yang C, Zhang S, Li J, Xiao Q, Huang W. Ginsenoside Rg1 Protects against Non-alcoholic Fatty Liver Disease by Ameliorating Lipid Peroxidation, Endoplasmic Reticulum Stress, and Inflammasome Activation. Biol Pharm Bull. 2018;41:1638-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 223. | Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N, Shiffman ML, Camargo M, Li G, Kersey K, Jia C, Zhu Y, Djedjos CS, Subramanian GM, Myers RP, Gunn N, Sheikh A, Anstee QM, Romero-Gomez M, Trauner M, Goodman Z, Lawitz EJ, Younossi Z; STELLAR-3; STELLAR-4 Investigators. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 224. | Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR; GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 225. | Harrison SA, Goodman Z, Jabbar A, Vemulapalli R, Younes ZH, Freilich B, Sheikh MY, Schattenberg JM, Kayali Z, Zivony A, Sheikh A, Garcia-Samaniego J, Satapathy SK, Therapondos G, Mena E, Schuppan D, Robinson J, Chan JL, Hagerty DT, Sanyal AJ. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72:816-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 226. | Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 227. | Frenette C, Kayali Z, Mena E, Mantry PS, Lucas KJ, Neff G, Rodriguez M, Thuluvath PJ, Weinberg E, Bhandari BR, Robinson J, Wedick N, Chan JL, Hagerty DT, Kowdley KV; IDN-6556-17 Study Investigators. Emricasan to prevent new decompensation in patients with NASH-related decompensated cirrhosis. J Hepatol. 2021;74:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 228. | Garcia-Tsao G, Bosch J, Kayali Z, Harrison SA, Abdelmalek MF, Lawitz E, Satapathy SK, Ghabril M, Shiffman ML, Younes ZH, Thuluvath PJ, Berzigotti A, Albillos A, Robinson JM, Hagerty DT, Chan JL, Sanyal AJ; IDN-6556-14 Investigators(‡). Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 229. | Teschke R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines. 2018;6:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 230. | Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 231. | Dou L, Shi X, He X, Gao Y. Macrophage Phenotype and Function in Liver Disorder. Front Immunol. 2019;10:3112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 232. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 233. | Gao C, Wang S, Xie X, Ramadori P, Li X, Liu X, Ding X, Liang J, Xu B, Feng Y, Tan X, Wang H, Zhang Y, Zhang H, Zhang T, Mi P, Li S, Zhang C, Yuan D, Heikenwalder M, Zhang P. Single-cell Profiling of Intrahepatic Immune Cells Reveals an Expansion of Tissue-resident Cytotoxic CD4(+) T Lymphocyte Subset Associated With Pathogenesis of Alcoholic-associated Liver Diseases. Cell Mol Gastroenterol Hepatol. 2025;19:101411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 234. | Li W, Amet T, Xing Y, Yang D, Liangpunsakul S, Puri P, Kamath PS, Sanyal AJ, Shah VH, Katz BP, Radaeva S, Crabb DW, Chalasani N, Yu Q. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: A prospective observational study. Hepatology. 2017;66:575-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 235. | Park KJ, Jin HM, Cho YN, Yoon JH, Kee SJ, Kim HS, Park YW. Altered Frequency, Activation, and Clinical Relevance of Circulating Innate and Innate-Like Lymphocytes in Patients With Alcoholic Liver Cirrhosis. Immune Netw. 2023;23:e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 236. | Zhang Y, Fan Y, He W, Han Y, Bao H, Yang R, Wang B, Kong D, Wang H. Persistent deficiency of mucosa-associated invariant T (MAIT) cells during alcohol-related liver disease. Cell Biosci. 2021;11:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 237. | Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, Shawcross D, Ryan JM, Evans A, Azarian S, Bajaj JS, Fagan A, Patel V, Mehta K, Lopez C, Simonova M, Katzarov K, Hadzhiolova T, Pavlova S, Wendon JA, Oo YH, Klenerman P, Williams R, Chokshi S. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67:918-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 238. | Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology. 2016;5:e98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 239. | Li W, Lin EL, Liangpunsakul S, Lan J, Chalasani S, Rane S, Puri P, Kamath PS, Sanyal AJ, Shah VH, Radaeva S, Crabb DW, Chalasani N, Yu Q. Alcohol Abstinence Does Not Fully Reverse Abnormalities of Mucosal-Associated Invariant T Cells in the Blood of Patients With Alcoholic Hepatitis. Clin Transl Gastroenterol. 2019;10:e00052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 240. | Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 241. | Couch RD, Dailey A, Zaidi F, Navarro K, Forsyth CB, Mutlu E, Engen PA, Keshavarzian A. Alcohol induced alterations to the human fecal VOC metabolome. PLoS One. 2015;10:e0119362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 242. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3883] [Article Influence: 970.8] [Reference Citation Analysis (3)] |
