Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.99134
Revised: December 19, 2024
Accepted: December 25, 2024
Published online: February 27, 2025
Processing time: 220 Days and 15.9 Hours
The hepatosplenic schistosomiasis (HSS) with portal hypertension can cause vascular complications such as hepatopulmonary syndrome (HPS). HPS increases the risk of mortality in patients with cirrhosis; however, there is no data on the mortality of patients with HSS and HPS.
To perform a survival analysis of patients with HPS related to cirrhotic and non-cirrhotic (schistosomiasis) portal hypertension.
From August 2023 to January 2024, medical records and the official mortality information service of 121 patients who participated in a cross-sectional study on HPS between 2010 and 2012 were analyzed. Survival curves were created using the Kaplan-Meier method, and comparisons were performed using the log-rank test. Cox regression models estimated the hazard ratios (HR).
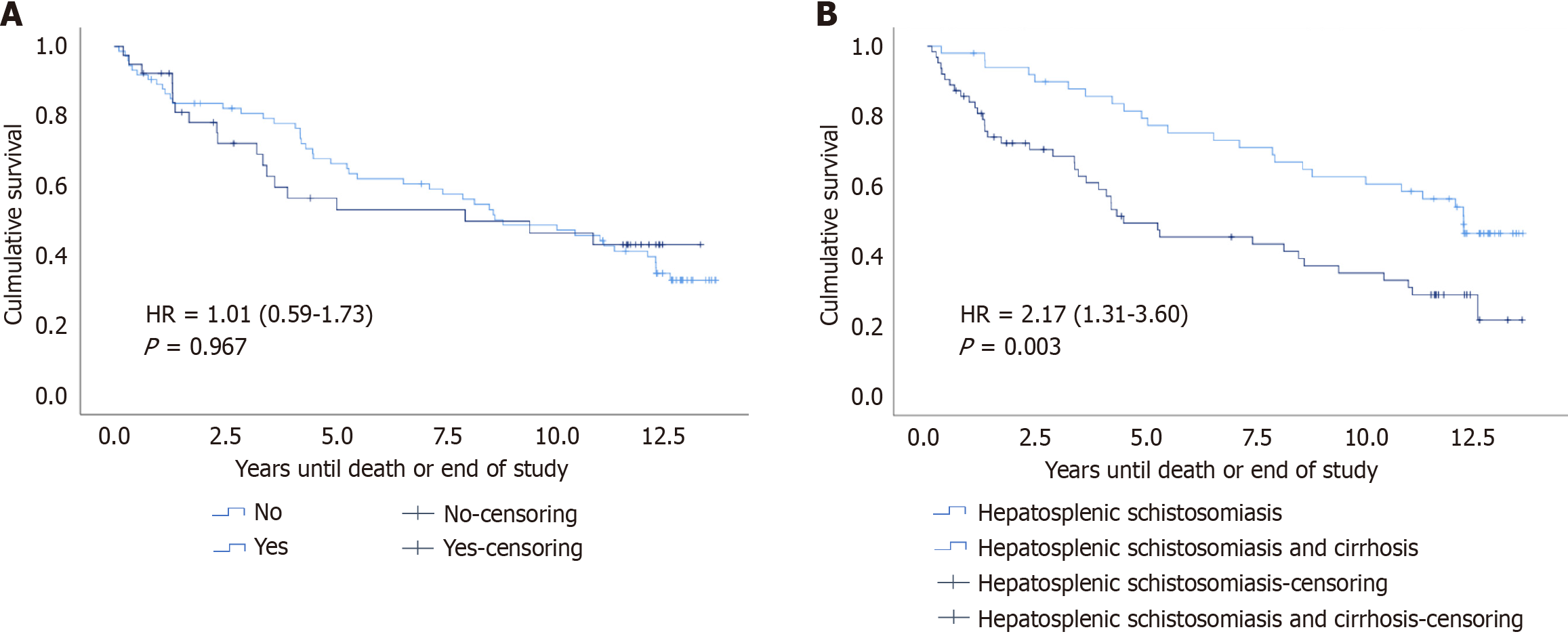
Overall, data of 113 patients were analyzed; most (55.8%) had HSS and concomitant cirrhosis (HSS/cirrhosis). Meanwhile, HPS was present in 39 (34.5%) patients. Death occurred in 65 patients [57.5%; 95% confidence interval (CI): 48%-67%. The average time to death was lower in those with HPS when compared to those without HPS (3.37 years vs 5.65 years; P = 0.017). According to the cause of liver disease, patients with HSS/cirrhosis died earlier, and their risk of death was twice as high compared with patients with HSS without cirrhosis (HR: 2.17; 95%CI: 1.3-3.60; P = 0.003). Meanwhile, there were no differences when comparing the two groups with and without HPS (HR: 1.01; 95%CI: 0.59-1.73; P = 0.967).
Patients with HSS and concomitant cirrhosis had a lower survival rate, but there was no difference in survival regardless of the presence of HPS.
Core Tip: Hepatopulmonary syndrome (HPS) increases the risk of mortality in patients with cirrhosis, but there is no data on the mortality of patients with hepatosplenic schistosomiasis. This study performed a survival analysis of patients with HPS related to cirrhotic and non-cirrhotic (schistosomiasis) portal hypertension. Patients who participated in a cross-sectional study on HPS 10 years ago were included, and survival analysis was performed using the Kaplan-Meier method. Our results showed that patients with hepatosplenic schistosomiasis and concomitant cirrhosis had lower survival compared with those without cirrhosis, but there was no difference in survival regardless of the presence of HPS.
- Citation: Rolim MM, Farsoun LG, Luna CF, Markman-Filho B, Querette P, Lopes EP, Domingues AL. Survival of patients with hepatopulmonary syndrome related to cirrhotic and non-cirrhotic (schistosomiasis) portal hypertension. World J Hepatol 2025; 17(2): 99134
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/99134.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.99134
Schistosomiasis is a neglected tropical disease (NTD) caused by the helminth of the genus Schistosoma spp. that affects approximately 260 million individuals worldwide[1]. In Brazil, Schistosomiasis mansoni is endemic and the second leading cause of death from NTD, with a prevalence of 1.5 million people[2]. Approximately 60% of persons infected with Schistosomiasis mansoni experience morbidity. Of these, up to 10% of cases progress to a severe form that is characterized by advanced periportal fibrosis and non-cirrhotic portal hypertension[3], known as hepatosplenic schistosomiasis (HSS), that develops without hepatocyte damage or impairment of liver function[4,5]. However, when HSS associated with other liver diseases, such as chronic viral, alcoholic, or fatty liver disease, hepatic changes occur, leading to disruption of the liver architecture, destruction of hepatocytes, and cirrhosis[6]. In patients with HSS, the presence of concomitant cirrhosis (HSS/cirrhosis) is related to higher morbidity and mortality[6].
One complication of portal hypertension is hepatopulmonary syndrome (HPS)[7]. HPS manifests with pulmonary vascular dilations that increase the alveolar-arterial oxygen gradient[8,9]. HPS can develop in patients with both cirrhotic and non-cirrhotic portal hypertension, such as in HSS, regardless of hepatocyte destruction and impaired liver function[10,11]. The incidence of HPS is higher in patients with portal hypertension due to HSS/cirrhosis compared to those with HSS alone (non-cirrhotic). Moreover, HPS develops more frequently in patients with cirrhosis and high model for end-stage liver disease scores[12,13]. When present, HPS confers worse prognosis and higher mortality in patients with chronic liver disease and portal hypertension[11-13]. Research on morbidity and mortality related to HPS mostly involved patients with cirrhosis[14-16]. Meanwhile, no studies have examined the mortality of patients with HSS. Thus, this study aimed to evaluate the mortality of patients with HPS with cirrhotic and non-cirrhotic (schistosomiasis) portal hypertension as well as performed a survival analysis using patient data of a study on HPS conducted approximately 10 years ago.
A retrospective study with survival analysis.
The sample was a convenience and non-probabilistic one that was composed of patients with HSS who participated in a previous cross-sectional study on HPS between 2010 and 2012. The previous study was conducted in the Hepatology Service of Hospital das Clínicas of the Universidade Federal de Pernambuco, Brazil. At the time of the previous study, all patients underwent ultrasonography to confirm the clinical and epidemiological diagnosis of HSS as well as upper gastrointestinal endoscopy to evaluate esophageal varices. The diagnosis of HSS/cirrhosis was based on clinical data and laboratory tests (elevated international normalized ratio and reduced serum albumin levels) and confirmed by liver biopsy or ultrasonography in those with chronic liver disease and positive hepatitis B or C viral markers, history of alcohol use, or metabolic dysfunction-associated steatotic liver disease. The diagnosis of HPS in these patients was based on the inference of pulmonary intravascular dilations by contrast transthoracic echocardiography together with an elevated alveolar-arterial oxygen gradient.
For this study, the medical records of the 121 patients from the previous study were initially assessed to verify their medical appointments and any notification of death. Simultaneously, this list was sent to the State Health Department’s Mortality Information Service to confirm the information. For each patient without a record of care in their chart, a registered message was sent by regular mail. Additionally, three telephone calls were made at different times, and a message via instant messenger (WhatsApp) was sent requesting information about the patient and, if still living, to visit the outpatient clinic.
A descriptive analysis was conducted to reveal the distribution of the variables. Initially, the mortality rate was verified, and Student’s t-test was used to compare the quantitative variables. In the bivariate analysis, logistic models were estimated with death as the dependent variable and sociodemographic and clinical characteristics as the independent variables. The strength of association between the independent and dependent variables was expressed by the odds ratio with its respective 95% confidence interval (CI). Finally, the Kaplan-Meier method was used to calculate the survival and risk curves, and the log-rank test was used to compare the curves. The risk ratio was calculated along with its 95%CI using Cox regression. To evaluate the proportional hazards assumption, Cox models with a time-dependent variable were estimated[17]. All conclusions were drawn at a 5% (chance) significance level. All analyses were conducted using Excel v16.31 and SPSS v25.0. Statistical review was performed by Carlos Feitosa Luna, from Statistics and Geoprocessing Center, Institute Aggeu Magalhães, Oswaldo Cruz Foundation, Recife, Pernambuco, Brazil.
This study was approved by the Research Ethics Committee of the Health Sciences Center of Universidade Federal de Pernambuco (CAAE 66964822.6.0000.5208, No. 5.968.435) on March 28, 2023.
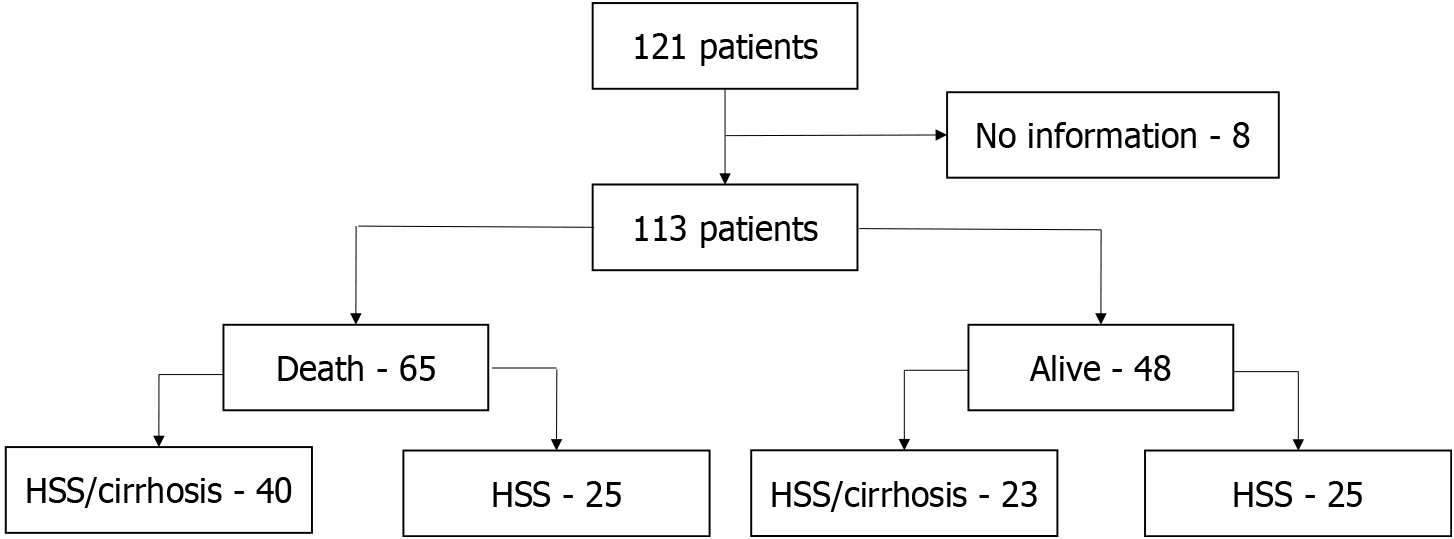
Of the 121 patients with HSS, 8 (6.61%) were excluded due to lack of data (Figure 1). As the study design was a case series, no sample size calculation was performed. The mean age at the time of death or at the end of the study was 63 ± 11.07 years (range, 33-94 years), and most of the participants were men (65/113; 57.5%) and had HSS/cirrhosis (63/113; 55.8%). Meanwhile, HPS was present in 39 (34.5%) participants. The participants were followed-up for 7.12 ± 4.88 years (range, 0.10-13.68 years). Overall, 65 (57.5%; 95%CI: 48%-67%) deaths occurred; most were men (60%) and aged > 50 years (92.3%). Death related to liver disease was noted in 38/65 (58.5%) patients; 24/40 (60%) with HSS/cirrhosis, and 14/25 (56%) with HSS without cirrhosis. When comparing patients who lived with those who died, there were no significant differences in terms of age, gender, cause of liver disease, or presence of HPS (Table 1).
| Characteristics | Death | OR | 95%CI | P value | |
| Yes | No | ||||
| Age (years), mean ± SD | 63.86 ± 9.97 | 61.83 ± 12.43 | 1.02 | 0.98-1.05 | 0.336 |
| Age group | |||||
| Up to 49 | 5 (35.7) | 9 (64.3) | 1.00 | - | - |
| 50-59 | 14 (63.6) | 8 (36.4) | 3.15 | 0.78-12.73 | 0.107 |
| 60-69 | 30 (61.2) | 19 (38.8) | 2.84 | 0.83-9.77 | 0.097 |
| 70-79 | 12 (52.2) | 11 (47.8) | 1.96 | 0.50-7.69 | 0.333 |
| 80 or more | 4 (80.0) | 1 (20.0) | 7.20 | 0.62-83.34 | 0.114 |
| Sex | |||||
| Female | 26 (54.2) | 22 (45.8) | 1.00 | - | - |
| Male | 39 (60.0) | 26 (40.0) | 1.27 | 0.60-2.70 | 0.535 |
| Etiology of liver disease | |||||
| HSS | 25 (50.0) | 25 (50.0) | 1.00 | - | - |
| HSS/cirrhosis | 40 (63.5) | 23 (36.5) | 1.74 | 0.82-3.70 | 0.151 |
| Hepatopulmonary syndrome | |||||
| No | 46 (62.2) | 28 (37.8) | 1.00 | - | - |
| Yes | 19 (48.7) | 20(51.3) | 0.58 | 0.26-1.27 | 0.171 |
Among the 65 patients who died, the average survival was significantly lower in those with HPS when compared to those without HPS (3.37 years vs 5.65 years, P = 0.017) (Table 2). According to the presence of HPS, survival analysis of the 113 patients revealed that half of the patients with and without HPS died within 7.98 years and 8.84 years, respectively, with no significant difference between the two groups (hazard ratios = 1.01, 95%CI: 0.59-1.73; P = 0.967) (Figure 2A). According to the etiology of liver disease (HSS with and without cirrhosis), survival analysis revealed that half of the patients with HSS with and without cirrhosis died within 4.51 years and 12.32 years, respectively. The mean survival was lower in those with HSS/cirrhosis (6.72 years vs 10.05 years, respectively), suggesting that the risk of mortality was twice as high in those with cirrhosis than in those without cirrhosis (hazard ratios = 2.17; 95%CI: 1.31-3.60; P = 0.003) (Figure 2B).
In this study, death occurred in more than half of the participants. Among those who died, the average time to the death was shorter in those with HPS. Survival curves showed that the time for half of the patients to die was three times shorter in the HSS/cirrhosis group compared to the HSS group. Another significant finding was that the survival of patients with HSS/cirrhosis was lower than that of patients with HSS without cirrhosis, and their risk of mortality was twice as high. Interestingly, there were no differences in survival regardless of the presence of HPS.
In a time series study conducted between 2000 and 2019 that evaluated mortality from NTD, schistosomiasis is reportedly the second most frequent cause of death among NTD in Brazil, the country with the highest number of deaths from NTD in Latin America, after Chagas Disease[18]. The authors also highlighted the Northeast region as accounting for more than half of the deaths from schistosomiasis. In our study, patients with HSS had a mortality rate of 57.5% during a follow-up period of 13.68 years. The higher incidence in men and patients aged ≥ 50 years reinforces the findings of an epidemiological study that assessed mortality from NTD. Additionally, the authors underscored the chronic nature of the disease as the reason for the higher mortality in individuals aged > 40 years[18]. In our study, all patients had HSS, which is the more severe form of schistosomiasis; of these patients, more than half had concomitant cirrhosis. Furthermore, there was a long period between HSS diagnosis and assessment for death. All these factors may have contributed to the high mortality rate in our study.
Survival curves revealed that 12 years and 4 years elapsed before for patients with HSS without and with cirrhosis, respectively, died. These results are similar to the findings of other studies that evaluated survival in patients with cirrhosis, showing a high mortality rate within a short follow-up period[6]. These results may also suggest that chronic damage to hepatocytes by viruses or alcohol, along with progression to liver failure, may have contributed to the lower survival of patients with HSS/cirrhosis.
In our study, survival analysis of the entire sample showed that HPS does not increase the risk of death. Even when survival was separately analyzed according to the presence or absence of HPS in patients with HSS with or without cirrhosis, no survival differences were observed. It is possible that the simple size in our study was insufficient to detect any differences. These findings are remarkable as they contradict what is known in the literature, wherein pulmonary vascular complications such as HPS worsen prognosis and increase mortality in patients with cirrhosis. HPS also results in greater severity and may contribute to eventual liver transplantation[12,15,19]. Most studies evaluating mortality in terms of the presence of HPS have focused on patients with cirrhosis who are candidates for liver transplantation[8,16]. Meanwhile, few studies have evaluated survival in patients with chronic liver disease who are not candidates for transplantation, and even fewer have analyzed these patients over a prolonged period[20]. It is possible that the high levels of portal pressure in patients in this study (all of whom had esophageal varices) played a more essential role in the cause of death compared to the presence of pulmonary vascular dilatation and altered gas exchange, which are components of the HPS triad. Furthermore, in this study, approximately 60% of patients who died had a cause of death related to liver disease, which was most likely due to digestive hemorrhage, progression to liver failure with ascites, spontaneous bacterial peritonitis, or encephalopathy. An analysis of the 65 patients who died during the study period showed that when comparing the average time to death in patients with and without HPS, the presence of HPS contributed to an earlier death. Thus, it is possible that the patients who died had higher levels of portal pressure, leading to serious complications such as HPS which could be associated with a shorter time from diagnosis to death.
Supporting the potential association between mortality and higher levels of portal hypertension, a multicenter prospective cohort study involving 85 patients and 146 patients with and without, respectively, showed that those with HPS developed more complications related to portal hypertension and had an increased risk of death[21]. In another study that followed 59 patients with liver cirrhosis for 6 months, 24 (40.67%) of whom had HPS, 27 (45.7%) deaths occurred during the observation period. Of these, 15 and 12 occurred in patients with and without HPS. The researchers concluded that the risk of death was nearly threefold higher in patients with HPS. However, HPS was not associated with increased mortality after adjusting for severity of liver disease, age, and gender[22].
This study has limitations. The study design did not permit causal inference. Furthermore, the absence of prior studies on the survival of patients with schistosomiasis and HPS made it inappropriate to calculate the sample using other possible designs. Another limitation was the retrospective collection of data and use of secondary data from death records. We attempted to improve the quality of death data by confirming them using an official death registration as well as trying to contact patients who were lost to follow-up. Finally, the isolation caused by the corona virus infectious disease-2019 pandemic may have made it difficult to follow-up and locate the eight patients not included in the final analysis.
The results of this showed that HPS does not impact the survival of patients with HSS with and without cirrhosis. It is important to underscore that the patients were not followed over time to ascertain whether those who initially did not have HPS developed it, or whether those with HSS developed cirrhosis during the follow-up period. Future cohort studies are more appropriate for assessing the survival of these patients as a long follow-up period would ensure a better analysis of the dynamics of disease progression and changes in patients’ clinical status. Consequently, more accurate insights should be obtained regarding factors potentially influencing the survival of these patients. Future research should examine potential mechanisms that may explain the unexpected outcomes observed in this study.
This study was conducted more than a decade after a diagnostic investigation for HPS in patients with cirrhotic and non-cirrhotic (schistosomiasis) portal hypertension. Although HSS and concomitant cirrhosis has a higher risk of mortality, there was no difference in survival regardless of the presence of HPS. Future prospective studies with a longer follow-up period and larger sample size are warranted to confirm our findings.
| 1. | World Health Organization. Status of schistosomiasis endemic countries: 2023. [cited 14 July 2024]. Available from: https://apps.who.int/neglected_diseases/ntddata/sch/sch.html?geog=0&indicator=i0&date=2022&bbox=234.07835000000003,62.897000000000006,234.07835000000003,90.59700000000002&printmode=true. |
| 2. | Ministério da Saúde. Monitoramento dos casos de arboviroses até a semana epidemiológica 45 de 2022. [cited 14 July 2024]. Avaliable from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no43/view. |
| 3. | Houlder EL, Costain AH, Cook PC, MacDonald AS. Schistosomes in the Lung: Immunobiology and Opportunity. Front Immunol. 2021;12:635513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Veiga ZST, Fernandes FF, Guimarães L, Piedade J, Pereira GHS. Natural History of Hepatosplenic Schistosomiasis (HSS) Non-Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis. Trop Med Infect Dis. 2023;8:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Malta KK, Palazzi C, Neves VH, Aguiar Y, Silva TP, Melo RCN. Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context. Microorganisms. 2022;10:2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Omar HH. Impact of chronic schistosomiasis and HBV/HCV co-infection on the liver: current perspectives. Hepat Med. 2019;11:131-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hudson D, Cançado GGL, Afzaal T, Malhi G, Theiventhiran S, Arab JP. Schistosomiasis: Hepatosplenic Disease and Portal Hypertensive Complications. Curr Hepatology Rep. 2023;22:170-181. [DOI] [Full Text] |
| 8. | Craciun R, Mocan T, Procopet B, Nemes A, Tefas C, Sparchez M, Mocan LP, Sparchez Z. Pulmonary complications of portal hypertension: The overlooked decompensation. World J Clin Cases. 2022;10:5531-5540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sayadi A, Duhaut L, Robert F, Savale L, Coilly A. [Hepatopulmonary syndrome]. Rev Med Interne. 2024;45:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 508] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 11. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 12. | Raevens S, Boret M, Fallon MB. Hepatopulmonary syndrome. JHEP Rep. 2022;4:100527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 13. | Kovvuri HLR, Karyampudi A, A SK. Hepatopulmonary syndrome. Indian J Gastroenterol. 2023;42:436-437. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Bommena S, Gerkin RD, Agarwal S, Raevens S, Glassberg MK, Fallon MB. Diagnosis of Hepatopulmonary Syndrome in a Large Integrated Health System. Clin Gastroenterol Hepatol. 2021;19:2370-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Premkumar M, Anand AC. Overview of Complications in Cirrhosis. J Clin Exp Hepatol. 2022;12:1150-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K, Kawut SM; Pulmonary Vascular Complications of Liver Disease Study Group. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Cox DR, Ookes D. Analysis of survival. 1st ed. New York: Chapman and Hall/CRC, 1984: 91-104. |
| 18. | Rocha MIF, Maranhão TA, da Frota MMC, de Araujo TKA, Veras E Silva WWS, Sousa GJB, Duarte Pereira ML, de Araujo Filho ACA. [Mortality from neglected tropical ciseases in Brazil in the 21st Century: Analysis of spatial and temporal trends and associated factorsMortalidad por enfermedades tropicales desatendidas en Brasil en el siglo XXI: análisis de tendencias espaciales y temporales y factores asociados]. Rev Panam Salud Publica. 2023;47:e146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Aragon Pinto C, Iyer VN, Albitar HAH, Anderson A, Cajigas H, Simonetto DA, Krowka MJ, DuBrock HM, Gallo de Moraes A. Outcomes of liver transplantation in patients with hepatopulmonary syndrome in the pre and post-MELD eras: A systematic review. Respir Med Res. 2021;80:100852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Pascasio JM, Grilo I, López-Pardo FJ, Ortega-Ruiz F, Tirado JL, Sousa JM, Rodriguez-Puras MJ, Ferrer MT, Sayago M, Gómez-Bravo MA, Grilo A. Prevalence and severity of hepatopulmonary syndrome and its influence on survival in cirrhotic patients evaluated for liver transplantation. Am J Transplant. 2014;14:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 21. | Kawut SM, Krowka MJ, Forde KA, Al-Naamani N, Krok KL, Patel M, Bartoli CR, Doyle M, Moutchia J, Lin G, Oh JK, Mottram CD, Scanlon PD, Fallon MB; Pulmonary Vascular Complications of Liver Disease Study Group. Impact of hepatopulmonary syndrome in liver transplantation candidates and the role of angiogenesis. Eur Respir J. 2022;60:2102304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Younis I, Sarwar S, Butt Z, Tanveer S, Qaadir A, Jadoon NA. Clinical characteristics, predictors, and survival among patients with hepatopulmonary syndrome. Ann Hepatol. 2015;14:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |