INTRODUCTION
Patients with psoriasis present an increased risk for steatosis and liver fibrosis compared to the general population. The pathophysiology of this association and the interplay of metabolic syndrome (MetS), the cumulative dose of methotrexate (MTX), and patatin-like phospholipase domain containing 3 (PNPLA3) and transmembrane 6 superfamily member 2 (TM6SF2) gene polymorphisms still need elucidation. The adequate classification of liver disease in psoriasis can provide effective treatment strategies for controlling metabolic risk factors and preventing fibrosis development. This review aimed to discuss psoriasis as a systemic inflammatory disease and raise a question regarding its adequate classification under the umbrella of steatotic liver disease (SLD), considering both the high prevalence of cardiometabolic factors as well as the influence of MTX in liver injury in this population.
THE NEW DEFINITION OF METABOLIC-DYSFUNCTION ASSOCIATED SLD
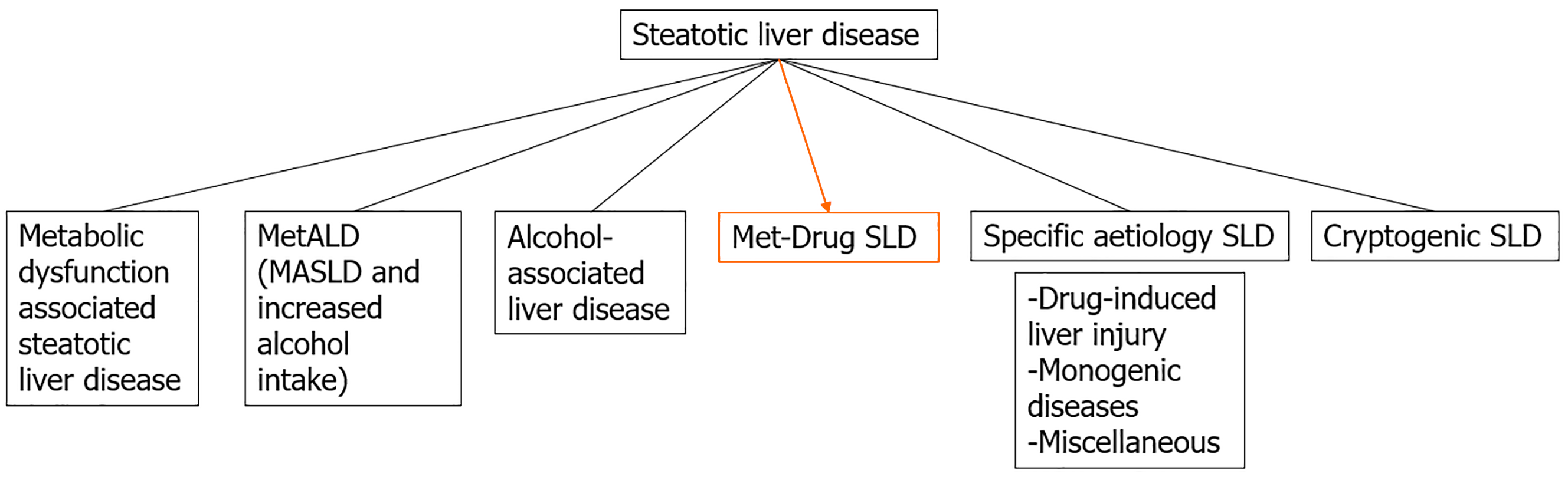
SLD, usually identified by image methods and defined histologically as macro or micro vesicular steatosis of more than 5% of hepatocytes, is associated with several etiologies and leads to different subcategories classification as recently proposed[1]. Among them, the previously named non-alcoholic fatty liver disease (NAFLD), now changed to metabolic-dysfunction associated SLD (MASLD), consists of liver macro vesicular steatosis with at least one cardiometabolic factor; alcohol-related liver disease (ALD) and the composite of both MASLD and ALD (MetALD) are also others etiologies. This way, SLD is currently an umbrella term that includes hepatic steatosis, which may be accompanied by inflammation and cellular injury (ballooning), with or without fibrosis, and finally, cirrhosis with inherent complications. Patients with metabolic-dysfunction-associated steatohepatitis and stage 2 fibrosis have an increased risk of liver-related morbidity and mortality[2]. Apart from these etiologies, there are less prevalent SLD that may be considered in certain circumstances[3] but with different pathophysiology (Figure 1), including drug-induced liver disease, monogenic diseases and miscellaneous.
Figure 1 Proposal of metabolic and drug-induced steatotic liver disease subcategory.
SLD: Steatotic liver disease; MASLD: Metabolic-dysfunction associated steatotic liver disease; MetALD: Metabolic-dysfunction associated steatotic liver disease and alcohol-related liver disease.
Regarding MASLD, the estimated prevalence in adults of 25% to 30%[2] has considerably increased and is now estimated at 38%[3]. As previously mentioned, the diagnostic criteria have been selected to align with cardiometabolic risk factors associated with insulin resistance, which have already been well-validated in the context of cardiovascular diseases[1]. Thus, insulin resistance still plays a central role in the disease, but other metabolic factors are also involved in its pathogenesis. Currently, individuals with steatosis and at least one of the cardio metabolic criteria, such as overweight, glucose intolerance, arterial hypertension or dyslipidemia, would be considered to have MASLD in the absence of a secondary cause (like drugs, malnutrition or monogenetic diseases), in individuals who drink little or no alcohol (defined as < 20 g/day for women and < 30 g/day for men)[1].
The liver is a key site of insulin action: It is the leading source of endogenous glucose production and the primary site of insulin degradation. The accumulation of hepatic lipids in MASLD impairs hepatic glucose and lipid metabolism, further increasing the risk of type-2 diabetes mellitus (T2DM) and cardiovascular disease, regardless of established risk factors. Additionally, MASLD, particularly steatohepatitis, may contribute to the low-grade inflammatory state through the systemic release of several markers of inflammation, oxidative stress and procoagulant factors. Therefore, the liver is not a “simple bystander” in this complex pathophysiology[4]. In this way, patients with T2DM, obesity and those with a family history of cirrhosis without an established etiology should undergo screening for advanced liver fibrosis, initially by calculating the fibrosis-4 score, followed by transient elastography (TE) in case of a fibrosis-4 score greater than 1.3. Liver biopsy is reserved for cases of diagnostic uncertainty, such as discordant or indeterminate results from non-invasive tests, discordance between non-invasive tests and clinical, radiographic or laboratory characteristics suggesting advanced fibrosis, or possible concurrent or concomitant diagnoses[2,5].
It should be noted that the spectrum of advanced fibrosis (stage F3) and cirrhosis (stage F4) is a continuum, and it is often not possible to differentiate the two stages clinically. It is more relevant to rule out or rule in clinically significant disease. All elastography techniques perform better in ruling out advanced fibrosis/cirrhosis than identifying early stages of liver fibrosis[6,7]. Advanced fibrosis is the beginning of the histological spectrum of compensated advanced chronic liver disease, which includes patients at risk of clinically significant portal hypertension and hepatic decompensations. The exclusion of advanced fibrosis reduces the risk for these outcomes, regardless of their etiology[7,8]. However, inpatients with psoriasis, the approach for screening advanced liver disease needs to be better defined. Additionally, as liver steatosis in this systemic disease may have both metabolic and drug-related compounds in individuals who are under long-term use of MTX, it is not clear where this patient should be classified under the umbrella of SLD. Due to the high prevalence of MASLD, additional etiologies have been combined, giving rise to new subcategories as MetALD, defined by women or men who drink more than 20/30 g of alcohol per day (or 140/210 g per week, respectively), but still less than 50/60 g of alcohol/day (350/420 g of alcohol/week). In this scenario, other subcategories might be considered, which will be discussed further[1].
PSORIASIS AS A SYSTEMIC INFLAMMATORY DISEASE AND THE PREVALENCE OF SLD
Psoriasis is a chronic, immune-mediated inflammatory skin disease associated with genetic and environmental factors. It is characterized by skin plaques resulting from epidermal hyperproliferation[9]. The prevalence in adults in Western countries is approximately 2%-4% and is associated with a loss of quality of life[10], even in mild cases, and increased mortality in severe cases. It equally affects both sexes and people of all ages, with peak incidence in early adulthood (20 years) and late adulthood (50 and 60 years). Most patients, approximately 80%, have limited disease (< 10% body surface area)[11], and approximately 10%-30% of patients with psoriasis develop psoriatic arthritis[9]. Currently, psoriasis is defined as the prototype of an inflammatory disease mediated by T cells, producing T helper cell-1 (Th-1) cytokines. Likewise, chronic inflammation via Th-1 activation is essential for MetS, T2DM and atherosclerosis pathophysiology. MetS is a combination of interconnected factors that directly increase the risk of coronary heart disease, other forms of atherosclerotic cardiovascular disease, and T2DM[12,13], with a prevalence in the Western world and adults of approximately 34%[13].
The prevalence of MetS in patients with psoriasis reaches 50%, which is twice as high in patients with psoriasis compared to control individuals without psoriasis[12]. Danielsen et al[14] conducted a population-based study confirming an increased prevalence of MetS in patients with psoriasis compared to controls. Interestingly, a different trend was observed between the sexes: A 3.8-fold higher probability of MetS was found in women < 30 years, with a decreasing odds ratio with increasing age. On the other hand, men presented a 1.35-fold higher odds ratio of MetS, regardless of age. MetS was more prevalent in men and women with psoriasis than those without psoriasis across all age groups[14]. Circulating levels of Th-1 cytokines, elevated in psoriasis, obesity and coronary artery disease, affect insulin signaling, adipogenesis and lipid metabolism[15]. Additionally, dysfunction of subcutaneous adipose tissue also contributes to the development of insulin resistance, and there is evidence that subcutaneous adipose tissue is dysfunctional directly beneath psoriatic skin lesions[16].
Inflammatory cytokines and hormones produced by conditions such as obesity, diabetes, and atherosclerosis may also influence the pathogenesis of psoriasis by increasing the susceptibility or severity of the disease. Tumor necrosis factor α, for example, is a Th-1 inflammatory cytokine secreted in adipose tissue and can lead to insulin resistance by inhibiting tyrosine kinase activity (of the insulin receptor) and activating peroxisome proliferator-activated receptor-gamma. It promotes epidermal proliferation and suppresses adiponectin secretion from adipocytes, an important anti-inflammatory molecule that regulates insulin sensitivity[15]. The low levels of adiponectin and high levels of leptin and other inflammatory cytokines produced by adipose tissue, such as interleukin-1 (IL-1), IL-6, and IL-17 in psoriasis, have repercussions on the pathogenesis of the cutaneous and metabolic disease as well as in MASLD[12,17,18].
The possibility of the existence of a “hepato-dermal axis” based on this evidence has been raised, where visceral adipose tissue would be the connection between the steatotic liver and psoriatic skin[19]. In the same way, liver steatosis, in addition to actively contributing to the severity of psoriasis by releasing pro-inflammatory cytokines, would also be one of the underlying mechanisms for atherogenesis[20]. The prevalence of steatosis, diagnosed by ultrasound, is notably higher in patients with psoriasis than in patients without psoriasis (47% vs 28%, P < 0.0001), even when controlling for age, sex, and body mass index (BMI)[20]. Subsequent similar findings also demonstrated the presence of steatosis (diagnosed by abdominal ultrasound) in 46.2% of psoriasis patients compared with 33.3% of controls (P = 0.005) on a large prospective population-based cohort study (part of the Rotterdam Study) in which 2292 participants aged > 55 years were included, of whom 5.1% had psoriasis. Psoriasis was significantly associated with MASLD even after adjustment for alcohol consumption, smoking and the presence of MetS [adjusted odds ratio (OR): 1.7, 95% confidence interval (CI): 1.1-2.6][21]. Subsequent data confirm the higher prevalence of steatosis diagnosed by imaging or histology in patients with psoriasis (occurring in up to 50%) when compared to controls, even after adjustment of its components[12,19,22].
In a meta-analysis evaluating the risk of MASLD in patients with psoriasis, including six controlled studies with patients without psoriasis (n = 267761), the pooled analysis indicated that patients with psoriasis had a two-fold risk of MASLD than controls, and the risk was increased in patients with more severe psoriasis or psoriatic arthritis. In subgroup analyses, variables such as BMI and T2DM did not have statistical differences in the presence of MASLD[23]. In a recent systematic review and meta-analysis of observational studies evaluating the association between psoriasis and MASLD, diagnosed by imaging or International Classification of Diseases codes, psoriasis was associated with MASLD (n = 11 studies, OR: 1.96, 95%CI: 1.70-2.26, I2 = 97%, P < 0.01). Patients with psoriasis and MASLD had a higher mean psoriasis area and severity index than those without MASLD[22].
A key consideration is validating all this data following SLD’s new definition and nomenclature, as these studies used the term NAFLD. However, addressing the impact of the new definition MASLD, there is evidence that 98% of existing records of patients with NAFLD would meet the new criteria for MASLD. Conceptually, patients with the previous definition NAFLD can now be considered fully within the category of MASLD[1]. It is also important to highlight that studies evaluating steatosis in psoriasis do not separate the alcohol use component in these patients.
Like steatosis prevalence, liver fibrosis is also more prevalent in patients with psoriasis compared to the general population, as shown in a cross-sectional study of elderly patients (n = 1535), where 4.7% had the diagnosis of psoriasis. The prevalence of advanced liver fibrosis in this group was 8.1% vs 3.6% (P = 0.05), defined by TE > 9.5 kPa[24]. Patients with psoriasis were twice as likely to have advanced liver fibrosis and four times more likely in the subgroup of patients with MASLD, even those without MTX use. Logistic regression analyses in this subgroup showed that patients with psoriasis had a 4-fold higher risk of advanced liver fibrosis than the reference population (OR: 4.2, 95%CI: 1.1-16.0). This risk remained 4-fold increased after adjustment for age, sex, alcohol consumption, aspartate aminotransferase level and MetS in a multivariable logistic regression model (OR: 4.1, 95%CI: 1.01-17.0)[24]. Despite study limitations regarding histological non-correlation and the elastography cutoff point, this populational study gives us the magnitude of the risk of liver fibrosis in patients with psoriasis, regardless of MTX use, when compared to the general population and population with MASLD. Recently, a cross-sectional study based on TE found a prevalence of 9% of advanced liver fibrosis in psoriasis patients with a high prevalence of MetS and no correlation with cumulative MTX dose[25].
PSORIASIS AND MASLD GENETICS
Based on the strong association between psoriasis and MASLD, the hypothesis of a hepato-dermal axis[19] and the central role of the expanded adipose tissue do not explain this frequent association independently of common metabolic factors. MASLD is a genetically complex disease, and polymorphisms in critical genes may determine MASLD susceptibility and disease progression independently of metabolic factors. The I148M (rs738409) polymorphism of the PNPLA3 has emerged as a major genetic determinant of MASLD, independent of BMI, T2DM, and alcohol use[26,27]. This association can be extended to histological severity, including an association with steatohepatitis, after adjustment for age, sex, BMI and insulin resistance[28]. Two meta-analyses exclusively in MASLD patients showed an increased risk for the entire spectrum of the disease in carriers of the PNPLA3 gene polymorphism: Steatosis (OR: 3.26, 95%CI: 2.73-3.90), steatohepatitis (OR: 3.26, 95%CI: 2.15-4.95)[29], and liver fibrosis (OR: 3.11, 95%CI: 2.66-3.65)[30]. The human TM6SF2 polymorphism is also associated with MASLD. This E167K variant (rs58542926) is also associated with MASLD and is independent of the effect of the I148M variant of PNPLA3, but also not associated with BMI, insulin resistance and alcohol consumption[31]. In a cohort of 1201 patients who underwent liver biopsy for suspected MASLD-related steatohepatitis, 188 individuals (13%) carried the E167K variant and had more severe steatosis, necroinflammation, ballooning and fibrosis (P < 0.05) and a higher risk of steatohepatitis (OR: 1.84, 95%CI: 1.23-2.79) and advanced fibrosis (OR: 2.08, 95%CI: 1.20-3.55) after adjustment for age, sex, BMI, insulin resistance and I148M variant (PNPLA3)[32]. A single study in individuals with psoriasis (n = 199) recently showed that the PNPLA3 G allele, but not the TM6SF2 polymorphism, impacted a 5-fold increased risk of advanced liver fibrosis and that MetS and T2DM confer a greater risk for steatosis and advanced fibrosis, respectively, but not MTX[25]. As previously stated, these findings highlight the close relation between psoriasis and MASLD, even regarding single nucleotide polymorphisms.
MTX AS A STEATOSIS-INDUCING DRUG AND HOW TO INTERPRET STEATOSIS IN PATIENTS WITH MASLD USING MTX
Historically, concerns about liver fibrosis inpatients with psoriasis precede the knowledge of the association between psoriasis and MASLD and even the description of SLD per se. MTX has been used for over 50 years as an effective treatment for psoriasis; it is a steatogenic drug with the potential to progress to steatohepatitis and liver fibrosis[33], and its use predates the era of randomized clinical trials. Decades after its use, and despite the introduction of immunobiological drugs, MTX remains an effective treatment in the therapeutic arsenal of psoriasis[34,35]. The first reports of liver fibrosis associated with MTX treatment were around the 1950s and 1960s, in children, when high doses were used to treat leukemia[36]. Subsequently, results from several series with histological follow-up identified cirrhosis in 25% of MTX-treated patients for psoriasis, preceded by macro vesicular steatosis and portal inflammation[37]. The histological classification of MTX-induced liver injury was formulated by Roenigk and was used by dermatologists from the 1970s until the mid-2000s[34].
The initial guidelines for monitoring MTX-induced liver injury, also developed by Roenigk et al[38-40] in 1972, were revised in 1988 and 1998. These guidelines recommended liver biopsy at the start of MTX treatment and after each cumulative dose of 1.5 g. Patients with moderate to severe fibrosis (Roenigk IIIb or IV) should discontinue the drug. However, at that time, there was no exclusion of viral liver diseases or high alcohol intake[41]. For the first time in 1998, the recommendations for pretreatment biopsy were relaxed[35]. In the early 2000s, Langman et al[42] were the first to observe that histopathologic features of MTX liver toxicity resembled non-alcoholic steatohepatitis and that it would be difficult to attribute the abnormal histology to MTX alone. This overlap suggested similar pathophysiological mechanisms that could result in liver injury in psoriasis with long-term MTX use and/or that pre-existing NAFLD (the definition used at that time), possibly aggravated by MTX, could be the primary cause of liver injury[42].
Aithal et al[43], in 2004, showed that with low-dose MTX therapy, progression to advanced liver fibrosis would be less frequent, with a probability of advanced fibrosis < 2.6% with a cumulative dose of up to 4 g. Rosenberg et al[44] found advanced fibrosis (using a classification already used for steatotic liver disease) in 38% of patients with metabolic risk factors, mainly T2DM and overweight (median cumulative MTX dose 1.6 g), compared to 9% in the group without risk factors (median cumulative MTX dose 2.1 g, P = 0.0012)[44]. In 2014, Maybury et al[45] conducted a systematic review of observational studies on MTX-induced liver fibrosis in patients with psoriasis, including only studies with more than two consecutive biopsies per patient, in an attempt to assess whether MTX increases the risk of liver fibrosis. They found a 22% increased risk of “any fibrosis” in the consecutive biopsy while using MTX. The cumulative dose and duration of MTX therapy were not associated with fibrosis/cirrhosis. The study concludes that MTX increases the risk of fibrosis, but not in all or not by itself, where MetS and its components or genetic factors would determine who would develop drug toxicity.
In 2023, the largest longitudinal cohort study with 999 patients (876 exposed to MTX, with a mean cumulative dose of 4.8 g and a mean treatment duration of 6years), 308 of whom diagnosed with psoriasis and the remainder with rheumatoid arthritis, found no association between the cumulative dose of MTX and advanced fibrosis (prevalence of 15.3% of advanced fibrosis with a cutoff point of 7.9 kPa, with a sensitivity of 91% to rule out advanced fibrosis)[46]. The risk factor most associated with increased liver stiffness was T2DM (OR: 3.19, 95%CI: 1.95-5.20, P < 0.001)[47]. Experimental studies show that MTX liver toxicity is acute and characterized by micro vesicular steatosis and microvascular injury, which do not mimic MASLD. In vitro, it has been shown that homocysteine and adenosine, two molecules that accumulate in hepatocytes exposed to high doses of MTX, can activate stellate cells and, therefore, fibrogenesis. However, their effect on inhibiting fibrinolysis is not known[48]. Given our current knowledge, it seems that MTX cannot induce chronic liver disease alone but has an indirect role in the progression of MASLD lesions through the induction of mitochondrial damage and the reduction of circulating and hepatic folate concentrations[34].
CONCLUSION
Screening for liver fibrosis could be advisable for all patients with psoriasis who are considered for treatment with MTX, as psoriasis increases the prevalence of MetS and steatosis. It is also worth noting that histological evaluation of the liver, while still the gold standard for evaluating liver fibrosis, has limitations regarding sampling errors, intra and inter-observer variability, and the risks of an invasive procedure. Based on several studies, liver fibrosis in patients with psoriasis can be assessed through liver stiffness by TE, as in MASLD. Starting MTX or continuing the drug once a TE evaluation suggests advanced fibrosis may be inadvisable but not prohibitive, considering that TE is an easy surveillance method and that poorly controlled psoriasis could progress to a life-threatening scenario. MTX remains an inexpensive medication, and immunobiological therapy may be a less available alternative with other potential health risks. PNPLA3 genotyping could be very useful.
Although it is well-defined that steatosis and fibrosis in psoriasis might be related to the inflammatory profile of the disease and the presence of MetS, they might be worsened by MTX in those who require long-term use for disease control. So far, this has not been evaluated in longitudinal studies that may accurately answer this question in the near future. Most studies on psoriasis, MetS, and MTX effects are sectional studies that do not allow for outcome inferences. However, we sought that a new classification of patients with MetS who have long-term use of an additional steatogenic drug, such as MTX in patients with psoriasis, might be discussed by the scientific community considering the same rationale as MetALD for those who use moderate alcohol but also have an adverse metabolic profile.
It might be a new subcategory of SLD that, similar to MetALD, could be called metabolic and drug-induced related SLD. The prototype of this categorization would be the combination of psoriasis, MetS and MTX use, allowing the development of longitudinal studies, aiming for a better characterization of the long-term impact of this association on liver damage, the evaluation of differentiated approaches and the proper management, which is yet not well established. So far, we recommend modifying the lifestyle and treatment of comorbidities for this subgroup of patients. In the long term, whether MTX should be maintained or not in patients with steatosis and MASLD is not well defined. If another group of steatogenic drugs might also fit into this branch, it still needs further clarification. Finally, more evidence and discussion will be required to confirm its plausibility and better guide surveillance and control of associated metabolic risk factors in this population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade B, Grade C
Novelty: Grade A, Grade A, Grade B, Grade B
Creativity or Innovation: Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade B, Grade C
P-Reviewer: Chen L; Wang PY S-Editor: Wei YF L-Editor: Filipodia P-Editor: Yu HG