| 243. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 244. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 557] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 245. | Liu L. Clinical features of hepatocellular carcinoma with hepatitis B virus among patients on Nucleos(t) ide analog therapy. Infect Agent Cancer. 2020;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 246. | Jayant K, Habib N, Huang KW, Podda M, Warwick J, Arasaradnam R. Immunological Basis of Genesis of Hepatocellular Carcinoma: Unique Challenges and Potential Opportunities through Immunomodulation. Vaccines (Basel). 2020;8:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 247. | Delhem N, Carpentier A, Moralès O, Miroux C, Groux H, Auriault C, Pancré V. [Regulatory T-cells and hepatocellular carcinoma: implication of the regulatory T lymphocytes in the control of the immune response]. Bull Cancer. 2008;95:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 248. | Taibi C, Vincenzi L, D’offizi G. Role of the Immune System in Hepatocellular Carcinoma. In: Ettorre GM, editor. Hepatocellular Carcinoma. Updates in Surgery. Cham: Springer, 2023. [DOI] [Full Text] |
| 249. | Nishida N. Role of Oncogenic Pathways on the Cancer Immunosuppressive Microenvironment and Its Clinical Implications in Hepatocellular Carcinoma. Cancers (Basel). 2021;13:3666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 250. | Xia Y, Brown ZJ, Huang H, Tsung A. Metabolic reprogramming of immune cells: Shaping the tumor microenvironment in hepatocellular carcinoma. Cancer Med. 2021;10:6374-6383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 251. | Yao RR, Li JH, Zhang R, Chen RX, Wang YH. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol. 2018;16:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 252. | Lawal G, Xiao Y, Rahnemai-Azar AA, Tsilimigras DI, Kuang M, Bakopoulos A, Pawlik TM. The Immunology of Hepatocellular Carcinoma. Vaccines (Basel). 2021;9:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 253. | Huang J, Wu Q, Geller DA, Yan Y. Macrophage metabolism, phenotype, function, and therapy in hepatocellular carcinoma (HCC). J Transl Med. 2023;21:815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 254. | Tian Z, Hou X, Liu W, Han Z, Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 255. | Yang H, Xuefeng Y, Jianhua X. Systematic review of the roles of interleukins in hepatocellular carcinoma. Clin Chim Acta. 2020;506:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 256. | Wang Y, Zhang T, Sun M, Ji X, Xie M, Huang W, Xia L. Therapeutic Values of Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma: Facts and Hopes. Cancers (Basel). 2021;13:5127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 257. | Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 258. | Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 259. | Medina-Echeverz J, Eggert T, Han M, Greten TF. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol Immunother. 2015;64:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 260. | Lu LC, Chang CJ, Hsu CH. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 261. | He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, Chen H, Zeng H. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 262. | Du G, Dou C, Sun P, Wang S, Liu J, Ma L. Regulatory T cells and immune escape in HCC: understanding the tumor microenvironment and advancing CAR-T cell therapy. Front Immunol. 2024;15:1431211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 263. | Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 264. | Akbulut Z, Aru B, Aydın F, Yanıkkaya Demirel G. Immune checkpoint inhibitors in the treatment of hepatocellular carcinoma. Front Immunol. 2024;15:1379622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 265. | Mandlik DS, Mandlik SK, Choudhary HB. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol. 2023;29:1054-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (3)] |
| 266. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 744] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 267. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1898] [Article Influence: 271.1] [Reference Citation Analysis (0)] |
| 268. | Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, Madrazo B, Kwon D, Garcia-Buitrago M, Martin P, Hosein PJ, Savaraj N. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125:3603-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 269. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 270. | Gu X, Huo J, Yu Z, Jiang J, Xu Y, Zhao L. Immunotherapy in hepatocellular carcinoma: an overview of immune checkpoint inhibitors, drug resistance, and adverse effects. Oncologie. 2024;26:9-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 271. | Yu SJ. Immunotherapy for hepatocellular carcinoma: Recent advances and future targets. Pharmacol Ther. 2023;244:108387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 272. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4698] [Article Influence: 939.6] [Reference Citation Analysis (2)] |
| 273. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 274. | Li X, Xia F. Immunotherapy for hepatocellular carcinoma: molecular pathogenesis and clinical research progress. Oncol Transl Med. 2023;206-212. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 275. | Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, Li Y, Jiang X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front Immunol. 2021;12:765101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 276. | Zhou M, Liu B, Shen J. Immunotherapy for hepatocellular carcinoma. Clin Exp Med. 2023;23:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 277. | Abushukair HM, Saeed A. Hepatocellular carcinoma and immunotherapy: Beyond immune checkpoint inhibitors. World J Gastrointest Oncol. 2022;14:1210-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 278. | Li M, Bhoori S, Mehta N, Mazzaferro V. Immunotherapy for hepatocellular carcinoma: The next evolution in expanding access to liver transplantation. J Hepatol. 2024;81:743-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 279. | Kim SJ, Cummins KC, Tsung A. Immunotherapy as a Complement to Surgical Management of Hepatocellular Carcinoma. Cancers (Basel). 2024;16:1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 280. | Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, Tsuneyama K, Nakanuma Y, Leung P, Ansari AA, Gershwin ME, Akashi K. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 281. | Harada K, Nakanuma Y. Innate immunity in the pathogenesis of cholangiopathy: a recent update. Inflamm Allergy Drug Targets. 2012;11:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 282. | Harada K, Nakanuma Y. Biliary innate immunity in the pathogenesis of biliary diseases. Inflamm Allergy Drug Targets. 2010;9:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 283. | Lan T, Qian S, Tang C, Gao J. Role of Immune Cells in Biliary Repair. Front Immunol. 2022;13:866040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 284. | Li Y, Leung PS, Zhang W, Zhang S, Liu Z, Kurth M, Patterson AD, Gershwin ME, Song J. Immunobiology of bile and cholangiocytes. J Autoimmun. 2025;151:103376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 285. | Verdier J, Luedde T, Sellge G. Biliary Mucosal Barrier and Microbiome. Viszeralmedizin. 2015;31:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 286. | Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 287. | Liu Q, He W, Tang R, Ma X. Intestinal homeostasis in autoimmune liver diseases. Chin Med J (Engl). 2022;135:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 288. | Prieto J, Banales JM, Medina JF. Primary biliary cholangitis: pathogenic mechanisms. Curr Opin Gastroenterol. 2021;37:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 289. | You H, Duan W, Li S, Lv T, Chen S, Lu L, Ma X, Han Y, Nan Y, Xu X, Duan Z, Wei L, Jia J, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the Diagnosis and Management of Primary Biliary Cholangitis (2021). J Clin Transl Hepatol. 2023;11:736-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 290. | Younossi ZM, Bernstein D, Shiffman ML, Kwo P, Kim WR, Kowdley KV, Jacobson IM. Diagnosis and Management of Primary Biliary Cholangitis. Am J Gastroenterol. 2019;114:48-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 291. | Wu P, Xie S, Cai Y, Liu H, Lv Y, Yang Y, He Y, Yin B, Lan T, Wu H. Causality of immune cells on primary sclerosing cholangitis: a bidirectional two-sample Mendelian randomization study. Front Immunol. 2024;15:1395513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 292. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on sclerosing cholangitis. J Hepatol. 2022;77:761-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 293. | Gulamhusein AF, Hirschfield GM. Pathophysiology of primary biliary cholangitis. Best Pract Res Clin Gastroenterol. 2018;34-35:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 294. | Tsuneyama K, Baba H, Morimoto Y, Tsunematsu T, Ogawa H. Primary Biliary Cholangitis: Its Pathological Characteristics and Immunopathological Mechanisms. J Med Invest. 2017;64:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 295. | Nguyen HH, Fritzler MJ, Swain MG. A Review on Biomarkers for the Evaluation of Autoimmune Cholestatic Liver Diseases and Their Overlap Syndromes. Front Mol Med. 2022;2:914505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 296. | Ma WT, Chen DK. Immunological abnormalities in patients with primary biliary cholangitis. Clin Sci (Lond). 2019;133:741-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 297. | Harada K, Nakanuma Y. Biliary innate immunity: function and modulation. Mediators Inflamm. 2010;2010:373878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 298. | Baba H, Sugitani A, Takahashi R, Kai K, Moritoki Y, Kikuchi K, Tsuneyama K. Immunopathology of Bile Duct Lesions of Primary Biliary Cirrhosis. In: Nakanuma Y, editor. Pathology of the Bile Duct. Singapore: Springer, 2017. [DOI] [Full Text] |
| 299. | Chen W, Wei Y, Xiong A, Li Y, Guan H, Wang Q, Miao Q, Bian Z, Xiao X, Lian M, Zhang J, Li B, Cao Q, Fan Z, Zhang W, Qiu D, Fang J, Gershwin ME, Yang L, Tang R, Ma X. Comprehensive Analysis of Serum and Fecal Bile Acid Profiles and Interaction with Gut Microbiota in Primary Biliary Cholangitis. Clin Rev Allergy Immunol. 2020;58:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 300. | Zhang L, Yang L, Chu H. Targeting Gut Microbiota for the Treatment of Primary Biliary Cholangitis: From Bench to Bedside. J Clin Transl Hepatol. 2023;11:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 301. | Kitahata S, Yamamoto Y, Yoshida O, Tokumoto Y, Kawamura T, Furukawa S, Kumagi T, Hirooka M, Takeshita E, Abe M, Ikeda Y, Hiasa Y. Ileal mucosa-associated microbiota overgrowth associated with pathogenesis of primary biliary cholangitis. Sci Rep. 2021;11:19705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 302. | Kaplan MM. Novosphingobium aromaticivorans: a potential initiator of primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2147-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 303. | Mohammed JP, Mattner J. Autoimmune disease triggered by infection with alphaproteobacteria. Expert Rev Clin Immunol. 2009;5:369-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 304. | Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 305. | Chung BK, Karlsen TH, Folseraas T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1390-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 306. | O'Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 307. | Peviani M, Cazzagon N, Gambato M, Bertin L, Zingone F, Savarino EV, Barberio B. Primary sclerosing cholangitis and inflammatory bowel disease: a complicated yet unique relationship. Minerva Gastroenterol (Torino). 2025;71:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 308. | Wang L, Cao ZM, Zhang LL, Li JM, Lv WL. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front Immunol. 2022;13:923599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 309. | Kamiya T, Ohtani N. The role of immune cells in the liver tumor microenvironment: an involvement of gut microbiota-derived factors. Int Immunol. 2022;34:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 310. | Pérez-Montes de Oca A, Julián MT, Ramos A, Puig-Domingo M, Alonso N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients. 2020;12:3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 311. | Wei W, Wong CC, Jia Z, Liu W, Liu C, Ji F, Pan Y, Wang F, Wang G, Zhao L, Chu ESH, Zhang X, Sung JJY, Yu J. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat Microbiol. 2023;8:1534-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 312. | Ganesan R, Thirumurugan D, Vinayagam S, Kim DJ, Suk KT, Iyer M, Yadav MK, HariKrishnaReddy D, Parkash J, Wander A, Vellingiri B. A critical review of microbiome-derived metabolic functions and translational research in liver diseases. Front Cell Infect Microbiol. 2025;15:1488874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 313. | Boicean A, Birlutiu V, Ichim C, Brusnic O, Onișor DM. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines. 2023;11:2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 314. | Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 933] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 315. | Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 316. | Ferreira I, Falcato F, Bandarra N, Rauter AP. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules. 2022;27:1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 317. | Pirault J, Bäck M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front Pharmacol. 2018;9:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 318. | Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 319. | Kim N, Shin HY. Deciphering the Potential Role of Specialized Pro-Resolving Mediators in Obesity-Associated Metabolic Disorders. Int J Mol Sci. 2024;25:9598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 320. | Liu X, Tang Y, Luo Y, Gao Y, He L. Role and mechanism of specialized pro-resolving mediators in obesity-associated insulin resistance. Lipids Health Dis. 2024;23:234. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 321. | Maciejewska-Markiewicz D, Stachowska E, Hawryłkowicz V, Stachowska L, Prowans P. The Role of Resolvins, Protectins and Marensins in Non-Alcoholic Fatty Liver Disease (NAFLD). Biomolecules. 2021;11:937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 322. | Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 323. | Clària J, Flores-Costa R, Duran-Güell M, López-Vicario C. Proresolving lipid mediators and liver disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:159023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 324. | Kotlyarov S, Kotlyarova A. Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System. Membranes (Basel). 2022;12:1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 325. | Ghanem SE, Elsabaawy MM, Abdelkareem MM, Helal ML, Othman W, Elsayed M, Elsabaawy DM, El Fert A. Evaluation of ABCA1 gene polymorphism as a prognostic index of fibrosis progression in NAFLD patients. Endocrinol Diabetes Metab. 2023;6:e394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 326. | Lyu J, Imachi H, Fukunaga K, Sato S, Kobayashi T, Dong T, Saheki T, Matsumoto M, Iwama H, Zhang H, Murao K. Role of ATP-binding cassette transporter A1 in suppressing lipid accumulation by glucagon-like peptide-1 agonist in hepatocytes. Mol Metab. 2020;34:16-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 327. | Zhang W, Lang R. Macrophage metabolism in nonalcoholic fatty liver disease. Front Immunol. 2023;14:1257596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 328. | Tian Y, Ni Y, Zhang T, Cao Y, Zhou M, Zhao C. Targeting hepatic macrophages for non-alcoholic fatty liver disease therapy. Front Cell Dev Biol. 2024;12:1444198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 329. | Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int J Mol Sci. 2023;24:1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 128] [Reference Citation Analysis (0)] |
| 330. | Li Z, Feng PP, Zhao ZB, Zhu W, Gong JP, Du HM. Liraglutide protects against inflammatory stress in non-alcoholic fatty liver by modulating Kupffer cells M2 polarization via cAMP-PKA-STAT3 signaling pathway. Biochem Biophys Res Commun. 2019;510:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 331. | Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 708] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 332. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |