Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.102152
Revised: November 23, 2024
Accepted: January 15, 2025
Published online: February 27, 2025
Processing time: 133 Days and 5.6 Hours
Various prognostic scores have been developed to predict mortality and response to steroids in alcoholic hepatitis (AH). We aimed to further validate and compare these scores, particularly pre-day 7 Lille scores, in addition to identifying reliable predictors of complications and mortality such as renal dysfunction and nutritional status.
To identify predictors of complications and mortality in AH, particularly focusing on demographics, renal involvement, underlying liver disease, and nutrition.
This is a retrospective analysis of patients admitted to a large urban tertiary care center with AH from 2020 to 2022. Receiver operating characteristics (ROC) curve analysis was conducted to compare established prognostic scores with Lille scores from day 3 to day 7 (LM3-7). Logistic regression equations were conducted to identify predictor variables.
Severe AH (SAH) as defined by Maddrey’s discriminant function ≥ 32 was diagnosed in 150 out of 425 patients with AH. LM3-7 had 28-day mortality rates in the responder group of 7%-11%, while in the non-responder group, mortality rates were approximately 38%-42%. LM3-7 had 90-day mortality rates in the responder group of 12% to 17%, while in the non-responder group, mortality rates were 48%-53%. Furthermore, all LM3-7 scores showed comparable efficacy in predicting mortality using ROC curve analysis; Area under ROC ranged from 0.771 to 0.802 for 28-day mortality and 0.743 to 0.809 for 90-day mortality. Regarding complications and mortality in AH, significant predictors included poor nutritional status, underlying cirrhosis, and acute renal dysfunction.
LM3-6 is as accurate as LM7 in predicting corticosteroid efficacy for 28-day and 90-day mortality in patients with SAH. Holding glucocorticoids early during the disease course can prevent unnecessary complications.
Core Tip: Our manuscript evaluates the utility of pre-day 7 Lille scores. Calculating the Lille score as early as day 3 can determine the need for glucocorticoids in patients with severe alcoholic hepatitis and potentially limit the side effects of unnecessary steroid therapy. Day 3-7 Lille scores all show comparable efficacy in predicting response to steroids and mortality.
- Citation: Yang K, Nallapeta N, Hossein-Javaheri N, Carlson A, Quigley B, Mahl T. Predictors and prognosticators of outcomes in alcoholic hepatitis: A retrospective single center study. World J Hepatol 2025; 17(2): 102152
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/102152.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.102152
Alcohol consumption can affect the liver in various ways, including but not limited to alcoholic fatty liver disease, alcoholic hepatitis (AH), and cirrhosis. Patients diagnosed with AH typically present with jaundice and history of heavy and recent alcohol use[1]. The term severe AH (SAH) refers to a subset of these patients who present with gross laboratory abnormalities, specifically with a Maddrey’s discriminant function (MDF) ≥ 32. Historically, this cutoff identifies patients who are at high risk of liver decompensation with mortality reaching up to 40% at 6 months[2]. The diagnosis of AH is based on both clinical and laboratory data, including heavy alcohol use > 40 g daily, jaundice in the last 3 months, and an aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio of at least 1.5[3]. As these patients often have poor outcomes, various prognostic models have been devised for risk stratification and guidance of treatment. These include the age-bilirubin-INR-creatinine (ABIC), model for end stage liver disease (MELD), and Glasgow AH score (GAHS)[4]. Furthermore, prior studies have identified specific risk factors for increased mortality in AH, such as acute kidney injury (AKI) and sepsis[5]. However, there is a paucity of data on other prevalent conditions seen in these patients. Consequently, we aimed to identify reliable predictors of complications and mortality in AH, particularly focusing on demographics, renal involvement, underlying liver disease, and nutrition. Specific areas of interest included cirrhosis, hepatorenal syndrome (HRS), and meal intake, among other data points described below. Glucocorticoids are the mainstay of treatment shown to improve 28-day survival in patients with SAH as noted in the Steroids or Pentoxifylline for AH (STOPAH) trial. Steroids are typically used in patients with SAH defined by MDF ≥ 32 without contraindications. Randomized controlled trials have shown a modest survival benefit at 28 days, although assessment of response to steroids usually occurs at 7 days according to the Lille Model (LM)[6]. A cutoff value (CUV) of 0.45 separates responders from non-responders, above which has been associated with a survival rate as low as 25%[7]. Given this data, patients would need to be exposed to 7 days of steroid therapy, which hypothetically includes longer hospitalizations, higher risk of infections, and other iatrogenic complications. This creates a need for earlier prognostic models to limit exposure to futile treatment. Two recent retrospective studies have evaluated the prognostic value of a day 4 Lille score (LM4) and have shown comparable accuracy in predicting 28-day and 90-day mortality[8,9]. However, these studies were limited by size and design, and would benefit from further validation. We aimed to assess the prognostic value of not just LM4, but Lille scores from day 3 to day 7 (LM3-7), with comparison to other established prognostic scores. Identification of non-responders should occur quickly and in a standardized fashion, to minimize unnecessary treatment and to guide alternative therapy.
This study is a retrospective analysis of patients admitted to Erie County Medical Center, a large urban tertiary care center, with AH from 2020 to 2022. The study protocol was approved by the appropriate institutional review board prior to commencement. All patients included in the study had jaundice within the last 3 months, alcohol use > 40 g daily, abstinence < 2 months, and suggestive liver chemistries. A subgroup of patients with SAH as defined by MDF ≥ 32 was separately analyzed. Data was extracted from the electronic medical record based on ICD-10 codes and patient charts were manually reviewed. Exclusion criteria were patients without a history of recent and heavy alcohol use, those with hepatocellular carcinoma or end-stage renal disease, and age < 18 and > 85.
Demographic data recorded were age, sex, race, and body mass index. Clinical and laboratory data collected on admission were history of cirrhosis, hepatitis C (HCV), hepatitis B, diabetes mellitus, hyperlipidemia, hypertension, chronic kidney disease (CKD), daily alcohol intake, AST, ALT, albumin, bilirubin, blood urea nitrogen (BUN), creatinine, international normalized ratio, prothrombin time (PT), sodium, and white blood cell count (WBC). Recorded complications during hospitalization included hepatic encephalopathy (HE), gastrointestinal bleeding (GIB), AKI, HRS, ascites, and bacterial infections. AKI was defined by > 1.5x or > 0.3 increase in baseline creatinine. Patients were screened for infections including chest x-ray, urinalysis, blood and urine cultures, and ascites fluid analysis when applicable. Other data points collected during chart review included length of stay (LOS), readmissions for alcohol related liver disease, nutrition consults, daily mean percentage meal intake (MPMI) during hospitalization, and mortality at 28 and 90 days. Laboratory data corresponding to day 3 to day 7 Lille scores were documented for patients receiving corticosteroids for SAH. All patients who fit the criteria for SAH were divided into those who did or did not receive corticosteroid therapy. All patients with SAH, who were candidates for corticosteroid therapy, received 40 mg of prednisolone/prednisone daily for up to 28 days, or until time of discontinuation. Lille scores were calculated for day 3 to day 7 biochemical data, with the formula obtained from www.lillemodel.com. Scores were also calculated for MDF, ABIC, MELD, and GAHS.
Primary endpoints of this study were mortality at 28 and 90 days. Secondary outcomes were analyzed as well, such as LOS and complications during hospitalization. Data were described as mean for continuous variables and number (%) for discrete variables. A series of logistic regression equations were conducted using a backwards step procedure such that each step removed the predictor variable accounting for the least variability until only those predictor variables that showed a significant relationship remained in the equation. In each analysis, the outcome variable of interest was regressed on demographic data, medical history, and clinical/biochemical data. Crosstabs were conducted to examine the relationships among certain independent and dependent variables as well. Area under receiver operating characteristic (AUROC) curve analysis was conducted to determine if ABIC, GAHS, MELD, MDF, and LM3 to LM7 scores predicted mortality at 28 and 90 days. Models with an AUROC ≥ 0.7 were considered to have good accuracy. Furthermore, 95% confidence intervals were calculated for each predictive test, and P < 0.05 was considered significant for each statistical test.
There were 425 patients on admission who fulfilled criteria for AH. SAH as defined by MDF ≥ 32 was diagnosed in 150/425 patients, out of which 126 received corticosteroid therapy. The most common contraindications to prednisolone were sepsis, septic shock, severe GIB, and extrahepatic organ failure. The baseline demographics and clinical data are described in (Table 1). The total group and the steroid group (SG) were largely similar in terms of demographics. When comparing complications between the total group and the SG, the latter had higher rates of HE, GIB, AKI, HRS, ascites, and bacterial infection. The SG also had longer LOS and higher mortality at both 28 and 90 days. We looked at predictors of outcomes for all patients admitted with AH. Specific areas examined were demographics and underlying cirrhosis, nutritional status, and renal involvement (Table 2).
| Full sample mean (SD/%) | Steroid sub-sample mean (SD/%) | |
| N | 425 | 126 |
| Age | 47.97 (11.95) | 49.17 (10.95) |
| Sex (female) | 32.0 | 30.2 |
| Race | White: 70.8; Black: 14.8; Other: 14.4 | White: 78.6; Black: 7.1; Other: 14.3 |
| BMI | 27.68 (6.63) | 29.17 (7.84) |
| History of cirrhosis | 56.7 | 86.5 |
| History of hep C | 13.6 | 12.7 |
| History of hep B | 2.4 | 1.6 |
| History of diabetes | 17.2 | 19.0 |
| History of hyperlipidemia | 24.7 | 19.0 |
| History of hypertension | 63.1 | 61.1 |
| History of chronic kidney disease | 9.4 | 11.1 |
| Length of stay (days) | 10.11 (8.43) | 14.44 (9.56) |
| Encephalopathy | 34.6 | 52.4 |
| GI bleeding | 19.8 | 30.2 |
| Acute kidney injury | 22.6 | 32.5 |
| Hepatorenal syndrome | 8.7 | 17.5 |
| Ascites | 24.0 | 43.7 |
| Infection | 30.2 | 42.1 |
| Readmission | 52.0 | 54.0 |
| Mortality at 28 days | 11.8 | 19.8 |
| Mortality at 90 days | 15.3 | 27.0 |
| Nutrition consult | 26.6 | 37.3 |
| Albumin day 1 | 3.55 (0.91) | 3.07 (0.74) |
| Albumin change day 1 to day 7 | No data | -0.25 (0.63) |
| Daily mean percent meal intake | 64.93 (25.62) | 55.05 (25.41) |
| Grams of alcohol per day | 210.25 (165.97) | 228.15 (174.79) |
| Standard drinks per day | 15.01 (11.85) | 16.30 (12.48) |
| Factor | Encephalopathy | GI bleeding | Acute kidney injury | Hepatorenal syndrome | Ascites | Infection | Readmission | 28 days mortality | 90 days mortality | Length of stay |
| Age | NS | NS | OR = 1.40, P = 0.002 (95%CI: 1.14-1.72) | NS | OR = 1.41, P < 0.001 (95%CI: 1.15- 1.73) | NS | NS | NS | NS | B = 0.12, P = 0.001 t = 3.27 (95%CI: 0.05-0.18) |
| Female sex | NS | NS | NS | NS | NS | OR = 2.12, P = 0.001 (95%CI: 1.34-3.35) | NS | NS | NS | NS |
| Black race | OR = 0.50, P = 0.037 (95%CI: 0.26-0.96) | OR = 0.41, P = 0.048 (95%CI: 0.17-0.99) | NS | NS | NS | OR = 0.20, P < 0.001 (95%CI: 0.08-0.49) | NS | NS | NS | NS |
| History of cirrhosis | OR = 3.86, P < 0.001 (95%CI: 2.45-6.08) | OR = 5.83, P < 0.001 (95%CI: 3.08-10.99) | OR = 2.75, P < 0.001 (95%CI: 1.65-4.58) | OR = 15.60, P < 0.001 (95%CI: 3.69-65.86) | NA | OR = 3.15, P < 0.001 (95%CI: 1.98-5.02) | NS | OR = 10.88, P < 0.001 (95%CI: 3.81-31.05) | OR = 7.98, P < 0.001 (95%CI: 3.53-18.04) | NS |
| Daily alcohol intake | NS | NS | NS | NS | NS | NS | OR = 1.15, P = 0.004 (95%CI: 1.05-1.27) | NS | NS | NS |
| History of chronic kidney disease | NS | NS | NS | NS | NS | NS | NS | NS | NS | B = 3.90, P = 0.005 t = 2.83 (95%CI: 1.19-6.60) |
| Acute kidney injury | OR = 1.79, P = 0.046 (95%CI: 1.01-3.15) | OR = 2.30, P = 0.002 (95%CI: 1.36-3.93) | NA | NA | OR = 4.10, P < 0.001 (95%CI: 2.26-7.46) | OR = 1.95, P = 0.024 (95%CI: 1.09-3.48) | NS | OR = 5.16, P < 0.001 (95%CI: 2.21-12.02) | OR = 3.94, P < 0.001 (95%CI: 1.86-8.37) | NS |
| Hepatorenal syndrome | OR = 3.22, P = 0.009 (95%CI: 1.34-7.70) | NS | NA | NA | OR = 3.67, P = 0.004 (95%CI: = 1.51- 8.95) | OR = 2.89, P = 0.015 (95%CI: 1.23-6.79) | OR = 0.07, P < 0.001 (95%CI: 0.03- 0.28) | OR = 9.09, P < 0.001 (95%CI: 3.52-23.51) | OR = 15.17, P < 0.001 (95%CI: 5.43-42.37) | NS |
| Nutrition consult | NS | OR = 0.44, P = 0.013 (95%CI: 0.23-0.84) | OR = 2.03, P < 0.001 (95%CI: 1.14-3.62) | NS | NA | NS | OR = 2.49, P < 0.001 (95%CI: 1.56-3.68) | NS | NS | NS |
| Percentage meal intake | OR = 0.86, P < 0.001 (95%CI: 0.78-0.94) | NS | OR = 0.79, P < 0.001 (95%CI: 0.71-0.88) | OR = 0.49, P < .001 (95%CI: 0.38-0.63) | NA | OR = 0.86, P = 0.002 (95%CI: 0.78-0.94) | NS | OR = 0.50, P < 0.001 (95%CI: 0.40-0.62) | OR = 0.54, P < 0.001 (95%CI: 0.46-0.65) | NS |
| Albumin day 1 | OR = 0.56, P < 0.001 (95%CI: 0.43-0.73) | OR = 0.36, P < 0.001 (95%CI: 0.26-0.50) | OR = 0.51, P < 0.001 (95%CI: 0.36-0.71) | OR = 0.14, P < 0.001 (95%CI: 0.05-0.34) | NA | OR = 0.47, P < 0.001 (95%CI: 0.35-0.63) | OR = 1.32, P = 0.016 (95%CI: 1.05-1.64) | OR = 0.17, P < 0.001 (95%CI: 0.08-0.37) | OR = 0.19, P < 0.001 (95%CI: 0.11-0.36) | NS |
In the first analysis examining encephalopathy as the outcome, significant predictors were non-black race, history of cirrhosis, lower MPMI, lower albumin at day 1, AKI, and HRS. Notably, among those without a history of cirrhosis, 19.6% developed encephalopathy, while 46.1% of cirrhotic patients developed encephalopathy. For every 10% increase in MPMI, patients were 1.17 times less likely to suffer HE. For every one unit increase in albumin, patients were 1.8 times less likely to suffer HE. In the analysis examining GIB as the outcome, significant predictors were non-black race, history of cirrhosis, lower day 1 albumin, lack of nutrition consultation, and AKI. Once again, history of cirrhosis had the greatest effect, with those patients being 5.8 times more likely to develop GIB. 30.2% of all patients with AH developed a bacterial infection while hospitalized, whereas the SG had an infection rate of 42.1%. When examining infection, significant predictors were female sex, nonblack race, history of cirrhosis, lower day 1 albumin, lower MPMI, AKI, and HRS. Females were 2.1 times more likely than males to have an infection. Among those without a history of cirrhosis, 17.5% developed an infection, while 39.8% of cirrhotic patients developed an infection. Analyzing AKI as the outcome, risk factors were older age, history of cirrhosis, nutrition consultation, lower day 1 albumin, and lower MPMI. For every 10-year increase in age, patients were 1.4 times more likely to have AKI. Among those with AKI, mean age was 51.25 years (SD = 12.14), while among those without AKI mean age was 47.01 years (SD = 11.74), t (423) = -3.09, P = 0.002. For every 10% increase in MPMI, patients were 1.3 times less likely to have an AKI. For every one unit increase in albumin, patients were 2 times less likely to have an AKI. Moreover, cirrhotic patients were 2.7 times more likely to have an AKI. For HRS, significant predictors were history of cirrhosis, lower albumin at day 1, and lower MPMI. Among those with no history of cirrhosis, 1.1% suffered HRS, while 14.5% of patients with cirrhosis suffered HRS. Other important secondary outcomes included LOS and readmission rates. Higher daily alcohol intake, nutrition consultation, higher day 1 albumin, and absence of HRS predicted a subsequent readmission for alcohol-related liver disease. Patients with HRS were 10.4 times less likely to be readmitted, likely since mortality was not accounted for. For LOS, significant predictors were older age, history of CKD, and AKI. Among those with a history of CKD, mean LOS was 14.28 days (SD = 10.70), while among those without CKD, mean hospital stay was 9.68 days (SD = 8.05), t (423) = -3.32, P < 0.001. Ultimately examining mortality at 28 and 90 days, the significant risk factors were identical and included history of cirrhosis, lower day 1 albumin, lower MPMI, AKI, and HRS. By 28 days, those with a history of cirrhosis were 10.9 times more likely to die. For every 10% increase in MPMI, patients were 2 times less likely to die. For every one unit increase in albumin, patients were 5.8 times less likely to die. Among those surviving, MPMI was 69.53 (SD = 21.91), while among those who died, MPMI was 25.59 (SD = 21.06), t (409) = 12.49, P < 0.001. Among those who survived, mean albumin was 3.69 (SD = 0.85), while among those who died, mean albumin was 2.50 (SD = 0.64), t (423) = 9.48, P < 0.001. Patients with AKI were 5.2 times more likely to die within 28 days, and those with HRS were 9.1 times more likely. The 90-day mortality rate for cirrhotic patients was 23.7%, vs 4.3% for those without cirrhosis. For every 10% increase in MPMI, patients were 1.8 times less likely to die, and for every one unit increase in albumin, patients were 5.2 times less likely to die. Among those with AKI, 44.8% passed away within 90 days, while only 6.7% of those without AKI died. Those with HRS were 15.2 times more likely to die within 90 days. 81.1% of those with HRS had died within 90 days vs only 9.0% of those without HRS.
LM scores for days 3 to 7 had similar means (0.34-0.35) and ranges (0.00 to 0.98). LM scores for each day identified approximately two thirds of the sample as responders (high 68.3% LM5, low 64.3% LM7) (Table 3). Daily LM scores showed high agreement, ranging from a low agreement rate of 79% (k = 0.70) between LM3 and LM7 to a high 99% (k = 0.95) agreement between LM4 and LM5. Average overall agreement was 91.7% (k =0.82) (Table 4). For SAH, there was a 19.8% 28-day mortality rate; however, the rates for responders and non-responders to treatment differed highly, and the difference varied depending on which index of response was used (Table 5). LM3-7 all had 28-day mortality rates in the responder group of approximately 10%, while in the non-responder group, mortality rates were approximately 40%. All 5 days on which LM was examined showed odds ratios indicating that lack of response was associated with a 5 to 9 times greater chance of mortality by 28 days. Additionally, there was a 27% 90-day mortality rate; however, like the 28-day mortality rates, the mortality rates for responders and non-responders to treatment differed highly and the difference varied depending on which index of response was used. LM3-7 all had 90-day mortality rates in the responder group of approximately 12% to 17%, while in the non-responder group, the mortality rates were approximately 50%. All 5 days on which LM was examined showed odds ratios indicating that lack of response was associated with 4.5 to 8 times greater chance of mortality by 90 days.
| Responders (%) | Non-responders (%) | Mean (SD) | Min/max | |
| LM3 | 84 (66.7) | 42 (33.3) | 0.35 (0.30) | 0.00/0.98 |
| LM4 | 85 (67.5) | 41 (32.5) | 0.35 (0.31) | 0.00/0.99 |
| LM5 | 86 (68.3) | 40 (31.7) | 0.34 (0.31) | 0.00/0.99 |
| LM6 | 85 (67.5) | 41 (32.5) | 0.34 (0.32) | 0.00/0.98 |
| LM7 | 81 (64.3) | 45 (35.7) | 0.35 (0.32) | 0.00/0.98 |
| LM4 | LM5 | LM6 | LM7 | |
| LM3 (%) | k = 0.87, 98 | k = 0.82, 92 | k = 0.70, 78 | k = 0.70, 79 |
| LM4 (%) | k = 0.95, 99 | k = 0.82, 92 | k = 0.79, 89 | |
| LM5 (%) | k = 0.87, 98 | k = 0.84, 95 | ||
| LM6 (%) | k = 0.86, 97 |
| 28 days mortality | 90 days mortality | |||||
| Responders | Non-responders | Odds ratio (95%CI) | Responders | Non-responders | Odds ratio (95%CI) | |
| LM3 (%) | 11 | 38 | 5.12 (2.02-13.00) | 17 | 48 | 4.55 (1.97 -10.47) |
| LM4 (%) | 11 | 39 | 5.40 (2.15-13.74) | 16 | 49 | 4.83 (2.09 -11.17) |
| LM5 (%) | 10 | 40 | 5.70 (2.24-14.55) | 16 | 50 | 5.14 (2.21 -11.96) |
| LM6 (%) | 9 | 41 | 6.81 (2.62-17.75) | 15 | 51 | 5.82 (2.48 -13.62) |
| LM7 (%) | 7 | 42 | 9.14 (3.29-25.34) | 12 | 53 | 8.11 (3.35 -19.64) |
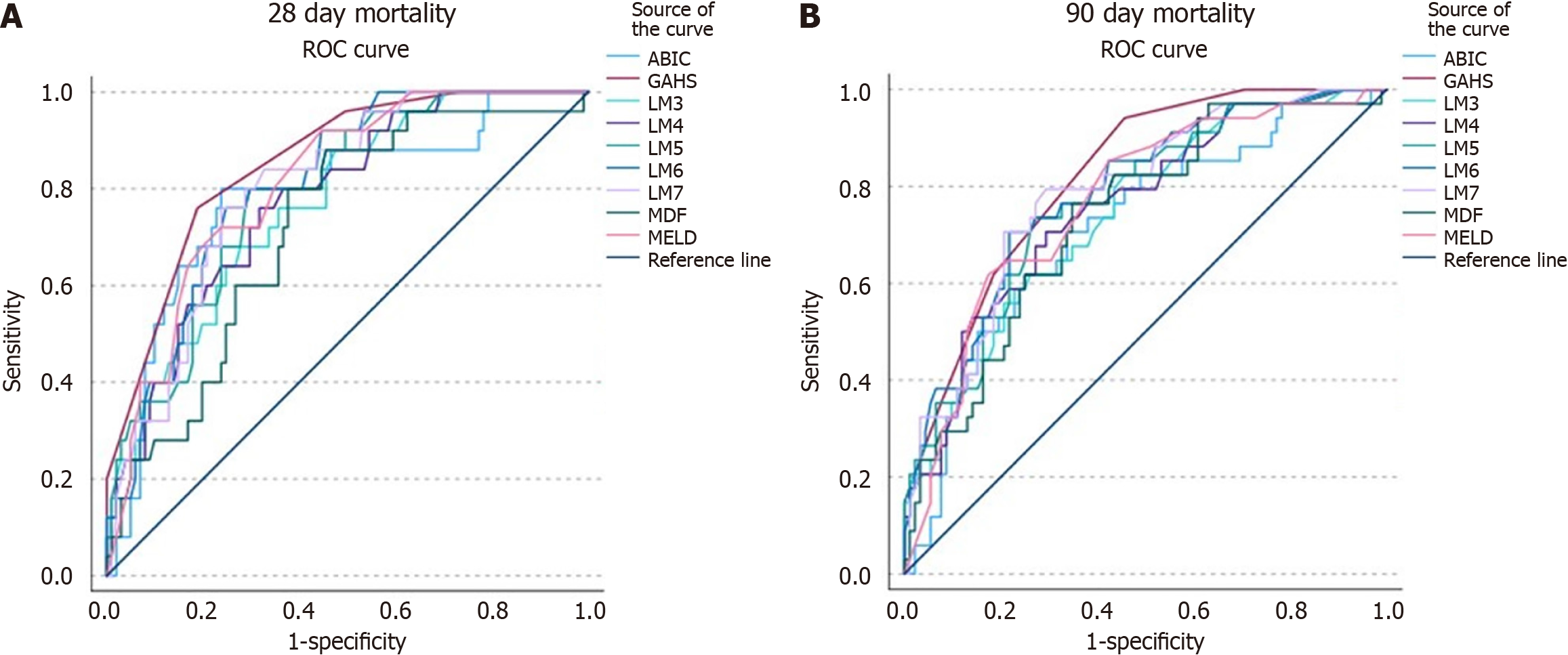
Using established CUVs (ABIC > 9, GAHS ≥ 9, MELD ≥ 21, MDF > 32, and LM ≥ 0.45), scores were compared based on the AUROC to determine best predictor of mortality (Figure 1). The AUROC for LM3-7 for 28-day mortality was 0.771, 0.778, 0.791, 0.809, and 0.802 in consecutive order (Table 6). The AUROC was 0.784 for ABIC, 0.855 for GAHS, 0.719 for MDF, and 0.812 for MELD. The LM3-7 AUROC for 90-day mortality was 0.741, 0.743, 0.756, 0.770, 0.787, 0.779, consecutively. Additionally, the AUROC was 0.784 for ABIC, 0.855 for GAHS, 0.719 for MDF, and 0.744 for MELD. In both 28-day and 90-day mortality, the best performer was the GAHS, which considers age, WBC, BUN, bilirubin, and PT. Furthermore, all LM3-7 scores showed comparable efficacy in predicting mortality.
| Test | 28 mortality | 90 days mortality | ||
| Area under ROC curve | 95%CI | Area under ROC curve | 95%CI | |
| LM3 | 0.771 | 0.676-0.866 | 0.743 | 0.649-0.837 |
| LM4 | 0.778 | 0.685-0.871 | 0.756 | 0.663-0.849 |
| LM5 | 0.791 | 0.703-0.880 | 0.770 | 0.680-0.860 |
| LM6 | 0.809 | 0.727-0.891 | 0.809 | 0.727-0.891 |
| LM7 | 0.802 | 0.718-0.886 | 0.785 | 0.698-0.872 |
| MDF | 0.719 | 0.614-0.824 | 0.735 | 0.640-0.831 |
| ABIC | 0.784 | 0.680-0.888 | 0.713 | 0.611-0.815 |
| GAHS | 0.855 | 0.780-0.930 | 0.820 | 0.745-0.894 |
| MELD | 0.81 | 0.729-0.895 | 0.767 | 0.676-0.858 |
In this study, we present pertinent factors that predicted outcomes in hospitalized patients with AH. They include underlying cirrhosis, development of AKI/HRS, and poor nutritional status. In patients with underlying liver disease such as cirrhosis or HCV, diagnosing AH can be challenging since there is often overlap between clinical and biochemical data for each disease. Additionally, these conditions frequently occur simultaneously. Obtaining a liver biopsy may be helpful in suspected AH with comorbid liver disease, but this is often unnecessary and rarely changes the diagnosis[10]. While prior studies on AH did not usually differentiate underlying cirrhosis, we specifically looked at cirrhosis as a predictor of outcomes in AH. Indeed, cirrhotic patients in our study were more likely to develop all types of complications such as HE, GIB, AKI, HRS, infection, and they were more likely to die by the 28-day and 90-day endpoints. Critically ill patients with AH who develop renal failure have increased mortality, as shown in one study[5]. Most studies including steroid use for SAH excluded patients with abnormal creatinine in the setting of AKI or CKD. It is unclear how patients with impaired renal function respond to steroid therapy for SAH. We aimed to explore this relationship by studying complications and outcomes of patients who developed AKI or HRS during their hospitalization for AH, as well as pre-existing CKD on admission. Our results showed that development of both AKI and HRS portended much worse outcomes in AH. Interestingly, history of CKD was only a significant predictor for longer LOS, and it did not affect other outcomes such as mortality. A prior study also demonstrated that decreased survival in the setting of AKI has been linked to higher rates of infections, which our data supports[11]. Therefore, it may be beneficial to terminate corticosteroid therapy if significant renal dysfunction occurs during hospitalization. In one study on patients with AH, severe protein calorie malnutrition was evident in almost all patients[12]. Nutritional support is essential for individuals with AH due to several reasons. Firstly, excessive alcohol consumption can interfere with nutrient absorption, increase nutrient excretion, and impair liver function. Secondly, indices of malnutrition correlate closely with clinical severity of liver disease[13]. While nutrition should be encouraged for these patients, invasive enteral nutrition does not actually improve mortality[14]. Even so, our retrospective study confirmed that greater nutritional status, defined here as higher albumin levels on admission and higher MPMI, was associated with improved outcomes including 28-day and 90-day mortality. When looking at our patients with SAH, we were able to demonstrate that all LM scores from day 3 to day 7 of corticosteroid therapy showed comparable efficacy in predicting mortality, as evidenced by their similar AUROC graphs. This substantiates previous studies that have suggested LM4 is as accurate as LM7 in predicting short term mortality. It is imperative to limit steroid use when contraindicated and to reduce risk of infection. Additionally, we note that the group receiving glucocorticoids for SAH had more infections when compared to those who did not receive steroids. Utilizing the LM earlier may be able to decrease preventable iatrogenic complications, LOS, and unnecessary treatment. In our study, other prognostic scores for 28 and 90-day mortalities were all comparable as well, although the GAHS was most predictive, which was initially proposed in 2005. Regardless, corticosteroids are the main treatment for SAH at this time. With a Lille score < 0.45 and no contraindications, undergoing 28 days of therapy with prednisolone is generally recommended[15]. Theoretically, corticosteroids reduce pro-inflammatory cytokines such as tumor necrosis factor-alpha and increase anti-inflammatory cytokines such as interleukin-10 and interleukin-6, which may tamper inflammation in SAH[16]. However, many patients are non-responders, and this study may help shift the paradigm that 7 days of steroid therapy are needed to assess for response. Furthermore, steroid therapy in SAH is generally contraindicated in patients with active infection, especially for septic patients. According to our results, a greater percentage of patients who underwent steroid therapy developed infections. Respiratory infection, spontaneous bacterial peritonitis, bacteremia, and urinary tract infection are among the most reported complications historically, which may be associated with an increased risk of mortality[17]. Moreover, use of steroids in patients with active GIB, acute pancreatitis, psychosis, uncontrolled hyperglycemia, and renal failure should be avoided. Despite their hepatoprotective properties, the efficacy of corticosteroid use in SAH has been conflicting. The STOPAH trial included 1103 patients with SAH who were randomized to placebo, prednisolone, pentoxifylline, or both prednisolone and pentoxifylline, and showed a trend towards improving 28-day mortality in the steroid arm that did not reach significance[18]. One meta-analysis in 2011 examined 5 trials involving 221 patients showed that glucocorticoid therapy was associated with a 20% mortality rate compared to 34% for placebo[7]. However, a subsequent study in 2017 involving 15 randomized trials including 1861 patients showed that mortality was not significantly different between patients treated with glucocorticoids and placebo[19]. Long term mortality data on SAH is much more limited, although have not shown mortality benefit[20]. Additional therapies with N-acetylcysteine have demonstrated improved one-month mortality if used as an adjuvant therapy to corticosteroids[21]. Although several other therapeutic agents are under investigation, none are yet approved for the management of AH[22]. Therefore, maximizing the benefits of current therapies may be the best approach to treat SAH at this time.
In conclusion, our study showed that LM3-6 is as accurate as LM7 in predicting corticosteroid efficacy for 28-day and 90-day mortality in patients with SAH. The decision to continue or hold glucocorticoids early during the disease course can prevent unnecessary treatment and medication-associated complications. However, our study is not free of limitation. Sample size may have resulted in decreased statistical power and statistical significance. Given the retrospective nature of the study based on a single institution registry and in the absence of a randomized-control cohort, interpretation of the data might have been influenced by biases. However, the authors of this study have tried to avoid any potential biases including sampling and selection biases. In addition, more than half of patients had a diagnosis of cirrhosis which would have made them more susceptible to in-hospital complications such as infection, encephalopathy, and ascites. As such, it is possible to argue that cirrhosis might independently influence LM3-7. Therefore, future studies should assess LM in two distinct groups of patients with and without cirrhosis for more accurate analysis. Finally, most patients did not have a liver biopsy to confirm AH; however, strict clinical criteria were used to diagnose SAH on admission and alternative diagnoses were ruled out.
| 1. | Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] [DOI] [Full Text] |
| 3. | Sharma P, Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl Gastroenterol Hepatol. 2020;5:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, Singhal S, Brind A, Haydon G, O'Grady J, Day CP, Hayes PC, Murray LS, Morris AJ. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Altamirano J, Fagundes C, Dominguez M, García E, Michelena J, Cárdenas A, Guevara M, Pereira G, Torres-Vigil K, Arroyo V, Caballería J, Ginès P, Bataller R. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65-71.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, Boleslawski E, Deltenre P, Canva V, Pruvot FR, Mathurin P. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 513] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 7. | Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 8. | Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J, Chavez-Araujo R, Prado V, de Lourdes Candolo-Martinelli A, Holanda-Almeida P, Becerra-Martins-de-Oliveira B, Fernandez-de-Almeida S, Bataller R, Caballeria J, Duarte-Rojo A. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol. 2017;112:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Foncea CG, Sporea I, Lupușoru R, Moga TV, Bende F, Șirli R, Popescu A. Day-4 Lille Score Is a Good Prognostic Factor and Early Predictor in Assessing Therapy Response in Patients with Liver Cirrhosis and Severe Alcoholic Hepatitis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Forrest E, Petts G, Austin A, Lloyd K, Wright M, Vergis N, Atkinson S, Masson S, Patch D, Quaglia A, Thursz M, Goldin R. The diagnostic and prognostic significance of liver histology in alcoholic hepatitis. Aliment Pharmacol Ther. 2021;53:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Sujan R, Cruz-Lemini M, Altamirano J, Simonetto DA, Maiwall R, Axley P, Richardson T, Desai V, Cabezas J, Vargas V, Kamath PS, Shah VH, Sarin SK, Bataller R, Singal AK. A Validated Score Predicts Acute Kidney Injury and Survival in Patients With Alcoholic Hepatitis. Liver Transpl. 2018;24:1655-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 260] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220-231. [PubMed] |
| 14. | Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, Hittelet A, Piquet MA, Laleman W, Orlent H, Lasser L, Sersté T, Starkel P, De Koninck X, Negrin Dastis S, Delwaide J, Colle I, de Galocsy C, Francque S, Langlet P, Putzeys V, Reynaert H, Degré D, Trépo E. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. 2016;150:903-10.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Saberi B, Dadabhai AS, Jang YY, Gurakar A, Mezey E. Current Management of Alcoholic Hepatitis and Future Therapies. J Clin Transl Hepatol. 2016;4:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27 Suppl 2:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, Mathurin P. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O'Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 558] [Article Influence: 55.8] [Reference Citation Analysis (1)] |
| 19. | Pavlov CS, Varganova DL, Casazza G, Tsochatzis E, Nikolova D, Gluud C. Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database Syst Rev. 2017;11:CD001511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, O'Grady JG, Akriviadis E, Sinakos E, Carithers RL Jr, Ramond MJ, Maddrey WC, Morgan TR, Duhamel A, Mathurin P. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018;155:458-468.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 21. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 22. | German MN, Musto J, Lucey MR. Novel treatments for alcoholic hepatitis. Curr Opin Gastroenterol. 2021;37:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |