Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.107378
Revised: April 23, 2025
Accepted: June 10, 2025
Published online: July 27, 2025
Processing time: 122 Days and 1.3 Hours
Liver regeneration (LR) following partial hepatectomy (PH) is a unique and complex physiological response that restores hepatic mass and function through tightly orchestrated cellular and molecular events. Traditionally viewed as a proliferation-driven process, LR is now understood to involve both hepatocyte hyperplasia and hypertrophy, triggered primarily by hemodynamic alterations such as increased portal pressure and shear stress. These promote LR through endothelial–hepatocyte communication via activation of Piezo1 - a mechanosensitive ion channel highly expressed in vascular endothelial cells. This channel is considered one of the potential upstream activators of molecular cascades including the interleukin (IL)-6/signal transducer and activator of transcription 3, tumour necrosis factor-alpha/nuclear factor-kappa B, Wnt/β-catenin, Hippo/ YAP, transforming growth factor-beta, and Notch pathways, which contribute variably to the proliferation, differentiation, or suppression of hepatic cells. Novel insights into the IL-22 and IL-33 signaling axes, bile acid and glutamine meta
Core Tip: This review synthesizes key triggers and molecular pathways of Liver regeneration after partial hepatectomy, emphasizing the central role of portal hemodynamics, cytokine signaling, and extracellular matrix remodeling. It highlights underexplored processes such as ductular reaction in granulation tissue, and the role of metabolic regulators like bile acids and glutamine. The paper outlines mechanisms of the termination of liver regenerative and proposes new perspectives for its therapeutic modulation in clinical settings.
- Citation: Korchilava B, Khachidze T, Megrelishvili N, Svanadze L, Kakabadze M, Tsomaia K, Jintcharadze M, Kordzaia D. Liver regeneration after partial hepatectomy: Triggers and mechanisms. World J Hepatol 2025; 17(7): 107378
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/107378.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.107378
Liver regeneration (LR) is a unique physiological process by which the liver restores its mass and function after injury or resection. Following partial hepatectomy (PH), LR unfolds as a complex and tightly regulated process involving portal hemodynamic shifts, activation of cytokines and growth factors, extracellular matrix (ECM) remodeling, and metabolic reprogramming. These events lead to hepatocyte hypertrophy and proliferation, ultimately restoring liver mass[1-4]. Unlike simple wound healing or stem cell-driven organ repair, liver regeneration after PH occurs predominantly through the coordinated activity of mature hepatocytes, without reliance on a stem cell niche. This remarkable capacity has made the liver a long-standing model for studying tissue regeneration, with direct relevance to hepatic surgery and trans
Over the past decades, our understanding of liver regeneration has evolved from a descriptive phenomenon into a highly orchestrated molecular cascade. It is now recognized that LR comprises multiple phases — priming, proliferation, hypertrophy, remodeling, and termination — each governed by overlapping regulatory networks. While classic studies emphasized hepatocyte proliferation as the central mechanism of liver mass restoration, more recent evidence highlights hypertrophy as a critical, compensatory mechanism, particularly in cases where proliferation is delayed or suppressed[5,6].
The rationale for this review stems from our longstanding research into liver anatomy, regeneration, and experimental hepatic surgery. We aim to synthesize emerging insights into the interplay between hypertrophy and hyperplasia, mechanosensitive triggers of regeneration, and the layered signaling pathways that ensure both liver mass restoration and proper termination. We also explore how these processes are influenced by anatomical configuration, experimental manipulation, and clinical outcomes.
Liver regeneration begins immediately following resection. The extent of resection varies: Minor hepatectomy typically removes < 30% of liver mass, while major hepatectomy involves ≥ 50%. Minor resections tend to induce hypertrophic regeneration, characterized by enlargement of existing hepatocytes. Major resections, by contrast, lead to hyperplastic regeneration, driven by both hypertrophy and cell proliferation[5,7]. However, hepatocyte hyperplasia is generally accepted as the dominant mechanism restoring liver mass after PH, with hypertrophy playing a supportive or transient role[8].
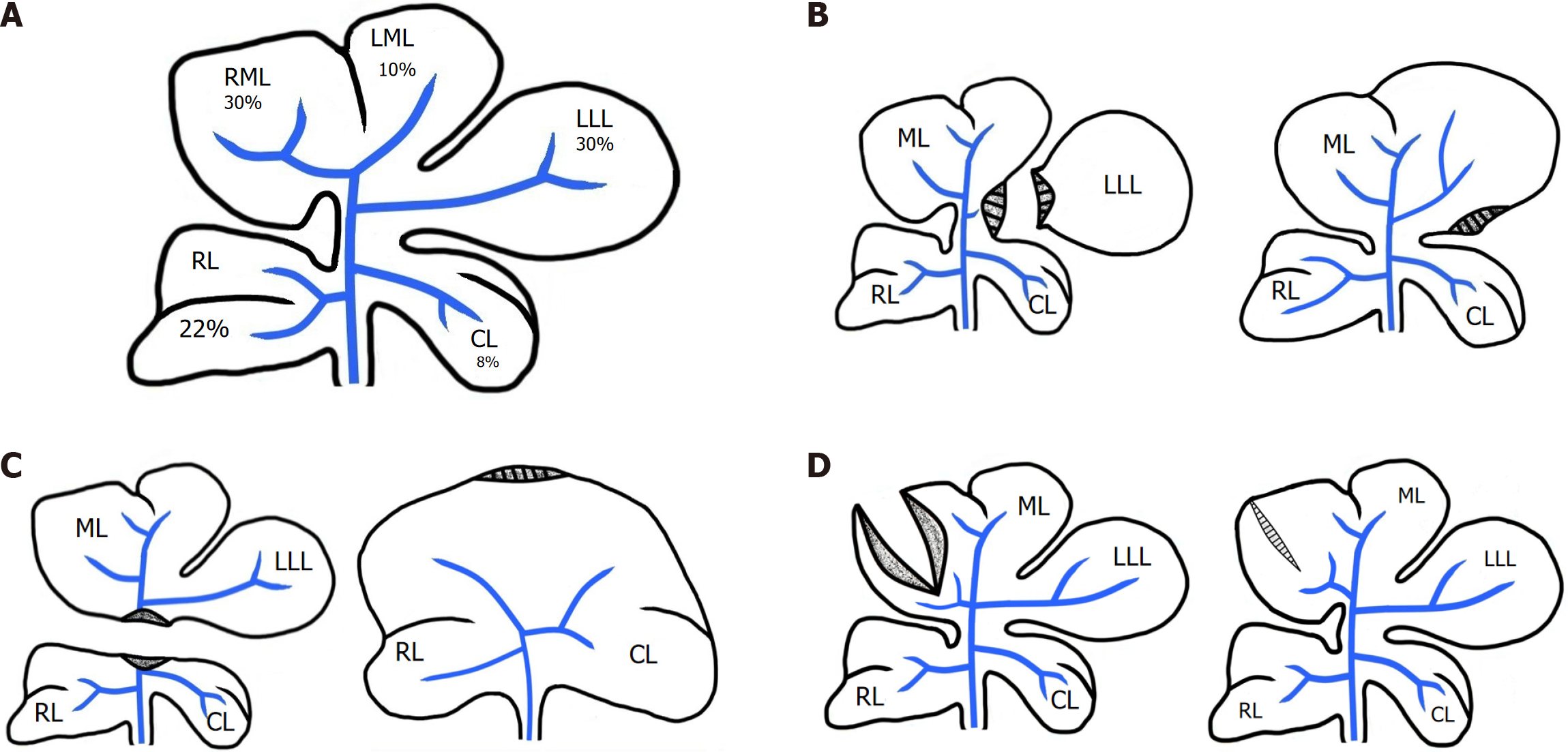
Notably, the cross-sectional area of dissected liver tissue may not correlate with the percentage of mass removed. In animal models with lobulated livers (e.g., rats, dogs) (Figure 1A), minor resections may paradoxically involve a larger dissection area than major resections (Figure 1B and C). Based on this, it is generally accepted that hyperplasia-driven regeneration occurs only when a significant portion of liver mass is lost, and is not dependent on the size of the dissection plane. While this concept holds in most experimental models, it warrants further evaluation and clarification[9].
In surgical practice, liver tissue disruption may occur even without resection. Experimental models demonstrate that dissection alone, without removal of mass, can elicit inflammatory responses and scar formation without inducing regeneration (Figure 1D)[10].
Our long-term studies, supported by literature data, indicate that the direction of regenerative processes following liver tissue damage is determined by changes in portal pressure and flow—which act as mechanical and physiological determinants[4,9,11,12].
In cases where liver injury occurs without resection (such as trauma or stab wounds) and portal circulation parameters remain unchanged, the regenerative response primarily consists of inflammatory and reparative processes.
In contrast, after resection more than 50% of the liver, the blood supply previously directed to the entire liver, is redistributed to the remaining liver tissue, resulting in increased pressure and flow. This triggers a cascade of cellular proliferation, leading to proliferative (hyperplastic) regeneration[4].
Conversely, when no more than 30% of the liver mass is resected, the intrahepatic portal pool is not reduced to an extent that would initiate mitotic activity. However, it is sufficient to induce hypertrophic regeneration[5].
Liver injury triggers a complex regenerative process, the nature of which is highly dependent on the type of liver damage, particularly how it affects the intrahepatic portal pool and portal blood hemodynamics.
Both models—liver tissue repair and LR - are mediated by growth factors and cytokines, which play a crucial role in these processes. During liver injury, the restorative process involves an inflammatory response, which is characterized by an immediate reaction to tissue damage, including vasoconstriction and vasodilation, mobilization of immune cells (such as macrophages and neutrophils), and the activation of proteins that facilitate cytokine and growth factor release to coordinate the repair process. At this stage, cytokines such as tumour necrosis factor (TNF) and interleukin (IL)-1 and IL-6 further promote the recruitment of immune cells and fibroblasts to the site of injury[13,14].
Next, the proliferative and migratory processes take place: As necrotic tissue degrades, the platelets become activated, accumulate at the wound site, and secrete growth factors, including platelet-derived growth factor, which stimulates fibroblasts and other cells to initiate tissue repair. Fibroblasts migrate to the injured area, producing collagen and other ECM components necessary for restoring tissue integrity. A reduction in oxygen supply at the wound site stimulates the release of vascular endothelial growth factor (VEGF), which promotes angiogenesis (the formation of new blood vessels), thereby enhancing the delivery of oxygen and nutrients to the injured liver[15].
At the final stage, granulation (fibrotic) tissue undergoes a remodeling phase and gains structural stability, including scar tissue formation. It was shown, that liver reparative regeneration (wound healing) is dependent on the ECM disruption. The adult liver responds to the dissection with inflammation and stellate cell activation, culminating in fibrosis characterized by collagen deposition. These findings suggest that ECM disruption leads not to regeneration but rather to scarring, similar to other mammalian organs[16].
Unlike the liver repair process, true LR occurs only after the loss of a significant portion of liver mass, such as following PH. In rodent models, experimental data show that liver mass is typically restored within 7-10 days after partial hepatectomy, with a peak in DNA synthesis occurring within the first 1-3 days. In contrast, in humans, complete liver mass recovery usually takes up to 3 months, although hepatocyte DNA synthesis—measured by radiolabel uptake or Ki-67 staining—reaches its peak between postoperative days 7 and 10[7,17].
The main research directions in LR include: Investigation of triggers (stimuli) that initiate regeneration; Examination of the molecular pathways regulating liver mass restoration and termination of regeneration; Study of structural changes (both cellular and tissue-level) occurring during and after LR.
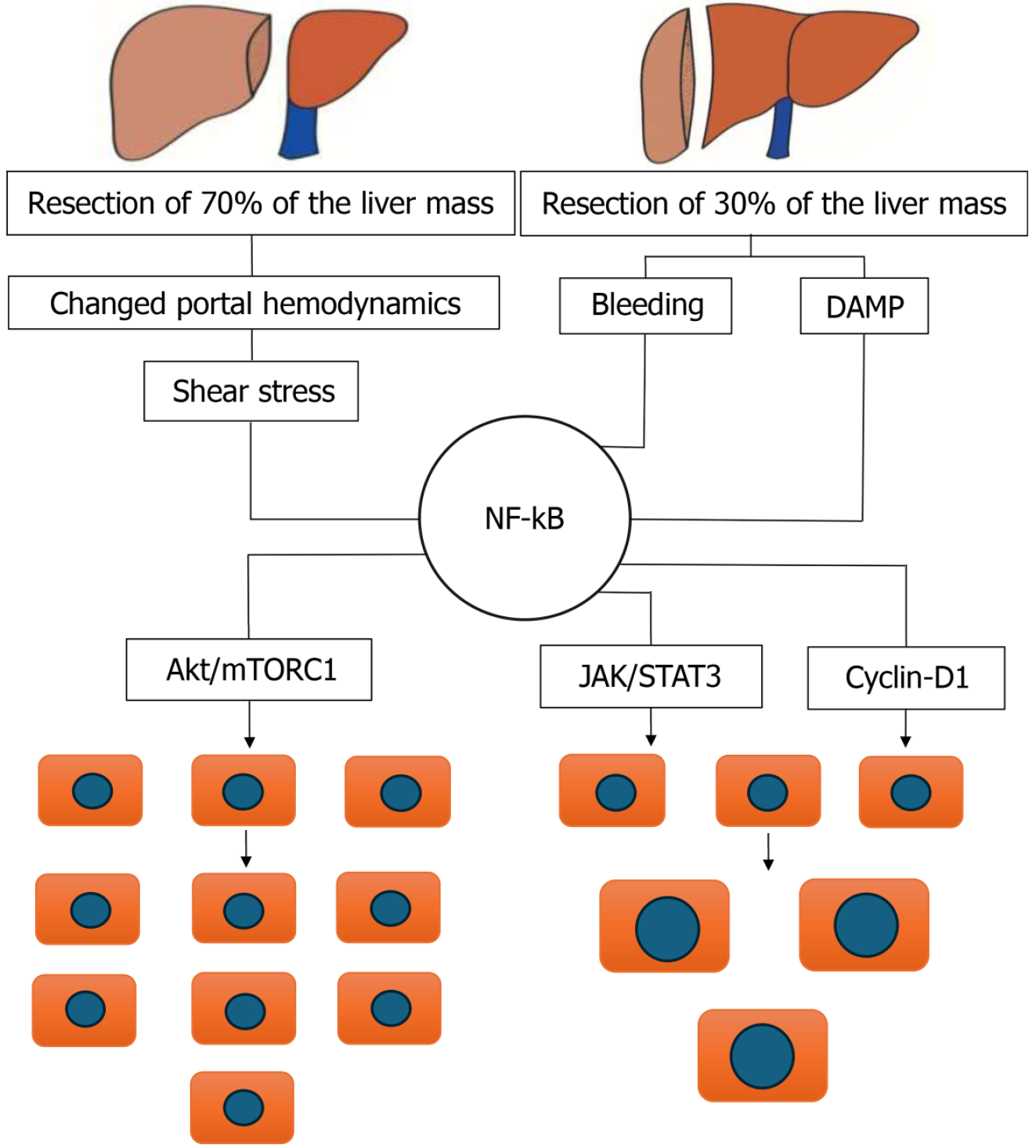
LR begins with the activation of cytokines and growth factors released following immediate reduction of the intrahepatic portal pool caused by the resection of a significant portion of liver tissue. The increased portal pressure and blood flow induce shear stress on endothelial cells within the portal vascular network[18]. It is shown, that portal hemodynamic changes influence LR through endothelial-hepatocyte communication. Particularly, the mechanical stress from altered blood flow activates Piezo1 - a mechanosensitive ion channel highly expressed in vascular endothelial cells (VECs)[19]. Piezo1, in turn, upregulates the expression and secretion of amphiregulin (AREG) and epiregulin (EREG) via the protein kinase C/extracellular signal-regulated kinases 1 and 2 (PKC/ERK1/2) signaling axis[4]. These ligands activate epidermal growth factor receptor (EGFR) signaling in hepatocytes driving their proliferation, especially in liver zones 1 and 2[4,20].
Experimental studies have demonstrated that following 50%–70% partial hepatectomy, the portal venous pressure (PVP) increases significantly—from a baseline of approximately 7–8 mmHg to 14–17 mmHg—reflecting increased sinusoidal shear stress that activates regenerative pathways. It was reported that after 70% PHx in rats, PVP rose from 8.4 ± 0.7 to 16.1 ± 1.3 mmHg, which correlated with hepatocyte proliferation observed within 24 hours postoperatively[18]. Similarly, Sato et al[21] found that acute portal hypertension induced by 70% resection enhanced [³H]-thymidine incorporation into hepatocyte DNA, supporting the concept that elevated shear stress serves as a regenerative trigger. Our previous conceptual work also proposed that portal hypertension (increased from 104 to 191 mmH2O) act through mechanotransduction pathways to stimulate non-parenchymal cell signaling, further initiating regeneration[11].
Importantly, while moderate elevations in portal pressure (14-17 mmHg) facilitate regeneration, excessive increases beyond approximately 20 mmHg, such as after 90% hepatectomy, are associated with liver sinusoidal endothelial damage, hemorrhagic necrosis, and post-hepatectomy liver failure (PHLF). This dual role underscores the need for a hemodynamic 'Goldilocks zone': Enough shear stress to trigger regeneration, but not so much as to induce vascular injury.
Clinical data support this concept: In a study by Allard et al[22], patients with post-hepatectomy portal pressure > 20 mmHg had a 48% rate of liver failure and 26% mortality, compared to just 8% and 2%, respectively, in patients with lower portal pressure. Similarly, Junrungsee et al[23] demonstrated that modulating portal flow via splenic artery ligation reduced post-hepatectomy liver failure from 22% to 7%. These findings are further supported by a systematic review (Gavriilidis et al[24]), which confirmed that portal hemodynamic control improves liver function and survival after resection.
Apart from the mechanical impact, changes in the ratio of portal and arterial blood supply result in reduced oxygen delivery to endothelial cells. In response, these cells release VEGF and nitric oxide (NO)[25-27], which enhance nutrient and oxygen supply to the remaining liver tissue and support the initiation of the regeneration process.
Liver resection remains the only curative option for many patients with malignant liver disease. However, its main limitation is the risk of PHLF, which occurs when the future liver remnant (FLR) is insufficient to sustain liver function[28].
To mitigate this risk, various approaches have been developed, including portal vein embolization (PVE), which promotes compensatory hypertrophy of the remaining liver; Hepatic vein deprivation and Liver venous deprivation (LVD) — a technique combining transhepatic portal and hepatic vein embolization. These procedures aim to precondition the liver prior to major hepatectomy, particularly in patients with insufficient FLR. LVD combines PVE with hepatic vein occlusion, leading to enhanced hemodynamic redistribution toward the FLR. Studies have demonstrated that LVD induces faster FLR growth and enables earlier liver resection compared to PVE alone[29-31].
The close relationship between changes in portal hemodynamics and LR is also supported by the studies on Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) - in both clinical and experimental settings. ALPPS is a surgical technique developed in 2007 by Schnitzbauer and colleagues, in which liver resection is performed in two stages. In the first stage, the portal vein branch supplying the portion of the liver to be resected is ligated, and partial liver dissection is performed. This results in a sudden increase in portal pressure within the liver portion that will remain, triggering intensive hyperplasia. In the second stage, the liver section excluded from portal circulation is resected, significantly reducing the risk of post-hepatectomy liver failure since the remaining liver has already undergone substantial regeneration. Research has shown that LR under conditions occurs significantly faster compared to standard hepatectomy[32]. However, benefits and risks of ALPPS are still under active investigation, as long-term outcomes remain controversial.
A unique opportunity to observe LR arises in living donor liver transplantation[7] In this setting, different portions of the same liver undergo regeneration in two different organisms. Furthermore, in the recipient, the transplanted liver regenerates under conditions where it lacks the innervation and lymphatic drainage[33]. Interest in such observations is growing due to the results of a recent study, according to which, the anatomical tracing and functional mapping revealed a neural circuit from noradrenergic neurons in the locus coeruleus to serotonergic neurons in the rostral medullary raphe region, which critically contributes to the inhibition of LR under chronic stress. In addition, hepatic sympathetic nerves were crucial for the inhibitory effect on LR by releasing norepinephrine (NE), which acts on the adrenergic receptor β2 (ADRB2) and blocks proinflammatory macrophage activation. The “brain in the liver” mediates the inhibition of LR under chronic stress through the hepatic sympathetic nerve/macrophage axis. Furthermore, the accumulation of NE released by hyperactivated hepatic sympathetic nerves can block the polarization of proinflammatory macrophages by acting on ADRB2, which is highly expressed on the macrophage membrane. These findings suggest that the NELC-5-HTrMR neural circuit plays a fundamental role in the homeostasis of LR[34].
Unfortunately, little attention has been paid to the results of experimental studies, that provide critical insights into LR mechanisms. It has been demonstrated, that orthotropic transplantation of liver from small size donor to larger size recipient causes the increase in transplant’s size[35]. In vice versa, the liver transplantation from the large to small animals causes the reduction of the graft size[36]. These data provides the additional confirmation that liver size regulation is primarily controlled by changes in portal pressure and blood flow dynamics[37].
Additionally, studies have shown that LR slows down if hepatectomy is performed after portocaval shunting[38,39]. Moreover, in experiments where the portal circulations of two animals are connected via a shunt, hepatectomy in one animal induces liver growth in the other, despite the absence of direct liver tissue damage[40,41]. This phenomenon is particularly intriguing, as it highlights the strong regulatory influence of portal hemodynamics on LR, independent of direct liver injury.
All of the aforementioned examples indicate that enhanced portal hemodynamics and shear stress serve as critical triggers for LR. However, studies have also demonstrated that excessive shear stress can lead to damage of liver sinusoidal endothelial cells (LSECs) and haemorrhagic necrosis[42]. For instance, following 80–90% liver resection, only a small fraction of operated animals survives[21].
Additionally, pharmacological reduction of postoperative portal pressure, such as with Terlipressin, has been shown to prevent hepatocyte necrosis and promote proliferative activity, ultimately improving survival rates in operated animals. These findings emphasize the critical importance of determining the optimal resection volume - and thus, the acceptable limits of portal pressure and flow alterations - to ensure effective proliferative LR[9].
The cytokines and growth factors involved in liver regeneration stimulate hepatocyte proliferation and angiogenesis, leading to the restoration of liver mass and function[37].
Key signaling molecules participating in LR include: IL-1 and IL-6, which act as a triggering cytokine for regeneration; hepatocyte growth factor (HGF), which stimulates hepatocyte proliferation; transforming growth factor-alpha and epidermal growth factor, which promote cell cycle activation; VEGF, which plays a critical role in angiogenesis; NO, which facilitates nutrient and oxygen supply to liver tissue[43].
However, most of these signaling molecules are expressed in all cases of liver tissue damage, regardless of whether the injury results from dissection (trauma, wounds) or resection. But, in the case of trauma or incision, the liver primarily focuses on repair, preventing infection, and restoring tissue integrity; in contrast, following partial resection, the liver “aims” not only to heal the wound but also to fully restore its mass. That being said, this assumption contains a philosophical misconception - the liver is not a “thinking” organ that “aims” and “decides" which process to initiate based on the type of injury. Thus, the liver’s response to injury depends not on a conscious “aim” and “decision” but rather on the biochemical and physiological processes triggered by the type and extent of the damage. Following PH, portal pressure and blood flow increase[21], including within the extrahepatic portal pool (e.g., the gastrointestinal tract and splenic veins). This enhances shear stress effects and promotes the release of growth factors, which play a critical role in hepatocyte proliferation and angiogenesis[44].
Studies also demonstrate that intestinal endotoxins contribute to the stimulation of LR. Following PH, there is an increased likelihood of gut microbial factors infiltrating portal circulation, including pathogen-associated molecular patterns (PAMPs) and lipopolysaccharides (LPS). As a result, the remaining liver tissue comes into direct contact with microbial endotoxins[7].
Both PAMPs and LPS activate Kupffer cells, which subsequently synthesize two key cytokines—IL-6[45] and tumour necrosis factor-alpha (TNF-α)[46]. TNF-α functions via both autocrine and paracrine mechanisms: It binds to TNF receptor 1 in hepatocytes; also interacts with TNF receptors on Kupffer cells, triggering a signaling cascade. This ligand-receptor interaction induces the synthesis and mobilization of other cytokines, including IL-6, which further stimulates hepatocyte proliferation through the nuclear factor-kappa B (NF-κB) signaling pathway.
The postulate that gut microbiota plays a role in LR is further supported by studies showing that antibiotic treatment impairs LR following PH. Research has demonstrated that the use of antibiotics reduces both liver mass recovery and the expression of proliferative markers - proliferating cell nuclear antigen and Bromodeoxyuridine (BrdU). It is speculated that antibiotics alter Kupffer cell tolerance and lead to hyperactivation of natural killer T cells, which in turn inhibits LR[47].
Another critical regulator of LR following PH is Interleukin-22 (IL-22). Studies have confirmed a significant increase in both IL-22 and its receptor (IL-22R) expression in the serum following 70% hepatectomy[48]. Blocking IL-22 has been shown to slow down LR, as evidenced by a decrease in DNA synthesis (reduced BrdU incorporation) and lower activation of signal transducer and activator of transcription 3 (STAT3), a key transcription factor involved in cell survival and proliferation. Conversely, IL-22 administration increases IL-6 and TGF-β levels, indicating that its regenerative effects are closely linked to these signaling pathways. Interestingly, blocking IL-22 does not completely suppress STAT3 activation, suggesting that LR is contributed by multiple regulatory mechanisms. These findings highlight the complex and interconnected nature of cytokine signaling in LR. IL-22, through its interaction with IL-6, TGF-β, and STAT3, plays a crucial role in hepatocyte proliferation and liver recovery. This suggests that IL-22 may have therapeutic potential, similar to the portal pressure-lowering effects of Terlipressin, in conditions where modulating LR is necessary[48].
Beyond IL-22, various other molecular factors and signaling pathways have been identified as active participants in hepatocyte survival and proliferation, angiogenesis, and liver mass recovery. These pathways collectively regulate the fine balance between liver repair and regenerative processes, further emphasizing the multifactorial nature of LR.
The Wnt/β-catenin signaling pathway is activated within LSECs in response to liver injury. Following tissue damage, β-catenin levels rise significantly, which may be linked to shear stress, and it plays a crucial role in transcription of genes essential for cell proliferation[49].
The elevation of β-catenin represents an early event in LR and significantly impacts the activation of the hepatocyte cell cycle. Importantly, the Wnt/β-catenin pathway regulates not only hepatocyte proliferation but also liver metabolism, detoxification, and immune responses. However, some studies indicate that excessive activation of Wnt/β-catenin and TGF-β during LR can disrupt the balance of the cell cycle and contribute to the formation of tumor cells. Individuals at particularly high risk include those with: Chronic viral hepatitis (Hepatitis B virus, Hepatitis C virus)[50], Non-alcoholic fatty liver disease and Alcoholic liver disease. Hyperactivation of the Wnt/β-catenin pathway is a key mechanism in the pathogenesis of hepatocellular carcinoma, as it promotes uncontrolled cell proliferation[51].
The activation of Wnt/β-catenin pathway might be also linked with Mesencephalic astrocyte-derived neurotrophic factor (MANF), a member of newly identified neurotrophic factors family, which extensively expresses in the liver and has demonstrated cytoprotective effects during endoplasmic reticulum stress and inflammation. MANF expression was up-regulated in a time-dependent manner, reaching the peak at 24 hours and 36 hours after 2/3 PH, respectively. However, MANF knockout delayed the peak proliferation period by 24h. MANF physically interacts with lipoprotein receptor-related protein 5 (LRP5) and β-catenin, two essential components of Wnt/β-catenin pathway. Specifically, as a cofactor, MANF binds to the extracellular segment of LRP5 to activate Wnt/β-catenin signaling. On the other hand, MANF interacts with β-catenin to stabilize cytosolic β-catenin level and promote its nuclear translocation, which further enhance the Wnt/β-catenin signaling[52].
The NF-κB signalling pathway is activated following liver injury and is closely linked to the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) and phosphoinositide 3-kinase/protein kinase B, PKB/ma
Studies have demonstrated that NF-κB inhibits hepatocyte apoptosis and accelerates LR by promoting the expression of pro-survival and anti-apoptotic genes[54]. This signalling pathway is essential for protecting hepatocytes from excessive damage and ensuring a rapid regenerative response.
However, The Akt/mTORC1 pathway is a key mediator of cell growth in many cellular systems, including certain settings of liver regeneration[55]. We therefore examined whether bleeding influences components of this pathway. Western blot analysis of liver extracts revealed that on POD1 after hepatectomy, phosphorylation of mTOR and S6 were markedly increased in rats subjected to PHx and bleeding compared to PHx only. To assess the functional significance of mTORC1 signaling for liver regeneration in rats subjected to PHx and bleeding, we treated additional rats with themTORC1 inhibitor rapamycin. This treatment resulted in a significant elimination of bleeding-induced hepatocyte hypertrophy along with inhibition of cell proliferation.
The Notch signalling system plays a crucial role in liver development, particularly in the differentiation of liver stem cells. However, its role in post-resection LR is relatively limited, as liver mass restoration after resection is primarily driven by the proliferation of residual hepatocytes rather than stem cell activation[56].
Nevertheless, in cases of PH involving a diseased liver (where hepatocytes are severely damaged), Notch signaling may be activated to facilitate the involvement of liver stem cells in the regenerative process.
Notch signaling is also considered a mediator of the Hippo/YAP (Yes-Associated Protein) pathway. The activation of YAP leads to an increase in Notch receptor expression, which in turn triggers Notch signaling and translocation of Notch intracellular domain into the nucleus[57]. Additionally, Notch can modulate STAT3 activity and influence the dedifferentiation of LSECs (liver sinusoidal endothelial cells), which is crucial for liver repair mechanisms[58].
A deeper understanding of these processes is critical for the management of liver diseases, as they offer potential therapeutic targets for enhancing LR[59].
The Hedgehog (Hh) signaling pathway regulates the expression of YAP-1, which in turn stimulates glutaminolysis and activates hepatic stellate cells (Ito cells). Additionally, Hh signaling prevents apoptosis of hepatocytes, biliary epithelial cells, and liver progenitor cells, playing an up-regulatory role in LR[60]. Several studies suggest that cholangiocarcinoma and other biliary tract malignancies are frequently associated with overactivation of the Notch and Hedgehog signaling pathways[61].
Epidermal growth factor and transforming growth factor-alpha play key roles in hepatocyte proliferation and survival. These growth factors activate the cell cycle and protect hepatocytes from a stressful environment, ensuring the continuation of the regenerative process[62].
Transforming growth factor-beta (TGF-β) is one of the central regulators of LR but exhibits a dual role: At low concentrations, it promotes regeneration; at high concentrations, it induces hepatocyte apoptosis and suppresses regeneration. TGF-β is considered the main termination mechanism of LR, ensuring that the process ceases when the liver reaches its normal size. Notably, its activity is closely linked to portal circulation parameters and shear stress[60].
The insulin-like growth factor (IGF) pathway significantly influences hepatocyte survival and proliferation, acting as a growth-promoting and protective factor. The potential of IGF as a therapeutic agent for enhancing LR is of growing research interest, particularly in conditions involving metabolic disturbances such as starvation or diabetes[63].
The fibroblast growth factor (FGF) signalling pathway - particularly FGF-1 and FGF-2—plays a crucial role in hepatocyte division and angiogenesis. These factors are essential for optimizing liver microcirculation and blood supply. Importantly, FGF-2 also participates in balancing liver fibrosis and regeneration, highlighting its dual role in hepatic homeostasis[64].
Recent studies have highlighted IL-33 as a key player in LR. IL-33 is activated via the suppression of tumorigenicity 2 (ST2) receptor and stimulates serotonin release into portal circulation. This mechanism promotes hepatocyte proliferation through the 5-Hydroxytryptamine Receptor 2A/Ribosomal Protein S6 Kinase, 70kDa (HTR2A/p70S6K) pathway[65]. Elevated IL-33 Levels and inhibition of C-C motif chemokine ligand 5 (CCL5) have been suggested as potential cytokine-based therapeutic strategies to enhance LR through immune modulation[66]. Forkhead box O3 (FoxO3a), activated by the CCL5/chemokine receptors 1&5 (CCL5/CCR1/CCR5) signaling axis, inhibits HGF production and promotes pro-inflammatory macrophage polarization, thereby restricting the liver's regenerative capacity. It is hypothesized that targeting the IL-33/ST2 pathway and modulating FoxO3a-driven macrophage polarization could serve as new therapeutic approaches for enhancing LR. For instance, Meloxicam - a selective cyclooxygenase-2 inhibitor, was identified as a pharmacological agent capable of enhancing LR, demonstrating the increase of EGFR expression, leading to a 70% improvement in LR following resection[67].
These findings emphasize the potential of targeted cytokine modulation and pharmacological therapies in optimizing liver recovery following hepatic injury or resection.
LR following PH is a complex and highly coordinated process involving hepatocyte proliferation, ECM reorganization, and metabolic remodeling[68].
Studies have demonstrated that cholesterol metabolism and bile acid signaling play key roles in LR. The addition of exogenous cholesterol activates Hypoxia-Inducible Factor 1-alpha (HIF-1α) and Nuclear Factor Erythroid 2-Related Factor 2 (Nrf-2) pathways, which enhance hepatocyte proliferation and survival[69].
Bile acids regulate liver growth via the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5) receptors[70], which stimulate hepatocyte division and protect liver tissue from inflammatory damage. These findings confirm the strong interconnection between liver metabolism and regeneration, suggesting that targeting these pathways could provide novel therapeutic strategies for enhancing post-resection liver recovery[67].
Glutamine (Gln), a critical amino acid, involved in energy metabolism and ECM formation, has been also identified as a key regulator of LR[71]. In studies using C57 Black 6 (C57BL/6) mice undergoing 70% hepatectomy, animals that received 0.6% Gln-enriched water before and after surgery exhibited accelerated liver mass recovery, particularly between 2 and 4 days after intervention. This effect was attributed to enhanced hepatocyte proliferation, increased amino acid metabolism [Glutamine Synthetase, Carbamoyl Phosphate Synthetase 1, ornithine transaminase (OAT)], and lipid synthesis activation. Further analysis revealed that OAT activation linked glutamine metabolism to ammonia detoxification and ECM remodeling[68].
Additionally, collagen deposition within the ECM significantly increased in glutamine-supplemented mice, particularly in the portal tract region. Hepatic steatosis was also more pronounced in these animals, indicating a regenerative adaptation response[68]. These data are consistent with the results of our study, which showed different regeneration dynamics depending on the zones of the liver acinus[72,73].
Thus, the emerging evidence underscores the importance of metabolic pathways in LR, with cholesterol, bile acids, and Gln metabolism playing significant roles in post-hepatectomy recovery. These insights provide potential therapeutic targets for optimizing LR and enhancing patient outcomes following major liver resections.
All of the above demonstrates that multiple signaling pathways and mechanisms are involved in LR. At the same time, they all act in a coordinated manner to ensure timely liver recovery and maintain the functional balance of the organism. An analysis of reparative and post-resection LR suggests that similar molecules and signaling pathways participate in both processes. However, both types of regeneration occur within a specific environment, characterized by appropriate blood pressure, temperature and pH.
The portal pressure and blood flow have already been discussed. In addition to pressure, temperature also plays a crucial role in LR. It is known that hypothermia is a promising approach for improving cellular regeneration, alongside transcription factors, microRNAs, epigenetic modifications, and DNA methylation. The effects of hypothermia on HGF and other factors production and their impact on pathways activated or inactivated following PH remain subjects of ongoing research[74]. However, the opposite opinions are also provided[75,76], indicating that the role of the temperature in LR following PH remains the subject of further investigation.
A change in pH during subsequent LR was also demonstrated. Studies dedicated to Small-for-Size liver resection and Flow Syndrome showed that following 75% liver resection, a decrease in pH was observed not only in the hepatic artery (from 7.377 to 7.322) and in the portal vein (from 7.325 to 7.273 in the case of), but also in the systemic circulation — in the carotid artery (from 7.381 to 7.335) and in the jugular vein (from 7.335 to 7.288). This confirms that the activation of the molecular reactions described above occurs in a low pH environment. Notably, such pH changes have not been observed during reparative regeneration following liver dissection[77].
These changes overlap with early swelling of the hepatocytes which represent a biophysical trigger that activates downstream proliferative genes and drives cells from the G0/G1 phase into the S-phase of the cell cycle. In fact, the intracellular alkalinization mediated by sodium hydrogen exchanger has been identified as a permissive signal for DNA synthesis and mitotic activity[78].
It can be assumed that after liver mechanical injury, the determination of the type of LR depends on physical (physiological) factors such as pressure, temperature, and pH, while the processes pass biochemically result in distinct morphological outcomes.
During liver regeneration, hepatocyte polyploidization represents one of the key cellular responses, especially in the context of hypertrophy. Polyploid hepatocytes, containing 4n, 8n, or higher chromosomal content, are frequently observed in morphological adaptation. This mechanism is often associated with the liver's attempt to rapidly restore metabolic capacity without undergoing complete cell division[79].
In addition, binucleated hepatocytes are commonly detected during liver regeneration. Their presence reflects ongoing mitotic activity that is not followed by cytokinesis. This phenomenon is often viewed as an intermediate state between hyperplasia and hypertrophy, illustrating the liver’s flexibility in maintaining regenerative momentum even under conditions where full cell division is limited or impaired[55].
Importantly, polyploidization is not only a form of cellular growth but is also implicated in maintaining genomic stability, buffering metabolic demands, and enhancing stress resistance. While diploid hepatocytes have been shown to dominate the early proliferative response following major hepatectomy, polyploid hepatocytes contribute to regeneration through selective division and hypertrophy, particularly under conditions where proliferation is delayed or impaired[80,81].
Thus, polyploidization and binucleation contribute to both the functional and structural reconstitution of hepatic tissue. The further understanding their role may add the valuable insight into the complicated mosaic of liver re
According to Miyaoka and Miyajima's calculations[55], the number of hepatocytes in the remaining lobes after 70% hepatectomy increases 1.6 times, and the mass of these lobes increases 2.4 times. Additionally, Wagenaar GT and co-authors[82] have shown that the porta-central distance increases 1.2-1.5 times after PH. These data prove that hepatocytes undergo not only hyperplasia but also the hypertrophy, which during their proliferation is carried out by the pathway[55]. However, in the same study, Miyaoka and Miyajima note that the process of hyperplasia and hypertrophy does not equally affect all hepatocytes and all lobes. Although radioactive nucleotides have shown hepatocytes entering the S phase of the cell cycle, DNA replication does not necessarily mean cell division. If all hepatocytes undergo S phase and divide, ploidy should remain constant after PH. However, ploidy is known to increase after PH. These results support the opinion of Fausto[83] presented the decades ago.
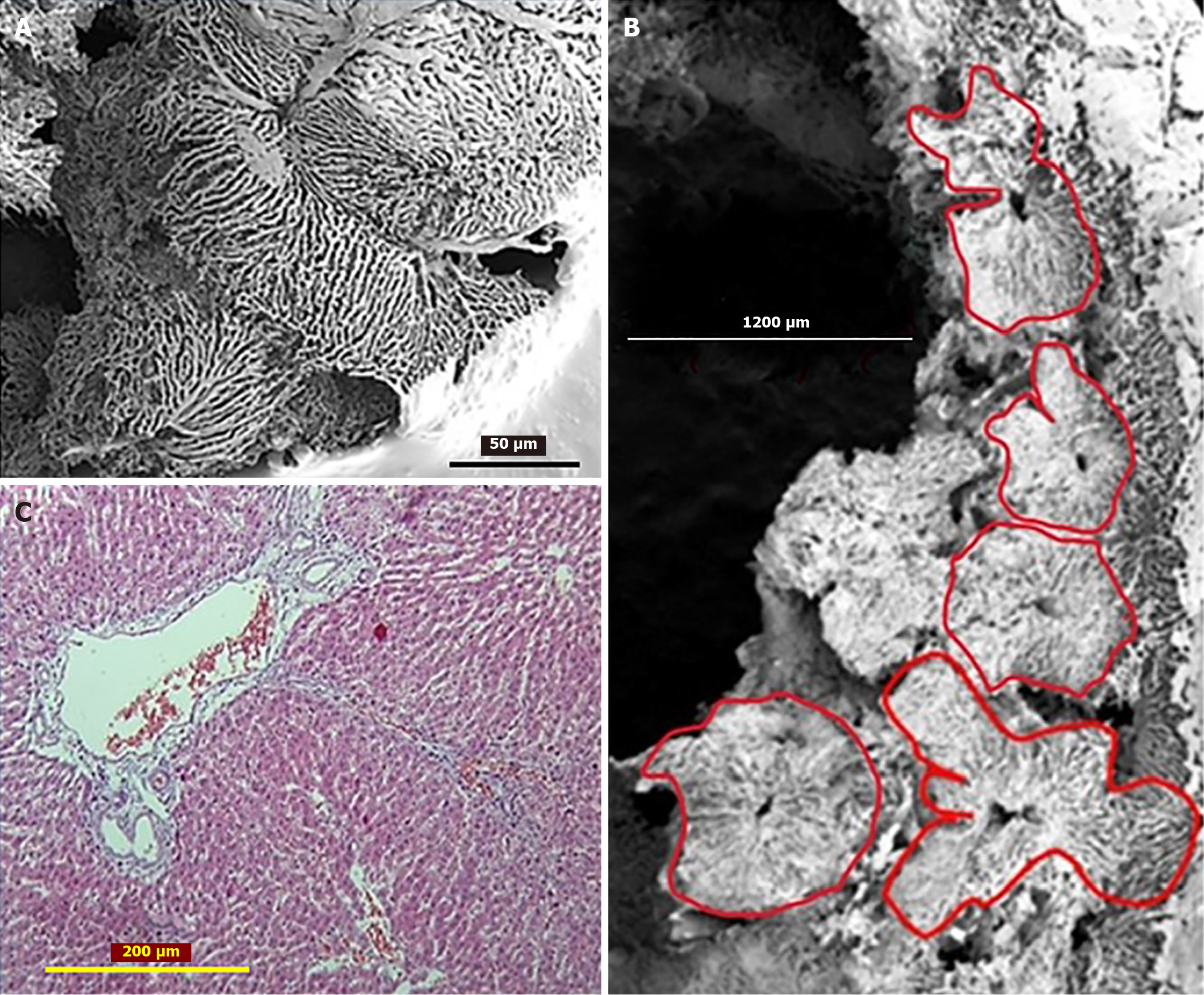
The above mentioned is supported by results of our experimental investigation of liver tissue, following two-thirds liver resection. The dynamic observations over two weeks of regeneration have revealed changes in hepatocyte size, shape, and the spatial architecture of the sinusoidal network. Analysis of hepatic acinar zones 1st and 3rd showed alterations in hepatocyte morphology and size, alongside the formation of new intercellular connections, including atypical membrane protrusions between adjacent hepatocytes with altered shapes (Figure 2). The scanning electron microscopy of corrosion casts revealed a sinusoidal network with a spatial architecture suggesting lobules of different sizes and shapes, including some that appeared to form by the fusion of two "normal" lobules (Figure 3A). In certain areas, vascular sprouting-like patterns were observed within hepatic vein tributaries and associated large sinusoids. The continued transformation of hepatocyte shape and size, as well as vascular network remodeling, contributes to alterations in the spatial organization of hepatic lobules[73].
Six months after PH, morphological studies of rat liver tissue confirm that LR is driven by both hepatocyte proliferation and hypertrophy. Hepatocytes and sinusoids become organized into remodeled lobules, exhibiting considerable variation in size and shape (Figure 3B). This raises the question of whether hyperplasia, hypertrophy, and lobular remodeling occur uniformly across all hepatocytes and lobules. Furthermore, it is possible that new lobules form during the remodeling process. The remodeled lobules (especially the “mega-lobules” with the sizes 1000-1200 µm) contain the transformed meshwork of the sinusoids, the part of which is dilated asymmetrically. This meshwork might have originated from the several portal venules (interlobular and/or inlet). The boundaries between the adjacent lobules (including mega-lobules) are widened and filled by connective tissue fibers, which gives the liver parenchyma a nodular look (Figure 3C)[72].
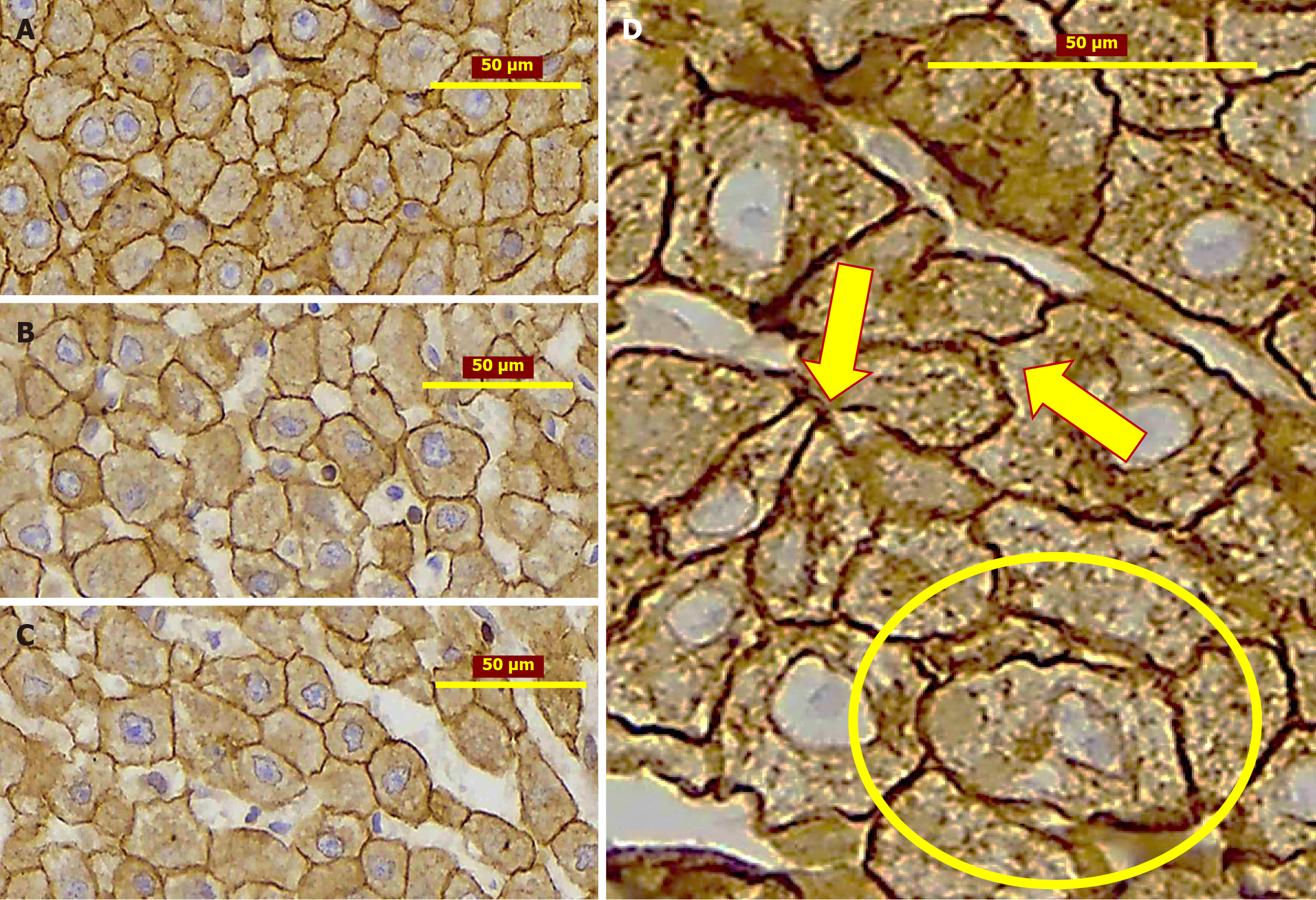
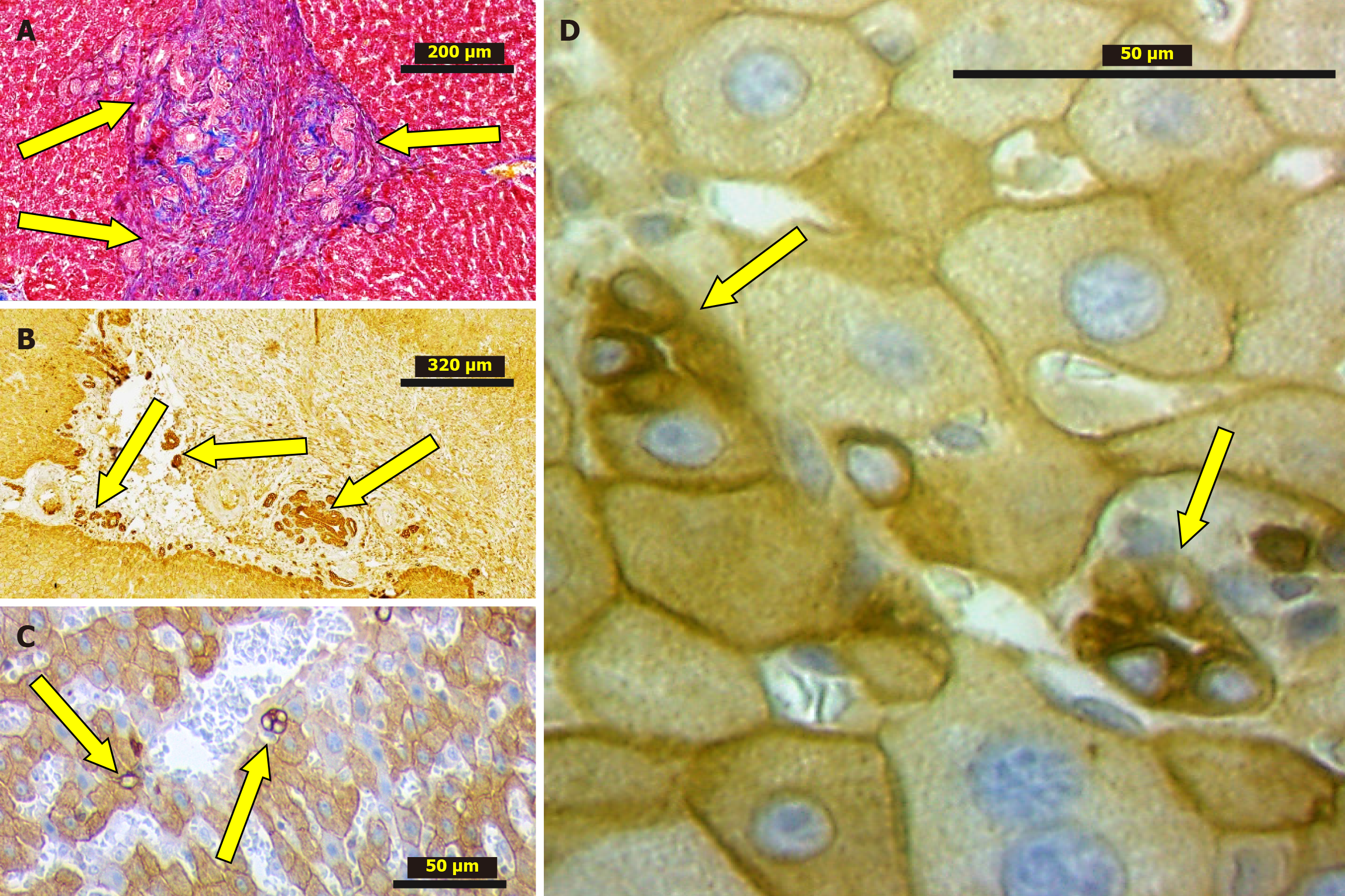
Interestingly, that in the liver tissue studied histologically (H&E, IHC) both after 2 weeks and 6 months from PH, the biliary structures were revealed. Their number is greater than in normal livers. Besides, the ductular profiles were observed not only in the portal tracts or periportal zones, but also within the liver lobules including the adjacent areas to the various caliber branches of the liver veins (Figure 4)[64]. These findings indicate that LR following PH alongside with other mechanisms involves ductular reaction (DR) as well. However, when considering disease models characterized by the development of a ductal reaction, PH is not included[74] suggesting that DRs are neglected in the process of post-resectional LR. It underlines that this feature, which was not described yet, requires dedicated research.
Based on these findings, it has been hypothesized that although liver mass is restored, in 7-10 days period, the regeneration process remains ongoing. This phenomenon requires further investigation because it could influence the search for and development of new therapeutic targets.
In clinical surgery, when the liver resection almost always results in mechanical liver tissue damage, including deuteriation of ECM and inflammatory response[2], the DR associated with local inflammation and ECM remodeling in the dissection area, are often revealed[84]. Similarly, the studies in C57BL/6J and Severe combined immunodeficiency mice also have shown that after 20%-30% resection of the liver mass, significant DRs were observed at the border of the injured area, peaking on postoperative days 2-3. Histological analysis of the liver revealed cytokeratin 19, Thy1 (CD90), A6, and epithelial cell adhesion molecule expression, indicating that these ductular structures originated from LPCs[85]. Interestingly, these DRs were absent 24 hours after a 20%–30% resection, suggesting that LPC activation occurs later than the initiation of hepatocyte proliferation in standard PH (66%-70%) models. ECM remodeling is also a critical factor in the amplification of DRs. These findings indicate that ECM remodeling provides the structural framework necessary for DRs and tissue repair mechanisms. Overall, DRs are a crucial component of LR, regulated by inflammatory cytokines and ECM remodeling. The ability of LPCs to respond to local injury highlights their therapeutic potential in the liver recovery process. Thus, it can be concluded that DRs play a significant role in LR following 20%–30% resection. These reactions, driven by the proliferation of LPCs, are essential for liver recovery, particularly in conditions where hepatocyte proliferation is impaired[84].
The extent to which DRs contribute to liver wound healing remains poorly studied. This is primarily due to technical challenges in obtaining clinical material (as there are practically no reports on biopsy findings from liver wound healing sites) and a lack of experimental models focusing on liver injury without tissue resection.
However, the fundamental research conducted by Ikeda et al[86] has convincingly demonstrated that bile ducts exhibit vectorial proliferation toward granulation tissue that forms following liver injury. Specifically, it has been shown that bile ducts proliferate within the granulation tissue that develops at the site of necrosis appearing after liver adhesion to a perforated duodenum. Three-dimensional reconstruction of histological slides confirmed that proliferative bile ducts originated from pre-existing bile ducts, were not connected to hepatocytes, and extended into the granulation tissue alongside newly forming blood vessels.
Considering the above, it can be proposed that DR occurs in both reparative LR (liver wound healing) and post-resectional regeneration, and its localization is expected to be adjacent to the dissection area. The DR following 20%-30% liver resection has already been discussed. Figure 4 represents our findings on DR in the granulation tissue surrounding adhesions developed after 2/3 liver resection among the adjusted remnant lobes as well as granulation tissue developed at the edge of resected lobe.
Thus, it can be inferred that LR, alongside other cellular mechanisms, also relies on bile duct proliferation. However, it might be concluded that DRs in both liver parenchyma and cut edge connective tissue following liver resection remains poorly studied, and further investigations - particularly utilizing three-dimensional reconstruction techniques - should play a significant role in achieving a better understanding of the complex process of LR.
Obviously, after PH, along with the restoration of liver mass and volume, it is important to analyze the process of so-called functional regeneration of the organ.
Functional regeneration refers to the ability of the liver to restore its metabolic, synthetic and detoxification functions. It is assessed by biochemical parameters (prothrombin time, albumin, bilirubin), clearance tests (e.g., indocyanine green), and enzymatic activity (e.g., cytochrome P-450). Studies indicate that after resection, liver function does not recover as fast as the regeneration of liver mass and volume. It is related to the terms needed for the correction of mitochondrial dysfunction, the metabolic demands of the organism, and changes in microcirculation[87].
While conventional biochemical markers such as bilirubin, INR, ALT, and albumin are routinely used to assess liver function after partial hepatectomy, these indicators often normalize quickly and may not fully reflect the true functional reserve of the regenerating liver. Consequently, more sensitive and dynamic assessment tools have been developed. Quantitative liver function tests such as the galactose elimination capacity and the 13C-methacetin breath test allow real-time evaluation of enzymatic function and correlate with hepatocellular metabolic integrity[88,89].
These tests have demonstrated that although biochemical markers may return to baseline within 7-10 days postoperatively, full functional recovery may extend over 90 to 180 days. Platelet count dynamics have also emerged as valuable surrogate markers, with early postoperative thrombocytopenia associated with delayed functional recovery and increased complication rates[90,91]. In experimental models, the recovery of liver function has been further characterized using stereological and immunohistochemical methods, including assessment of hepatocyte proliferation via Ki-67 staining and β-catenin signaling[92]. Collectively, these data emphasize the need to distinguish between biochemical normalization and true functional regeneration in both research and clinical contexts[93].
When the liver restores its mass and size following PH, regeneration ceases automatically. The LR process is naturally regulated to maintain a balance between regeneration and cell cycle termination. If this regulation is disrupted, the liver may either fail to regenerate effectively (as seen in cirrhosis) or continue pathological proliferation, increasing the risk of tumor development[94]. This means that LR can become either ineffective or pathological.
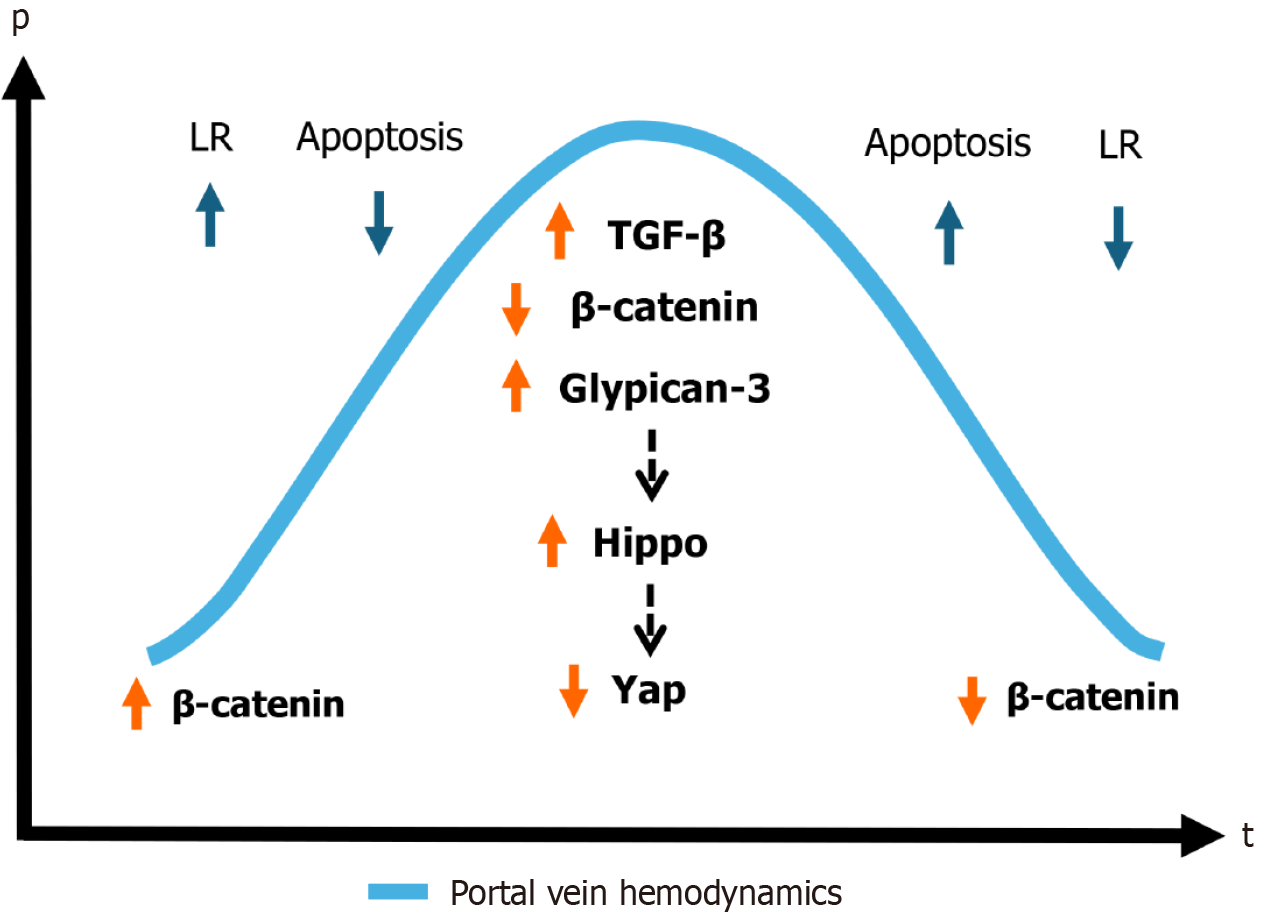
Currently, it is believed that termination of LR after PH, when liver mass returns to normal, occurs through several mechanisms: LR does not cease abruptly but rather follows a regulated sequence, coinciding with the normalization of portal pressure and blood flow. This process is accompanied by: TGF-β activation: TGF-β is the primary suppressor of LR, becoming active when the liver reaches the required mass. Its function is to halt the cell cycle and stimulate apoptosis in hepatocytes that are no longer needed for regeneration[95].
Wnt/β-catenin pathway activation: Wnt/β-catenin activity increases during regeneration; however, once the liver attains its required size, β-catenin levels decrease, which facilitates cell cycle arrest. This pathway plays a critical role in regulating liver mass and hemodynamics.
Hippo-YAP signaling pathway: Hippo signaling is activated when the liver reaches its normal size, suppressing YAP activity and thereby reducing cell proliferation. YAP inhibition is one of the main mechanisms terminating LR[96]. Glypican-3 (GPC3) plays a key role in stopping regeneration, as it stimulates Hippo signaling and blocks YAP activity. Once LR is complete, maintaining high levels of GPC3 prevents uncontrolled hepatocyte proliferation[97]. Interestingly, some forms of liver cancer are associated with GPC3 inhibition, which leads to reactivated cell proliferation. Additionally, elevated concentrations of IL-2 and IL-18 contribute to inhibiting liver growth and downregulating regenerative pro
Although there is no single mechanism that fully explains this phenomenon, several interrelated regulatory systems are believed to contribute. They are: Portal hemodynamic normalization; activation of inhibitory signaling pathways; metabolic feedback mechanisms (restoration of liver metabolic function); spatiotemporal reorganization of lobular spatial architecture.
The phrase “achieving the balance” refers to the gradual, coordinated downregulation of regenerative signals and progressive re-establishment of hepatic homeostasis due to normalization of the portal hemodynamics via the restoration of the compliance among the portal inflow and the volume of the intrahepatic portal pool. Rather than a single “off switch”, termination of liver regeneration occurs via redundant and overlapping pathways—including TGF-β, Hippo-YAP, and Wnt/β-catenin, GPC3 activation - each contributing to halting hepatocyte proliferation and suppressing further liver regrowth[96,100]. However, it should be noted that termination of proliferation does not equal termination of regeneration, as lobular remodeling and functional re-equilibration may continue for weeks to months.
We support the idea that liver regeneration halts when a physiological balance is reestablished between the metabolic and synthetic demands of the organism and the liver’s capacity to fulfill them. Furthermore, if this balance is disrupted again - for example, following a repeat resection of a previously regenerated liver - regeneration is re-initiated using the same mechanisms and continues until liver mass is once again restored, as we have shown in our experimental model[72].
Taking all the above into account, it can be concluded that LR following PH is a dynamic, multifactorial process governed by portal hemodynamics, cytokine signaling, metabolic reprogramming, ECM remodeling, and hepatocyte proliferation, hypertrophy, and polyploidization.
Recent insights highlight the importance of mechanical cues such as portal pressure changes and shear stress, as well as metabolic signals from bile acids, cholesterol, and glutamine in modulating regenerative pathways. Furthermore, the structural remodeling of liver architecture and the spatiotemporal dynamics of regeneration underscore the complexity of this adaptive process.
However, there is no single factor or pathway that alone can fully orchestrate liver regeneration or its termination. This is a redundant and compensatory process: If one pathway is inhibited, others may compensate to ensure (1) Effective liver regeneration; or (2) Timely termination of the regenerative response.
For instance, whether NF-κB activation results in hepatocyte hyperplasia (proliferation) or hypertrophy (cell growth) depends on the regenerative microenvironment. Under conditions of altered perfusion and nutrient delivery, NF-κB synergizes with the JAK/STAT3 and Cyclin D1 pathways to promote cell cycle progression, thereby favoring hyper
However, Akt/mTORC1 pathways activation might be caused also by Piezo1 - a mechanosensitive ion channel, highly expressed in VECs after major liver resection. Piezo1 via the PKC/ERK1/2 signaling axis upregulates the secretion of AREG and EREG which activates EGFR signaling in hepatocytes driving their proliferation (Figure 5).
Likewise, the termination of LR is also a multifactorial process regulated by multiple interacting pathways (Figure 6). Termination is reinforced by TGF-β, Hippo-YAP, and Wnt/β-catenin downregulation, which inhibit proliferation and promote return to hepatocyte quiescence once sufficient liver mass has been restored. However, even mTOR inhibitors, which strongly suppress cell proliferation, cannot completely halt regeneration alone, but merely slow it down.
This postulate supports our view that termination of liver mass regrowth is not equivalent to the termination of liver regeneration, as spatial remodeling of liver architecture—such as changes in hepatocyte shape, size, and sinusoidal alignment—continues beyond volumetric restoration. This highlights the extraordinary plasticity of liver tissue.
Continued investigation, particularly into underexplored mechanisms such as DRs in liver parenchyma and scar tissue, may reveal novel strategies to enhance regeneration and functional recovery. These advances hold great promise for the development of targeted therapies in liver surgery and transplantation.
This review focuses on the general principles, triggers, and mechanisms of liver regeneration following partial hepatectomy in both experimental and clinical contexts. While we acknowledge the growing recognition of interindividual variability—including differences in regenerative capacity related to age, sex, and chronic liver disease status—a comprehensive discussion of these factors was beyond the intended scope of this manuscript. Age-associated delays in DNA synthesis, impaired regenerative responses in chronic liver disease, and other patient-specific modifiers represent an important and evolving field of study that warrants focused investigation. These limitations are acknowledged, and we underscore the need for future scientific papers specifically dedicated to personalized approaches in liver re
| 2. | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13. [PubMed] [DOI] [Full Text] |
| 3. | Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40-55. [PubMed] [DOI] [Full Text] |
| 4. | Hu Y, Du G, Li C, Wang R, Liu J, Wang Y, Dong J. EGFR-mediated crosstalk between vascular endothelial cells and hepatocytes promotes Piezo1-dependent liver regeneration. Genes Dis. 2025;12:101321. [PubMed] [DOI] [Full Text] |
| 5. | Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166-1175. [PubMed] [DOI] [Full Text] |
| 6. | Matot I, Nachmansson N, Duev O, Schulz S, Schroeder-Stein K, Frede S, Abramovitch R. Impaired liver regeneration after hepatectomy and bleeding is associated with a shift from hepatocyte proliferation to hypertrophy. FASEB J. 2017;31:5283-5295. [PubMed] [DOI] [Full Text] |
| 7. | Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int J Mol Sci. 2020;21:8414. [PubMed] [DOI] [Full Text] |
| 8. | Marongiu F, Marongiu M, Contini A, Serra M, Cadoni E, Murgia R, Laconi E. Hyperplasia vs hypertrophy in tissue regeneration after extensive liver resection. World J Gastroenterol. 2017;23:1764-1770. [PubMed] [DOI] [Full Text] |
| 9. | Fahrner R, Patsenker E, de Gottardi A, Stickel F, Montani M, Stroka D, Candinas D, Beldi G. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation. 2014;97:892-900. [PubMed] [DOI] [Full Text] |
| 10. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [PubMed] [DOI] [Full Text] |
| 11. | Kordzaia D. Portal and Biliary Hypertension as the Cells Proliferation Trigger (Landmarks for Future Investigations). Bulletin of the Georgian National Academy of Sciences. 2009;3:181-190. |
| 12. | Inauri N, Tsomaia K, Ghirdaladze A, Chkhaidze Z, Khodeli N, Chanukvadze I, Kakabadze Z, Kordzaia D, Kiladze M. Challenges and perspectives of surgical treatment of liver failure. Current status and last achievements in Georgia. Ann Ital Chir. 2021;92:595-603. [PubMed] |
| 13. | Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777-784. [PubMed] [DOI] [Full Text] |
| 14. | Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160-171. [PubMed] [DOI] [Full Text] |
| 15. | Jin Y, Guo YH, Li JC, Li Q, Ye D, Zhang XX, Li JT. Vascular endothelial growth factor protein and gene delivery by novel nanomaterials for promoting liver regeneration after partial hepatectomy. World J Gastroenterol. 2023;29:3748-3757. [PubMed] [DOI] [Full Text] |
| 16. | Masuzaki R, Zhao SR, Csizmadia E, Yannas I, Karp SJ. Scar formation and lack of regeneration in adult and neonatal liver after stromal injury. Wound Repair Regen. 2013;21:122-130. [PubMed] [DOI] [Full Text] |
| 17. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692-694. [PubMed] [DOI] [Full Text] |
| 18. | Niiya T, Murakami M, Aoki T, Murai N, Shimizu Y, Kusano M. Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg. 1999;6:275-280. [PubMed] [DOI] [Full Text] |
| 19. | Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87:1162-1179. [PubMed] [DOI] [Full Text] |
| 20. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [PubMed] [DOI] [Full Text] |
| 21. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [PubMed] [DOI] [Full Text] |
| 22. | Allard MA, Adam R, Bucur PO, Termos S, Cunha AS, Bismuth H, Castaing D, Vibert E. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann Surg. 2013;258:822-9; discussion 829. [PubMed] [DOI] [Full Text] |
| 23. | Junrungsee S, Vipudhamorn W, Lapisatepun W, Thepbunchonchai A, Chotirosniramit A, Lapisatepun W, Ko-Iam W. Portal flow modulation by splenic artery ligation to prevent posthepatectomy liver failure: A randomized controlled trial. Surgery. 2025;109351. [PubMed] [DOI] [Full Text] |
| 24. | Gavriilidis P, Hammond JS, Hidalgo E. A systematic review of the impact of portal vein pressure changes on clinical outcomes following hepatic resection. HPB (Oxford). 2020;22:1521-1529. [PubMed] [DOI] [Full Text] |
| 25. | Lu J, Zhao YL, Zhang XQ, Li LJ. The vascular endothelial growth factor signaling pathway regulates liver sinusoidal endothelial cells during liver regeneration after partial hepatectomy. Expert Rev Gastroenterol Hepatol. 2021;15:139-147. [PubMed] [DOI] [Full Text] |
| 26. | Golse N, Bucur PO, Adam R, Castaing D, Sa Cunha A, Vibert E. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg. 2013;17:593-605. [PubMed] [DOI] [Full Text] |
| 27. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [PubMed] [DOI] [Full Text] |
| 28. | Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3:238-246. [PubMed] [DOI] [Full Text] |
| 29. | Haddad A, Khavandi MM, Lendoire M, Acidi B, Chiang YJ, Gupta S, Tam A, Odisio BC, Mahvash A, Abdelsalam ME, Lin E, Kuban J, Newhook TE, Tran Cao HS, Tzeng CD, Huang SY, Vauthey JN, Habibollahi P. Propensity Score-Matched Analysis of Liver Venous Deprivation and Portal Vein Embolization Before Planned Hepatectomy in Patients with Extensive Colorectal Liver Metastases and High-Risk Factors for Inadequate Regeneration. Ann Surg Oncol. 2025;32:1752-1761. [PubMed] [DOI] [Full Text] |
| 30. | Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, Fabre JM, Quenet F, Herrero A, Panaro F, Baudin G, Ramos J. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26:4259-4267. [PubMed] [DOI] [Full Text] |
| 31. | Bockhorn M, Benkö T, Opitz B, Sheu SY, Sotiropoulos GC, Schlaak JF, Broelsch CE, Lang H. Impact of hepatic vein deprivation on liver regeneration and function after major hepatectomy. Langenbecks Arch Surg. 2008;393:527-533. [PubMed] [DOI] [Full Text] |
| 32. | Yang X, Yang C, Qiu Y, Shen S, Kong J, Wang W. A preliminary study of associating liver partition and portal vein ligation for staged hepatectomy in a rat model of liver cirrhosis. Exp Ther Med. 2019;18:1203-1211. [PubMed] [DOI] [Full Text] |
| 33. | Partsakhashvili D, Chkhaidze Z, Khodeli N, Pilishvili O, Jangavadze M, Kordzaia D. Experimental liver autotransplantation with novel scheme of veno-venous bypass as a model of liver denervation and delymphatization. Transplant Proc. 2013;45:1739-1742. [PubMed] [DOI] [Full Text] |
| 34. | Zhou Y, Lin X, Jiao Y, Yang D, Li Z, Zhu L, Li Y, Yin S, Li Q, Xu S, Tang D, Zhang S, Yu W, Gao P, Yang L. A brain-to-liver signal mediates the inhibition of liver regeneration under chronic stress in mice. Nat Commun. 2024;15:10361. [PubMed] [DOI] [Full Text] |
| 35. | Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M. Baboon-to-human liver transplantation. Lancet. 1993;341:65-71. [PubMed] [DOI] [Full Text] |
| 36. | Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Barone M, Van Thiel DH, Starzl TE. Regulation of liver size and regeneration: importance in liver transplantation. Transplant Proc. 1988;20:494-497. [PubMed] |
| 37. | Rabbany SY, Rafii S. Blood flow forces liver growth. Nature. 2018;562:42-43. [PubMed] [DOI] [Full Text] |
| 38. | Hata Y, Yoshikawa Y, Une Y, Sasaki F, Nakajima Y, Takahashi H, Namieno T, Uchino J. Liver regeneration following portacaval shunt in rats: 3',5'-cyclic AMP changes in plasma and liver tissue. Res Exp Med (Berl). 1992;192:131-136. [PubMed] [DOI] [Full Text] |
| 39. | Oura T, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Uno M, Goto R, Watanabe M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Does the permanent portacaval shunt for a small-for-size graft in a living donor liver transplantation do more harm than good? Am J Transplant. 2008;8:250-252. [PubMed] [DOI] [Full Text] |
| 40. | Fisher B, Szuch P, Levine M, Fisher ER. A portal blood factor as the humoral agent in liver regeneration. Science. 1971;171:575-577. [PubMed] [DOI] [Full Text] |
| 41. | Moolten FL, Bucher NL. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science. 1967;158:272-274. [PubMed] [DOI] [Full Text] |
| 42. | Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol. 2017;66:212-227. [PubMed] [DOI] [Full Text] |
| 43. | Huang W, Han N, Du L, Wang M, Chen L, Tang H. A narrative review of liver regeneration-from models to molecular basis. Ann Transl Med. 2021;9:1705. [PubMed] [DOI] [Full Text] |
| 44. | Uda Y, Hirano T, Son G, Iimuro Y, Uyama N, Yamanaka J, Mori A, Arii S, Fujimoto J. Angiogenesis is crucial for liver regeneration after partial hepatectomy. Surgery. 2013;153:70-77. [PubMed] [DOI] [Full Text] |
| 45. | Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674-682. [PubMed] [DOI] [Full Text] |
| 46. | Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785-797. [PubMed] [DOI] [Full Text] |
| 47. | Wu X, Sun R, Chen Y, Zheng X, Bai L, Lian Z, Wei H, Tian Z. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology. 2015;62:253-264. [PubMed] [DOI] [Full Text] |
| 48. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [PubMed] [DOI] [Full Text] |
| 49. | Zou G, Park JI. Wnt signaling in liver regeneration, disease, and cancer. Clin Mol Hepatol. 2023;29:33-50. [PubMed] [DOI] [Full Text] |
| 50. | Chen Q, Lu X, Zhang X. Noncanonical NF-κB Signaling Pathway in Liver Diseases. J Clin Transl Hepatol. 2021;9:81-89. [PubMed] [DOI] [Full Text] |
| 51. | Chua M, Ma L, Wei W, So S. WNT/β-catenin pathway activation in hepatocellular carcinoma: a clinical perspective. GICTT. 2014;. [DOI] [Full Text] |
| 52. | Liang Y, Mei Q, He E, Ballar P, Wei C, Wang Y, Dong Y, Zhou J, Tao X, Qu W, Zhao M, Chhetri G, Wei L, Shao J, Shen Y, Liu J, Feng L, Shen Y. MANF serves as a novel hepatocyte factor to promote liver regeneration after 2/3 partial hepatectomy via doubly targeting Wnt/β-catenin signaling. Cell Death Dis. 2024;15:681. [PubMed] [DOI] [Full Text] |
| 53. | Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228-6244. [PubMed] [DOI] [Full Text] |
| 54. | Papa S, Bubici C, Zazzeroni F, Franzoso G. Mechanisms of liver disease: cross-talk between the NF-kappaB and JNK pathways. Biol Chem. 2009;390:965-976. [PubMed] [DOI] [Full Text] |
| 55. | Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8. [PubMed] [DOI] [Full Text] |
| 56. | Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247-257. [PubMed] [DOI] [Full Text] |
| 57. | Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, Park O, Ishitani T, Jho EH, Gao B, Yang Y. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137-152. [PubMed] [DOI] [Full Text] |
| 58. | Duan JL, Ruan B, Yan XC, Liang L, Song P, Yang ZY, Liu Y, Dou KF, Han H, Wang L. Endothelial Notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology. 2018;68:677-690. [PubMed] [DOI] [Full Text] |
| 59. | Yue Z, Ruan B, Duan J, Han H, Wang L. The role of the Notch signaling pathway in liver injury and repair. J Bio-X Res. 2018;01:95-104. [DOI] [Full Text] |
| 60. | Kiseleva YV, Antonyan SZ, Zharikova TS, Tupikin KA, Kalinin DV, Zharikov YO. Molecular pathways of liver regeneration: A comprehensive review. World J Hepatol. 2021;13:270-290. [PubMed] [DOI] [Full Text] |
| 61. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [PubMed] [DOI] [Full Text] |
| 62. | Bhushan B, Michalopoulos GK. Role of epidermal growth factor receptor in liver injury and lipid metabolism: Emerging new roles for an old receptor. Chem Biol Interact. 2020;324:109090. [PubMed] [DOI] [Full Text] |
| 63. | Hoffmann K, Nagel AJ, Tanabe K, Fuchs J, Dehlke K, Ghamarnejad O, Lemekhova A, Mehrabi A. Markers of liver regeneration-the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20:31. [PubMed] [DOI] [Full Text] |
| 64. | Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215-266. [PubMed] [DOI] [Full Text] |
| 65. | Wen Y, Emontzpohl C, Xu L, Atkins CL, Jeong JM, Yang Y, Kim K, Wu C, Akira S, Ju C. Interleukin-33 facilitates liver regeneration through serotonin-involved gut-liver axis. Hepatology. 2023;77:1580-1592. [PubMed] [DOI] [Full Text] |
| 66. | Bawazeer MA, Theoharides TC. IL-33 stimulates human mast cell release of CCL5 and CCL2 via MAPK and NF-κB, inhibited by methoxyluteolin. Eur J Pharmacol. 2019;865:172760. [PubMed] [DOI] [Full Text] |
| 67. | Liu Q, Wang S, Fu J, Chen Y, Xu J, Wei W, Song H, Zhao X, Wang H. Liver regeneration after injury: Mechanisms, cellular interactions and therapeutic innovations. Clin Transl Med. 2024;14:e1812. [PubMed] [DOI] [Full Text] |
| 68. | Yen CC, Yen CS, Tsai HW, Yeh MM, Hong TM, Wang WL, Liu IT, Shan YS, Yen CJ. Second harmonic generation microscopy reveals the spatial orientation of glutamine-potentiated liver regeneration after hepatectomy. Hepatol Commun. 2025;9:e0640. [PubMed] [DOI] [Full Text] |
| 69. | Kaminsky-Kolesnikov Y, Rauchbach E, Abu-Halaka D, Hahn M, García-Ruiz C, Fernandez-Checa JC, Madar Z, Tirosh O. Cholesterol Induces Nrf-2- and HIF-1α-Dependent Hepatocyte Proliferation and Liver Regeneration to Ameliorate Bile Acid Toxicity in Mouse Models of NASH and Fibrosis. Oxid Med Cell Longev. 2020;2020:5393761. [PubMed] [DOI] [Full Text] |
| 70. | Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554-G573. [PubMed] [DOI] [Full Text] |
| 71. | Wu C. Glutamine synthetase. IV.ITS formation in rat liver following partial hepatectomy and during repletion. Arch Biochem Biophys. 1964;106:402-409. [PubMed] [DOI] [Full Text] |
| 72. | Tsomaia K, Patarashvili L, Karumidze N, Bebiashvili I, Azmaipharashvili E, Modebadze I, Dzidziguri D, Sareli M, Gusev S, Kordzaia D. Liver structural transformation after partial hepatectomy and repeated partial hepatectomy in rats: A renewed view on liver regeneration. World J Gastroenterol. 2020;26:3899-3916. [PubMed] [DOI] [Full Text] |
| 73. | Tsomaia K, Azmaipharashvili E, Gvidiani S, Bebiashvili I, Gusev S, Kordzaia D. STRUCTURAL CHANGES IN RATS' LIVER DURING THE FIRST 2 WEEKS FOLLOWING 2/3 PARTIAL HEPATECTOMY. Georgian Med News. 2021;134-141. [PubMed] |
| 74. | Marzoog BA. Systemic and local hypothermia in the context of cell regeneration. Cryo Letters. 2022;43:66-73. [PubMed] [DOI] [Full Text] |
| 75. | Oka Y, Akagi Y, Kinugasa T, Ishibashi N, Iwakuma N, Shiratsuchi I, Shirouzu K. Heat-shock pre-treatment reduces liver injury and aids liver recovery after partial hepatectomy in mice. Anticancer Res. 2013;33:2887-2894. [PubMed] |
| 76. | Wolf JH, Bhatti TR, Fouraschen S, Chakravorty S, Wang L, Kurian S, Salomon D, Olthoff KM, Hancock WW, Levine MH. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transpl. 2014;20:376-385. [PubMed] [DOI] [Full Text] |
| 77. | Golriz M, Abbasi S, Fathi P, Majlesara A, Brenner T, Mehrabi A. Does acid-base equilibrium correlate with remnant liver volume during stepwise liver resection? Am J Physiol Gastrointest Liver Physiol. 2017;313:G313-G319. [PubMed] [DOI] [Full Text] |
| 78. | Li T, Tuo B. Pathophysiology of hepatic Na(+)/H(+) exchange (Review). Exp Ther Med. 2020;20:1220-1229. [PubMed] [DOI] [Full Text] |
| 79. | Donne R, Saroul-Aïnama M, Cordier P, Celton-Morizur S, Desdouets C. Polyploidy in liver development, homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:391-405. [PubMed] [DOI] [Full Text] |
| 80. | Wilkinson PD, Delgado ER, Alencastro F, Leek MP, Roy N, Weirich MP, Stahl EC, Otero PA, Chen MI, Brown WK, Duncan AW. The Polyploid State Restricts Hepatocyte Proliferation and Liver Regeneration in Mice. Hepatology. 2019;69:1242-1258. [PubMed] [DOI] [Full Text] |
| 81. | Matsumoto T, Wakefield L, Tarlow BD, Grompe M. In Vivo Lineage Tracing of Polyploid Hepatocytes Reveals Extensive Proliferation during Liver Regeneration. Cell Stem Cell. 2020;26:34-47.e3. [PubMed] [DOI] [Full Text] |
| 82. | Wagenaar GT, Chamuleau RA, Pool CW, de Haan JG, Maas MA, Korfage HA, Lamers WH. Distribution and activity of glutamine synthase and carbamoylphosphate synthase upon enlargement of the liver lobule by repeated partial hepatectomies. J Hepatol. 1993;17:397-407. [PubMed] [DOI] [Full Text] |
| 83. | Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3:219-234. [PubMed] [DOI] [Full Text] |
| 84. | Suzuki Y, Katagiri H, Wang T, Kakisaka K, Kume K, Nishizuka SS, Takikawa Y. Ductular reactions in the liver regeneration process with local inflammation after physical partial hepatectomy. Lab Invest. 2016;96:1211-1222. [PubMed] [DOI] [Full Text] |
| 85. | Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390-393. [PubMed] [DOI] [Full Text] |
| 86. | Ikeda Y, Nakagawa K, Nakamura T, Arakawa T, Kobayashi K, Kaneda K. Bile ductular proliferation in the granulation tissue of the rat liver: Histological and serial reconstruction studies. Biomed Res. 1997;18:113-127. [DOI] [Full Text] |
| 87. | Szijártó A, Fülöp A. Triggered liver regeneration: from experimental model to clinical implications. Eur Surg Res. 2015;54:148-161. [PubMed] [DOI] [Full Text] |
| 88. | Nadalin S, Testa G, Malagó M, Beste M, Frilling A, Schroeder T, Jochum C, Gerken G, Broelsch CE. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024-1029. [PubMed] [DOI] [Full Text] |
| 89. | Lock JF, Malinowski M, Seehofer D, Hoppe S, Röhl RI, Niehues SM, Neuhaus P, Stockmann M. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297-1304. [PubMed] [DOI] [Full Text] |
| 90. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [PubMed] [DOI] [Full Text] |
| 91. | Takahashi K, Kurokawa T, Oshiro Y, Fukunaga K, Sakashita S, Ohkohchi N. Postoperative Decrease in Platelet Counts Is Associated with Delayed Liver Function Recovery and Complications after Partial Hepatectomy. Tohoku J Exp Med. 2016;239:47-55. [PubMed] [DOI] [Full Text] |
| 92. | Lund A, Andersen KJ, Meier M, Pedersen MI, Knudsen AR, Kirkegård J, Mortensen FV, Nyengaard JR. Biochemical and morphological responses to post-hepatectomy liver failure in rats. Sci Rep. 2023;13:13544. [PubMed] [DOI] [Full Text] |
| 93. | Helling TS. Liver failure following partial hepatectomy. HPB (Oxford). 2006;8:165-174. [PubMed] [DOI] [Full Text] |
| 94. | Álvarez-Mercado AI, Caballeria-Casals A, Rojano-Alfonso C, Chávez-Reyes J, Micó-Carnero M, Sanchez-Gonzalez A, Casillas-Ramírez A, Gracia-Sancho J, Peralta C. Insights into Growth Factors in Liver Carcinogenesis and Regeneration: An Ongoing Debate on Minimizing Cancer Recurrence after Liver Resection. Biomedicines. 2021;9. [PubMed] [DOI] [Full Text] |
| 95. | Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P; IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016;283:2219-2232. [PubMed] [DOI] [Full Text] |
| 96. | Nguyen-Lefebvre AT, Selzner N, Wrana JL, Bhat M. The hippo pathway: A master regulator of liver metabolism, regeneration, and disease. FASEB J. 2021;35:e21570. [PubMed] [DOI] [Full Text] |
| 97. | Liu B, Bell AW, Paranjpe S, Bowen WC, Khillan JS, Luo JH, Mars WM, Michalopoulos GK. Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice. Hepatology. 2010;52:1060-1067. [PubMed] [DOI] [Full Text] |
| 98. | Tanaka N, Yamamoto H, Tatemoto A, Urabe T, Orita K. Regulation of liver regeneration by interleukin-2 and its inhibitors: cyclosporine A and FK 506. Int J Immunopharmacol. 1993;15:211-218. [PubMed] [DOI] [Full Text] |
| 99. | Ma T, Zhang Y, Lao M, Chen W, Hu Q, Zhi X, Chen Z, Bai X, Dang X, Liang T. Endogenous Interleukin 18 Suppresses Liver Regeneration After Hepatectomy in Mice. Liver Transpl. 2020;26:408-418. [PubMed] [DOI] [Full Text] |
| 100. | Russell JO, Monga SP. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol. 2018;13:351-378. [PubMed] [DOI] [Full Text] |