Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.101724
Revised: January 18, 2025
Accepted: March 4, 2025
Published online: March 27, 2025
Processing time: 182 Days and 13.3 Hours
Yinchenhao decoction (YCHD) is a traditional Chinese medicine widely used to treat liver damage caused by obstructive jaundice (OJ). Although YCHD has demonstrated protective effects against liver damage, reduced apoptosis, and mitigated oxidative stress in OJ, the precise molecular mechanisms involved remain poorly understood.
To investigate the beneficial effects of YCHD on OJ and elucidate the underlying mechanisms.
The active constituents of YCHD were identified using liquid chromatography-tandem mass spectrometry, and their potential targets for OJ treatment were predicted through network pharmacology. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses were performed. An OJ rat model was established by common bile duct ligation. Rats were divided into three groups: Sham surgery (S Group), model (O Group), and YCHD (Y Group). YCHD was administered to Group Y for one week. Bilirubin levels, liver function parameters, and bile acid concentrations in blood and urine were measured by enzyme-linked immunosorbent assay. The bile acid renal clearance rate (Clr) was calculated. Histopathological evaluation of liver and kidney tissues was performed using hematoxylin-eosin staining. Western blotting was utilized to assess the expression of key bile acid metabolism and transport proteins in both liver and kidney tissues. The expression of the constitutive androstane receptor (CAR) and its nuclear localization were evaluated by immunohistochemistry. Molecular docking studies identified the epidermal growth factor receptor (EGFR) as a potential target of YCHD's active components. An OJ cell model was created using human liver (L02) and renal tubular epithelial (HK-2) cells, which were treated with YCHD-containing serum. Western blotting and immunofluorescence assays were employed to evaluate CAR expression and its nuclear localization in relation to EGFR activation.
Network analysis identified the EGFR signaling pathway as a key mechanism through which YCHD exerts its effects on OJ. In vivo experiments showed that YCHD improved liver function, reduced OJ-induced pathology in liver and kidney tissues, and decreased serum bile acid content by enhancing bile acid Clr and urine output. YCHD also increased CAR expression and nuclear heterotopy, upregulating proteins involved in bile acid metabolism and transport, including CYP3A4, UGT1A1, MRP3, and MRP4 in the liver, and MRP2 and MRP4 in the kidneys. In vitro, YCHD increased CAR expression and nuclear heterotopy in L02 and HK-2 cells, an effect that was reversed by EGFR agonists.
YCHD enhances bile acid metabolism in the liver and promotes bile acid excretion in the kidneys, ameliorating liver damage caused by OJ. These effects are likely mediated by the upregulation of CAR and its nuclear translocation.
Core Tip: Yinchenhao decoction (YCHD) alleviates obstructive jaundice-induced liver damage by enhancing bile acid metabolism and excretion. This study reveals that YCHD modulates the epidermal growth factor receptor signaling pathway, upregulates the constitutive androstane receptor, and promotes its nuclear localization, contributing to improved liver and kidney function. These findings provide insights into the molecular mechanisms behind YCHD's therapeutic effects on obstructive jaundice.
- Citation: Liu JJ, Mei HW, Jing YY, Li ZL, Wu SG, Yuan HX, Zhang XB. Yinchenhao decoction alleviates obstructive jaundice liver injury by modulating epidermal growth factor receptor and constitutive androstane receptor signaling. World J Hepatol 2025; 17(3): 101724
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/101724.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.101724
Obstructive jaundice (OJ) is frequently caused by mechanical obstructions in the bile duct, including stones, biliary obstruction, or tumor compression. These obstructions result in bile stagnation, triggering pathological changes in the liver, bile ducts, and the entire organism. Liver damage in OJ is closely linked to the accumulation of cytotoxic bile acids, which induce liver cell injury through inflammatory responses and the activation of mitochondrial[1], endoplasmic reticulum[2], and death receptor[3] pathways. The buildup of bile acids is a primary factor in liver cell damage, leading to apoptosis or necrosis.
In biliary obstruction, the normal flow of bile acids in the hepatic-intestinal circulation is disrupted. Consequently, bile acid excretion from liver cells increases, and excess bile acids are actively transported to the urine for elimination via renal tubules[4]. However, these compensatory mechanisms are often insufficient to counteract cholestasis-induced liver damage. The limited efficacy of current treatments for cholestatic conditions has prompted the investigation of alternative therapeutic strategies. Traditional Chinese medicine (TCM), with its rich history in managing jaundice progression and preserving liver function, offers promising potential. Nevertheless, additional research is required to fully understand the mechanisms underlying these therapeutic effects.
The constitutive androstane receptor (CAR) is crucial in regulating bile acid metabolism and maintaining bile acid homeostasis. CAR facilitates gene expression in bile acid metabolism and transport, promoting their elimination. This regulation includes essential processes such as bile acid hydroxylation (Phase I), where the cytochrome P450 family 3 subfamily A member 4 (CYP3A4) enzyme converts bile acids into more hydrophilic, less toxic forms[5]. Additionally, CYP3A4 expression is regulated by CAR[6].
In Phase II, bile acid conjugation is catalyzed by UDP glucuronosyltransferase family 1 member A1 (UGT1A1), a key step in bile acid detoxification. Conjugation increases bile acid polarity, enhancing water solubility and facilitating renal excretion[7]. CAR also regulates UGT1A1, which is essential for bile acid detoxification[8]. Beyond Phase I and II detoxification, CAR actively mediates bile acid transport in liver and renal tubular cells by regulating the expression of multidrug resistance-associated proteins (MRPs)[9]. Thus, CAR is crucial for liver cell detoxification[9,10].
In OJ-related bile stasis, the activation of CAR and the subsequent induction of Phase I and II metabolic enzymes and active transporters play a critical role in promoting bile acid clearance, restoring bile acid flow, and alleviating the effects of bile stasis[11].
The epidermal growth factor receptor (EGFR) is a key membrane receptor that regulates the dephosphorylation of CAR. Epidermal growth factor (EGF) inhibits CAR dephosphorylation and nuclear translocation in mouse liver cells[12]. Upon binding with the receptor of activated protein kinase C 1 (RACK1), cytoplasmic protein phosphatase 2A (PP2A) dephosphorylates CAR, a necessary step for its nuclear translocation. When EGFR is activated by EGF, the phosphory
Yinchenhao decoction (YCHD), a renowned TCM formula documented in the Treatise on exogenous febrile diseases, is composed of Yinchen (Artemisiae Scopariae Herba), Zhizi (Gardenia jasminoides Ellis), and Dahuang (Radix Rhei et Rhizoma). It is commonly prescribed for the treatment of damp-heat jaundice. Our research group has confirmed the therapeutic effectiveness of YCHD in treating OJ through early-stage clinical trials[15]. Previous studies have identified the key components and unique properties of YCHD, highlighting its diverse pharmacological effects, including anti-inflammatory activity, liver function preservation, and protection against liver cell apoptosis[16,17]. YCHD has been shown to enhance the Nrf2 expression and promote its translocation into the nucleus[18]. This process regulates the expression of endothelial and inducible nitric oxide synthase, reducing excessive nitric oxide levels. Additionally, YCHD activates downstream expressions of reduced glutathione and NAD(P)H-quinone dehydrogenase 1, offering protection against oxidative damage in liver tissue. Furthermore, YCHD improves liver function and reduces liver cell apoptosis by inhibiting the activation of the protein kinase RNA-like endoplasmic reticulum kinase/C/EBP homologous protein/growth arrest and DNA damage-inducible protein 34 (PERK/CHOP/GADD34) pathway while restoring the balance of the B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein ratio[19]. Additionally, YCHD significantly influences various proteins, including sodium taurocholate co-transporter peptide, multidrug-resistant protein 1, bile salt export pump, organic cation transporter 1, and organic anion transporter 1A2, further enhancing its therapeutic potential[20].
The underlying mechanisms remain unclear despite YCHD significantly alleviating clinical symptoms and pathological damage linked to OJ. This study explored the function of the EGFR/CAR pathway in mediating the therapeutic effects of YCHD on OJ for the first time. Our goal was to provide novel insights that support its clinical application. Additionally, we identified the EGFR/CAR pathway as a potential novel therapeutic target for the pre
Yinchen, Zhizi, and Dahuang were sourced from the TCM Pharmacy at Tianjin Medical University Nankai Hospital. Diagnostic kits for total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bile acid were purchased from the China National Institute of Biochemistry. Antibodies against CAR, UGT1A1, and CYP450 3A4 were obtained from Abcam (Shanghai, China), and anti-phospho EGFR from Affinity (Jiangsu, China). β-actin was from Proteintech Group (Wuhan, China), and secondary antibodies from Beijing Zhongshan Gold Bridge Biotechnology and Proteintech. PVDF membrane, ECL reagent kit, and RIPA lysis buffer were obtained from Millipore (Shanghai, China). Paraformaldehyde, poly-L-lysine, 3,3'-diaminobenzidine (DAB) staining kit, and hema
YCHD granules were purchased from the Granule Pharmacy of Tianjin Integrated Traditional Chinese and Western Medicine Hospital. The preparation followed the guidelines outlined in the Shanghan Lun, combining specific pro
YCHD (2 mg) was dissolved in 1 mL of 50% methanol by vortexing, sonication, and centrifugation at 4 °C, 14000 rpm for 20 minutes (Eppendorf 5804R). The resulting supernatant was used for liquid chromatography-tandem mass spec
The LC system used was a Vanquish UHPLC (Thermo Fisher Scientific) paired with an ACQUITY UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 µm). A 2 μL injection volume was used, with the column set to 35 °C and the sample chamber at 4 °C. The mobile phase consisted of acetonitrile (Phase A) and a 0.1% (v/v) formic acid aqueous solution (Phase B) with a flow rate of 0.3 mL/minute. Elution followed an initial gradient from 0 to 24 minutes (5%-100% A), a transition at 24-26.5 minutes to 100% A, a gradient from 26.5 to 27 minutes (100%-5% A), and then held at 5% A from 27 to 30 minutes. The mass spectrometer used was the Thermo Fisher Orbitrap Exploris 120 (O-Exitive) with a high-energy electrospray ionization source. Data acquisition was performed in Full-MS/dd-MS2 mode, collecting both positive and negative ions in the 100-1500 Da range at a resolution of 60000. The following settings were used: Spray voltage 3 kV, capillary tube temperature 320 °C, auxiliary temperature 350 °C, collision energy (NCE) 10, 30, 50 V, sheath gas 35, and auxiliary gas 10.
Qualitative analysis via LC-MS/MS was conducted to compare blank plasma, mixed herbal extracts, plasma samples from the treatment and control groups, and the control solution. Ion peaks at m/z were accurately extracted using Xcalibur software for substance identification.
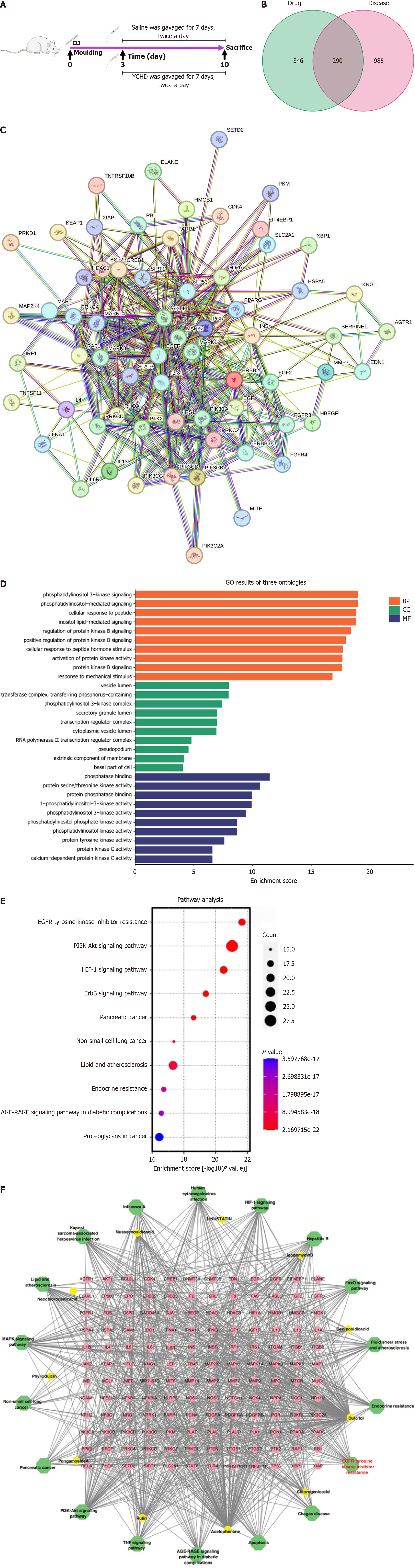
Incorporating YCHD data into blood component analysis via GeneCards (https://www.genecards.org/) and UniProt (https://www.uniprot.org/) enables predictive identification of potential targets. A systematic search was conducted in GeneCards using "obstructive jaundice" as the primary search term. All relevant genes were identified, downloaded, and integrated, ensuring comprehensive inclusion of genetic information related to OJ. Protein-protein interaction (PPI) analysis explored the integration of YCHD data with existing PPI networks. The overlap between YCHD data and PPI networks was visualized utilizing Venn diagrams (https://bioinfogp.cnb.csic.es/tools/venny/). Additionally, the STRING database (https://cn.string-db.org/), a tool for retrieving interaction genes/proteins, was used for further analysis. STRING analysis was limited to Homo sapiens, with a confidence score threshold of 0.7, identifying 65 significant PPIs. Gene Ontology (GO) enrichment analysis was conducted utilizing the GO database (https://www.gene
SPF-grade Sprague-Dawley (SD) rats, weighing between 200-230 g, were obtained from Beijing HFK Bioscience (license No. SYXK Jin 2020-0008) and housed at the Laboratory Animal Center of Tianjin Nankai Hospital, following ethical guidelines. Prior to the study, the rats were acclimated for two weeks in a controlled environment with temperatures of 22-24 °C, a 12-hour light/dark cycle, and humidity at 60%-65%. The rats had free access to food and water during the acclimatization period.
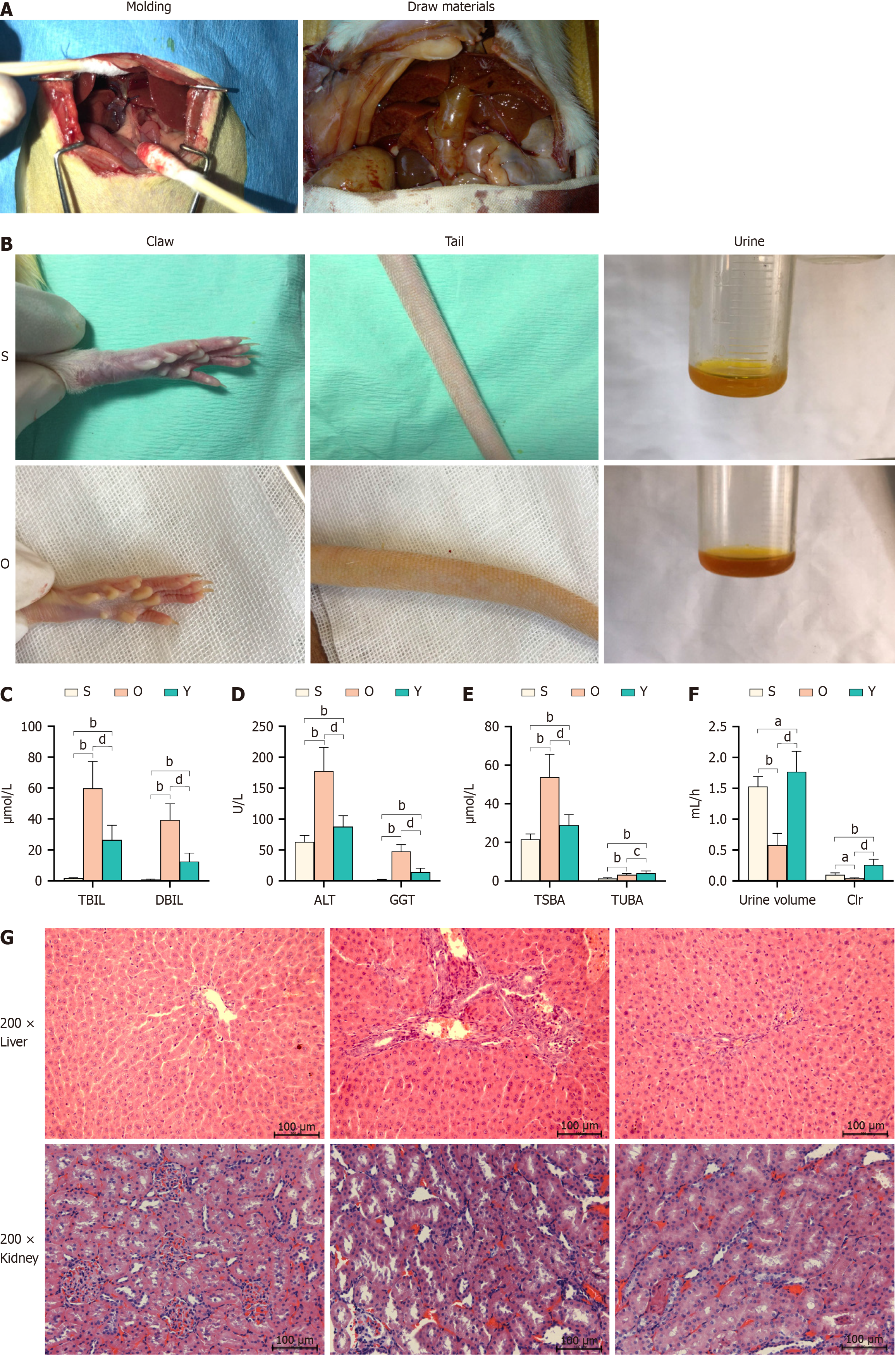
Twenty-four healthy rats were randomly allocated into three groups (n = 8): Sham (Group S), OJ model (Group O), and YCHD-treated (Group Y). OJ was induced in Groups O and Y by double ligation of the common bile duct, confirmed by jaundice appearance 5 days post-surgery. In Group S, the common bile duct was left intact as a control. Group Y rats received YCHD via gavage (1.8 mL/kg) twice daily for one week, while Groups S and O were administered an equivalent volume of physiological saline by intragastric administration. On the 12th day after surgery, urine was collected for 6 hours using a metabolic cage to assess changes in urine output. All rats were euthanized by decapitation, and liver, kidney, and blood samples were collected for further analysis (Figure 1A). The experiments adhered to the "Guide for the Care and Use of Laboratory Animals" by the National Institutes of Health. The experimental protocol was approved by the Animal Research Committee of Tianjin Medical University Nankai Hospital (approval No. NKYY-DWLL-2023-073).
Blood samples were centrifuged at 3000 rpm for 10 minutes to separate the serum. Serum levels of total TBIL, DBIL, ALT, AST, total serum bile acid (TSBA), and total urinary bile acid (TUBA), were quantified. Renal clearance rate (Clr) of bile acids was calculated using the formula Clr = U × V/C, where Clr represents the clearance rate in milliliters per hour, V is the urine volume per hour (mL/hour), U is the concentration of the substance in urine (mg/L), and C is the concentration of the substance in the blood (mg/L).
Tissue sections were processed for hematoxylin and eosin staining as well as immunofluorescence staining. Immunohistochemistry (IHC) was conducted following standard protocols[21]. Tissue slides were dewaxed, rehydrated, and underwent antigen retrieval. Endogenous peroxidase activity was quenched by incubation in a 3% hydrogen peroxide solution. The slides were then sealed and incubated overnight at 4 °C with a primary antibody against CAR.
The next day, the slides were treated with a polymer-horseradish peroxidase-conjugated anti-rabbit secondary antibody. The sections were stained with DAB and counterstained with hematoxylin. After dehydration, the slides were mounted with glass coverslips. To assess CAR protein expression in liver tissue, the average optical density (AOD) was analyzed using ImageJ software.
Three randomly selected fields per slide were examined, and the number of cells with positive nuclear marker expression was counted. The results were reported as the percentage of positively stained cells compared to the total number of cells in each field.
Proteins were extracted using RIPA lysis buffer with proteinase inhibitors, and concentrations were measured using the BCA assay. Samples were separated by electrophoresis, transferred to membranes, and incubated with primary antibodies: Anti-CAR (1:1000), anti-CYP450 3A4 (1:500), anti-UGT1A1 (1:500), anti-MRP4 (1:1000), anti-MRP3 (1:1000), anti-MRP2 (1:1000), anti-p-EGFR (1:1000), and anti-β-actin (1:5000). After washing with TBST, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies. Protein bands were visualized utilizing electrochemiluminescence, and relative protein expression was assessed by normalizing the AOD of the target protein to β-actin using ImageJ software.
For the preparation of OJ serum, male SD rats weighing approximately 200 g were selected. After inducing OJ as previously described, the rats were treated with daily physiological saline for 7 days. Serum was collected via abdominal aorta sampling.
For the preparation of medicated serum from YCHD-treated rats, the equivalent dosage of YCHD was calculated based on clinical standards (3.6 mL/kg/day). Rats received YCHD at a dosage ten times the calculated amount via gavage twice daily for 7 consecutive days.
Two hours after the final dose, blood was collected from the inferior vena cava of YCHD-treated rats. The serum was isolated by centrifuging the blood at 3000 rpm for 15 minutes, heat-inactivated at 56 °C for 30 minutes, and filtered through a 0.22 μm microporous membrane to remove bacterial contamination. The serum was then stored at -80 °C for future use, ensuring high-quality medicated serum for subsequent experiments.
Human normal proximal renal tubular epithelial cells (HK-2) were cultured in DMEM/F12 basal medium enriched with 10% FBS and 1% penicillin-streptomycin. Human normal liver cells (L02) were cultured in RPMI 1640 medium with 10% FBS and 1% penicillin-streptomycin. Both cell lines were incubated at 37 °C in a 5% CO2 humidified incubator.
All cell groups underwent a 48-hour standard incubation before treatment, and relevant indicators were assessed 24 hours after administration. The following groups were included in the treatment: The model group (O group, 5% OJ serum + 10% FBS); the Chinese medicine-treated group (HY group, 5% OJ serum + 5% YCHD-containing serum + 5% FBS); and the EGFR agonist + TCM-treated group (HY + A group; 5% OJ + 5% YCHD-containing serum + 5% FBS + 10 μM NSC228155).
Cells were fixed in 4% paraformaldehyde for 30 minutes, permeabilized with 0.1% Triton X-100 in PBS for 10 minutes, and blocked with 5% normal goat serum for 30 minutes at room temperature (20-25 °C). The cells were incubated overnight at 4 °C with rabbit anti-CAR antibody, followed by a 1-hour incubation with the secondary antibody (594 goat anti-rabbit IgG) at room temperature. After washing with PBS, the slides were counterstained with DAPI for 5 minutes. High-resolution images were captured using a laser-scanning confocal microscope (Olympus BX53, Tokyo, Japan).
Molecular docking simulations were performed to investigate protein-ligand interactions and assess binding affinities. Ligand structures from YCHD were converted into the mol2 format, while the protein structure of EGFR (PDB No. 5UGB) was retrieved from the RCSB Protein Data Bank (https://www.rcsb.org/). Docking simulations were carried out using Molecular Operating Environment (MOE) 2022.02 software (Computing Group ULC, Montreal, QC, Canada), enabling precise analysis of binding affinities. This approach facilitates the identification of potential therapeutic targets and supports drug discovery efforts. The top four docking poses were analyzed in detail for further insights.
Data were analyzed utilizing SPSS version 26.0 software (IBM, Chicago, IL, United States). Results are reported as mean ± SE. One-way analysis of variance was used to compare measurement data among multiple groups, with post-hoc pairwise comparisons performed using the LSD-t test. A P value < 0.05 was considered statistically significant.
LC-MS/MS was employed to identify 49 active chemical components in YCHD (Table 1) and 11 active components detected in the blood of rats administered YCHD orally (Table 2). These components include Dulcitol, Geniposidic acid, Pongamoside A, Mussenosidic acid, Linustatin, Neochlorogenic acid, Chlorogenic acid, Phyllodulcin, Acetophenone, Ieodomycin D, and Rutin.
| No. | tR (minute) | Formula | Adduct | m/z | Name |
| 1 | 0.73 | C7H6Cl2 | (M-H)- | 159.8779 | 2,6-Dichlorotoluene |
| 2 | 0.78 | C9H20N2O2 | (M+H)+ | 189.1601 | N6,N6,N6-Trimethyl-L-lysine |
| 3 | 0.80 | C6H14N4O2 | (M+H)+ | 175.1191 | L-Arginine |
| 4 | 0.83 | C4H9NO2 | (M+H)+ | 104.1076 | D(-)-2-Aminobutyric acid |
| 5 | 0.85 | C4H9NO3 | (M+H)+ | 120.0657 | L-(-)-Threonine |
| 6 | 0.85 | C4H9NO2 | (M+H)+ | 104.1076 | N,N-Dimethylglycine |
| 7 | 0.85 | C4H8N2O3 | (M+H)+ | 133.0608 | L-(-)-Asparagine |
| 8 | 0.87 | C6H14O6 | (M-H)- | 181.0712 | Dulcitol |
| 9 | 0.87 | C6H12O7 | (M-H)- | 195.0503 | Gluconic acid |
| 10 | 0.90 | C12H22O11 | (M-H)- | 341.1092 | α,α-Trehalose |
| 11 | 0.90 | C6H10O8 | (M-H)- | 209.0297 | D-Saccharic acid |
| 12 | 0.92 | C5H9NO2 | (M+H)+ | 116.0709 | D-(+)-Proline |
| 13 | 0.93 | C7H12O6 | (M-H)- | 191.0553 | D-(-)-Quinic acid |
| 14 | 1.35 | C9H11NO3 | (M+H)+ | 182.0815 | L-tyrosine |
| 15 | 1.53 | C10H13N5O4 | (M+H)+ | 268.1043 | Adenosine |
| 16 | 1.78 | C6H10O5 | (M-H)- | 161.0447 | Diethylpyrocarbonate |
| 17 | 2.81 | C16H22O10 | (M+H)+ | 375.1287 | Geniposidic acid |
| 18 | 2.88 | C9H17NO5 | (M+H)+ | 220.1183 | vitamin b5 |
| 19 | 3.19 | C23H20O9 | (M-H)- | 439.1012 | Pongamoside A |
| 20 | 3.41 | C7H6O4 | (M-H)- | 155.0341 | 3,4-Dihydroxybenzoic acid |
| 21 | 3.41 | C16H24O10 | (M-H)- | 375.1297 | Mussaenosidic acid |
| 22 | 3.52 | C16H27NO11 | (M-H)- | 408.1516 | LINUSTATIN |
| 23 | 3.55 | C16H18O9 | (M-H)- | 353.0878 | Neochlorogenic acid |
| 24 | 3.75 | C11H9NO2 | (M+H)+ | 188.0709 | 3-Indoleacrylic acid |
| 25 | 4.38 | C9H6O3 | (M+H)+ | 162.9982 | 7-Hydroxycoumarine |
| 26 | 4.38 | C16H18O9 | (M+H)+ | 355.1026 | Chlorogenic acid |
| 27 | 4.97 | C17H24O10 | (M+H)+ | 389.1229 | Geniposide |
| 28 | 4.97 | C9H8O2 | (M+H)+ | 149.0600 | Phyllodulcin |
| 29 | 4.97 | C8H8O | (M+H)+ | 121.0650 | Acetophenone |
| 30 | 4.97 | C10H10O4 | (M+H)+ | 195.0652 | Acetovanillin |
| 31 | 4.97 | C11H14O5 | (M+H)+ | 227.1039 | Genipin |
| 32 | 4.97 | C8H7N | (M+H)+ | 118.0656 | Indole |
| 33 | 4.97 | C9H10O3 | (M+H)+ | 167.0706 | 3',4'-Dihydroxyphenylacetone |
| 34 | 5.41 | C18H24N4O5 | (M-H)- | 375.1659 | N-Acetylglycyl-N-[(2S)-5-amino-1,5-dioxo-2-pentanyl]-L-phenylalaninamide |
| 35 | 6.17 | C10H16O3 | (M-H)- | 183.1017 | Ieodomycin D |
| 36 | 6.20 | C27H30O16 | (M-H)- | 609.1463 | Rutin |
| 37 | 6.52 | C21H20O12 | (M+H)+ | 465.1032 | Quercetin-3β-D-glucoside |
| 38 | 6.66 | C11H12O5 | (M-H)- | 223.0609 | Sinapinic acid |
| 39 | 7.28 | C9H8O3 | (M+H)+ | 165.0546 | 4-Coumaric acid |
| 40 | 7.28 | C21H24O11 | (M+H)+ | 453.1671 | Aspalathin |
| 41 | 7.33 | C44H64O24 | (M-H)- | 975.3730 | Crocin |
| 42 | 7.40 | C25H24O12 | (M-H)- | 515.1195 | Isochlorogenic acid A |
| 43 | 7.40 | C25H24O12 | (M-H)- | 515.1195 | 4,5-Dicaffeoylquinic acid |
| 44 | 8.18 | C28H34O14 | (M+H)+ | 595.3627 | Fumotonaringin |
| 45 | 7.46 | C33H42O18 | (M+H)+ | 727.2448 | Naringenin 7-O-(2",6"-di-O-alpha-rhamnopyranosyl)-beta-glucopyranoside |
| 46 | 8.14 | C27H28O13 | (M+H)+ | 561.1606 | Cassiaoccidentalin A |
| No. | tR (minute) | Formula | Adduct | m/z | Name |
| 1 | 0.87 | C6H14O6 | (M-H)- | 181.0712 | Dulcitol |
| 2 | 2.81 | C16H22O10 | (M+H)+ | 375.1287 | Geniposidic acid |
| 3 | 3.19 | C23H20O9 | (M-H)- | 439.1012 | Pongamoside A |
| 4 | 3.41 | C16H24O10 | (M-H)- | 375.1297 | Mussaenosidic acid |
| 5 | 3.52 | C16H27NO11 | (M-H)- | 408.1516 | Linustatin |
| 6 | 3.55 | C16H18O9 | (M-H)- | 353.0878 | Neochlorogenic acid |
| 7 | 4.38 | C16H18O9 | (M+H)+ | 355.1026 | Chlorogenic acid |
| 8 | 4.97 | C9H8O2 | (M+H)+ | 149.0600 | Phyllodulcin |
| 9 | 4.97 | C8H8O | (M+H)+ | 121.0650 | Acetophenone |
| 10 | 6.17 | C10H16O3 | (M-H)- | 183.1017 | Ieodomycin D |
| 11 | 6.20 | C27H30O16 | (M-H)- | 609.1463 | Rutin |
The GeneCards, OMIM, DrugBank, and PharmGKB databases were used to retrieve 1301 unique targets related to OJ. Among these, 293 targets overlapped with the active ingredients found in YCHD (Figure 1B). A PPI network was performed from the overlapping targets identified in the STRING database, consisting of 65 nodes and 382 interactions (Figure 1C). The differentially expressed genes likely participate in proliferation, anti-apoptosis, inflammatory responses, and hypoxia adaptation. Their products may act through cell surface receptor-mediated signaling (e.g., ERK/MAPK, PI3K/AKT pathways) and extracellular interactions (e.g., cytokines, integrins). These findings align with biological contexts such as cancer progression, immune activation, or tissue repair (Figure 1D). Functional annotation of the core targets revealed significant associations with the EGFR and PI3K-Akt signaling pathways (Figure 1E). Further analysis led to the creation of a component-target-pathway-disease network, which included 175 nodes (11 components, 144 overlapping targets, and 20 pathways) and 957 edges (Figure 1F). Network analysis suggested that the potential mechanism of YCHD in treating OJ may involve modulation of the EGFR signaling pathway.
Five days after the double ligation of the common bile duct, rats exhibited significant changes, including an increase in the diameter of the bile duct (Figure 2A), yellowing of the claws and tail, reduced motor activity and appetite, yellow-brown discoloration of urine, and decreased urine output (Figure 2B). Group Y showed a significant decrease in bilirubin levels (Figure 2C), as well as lower ALT and AST values (Figure 2D) compared to group O (P < 0.01). Furthermore, YCHD markedly increased urine output (P < 0.01) and enhanced the Clr rate of bile acids (P < 0.01) in rats with OJ (Figure 2E), leading to a reduction in the total serum bile acid content (P < 0.01; Figure 2F). Histopathological analysis confirmed these beneficial effects (Figure 2G). Rats in the O group exhibited proliferative small bile ducts, fibrous tissue cell proliferation in the portal area, occasional bile duct dilation, and infiltration of neutrophils and lymphocytes in the liver. Some liver cells showed signs of degeneration and necrosis, while renal tissue exhibited swelling and focal damage to renal tubular epithelial cells, with infiltration of neutrophils and lymphocytes. YCHD treatment effectively alleviated these injuries in both liver and kidney tissues, suggesting its potential to mitigate liver damage and reduce bile acid accumulation in rats with OJ.
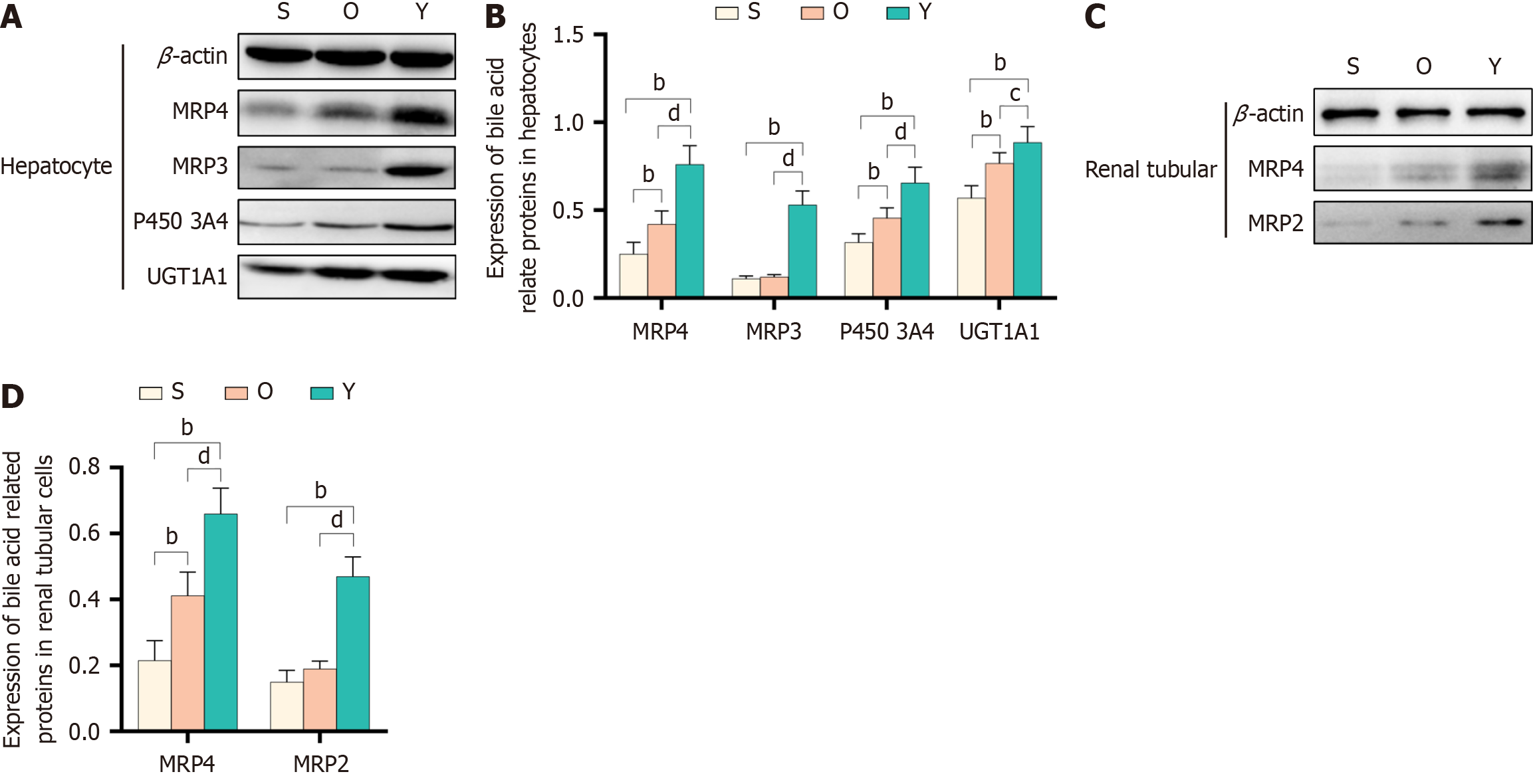
Western blot analysis revealed that the expression of P4503A4, UGT1A1, MRP3, and MRP4 were higher in the liver cells of rats from the Y groups compared to those from the O group (Figure 3A and B). Additionally, YCHD treatment led to an upregulation of MRP2 and MRP4 expression in the renal tubular cells of rats with OJ (Figure 3C and D). These findings indicate that YCHD promotes the expression of proteins involved in bile acid metabolism and excretion in the liver and kidneys, thereby reducing bile acid accumulation and enhancing their effective removal.
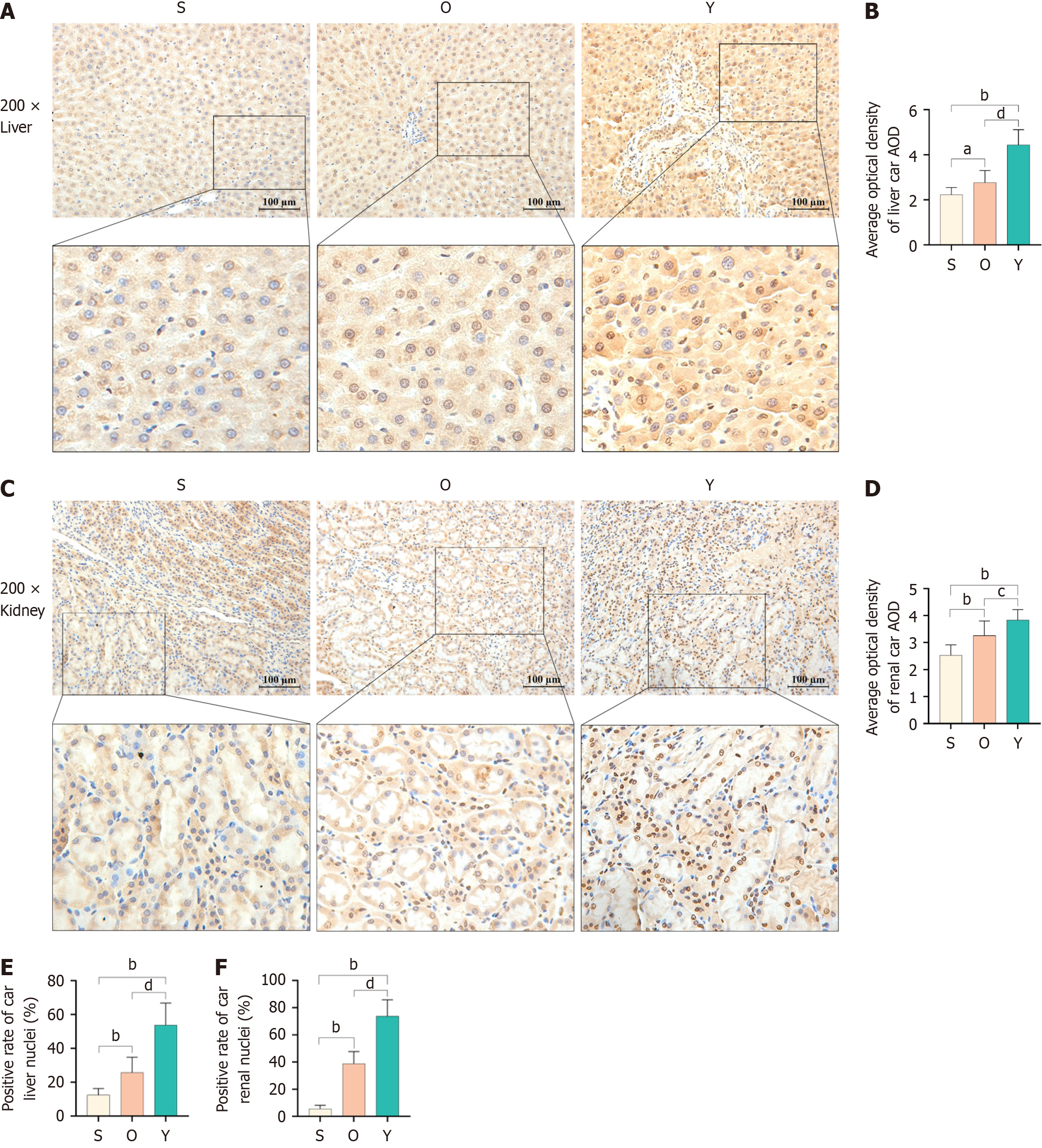
IHC staining of the liver (Figure 4A and B) and kidney tissues (Figure 4C and D) demonstrated a significant upregulation of CAR expression and increased nuclear localization in the Y group compared to the O group. These results suggest that YCHD activates CAR.
Moreover, the ratio of nuclear to cytoplasmic CAR was higher (P < 0.01) in the Y group than in the O group, both in the liver (Figure 4E) and kidney tissues (Figure 4F). Overall, YCHD significantly enhanced CAR expression and promoted its nuclear localization in both liver and kidney tissues, leading to an increased proportion of CAR in the nucleus compared to the cytoplasm in treated rats.
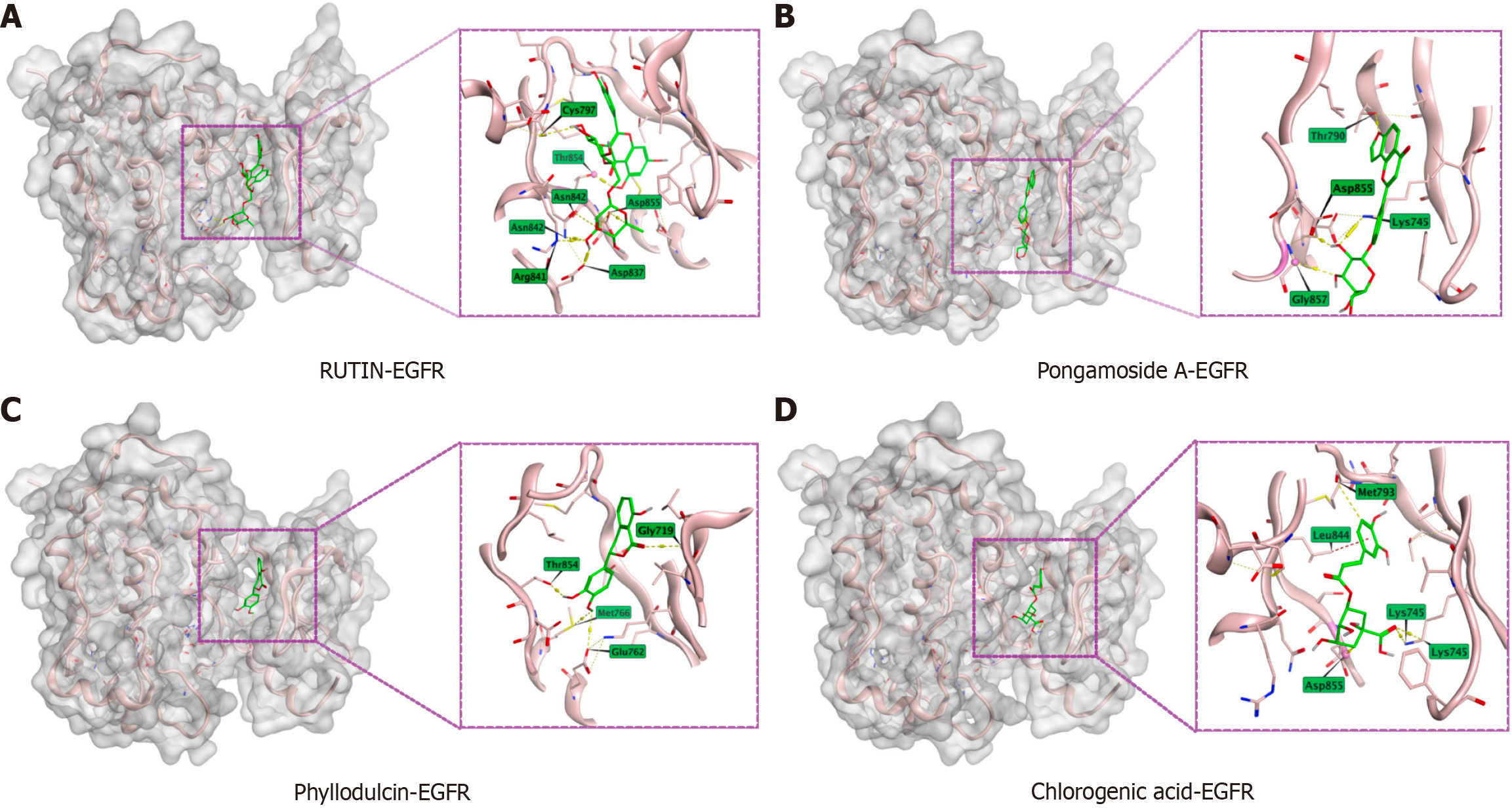
Building on previous network pharmacology findings, molecular docking simulations were performed using MOE 2002 software to verify the interactions between the primary active components of YCHD and the EGFR protein. Rutin (Figure 5A), Pongamoside A (Figure 5B), Phyllodulcin (Figure 5C), and Chlorogenic acid (Figure 5D) all effectively docked with EGFR at specific binding sites, exhibiting the lowest binding energies.
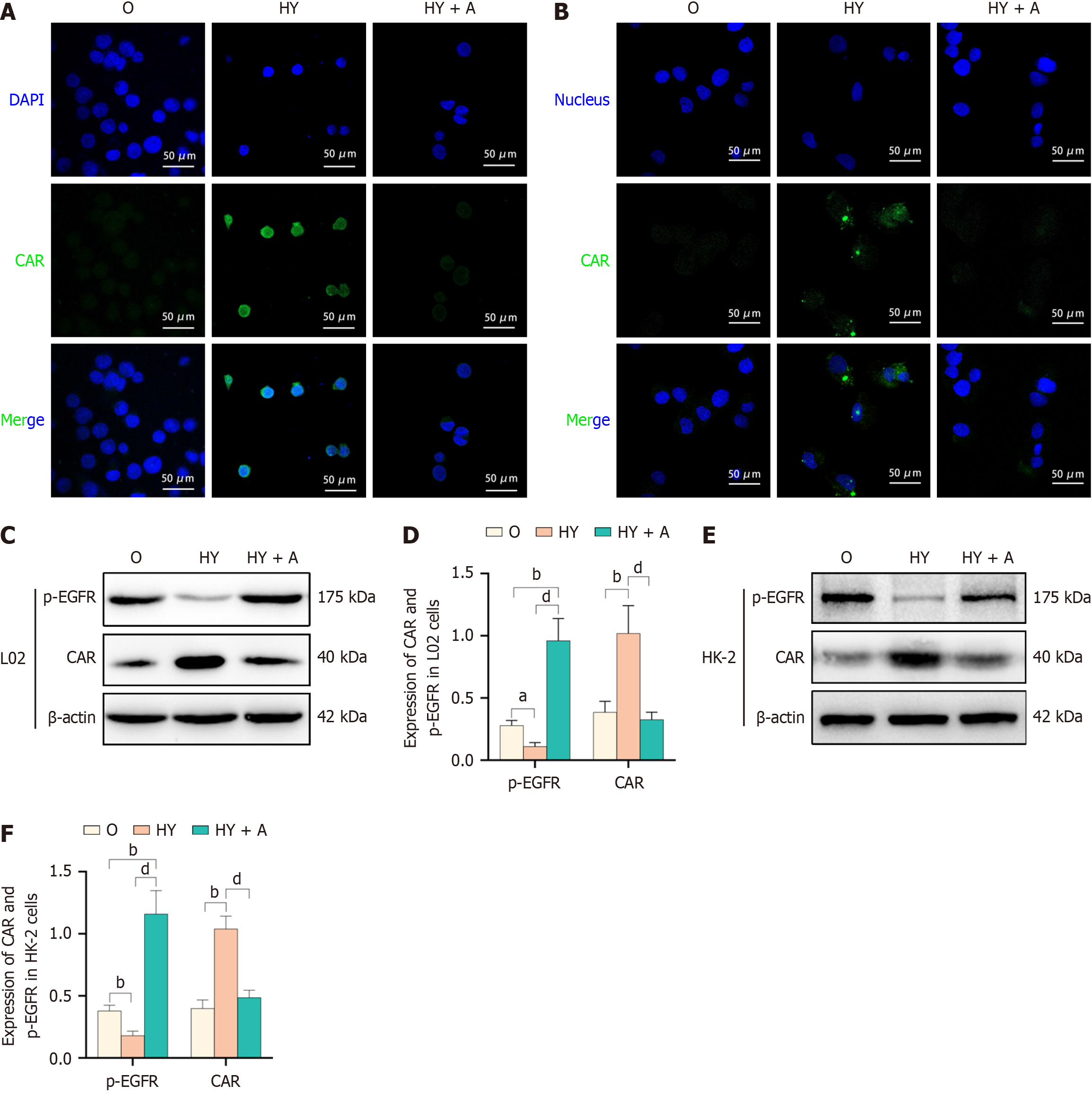
Immunofluorescence analysis of L02 cells revealed a significant increase in CAR expression and its nuclear localization following YCHD administration. These cells displayed enhanced green fluorescence and greater brightness in the nuclei compared to the model group. The addition of an EGFR agonist to the culture medium reversed these effects, suggesting a critical role for EGFR in CAR nuclear localization (Figure 6A). Similar observations were made in HK-2 epithelial cells (Figure 6B). These findings highlight YCHD’s ability to increase CAR expression and induce its nuclear localization in both liver and renal tubular epithelial cells through EGFR modulation. Western blot analysis of p-EGFR and CAR expression in L02 cells further supported these results (Figure 6C). YCHD reduced p-EGFR levels while increasing CAR expression. However, in the presence of an EGFR agonist, these effects of YCHD on p-EGFR and CAR expression were reversed (Figure 6D). Similar results were observed in HK-2 cells (Figure 6E and F). Overall, YCHD enhances CAR expression by inhibiting EGFR phosphorylation in liver cells, and EGFR activation can counteract YCHD’s regulatory effects on CAR.
OJ refers to the blockage of the left and right hepatic ducts, resulting in reduced bile excretion and substantially increased pressure within the biliary system due to bile stasis[22]. Conversely, cholestasis is characterized by impaired bile secretion and excretion, resulting in a deficiency in intestinal bile and potential accumulation of toxic bile acids in the liver and systemic circulation[23]. This accumulation can contribute to liver injury and fibrosis[24].
Cholestasis is categorized into two types: The first involves dysfunction at the liver cell level, impairing bile formation and/or secretion; the second involves impaired bile secretion and excretion at the bile duct level[25,26]. Deficient bile formation at the liver cell level can arise from genetic transporter defects, drug or hormone exposure, or inflammation[27,28]. Impaired bile secretion and excretion at the bile duct level, often caused by obstructions or inflammation, are most commonly observed in OJ[29,30].
Given the pathophysiological challenges posed by OJ, new therapeutic approaches are urgently needed. In this study, we explored how YCHD improves OJ in rats by modulating the EGFR/CAR signaling pathway, providing insight into the mechanism of YCHD's action. Plants and their derivatives have long been utilized as functional foods and TCMs, with a rich historical record of use in treating various ailments[31]. YCHD, a TCM, has been employed for centuries as a remedy for OJ, and our previous research has demonstrated its clinical efficacy[19]. Consistent with its clinical effectiveness, our study demonstrated that YCHD significantly reduced jaundice indicators and mitigated liver damage in rats, further validating its therapeutic potential in the treatment of OJ.
Nuclear receptors are crucial in regulating the metabolism of various substances, including bile acids and pharmaceuticals[32]. Recent studies have illuminated the mechanisms by which nuclear receptors regulate bile acid metabolism[33,34]. Specifically, the CAR and pregnane X receptor (PXR)[35] function as sensors for potentially toxic byproducts, while also acting as key regulators to maintain the dynamic balance of bile acids[36]. These receptors modulate many genes involved in drug and xenobiotic metabolism, including phase I and II drug-metabolizing enzymes and transporters[37,38].
Initially recognized for their broad biosensing functions, CAR and PXR are also crucial in lipid metabolism, glucose homeostasis, and inflammation[39]. Consequently, novel compounds targeting CAR hold great potential for therapeutic interventions in OJ, offering the promise of efficacy with minimal side effects. Our study found that YCHD significantly upregulated the expression of P4503A4, UGT1A1, MRP3, and MRP4 in the liver cells of rats with OJ. Additionally, YCHD treatment enhanced the expression of MRP2 and MRP4 in the renal tubular cells of these rats. These results suggest that YCHD induces the expression of proteins involved in bile acid metabolism and excretion in the liver and kidneys, ultimately reducing bile acid accumulation in rats with OJ.
The pivotal step in CAR activation involves its nuclear heterotopy, a constitutively active nuclear receptor[40]. CAR activation is regulated through phosphorylation at the Thr-38 residue. Phosphorylated CAR remains sequestered in the cytoplasm, while PP2A dephosphorylates CAR, enabling its translocation to the nucleus[41]. In the nucleus, CAR forms a heterodimer with retinoid X receptor α and binds to the phenobarbital-responsive enhancer module, triggering the transcriptional activation of target genes. Therefore, nuclear heterotopy is a key step in CAR activation. CAR regulates several genes, including CYP3A, UGT1A1, SULT2A1, and members of the MRP family (MRP2, MRP3, MRP4)[42]. Our research demonstrated that YCHD enhances the expression of proteins involved in bile acid metabolism and excretion by increasing CAR expression and promoting its nuclear heterotopy in liver and kidney tissues.
EGFR, a membrane receptor, plays a critical role in regulating the dephosphorylation of the nuclear receptor CAR. By analyzing the interaction between YCHD targets and OJ, along with molecular docking studies of key YCHD components and EGFR, we identified that quercetin, β-sitosterol, and isorhamnetin can effectively bind to the extracellular region of EGFR. These findings, supported by prior research, suggest that YCHD may modulate the nuclear receptor activation pathway by regulating the EGFR signaling pathway[18].
When EGF activates EGFR, it triggers phosphorylation of Src kinase-dependent RACK1 at Tyr-52[13], disrupting the interaction between CAR, RACK1, and PP2A. As a result, CAR is prevented from dephosphorylation and remains sequestered in the cytoplasm[43]. Additionally, EGF-induced EGFR activation increases mitogen-activated protein kinase (MEK) activity, which subsequently phosphorylates and activates extracellular signal-regulated kinase (ERK)[44]. The activated ERK then interacts with signaling peptides near the C-terminus of CAR, facilitating CAR's translocation into the nucleus. Inhibition of the MEK/ERK pathway leads to ERK dephosphorylation and inactivation, promoting CAR’s nuclear translocation[45]. Inhibiting EGFR activation promotes the interaction between non-phosphorylated RACK1, CAR, and PP2A, alleviating the ERK-induced inhibition of CAR nuclear localization[46]. As a result, CAR translocates to the nucleus and initiates transcriptional activation. Our results show that YCHD significantly enhances CAR expression and induces nuclear heterotopia in liver and renal tubular epithelial cells affected by OJ. However, the administration of an EGFR agonist effectively blocked these effects, especially the nuclear heterotopia of CAR.
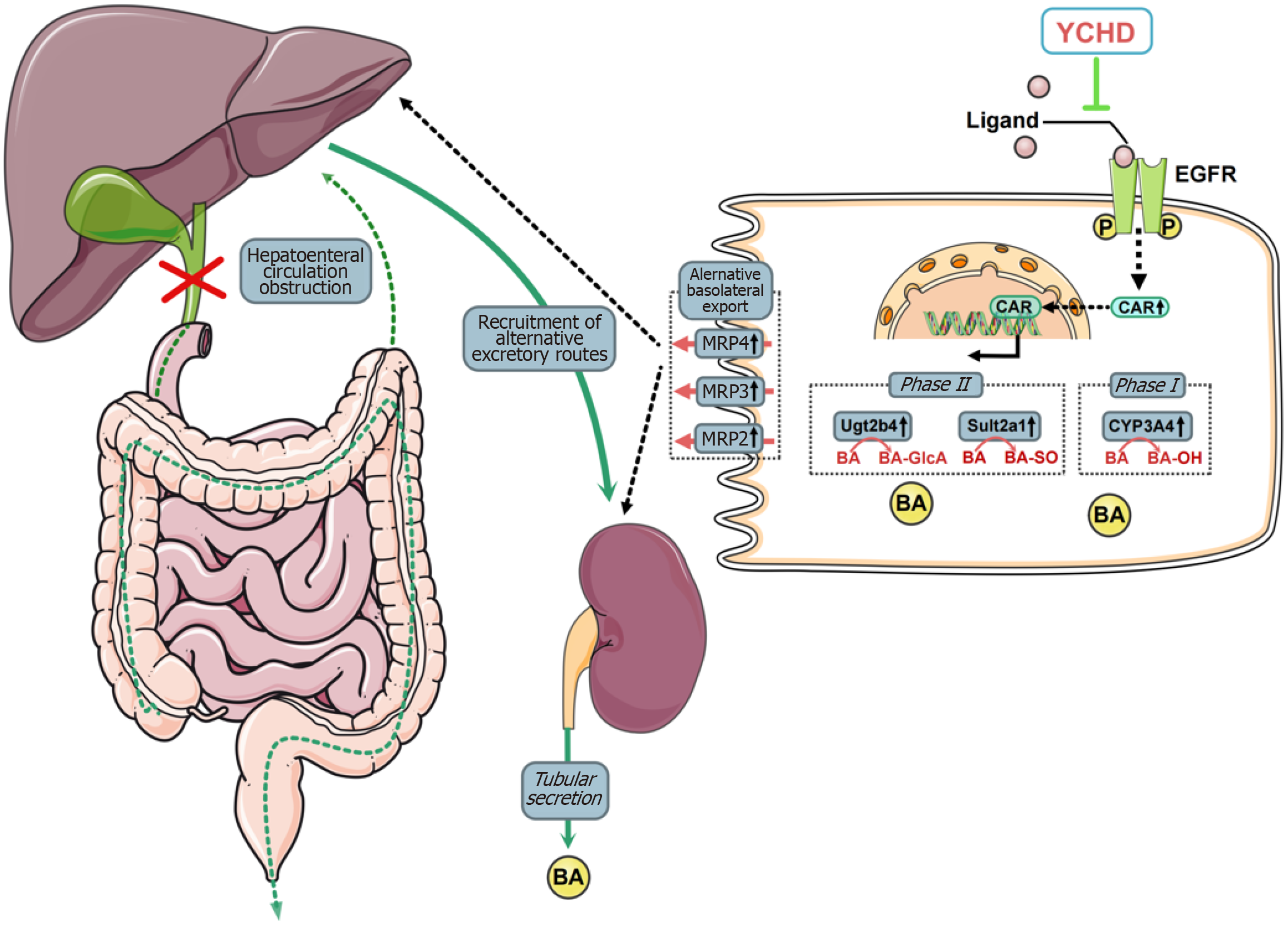
This study highlights the therapeutic potential of YCHD in mitigating liver injury induced by OJ. YCHD enhances bile acid metabolism and active transport in liver cells while promoting bile acid excretion by proximal renal tubular epithelial cells. The therapeutic effects of YCHD are attributed to its inhibition of EGFR activation, which leads to increased CAR expression and facilitates its nuclear translocation (Figure 7).
| 1. | Liu Q, Li BS, Song YJ, Hu MG, Lu JY, Gao A, Sun XJ, Guo XM, Liu R. Hydrogen-rich saline protects against mitochondrial dysfunction and apoptosis in mice with obstructive jaundice. Mol Med Rep. 2016;13:3588-3596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Huang YH, Yang YL, Huang FC, Tiao MM, Lin YC, Tsai MH, Wang FS. MicroRNA-29a mitigation of endoplasmic reticulum and autophagy aberrance counteracts in obstructive jaundice-induced fibrosis in mice. Exp Biol Med (Maywood). 2018;243:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Abe T, Arai T, Ogawa A, Hiromatsu T, Masuda A, Matsuguchi T, Nimura Y, Yoshikai Y. Kupffer cell-derived interleukin 10 is responsible for impaired bacterial clearance in bile duct-ligated mice. Hepatology. 2004;40:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Trauner M, Wagner M, Fickert P, Zollner G. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J Clin Gastroenterol. 2005;39:S111-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Vyhlidal CA, Bi C, Ye SQ, Leeder JS. Dynamics of Cytosine Methylation in the Proximal Promoters of CYP3A4 and CYP3A7 in Pediatric and Prenatal Livers. Drug Metab Dispos. 2016;44:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Shizu R, Osabe M, Perera L, Moore R, Sueyoshi T, Negishi M. Phosphorylated Nuclear Receptor CAR Forms a Homodimer To Repress Its Constitutive Activity for Ligand Activation. Mol Cell Biol. 2017;37:e00649-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Garcia M, Thirouard L, Monrose M, Holota H, De Haze A, Caira F, Beaudoin C, Volle DH. Farnesoid X receptor alpha (FXRα) is a critical actor of the development and pathologies of the male reproductive system. Cell Mol Life Sci. 2019;76:4849-4859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Hu X, Li M, Zhang C, Pang S. Constitutive Androstane Receptor-Mediated Inhibition of Metformin on Phase II Metabolic Enzyme SULT2A1. Int J Endocrinol. 2021;2021:8867218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yu XQ, Xue CC, Wang G, Zhou SF. Multidrug resistance associated proteins as determining factors of pharmacokinetics and pharmacodynamics of drugs. Curr Drug Metab. 2007;8:787-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Garcia M, Thirouard L, Sedès L, Monrose M, Holota H, Caira F, Volle DH, Beaudoin C. Nuclear Receptor Metabolism of Bile Acids and Xenobiotics: A Coordinated Detoxification System with Impact on Health and Diseases. Int J Mol Sci. 2018;19:3630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923-G932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, Negishi M. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 2013;6:ra31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Koike C, Moore R, Negishi M. Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol. 2007;71:1217-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhang JH, Shang HT, Liu JJ, Hao CF, Zhang DL, Han SW, Li ZL. [Effect of Yinchen Wuling Powder on Postoperative Liver Function Injury in Obstructive Jaundice Patients with Dampness Predominating over Heat Pattern:A Randomized Controlled Trial]. Zhongyi Zazhi. 2022;5:539-543. [DOI] [Full Text] |
| 16. | Luo S, Huang M, Lu X, Zhang M, Xiong H, Tan X, Deng X, Zhang W, Ma X, Zeng J, Efferth T. Optimized therapeutic potential of Yinchenhao decoction for cholestatic hepatitis by combined network meta-analysis and network pharmacology. Phytomedicine. 2024;129:155573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Sun H, Zhang AH, Yang L, Li MX, Fang H, Xie J, Wang XJ. High-throughput chinmedomics strategy for discovering the quality-markers and potential targets for Yinchenhao decoction. Phytomedicine. 2019;54:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Liu JJ, Xu Y, Chen S, Hao CF, Liang J, Li ZL. The mechanism of Yinchenhao decoction in treating obstructive-jaundice-induced liver injury based on Nrf2 signaling pathway. World J Gastroenterol. 2022;28:4635-4648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (5)] |
| 19. | Wu YL, Li ZL, Zhang XB, Liu H. Yinchenhao decoction attenuates obstructive jaundice-induced liver injury and hepatocyte apoptosis by suppressing protein kinase RNA-like endoplasmic reticulum kinase-induced pathway. World J Gastroenterol. 2019;25:6205-6221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Qian Z, Huang C, Shen C, Meng X, Chen Z, Hu T, Li Y, Li J. The permeability characteristics and interaction of the main components from Zhizi Bopi decoction in the MDCK cell model. Xenobiotica. 2016;46:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 488] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Temesgen R, Abebe H, Abera Y. Hepatobiliary and Pancreatic Duct Ascariasis: An Unusual Cause of Obstructive Jaundice and Severe Acute Cholangitis. Int Med Case Rep J. 2022;15:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med. 2003;9:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 700] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 25. | Squires JE, McKiernan P. Molecular Mechanisms in Pediatric Cholestasis. Gastroenterol Clin North Am. 2018;47:921-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Yuan ZQ, Li KW. Role of farnesoid X receptor in cholestasis. J Dig Dis. 2016;17:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Han D, Kim H, Kim S, Le QA, Han SY, Bae J, Shin HW, Kang HG, Han KH, Shin J, Park HW. Sestrin2 protects against cholestatic liver injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis. Exp Mol Med. 2022;54:239-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Zou M, Wang A, Wei J, Cai H, Yu Z, Zhang L, Wang X. An insight into the mechanism and molecular basis of dysfunctional immune response involved in cholestasis. Int Immunopharmacol. 2021;92:107328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Guglielmi FW, Regano N, Mazzuoli S, Fregnan S, Leogrande G, Guglielmi A, Merli M, Pironi L, Penco JM, Francavilla A. Cholestasis induced by total parenteral nutrition. Clin Liver Dis. 2008;12:97-110, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Qiu J. When the East meets the West: the future of traditional Chinese medicine in the 21st century. Natil Sci Rev. 2015;2:377-380. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Bae SDW, Nguyen R, Qiao L, George J. Role of the constitutive androstane receptor (CAR) in human liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Pustylnyak YA, Gulyaeva LF, Pustylnyak VO. Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation. Int J Mol Sci. 2020;21:6735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Oliviero F, Lukowicz C, Boussadia B, Forner-Piquer I, Pascussi JM, Marchi N, Mselli-Lakhal L. Constitutive Androstane Receptor: A Peripheral and a Neurovascular Stress or Environmental Sensor. Cells. 2020;9:2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Kilavuz H, Turan U, Yoldas A, Tolun FI, Tanriverdi B, Yaylali A, Yaman A, Yener MK, Irkorucu O. The effect of Farnesoid X receptor agonist tropifexor on liver damage in rats with experimental obstructive jaundice. Acta Cir Bras. 2021;36:e360902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Inouye Y. [Structure and Function of the Nuclear Receptor Constitutive Androstane Receptor]. Yakugaku Zasshi. 2016;136:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Kodama S, Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab Rev. 2006;38:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Fahrbach SE, Smagghe G, Velarde RA. Insect nuclear receptors. Annu Rev Entomol. 2012;57:83-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | van Golen RF, Olthof PB, de Haan LR, Coelen RJ, Pechlivanis A, de Keijzer MJ, Weijer R, de Waart DR, van Kuilenburg ABP, Roelofsen J, Gilijamse PW, Maas MA, Lewis MR, Nicholson JK, Verheij J, Heger M. The pathophysiology of human obstructive cholestasis is mimicked in cholestatic Gold Syrian hamsters. Biochim Biophys Acta Mol Basis Dis. 2018;1864:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Zhu G, Feng F. UPLC-MS-based metabonomic analysis of intervention effects of Da-Huang-Xiao-Shi decoction on ANIT-induced cholestasis. J Ethnopharmacol. 2019;238:111860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Shizu R, Min J, Sobhany M, Pedersen LC, Mutoh S, Negishi M. Interaction of the phosphorylated DNA-binding domain in nuclear receptor CAR with its ligand-binding domain regulates CAR activation. J Biol Chem. 2018;293:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102:2063-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Kawase A, Mukai H, Tateishi S, Kuroda S, Kazaoka A, Satoh R, Shimada H, Sugiura R, Iwaki M. Protein Kinase N Family Negatively Regulates Constitutive Androstane Receptor-Mediated Transcriptional Induction of Cytochrome P450 2b10 in the Livers of Mice. J Pharmacol Exp Ther. 2021;379:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem. 2016;16:1134-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 46. | Osabe M, Negishi M. Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. J Biol Chem. 2011;286:35763-35769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |