Published online Feb 27, 2025. doi: 10.4254/wjh.v17.i2.101741
Revised: November 28, 2024
Accepted: December 25, 2024
Published online: February 27, 2025
Processing time: 148 Days and 6.7 Hours
Acute drug-induced liver injury (DILI) events caused by chronic liver disease are relatively common. Some researchers believe that nonalcoholic fatty liver (NAFL) increases the overall risk of DILI. The clinical characteristics and prognosis of DILI in the context of NAFL disease (NAFLD) are still unclear. Therefore, hospitalized patients with NAFLD combined with DILI at the Tianjin Second People's Hospital were included in this study. The clinical manifestations, classifications, severities, laboratory indicators, and clinical outcomes of the enrolled patients were analyzed, and the clinical characteristics and prognoses of the NAFL + DILI patients were evaluated.
To investigate the clinical characteristics and prognosis of DILI in the context of NAFL.
Eighty-nine patients diagnosed with DILI and 110 patients diagnosed with both DILI and NAFL at the Tianjin Second People's Hospital were enrolled. Clinical data, including demographic characteristics, clinical features, laboratory test results, pathology findings, autoantibody titers, suspected drugs, and outcomes, were collected from the two groups of patients. All enrolled patients were followed up to determine the liver function recovery time.
Compared with the patients in the DILI group, those in the NAFL + DILI group had higher body mass indices; Controlled Attenuation Parameter scores; and triglyceride, total cholesterol, low-density lipoprotein, and insulin levels. The levels of the cytokines interleukin-4 and complement complement c3 (C3) were also greater in the NAFL + DILI group than in the DILI group. The proportions of patients with cholestatic-type DILI (16.4% vs 4.5%), cholestasis seen on pathoscopy (40.9% vs 25.8%), grade 2 or above DILI (48.18% vs 40.45%), and a recovery time for liver function ranging from 90 to 180 days (30.6% vs 15.5%) were greater in the NAFL + DILI group than in the DILI group. All of the abovementioned differences between the groups were statistically significant (P < 0.05). The autoantibody positivity rates did not significantly differ between the two groups (P > 0.05), and the proportions of patients who progressed to chronic drug hepatitis or autoimmune hepatitis were not significantly different between the two groups (both P > 0.05).
In the context of NAFL, DILI is more likely to be cholestatic, with a greater degree of liver injury, a longer recovery time, and more pronounced expression of immune factors.
Core Tip: In this retrospective study, we explored the clinical features and prognosis of patients with drug-induced liver injury (DILI) in the setting of nonalcoholic fatty liver (NAFL). We recruited a total of 199 patients diagnosed with DILI by clinical and biochemical indicators. The number of patients diagnosed with NAFL by pathologic biopsy, ultrasound, and computed tomography was 110, and the number of patients without NAFL was 89. DILI resulting from NAFL had more pronounced metabolic index abnormalities, was more prone to cholestasis, had a greater degree of hepatic injury, had a longer recovery time, and may involve more pronounced immunologic factors.
- Citation: Zhao Y, Li JZ, Liu YG, Zhu YJ, Zhang Y, Zheng WW, Ma L, Li J, Wang CY. Clinical features and prognosis of drug-induced liver injury in patients with non-alcoholic fatty liver. World J Hepatol 2025; 17(2): 101741
- URL: https://www.wjgnet.com/1948-5182/full/v17/i2/101741.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i2.101741
Drug-induced liver injury (DILI) is a serious and common side effect of prescription and nonprescription drugs. DILI occurs when drugs, including chemical drugs, biological products, Chinese patent medicines, traditional Chinese medicines, natural products, health products, dietary supplements, other metabolic products, excipients, contaminants, and impurities, cause damage to the liver. More than 1000 drugs have been reported to cause liver injury, and the annual incidence rate of DILI in China is increasing and is currently at least 23.8 per 100000 people[1].
Nonalcoholic fatty liver (NAFL) disease (NAFLD) is one of the most common chronic liver diseases worldwide and is closely related to metabolic syndrome and cardiovascular diseases. Recent research has shown that the prevalence of NAFLD in the adult population in Asia is 30.5%[2]. NAFLD is often associated with hyperlipidemia, hypertension, and type 2 diabetes. Some individuals with NAFLD inevitably experience DILI when they receive treatment for other diseases.
Acute DILI events in patients with chronic liver disease are relatively common[3]. In China, approximately 23% of DILI patients have chronic liver disease, mainly chronic hepatitis C and NAFLD. Some researchers believe that NAFL increases the overall risk of DILI[4]; however, the risk of DILI is not increased by statin drugs in chronic liver disease patients, including those with NAFLD. The clinical characteristics and prognosis of DILI in the context of NAFLD are still unclear. Therefore, hospitalized patients with NAFLD combined with DILI at the Tianjin Second People's Hospital were included in this study. The clinical manifestations, classifications, severities, laboratory indicators, and clinical outcomes of the enrolled patients were analyzed, and the clinical characteristics and prognoses of the NAFL + DILI patients were evaluated. This study provides a basis for the clinical diagnosis and treatment of this patient population.
A substantial number of studies were previously conducted from a singular perspective of heightened risk of DILI. However, this work addresses the limitations of this single perspective by examining DILI in the context of NAFL through a comprehensive array of clinical data, biochemical indices, and liver biopsy results. Additionally, this work fills a crucial data gap in the study of DILI in the context of chronic liver disease over the past five years.
In total, 199 patients who were diagnosed with DILI through clinical and biochemical indicators from January 2018 to March 2023 at the Tianjin Second People's Hospital were included, among which 89 patients were diagnosed with DILI only, and 110 patients were diagnosed with NAFL combined with DILI. The diagnosis of DILI referred to the “Guidelines for the Diagnosis and Treatment of Pharmacologic Liver Injury”[5]. The diagnosis of simple fatty liver refers to the “Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China)”[6]. In this study, the diagnosis of simple fatty liver was confirmed mainly via pathological biopsy, and a small portion of the patients were diagnosed via ultrasound or computed tomography. Simple fatty liver is an early manifestation of NAFLD, as confirmed by pathological diagnosis, with macrovesicular or macrovesicular predominant steatosis involving more than 5% of hepatocytes, which may be accompanied by mild nonspecific inflammation. The exclusion criteria were as follows: (1) Had viral liver diseases, including hepatitis A virus, hepatitis E virus, hepatitis B virus, hepatitis C virus, cytomegalovirus, or Epstein–Barr virus infection; (2) Had other coexisting liver diseases, such as alcoholic hepatitis, autoimmune hepatitis, nonalcoholic fatty hepatitis, nonalcoholic fatty cirrhosis, primary biliary cholangitis, primary sclerosing cholangitis, ischemic hepatitis, congestive hepatopathy, congenital liver abnormalities, parasitic infections, cirrhosis, or liver cancer; and (3) Important data, such as medication history and clinical outcome data, were missing.
Data collection: Patients were confirmed to have DILI after preliminary screening of data by two senior pathologists from the Pathology Department of the Tianjin Second People’s Hospital, and those who did not meet the criteria were excluded after the review of their clinical cases and laboratory test results. Relevant patient information, including demographic characteristics (age, sex, body mass index, etc.), pathological characteristics, laboratory indicators, autoantibody titers, medication history, clinical classification of DILI, severity grading, time to liver function recovery, follow-up liver function results, and changes in condition, was collected.
DILI assessment: The evaluation of DILI involved clinical staging, which was based on the first available liver biochemistry test results. The R value was calculated as follows: R value = [measured value of alanine aminotransferase (ALT)/limit of normal value of ALT]/[measured value of alkaline phosphatase (ALP)/upper limit of normal value of ALP]. Acute DILI was classified into the following three categories: (1) The hepatocellular type, r ≥ 5; (2) The cholestatic type, r ≤ 2; and (3) The mixed type, 2 < r < 5. DILI severity was categorized into grades 1-5 according to the established guidelines for the diagnosis and treatment of DILI.
All enrolled patients were followed up until December 31, 2023. Data on cardiac, hepatic, and renal function parameters; lipid levels; immune function parameters; abdominal ultrasound findings; and liver stiffness were collected from the patients’ follow-up records. If the patient's condition changed and a second liver pathology examination was needed, the findings of the subsequent examination were collected.
The occurrence of endpoint events (return to normal liver function, death due to any cause, a diagnosis of autoimmune hepatitis, chronic liver injury, or the end of this study) was monitored. Chronic disease was defined as the failure of serum aspartate aminotransferase (AST), ALT, ALP, and total bilirubin (TBil) levels to return to normal six months after the onset of DILI or as the presence of imaging and histological evidence of portal hypertension or chronic liver injury.
SPSS 25.0 software was used for the statistical analyses. Normally distributed continuous data are presented as the means ± SD, and nonnormally distributed data are presented as the medians (Q1, Q3). Comparisons between groups were performed via the Mann-Whitney nonparametric test. Categorical data are presented as the frequencies (%), and the χ2 test was used for intergroup comparisons. The Mann-Whitney nonparametric test was used for intergroup comparisons of ranked variables. A P value < 0.05 was considered to indicate statistical significance.
Among the 326 patients who were suspected of having DILI according to the RUCAM scoring system and liver biopsy findings, 199 patients were included on the basis of the above study criteria, including 89 patients in the DILI group and 110 patients in the NAFL + DILI group.
The median age of the patients in the DILI group was 51 years, whereas that of the patients in the NAFL + DILI group was 55 years. The proportions of female patients in the DILI and NAFL + DILI groups were 78.7% (70/89) and 77.3% (85/110), respectively, with no significant difference in demographic characteristics between the groups. There were no statistically significant differences in liver stiffness or smoking history between the NAFL + DILI and DILI groups. However, the patients in the NAFL+DILI group had greater body mass indexes (BMIs) [25.4 (23.25, 28) vs 23.55 (21.15, 25.83) kg/m², P = 0.019] and Controlled Attenuation Parameter (CAP) scores [237 (195.5, 282) vs 192 (171.5, 235.5), P = 0; Table 1].
| DILI (n = 89) | DILI + NAFL (n = 110) | P value | |
| Age (year) | 51 (42, 58.5) | 55 (43.75, 59.25) | 0.224 |
| Females (number) | 70 (78.7) | 85 (77.3) | 0.816 |
| BMI (kg/cm²) | 23.55 (21.15, 25.83) | 25.4 (23.25, 28) | 0.019 |
| Liver stiffness (kPa) | 9.8 (7.8, 12.95) | 10.4 (6.65, 14.8) | 0.541 |
| CAP (Db/m) | 192 (171.5, 235.5) | 237 (195.5, 282) | 0 |
| Smoking history (number) | 12 (13.5) | 17 (15.1) | 0.485 |
Compared with the patients in the DILI group, a significantly lower proportion of patients in the NAFL + DILI group had symptoms such as a poor appetite and nausea (Table 2).
| DILI (n = 89) | DILI + NAFL (n = 110) | P value | |
| Urine color deepens | 51 (57.3) | 61 (55.45) | 0.794 |
| Fatigue | 43 (48.31) | 48 (43.64) | 0.51 |
| Poor appetite | 47 (52.81) | 42 (38.18) | 0.039 |
| Nausea | 14 (15.73) | 6 (5.45) | 0.017 |
| Bloating | 6 (6.74) | 11 (10) | 0.414 |
| Abdominal pain | 7 (7.87) | 6 (5.45) | 0.494 |
| Fever | 4 (4.49) | 5 (4.55) | 0.986 |
| Pruritic | 1 (1.12) | 1 (0.91) | 1 |
As shown in Table 3, drugs that cause liver injury are classified into ten categories as follows: Herbal and proprietary Chinese medicines; dietary supplements; and Western medicines, such as nonsteroidal anti-inflammatory drugs, antibiotics, cardiovascular drugs, antineoplastic drugs, antiulcer drugs, psychotropic drugs, hormone antagonists, and other unclassified drugs. The DILI group included 66 drugs from 7 classes, whereas the NAFL + DILI group included 96 drugs from 9 classes. Patients in the NAFL + DILI group received more classes of drugs causing liver injury than did patients in the DILI group.
| Medication history | DILI | DILI + NAFL |
| Chinese medicine, proprietary Chinese medicine | Lianhua Qingdian (Mianma Guanzhong) | Gynostemma Total Glycosides Tablets |
| Warm Stomach Shule (Astragalus, Yanhusuo) | Gastrointestinal An | |
| Danlu Tongdu Tablet (Salvia miltiorrhiza, Astragalus membranaceus, Yanhuosuo) | Kun Bao Wan (Prepared He Shou Wu, Astragalus) | |
| Xian Ling Bone Fructus Capsule (Radix et Rhizoma Polygoni Multiflori, Salviae Miltiorrhizae) | Uterine Blood Ning | |
| Milk fetish elimination (Poria cocos) | Shen Xiang Shu Yu (Yan Hu Suo, Chai Hu) | |
| Lingbao Heart Protecting Tablets (Salvia miltiorrhiza) | Gentiana Macrophyllae Capsules | |
| Nourishing Heart Tablets (Astragalus, Yanhusuo, Salvia Miltiorrhiza) | Panax ginseng powder | |
| Golden Chamber Kidney Qi Pills (Zelda) | Bailemian (Containing Shouwu Teng) | |
| Rejuvenation (Zedoary, Shouwu Teng) | Dan Hong (Salvia miltiorrhiza) | |
| Bailomian (Shouwu Teng) | Moistening Dryness and Relieving Itching Capsule (He Shouwu, Prepared He Shouwu) | |
| Zidan Blood Capsules (Panax ginseng saponin, Radix et Rhizoma Ginseng) | Salvia divinorum Drops (Salvia divinorum) | |
| Bai Cao Yishou Tea (Astragalus) | Exquisite Yin Qiao Tablets | |
| Ganoderma Lucidum Spore Powder | Tongxin Nourishing Heart (Prepared He Shouwu) | |
| Brain Heart (Astragalus, Salvia Miltiorrhiza, Scorpion, Leech) | Di Ao Heart Blood Kang | |
| Jia Wei Yi Puan (Mentha piperita, Chai Hu) | Mudan Granules (Huang's, Yanhuisuo, Panax ginseng, Paeonia lactiflora, Paeonia lactiflora, Salvia miltiorrhiza, Chuan Gong, Safflower, Sumac, Chickweed) | |
| Antelope Clear Lung Capsule (Smallpox Powder, Mint) | Xintong Oral Liquid (Astragalus, He Shouwu, Salvia Miltiorrhiza) | |
| Jumbo Pills (Clove) | Lipitor (Zedoary) | |
| Osteoporosis (Astragalus, Danshen) | Niu Huang Blood Pressure Reducing Pill (Astragalus, Mentha piperita) | |
| Deer Chuan Activating Capsule (Scorpion, Yanhuisuo) | Dendrobium Nightshade Pill | |
| Stomach-clearing Huanglian Pill (Smallpox Powder, Astragalus) | Gynostemma Capsules (Radix et Rhizoma Ginseng, Radix et Rhizoma Serpentae, Radix et Rhizoma Pinelliae, Radix et Rhizoma Bitterwood, Pericarpium Laminariae, Icicle, Menthol) | |
| Pu Shen Capsule (He Shou Wu, Pu Huang) | Sanwu Capsule (Shouwu Teng) | |
| Huo Xiang Zheng Qi Capsule (Semen Armeniacae) | Lianhua Qingdian (Mianma Guanzhong) | |
| Nourishing Blood and Nourishing Kidney Pill (Prepared He Shouwu) | Wind-dispersing and pain-relieving granules (Chai Hu) | |
| Nourishing the Heart by Nourishing the Veins (Prepared He Shouwu) | Blue Scutellaria Oral Liquid (Astragalus) | |
| Compound Danshen Drop Pills (Salvia divinorum) | Shu Shen Ling (Shou Wu Teng) | |
| Tangerine Red Phlegm and Cough Liquid (Semen Huperzia) | Nourishing Blood and Clearing Brain (Yanhuosuo) | |
| Gui Zhi Fu Ling Capsules | Compound Rheumatism Ning | |
| Betel Nut Si-Xie Tablet | Inibi Tablets | |
| Shuanghuanglian (Astragalus) | Inibalance Pills (Roasted Astragalus, Chai Hu) | |
| Hematoxylin (Chai Hu) | Qingdianxie Tablet (Smallpox Powder, Astragalus, Chai Hu) | |
| Pu Shen Capsule (He Shou Wu, Ze Xie) | Dong Ling Cao Tablet | |
| Ginkgo Biloba Ketone Ester Dispersible Tablets | Tongxuanliang Pill (Semixia, Astragalus) | |
| Muxiang Shunqi | Tiger Balm Tablets | |
| Liu Wei Di Huang Pill | Gui Long Tendon & Bone Ning (Salvia miltiorrhiza) | |
| Yanshou Tablets | Golden Throat Pills (Acacia Pi) | |
| Heze | Zachong Thirteen Flavor Pills (Clove) | |
| Shouwu Teng | Shu Brain Xin Drops | |
| Lei Gong Teng | Xihuang Pills | |
| Saffron | Zijin Long Tablets (Astragalus and Salvia Miltiorrhiza) | |
| Zedoary | Bornin (Roasted Astragalus) | |
| He Shou Wu | Zhen's Fu Zheng (Astragalus) | |
| Pingxiao Capsules | ||
| Yin Qiao Tablet | ||
| Pientzehuang Tablet | ||
| Formula: (1) Fried Sophora japonica, Fried Bitter Almond, Pueraria Mirifica, Paeoniae Alba, Cortex Pseudostellariae, Peony Peel, Roasted Licorice, Ascorbic Acid, Gypsum, Ephedra, Cinnamon Branches, Cicada Shell | ||
| (2) Forsythia, Rhizoma Atractylodis Macrocephalae, Roasted Epimedium, Cinnamon Branches, Folium Artemisiae, Hei Shun Piece, Jujubes, Ginger, Sequelae, Wine Huang Cen, Zingiber officinale, Burnt Hawthorn, Taraxacum officinale | ||
| (3) Lycium barbarum, licorice, Radix Fructus Fructus, Fructus Chrysanthemi, Semen Coicis, Mulberry Leaf, Gardenia Jasminoides, Oyster, Cassia Seed, Lotus Seed | ||
| (4) Gypsum, Zhi Mu, Radix et Rhizoma Ginseng, Pollen, Poria cocos, Morus alba, Zhe Bei, Xuan Shen, Ophiopogon, Chrysanthemum, Radix et Rhizoma Sheng Di | ||
| Salvia miltiorrhiza | ||
| Radix Bupleurum Chinense | ||
| He Shou Wu | ||
| Psoralea rubra | ||
| Senna | ||
| Xiaku Cao Cao Cream | ||
| Deer Whip Cream | ||
| Ginseng | ||
| Lei Gong Teng | ||
| Scorpion | ||
| Leech | ||
| Zezhi diarrhea (used in TCM) | ||
| Acanthopanax Senticosus | ||
| Dragon's blood exhaust | ||
| Dandelion | ||
| Western medicine | ||
| Anti-infective drugs | Cefoperazone sodium sulbactam sodium, furotoxin, levofloxacin, azithromycin, amoxicillin, clindamycin, isoniazid, rifampicin, streptomycin, bispyrazinamide | Levofloxacin, furotoxin, amoxicillin, itraconazole, roxithromycin |
| Nonsteroidal anti-inflammatory drug | Loxoprofen, Lovenox Extended-Release Tablets, Acetaminophen | Saxifrage, aminopyrine, aminocaprofen tablets, cotrimoxazole, nimesulide |
| Cardiovascular drugs | Fluvastatin, Rosuvastatin, Propafenone | Amlodipine benzenesulfonate, aliskatam, nifedipine, irbesartan hydrochlorothiazide, valsartan, felodipine, trandolazine, atorvastatin, simvastatin, acyclovir, atorvastatin, simvastatin, acyclovir, propafenone |
| Antitumor drugs | ||
| Antiulcer drug | Omeprazole (antifungal agent) | Lansoprazole, Rabeprazole |
| Psychotropic drugs | Flunarizine hydrochloride, betahistine mesylate, clonazepam, trazodone | |
| Hormone antagonists | Letrozole, exemestane, tamoxifen citrate | |
| Other | Mothballs (naphthalene or p-dichlorobenzene), toluene | Glucosamine, analgesic injections (ketorolac tromethamine, resorcinol), eteplirisone hydrochloride tablets, methimazole |
| Dietary supplement | Amway Health Care, Herbalife, Jens United States | Bovine Vitamin Tablets, Enzymes, Glutamine |
Compared with those in the DILI group, the patients in the NAFL + DILI group had higher triglyceride, cholesterol, low-density lipoprotein, and insulin levels (P < 0.05). There were no statistically significant differences in AST, ALT, gamma-glutamyl transferase, ALP, total bile acid, TBil, indirect bilirubin, direct bilirubin, or high-density lipoprotein levels between the NAFL + DILI and DILI groups (P > 0.05; Table 4).
| DILI (n = 89) | DILI + NAFL (n = 110) | P value | |
| ALT (U/L) | 765.7 (360.25, 1016.35) | 523.35 (169.5, 1078.5) | 0.069 |
| AST (U/L) | 471.4 (259.9, 658.2) | 438.5 (127.5, 920.2) | 0.715 |
| γ-GT (U/L) | 169 (101.3, 246.1) | 202 (97.5, 391.73) | 0.071 |
| ALP (U/L) | 155.8 (111, 189.85) | 129.3 (93, 168.15) | 0.509 |
| LDH (IU/L) | 259 (220.5, 309) | 228 (179, 306) | 0.511 |
| TBA (μmol/L) | 33 (7, 136) | 36.75 (4.85, 142.03) | 0.243 |
| TBiL (μmol/L) | 59.5 (21.95, 136.3) | 74.1 (19.4, 202.63) | 0.265 |
| DBIL (μmol/L) | 19.6 (6.6, 88.6) | 43.4 (4.7, 122.35) | 0.965 |
| IBIL (μmol/L) | 20.4 (11.65, 29.9) | 17.7 (10.43, 35.15) | 0.651 |
| TP (g/L) | 68.55 ± 7.78 | 69.45 ± 6.86 | 0.342 |
| ALB (g/L) | 40.29 ± 4.46 | 40.00 ± 5.07 | 0.674 |
| TG (mmol/L) | 1.57 (1, 2.195) | 1.805 (1.435, 2.55) | 0.006 |
| CHO (mmol/L) | 4.15 (3.51, 4.96) | 4.58 (3.9, 5.43) | 0.003 |
| LDL (mmol/L) | 2.29 (1.87, 2.82) | 2.78 (2.03, 3.4) | 0.008 |
| HDL (mmol/L) | 1.1 (0.87,1.46) | 1.14 (0.8,1.39) | 0.292 |
| GLU (mmol/L) | 5.34 (4.95, 5.79) | 5.59 (4.94, 6.38) | 0.113 |
| HbA1C (%) | 5.7 (5.3, 7.9) | 6.5 (5.83, 7.08) | 0.291 |
| Insulin (μIU/mL) | 9.62 (6.18, 13.14) | 12.87 (9.96, 19.4) | 0.022 |
On the basis of the liver function parameters available at the time of DILI diagnosis, the DILI types were categorized into hepatocellular, mixed, and cholestatic types, accounting for 88.8% (79/89), 6.7% (14/27) and 4.5% (4/89), respectively. In the NAFL + DILI group, the types of DILI were also categorized into hepatocellular, cholestatic, and mixed types, accounting for 70% (77/110), 16.4% (18/110), and 13.6% (15/110), respectively. The proportion of patients with the cholestatic type in the NAFL+DILI group was greater than that in the DILI group [16.4% (18/110) vs 4.5% (4/89), P = 0.008], whereas the proportion of patients with the hepatocellular injury type in the DILI group was greater than that in the NAFL+DILI group [88.8% (79/89) vs 70% (77/110), P = 0.001; Table 5].
| Drug-induced liver injury types | DILI (n = 89) | DILI + NAFL (n = 110) | P value |
| Hepatocellular | 79 (88.8) | 77 (70) | 0.001 |
| Cholestatic types | 4 (4.5) | 18 (16.4) | 0.008 |
| Mixed | 6 (6.7) | 15 (13.6) | 0.115 |
The proportion of patients with grade 2 or above liver injury was greater in the NAFLD + DILI group than in the DILI group [48.18% (53/110) vs 40.45% (36/89), P = 0.023; Table 6].
| Grade | DILI (n = 89) | DILI + NAFL (n = 110) | P value |
| ≤ grade 2 | 53 (59.55) | 57 (51.82) | 0.023 |
| > grade 2 or above | 36 (40.45) | 53 (48.18) |
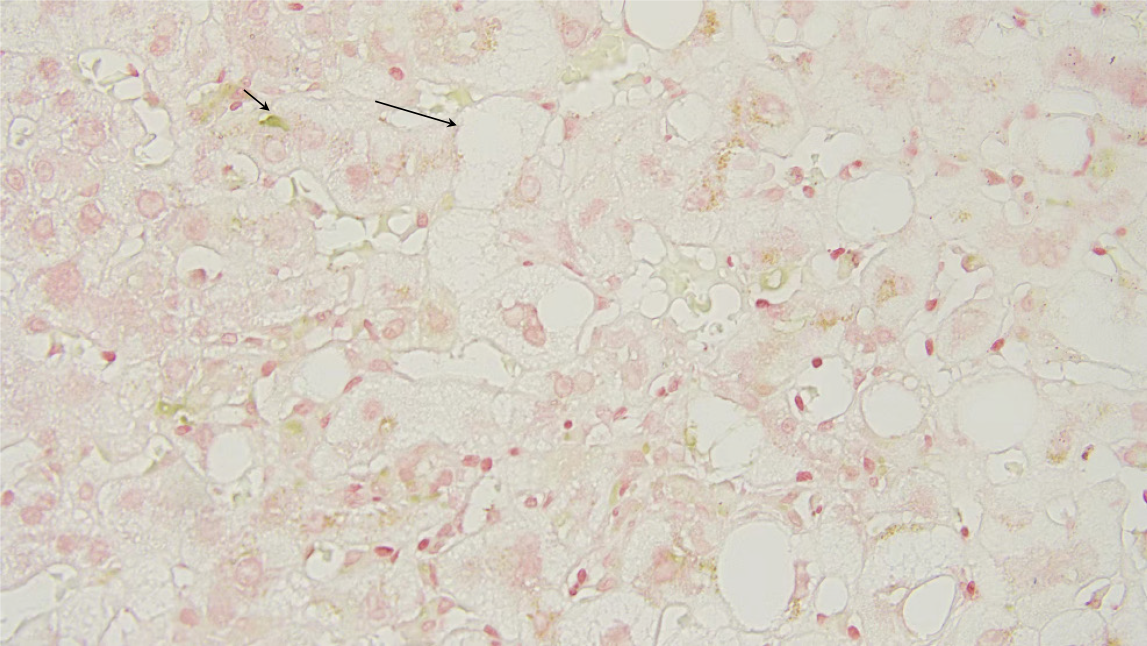
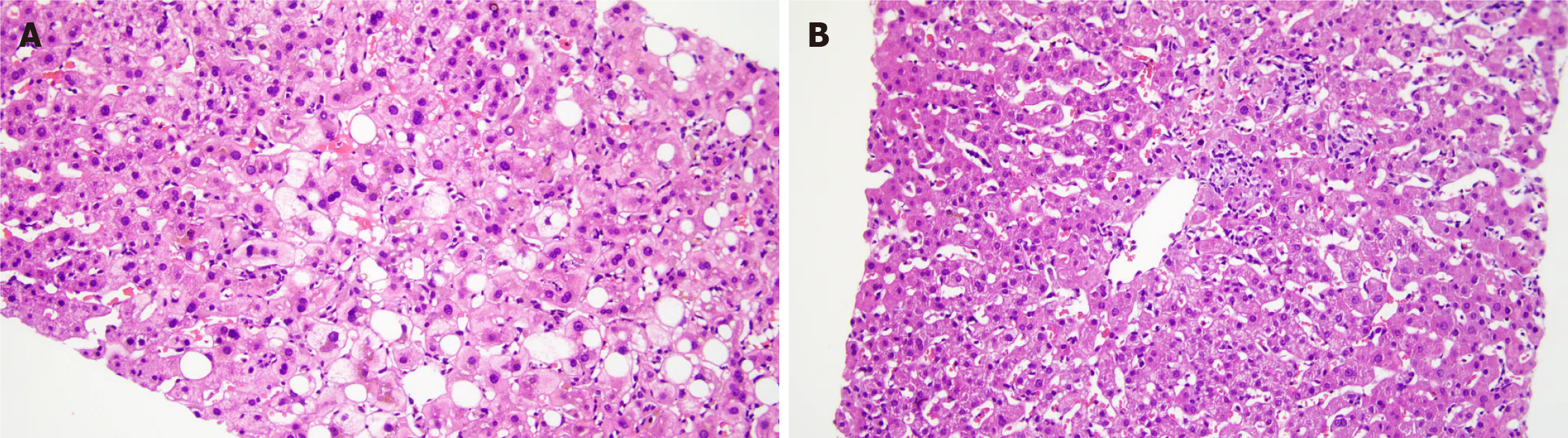
According to the liver biopsy pathology results, the proportions of fatty degeneration, vacuolar degeneration, and hepatocellular cholestasis were greater in the NAFL + DILI group than in the DILI group. There were no statistically significant differences in other pathological manifestations between the groups (Table 7). Figure 1 shows the liver pathology biopsy images of patients with DILI + NAFL, in which obvious cholestasis and vacuolar degeneration could be observed. Figure 2A shows the HE staining images of patients with DILI + NAFL, and Figure 2B shows the HE staining images of patients with DILI.
| DILI (n = 89) | DILI + NAFL (n = 110) | P value | |
| Hepatocellular carcinoma nodule | 6 (6.7) | 3 (2.7) | 0.312 |
| Lipoatrophy | 39 (43.8) | 77 (70) | 0 |
| Brownish-yellow granular deposits | 10 (11.2) | 13 (11.8) | 0.898 |
| Hepatocellular cholestasis | 23 (25.8) | 45 (40.9) | 0.026 |
| Vacuolar degeneration | 7 (7.9) | 22 (20) | 0.016 |
| Spotty necrosis | 88 (98.9) | 105 (95.5) | 0.16 |
| Apoptotic body | 61 (68.5) | 72 (65.5) | 0.646 |
| Inflammatory cell infiltration in the hepatic sinusoids | 22 (24.7) | 29 (26.4) | 0.792 |
| Hepatic sinusoidal dilatation and stasis | 1 (1.1) | 3 (2.7) | 0.769 |
| Pericarditis of the central vein | 64 (72.7) | 75 (68.2) | 0.487 |
| Epithelioid granuloma | 2 (2.3) | 5 (4.5) | 0.636 |
| Bridging necrosis | 15 (17) | 12 (10.9) | 0.211 |
| Phagocytic wax-like deposits | 39 (44.3) | 34 (30.9) | 0.052 |
| Lobular fusion necrosis | 1 (1.1) | 3 (2.7) | 0.778 |
| Expansion of the catchment area | 87 (97.8) | 107 (97.3) | 0.83 |
| Inflammatory cell infiltration in the confluent area | 88 (98.9) | 107 (97.3) | 0.423 |
| Microcystic bile ducts | 76 (85.4) | 88 (80) | 0.32 |
| Interlobular bile duct lesions | 4 (4.5) | 9 (8.2) | 0.461 |
| Boundary board damage | 80 (89.9) | 91 (82.7) | 0.149 |
| Fibrous tissue proliferation | 79 (88.8) | 105 (95.5) | 0.075 |
| Fiber gap formation | 1 (1.1) | 3 (2.7) | 0.769 |
| Lobular structural disorder | 1 (1.1) | 4 (3.6) | 0.502 |
| Iron staining is positive | 10 (11.2) | 12 (10.9) | 0.942 |
| Accompanied by an immune response | 26 (29.2) | 23 (24.6) | 0.176 |
The differences in the autoantibody titers between the NAFL + DILI and DILI groups were not statistically significant (P > 0.05; Table 8).
| Autoantibody | DILI (n = 54) | DILI + NAFL (n = 78) | P value |
| Antinuclear antibody ANA | 33 (61.1) | 50 (64.1) | 0.727 |
| Anti-mitochondrial Antibodies AMA | 2 (3.7) | 2 (2.6) | 0.707 |
| Anti-smooth muscle antibodies ASMA | 1 (1.9) | 3 (3.8) | 0.888 |
| Anti-dsDNA antibody | 0 (0) | 1 (1.3) | 1 |
| Anti-dsDNA antibody | 3 (3.2) | 1 (2.3) | 0.372 |
| Anti-U1-snRNP antibody | 2 (3.7) | 0 (0) | 0.166 |
| Anti-SSA/Ro60 antibody | 5 (9.3) | 5 (6.4) | 0.543 |
| Anti-SSB/Ro52 antibody | 5 (9.3) | 3 (3.8) | 0.363 |
| Anti-SSB/la antibody | 2 (3.7) | 2 (2.6) | 0.707 |
| Anti-Scl-70 antibody | 1 (1.9) | 0 (0) | 0.409 |
| Anti-matrix antibody | 0 (0) | 0 (0) | |
| Anti-Jo-1 antibody | 0 (0) | 0 (0) | |
| Anti-Ribosomal P0 Antibody | 0 (0) | 0 (0) | |
| P-ANCA | 0 (0) | 0 (0) | |
| Atypical P-ANCA | 0 (0) | 0 (0) | |
| Antiproteinase 3, PR3 | 0 (0) | 0 (0) | |
| Antiperoxidase, MPO | 0 (0) | 0 (0) | |
| Anti-histone antibody | 0 (0) | 0 (0) | |
| Anti-mitochondrial M2 antibody | 0 (0) | 2 (2.6) | 0.513 |
| Anti-nucleotide protein anti-Sp100 | 0 (0) | 2 (2.6) | 0.513 |
| Anti-liver and kidney microparticle antibody LKM1 | 0 (0) | 1 (1.3) | 1 |
| Anti-nuclear envelope antibody gp210 | 1 (1.9) | 0 (0) | 0.409 |
| Anti-hepatocyte lysate antibody LC1 | 0 (0) | 0 (0) | |
| Anti-soluble liver antigen SLA | 0 (0) | 0 (0) | |
| Serum perinuclear factor APF | 0 (0) | 0 (0) | |
| Antikeratin antibody AKA | 0 (0) | 0 (0) | |
| Anticyclic citrullinated peptide ACCP | 1 (1.9) | 1 (1.3) | 1 |
Cytokines: The level of interleukin (IL)-4 was greater in the NAFL + DILI group than in the DILI group (P < 0.05). However, there were no statistically significant differences in the levels of interferon, tumor necrosis factor, IL-2, IL-6, IL-10, or IL-17 between the NAFL + DILI and DILI groups (P > 0.05; Table 9).
| Immunity | DILI (n = 31) | DILI + NAFL (n = 39) | P value |
| Interferon (pg/mL) | 1.46 (0.67, 3.43) | 2.29 (1.58, 3.14) | 0.149 |
| Tumor necrosis factor (pg/mL) | 0.86 (0.48, 1.48) | 1.09 (0.62, 2.27) | 0.163 |
| IL-2 (pg/mL) | 0 (0, 0.93) | 0.4 (0, 2.56) | 0.092 |
| IL-4 (pg/mL) | 0.56 (0.28, 1.69) | 1.6 (0.82, 2.69) | 0.007 |
| IL-6 (pg/mL) | 4.65 (1.82, 6.39) | 5.11 (2.79, 9.54) | 0.144 |
| IL-10 (pg/mL) | 4.16 (2.85, 11.95) | 4.28 (2.22, 9.91) | 0.92 |
| IL-17 (pg/mL) | 0.91 (0.38, 1.95) | 1.1 (0.6, 2.35) | 0.274 |
Immunological function: Compared with the DILI group, the NAFL + DILI group had a greater level of complement C3 (P = 0.02), whereas the immunoglobulin M (IgM) level was lower in the NAFL + DILI group than in the DILI group (P = 0.047). There were no statistically significant differences between the groups in terms of immunoglobulin G (IgG), complement C4, or immunoglobulin A levels (P > 0.05; Table 10).
| DILI (n = 76) | DILI + NAFL (n = 98) | P value | |
| C3 (g/L) | 1.165 (0.99, 1.38) | 1.27 (1.15, 1.4) | 0.02 |
| IgG (g/L) | 13.3 (11.21, 16.09) | 13.44 (10.84, 15.53) | 0.714 |
| C4 (g/L) | 0.25 (0.19, 0.31) | 0.26 (0.22, 0.31) | 0.244 |
| IgM (g/L) | 1.01 (0.83, 1.48) | 0.94 (0.72, 1.30) | 0.047 |
| IgA (g/L) | 2.22 (1.89, 2.79) | 2.43 (2.01, 3.35) | 0.369 |
The proportions of patients receiving steroid treatment were not significantly different between the DILI and NAFL + DILI groups (Table 11). However, a greater proportion of patients in the NAFL + DILI group recovered liver function between 90 and 180 days [30.6% (19/62) vs 15.5% (9/58), P = 0.05]. There were no statistically significant differences between the groups in the proportions of patients with liver function recovery at other time intervals (Table 12). There were no statistically significant differences between the groups in the probability of eventual progression to chronic DILI (Table 13) or autoimmune hepatitis (Table 14) at follow-up (P= 0.868).
| DILI (n = 89) | DILI + NAFL (n = 110) | P value | |
| Hormone use (number) | 13 (14.6) | 12 (10.9) | 0.434 |
| Normalized liver function | DILI (n = 58) | DILI + NAFL (n = 62) | P value |
| D ≤ 7 | 4 (6.9) | 2 (3.2) | 0.615 |
| 7 < D ≤ 14 | 2 (3.4) | 1 (1.6) | 0.953 |
| 14 < D ≤ 30 | 5 (8.6) | 1 (1.6) | 0.18 |
| 30 < D ≤ 90 | 17 (29.3) | 11 (17.7) | 0.134 |
| 90 < D ≤ 180 | 9 (15.5) | 19 (30.6) | 0.05 |
| Normalized liver function | DILI (n = 58) | DILI + NAFL (n = 62) | P value |
| > 180/chronicization | 21 (36.2) | 28 (45.2) | 0.319 |
| DILI (n = 58) | DILI + NAFL (n = 62) | P value | |
| Development of autoimmune hepatitis (number) | 6 (10.3) | 7 (11.3) | 0.868 |
DILI refers to liver damage caused by the use of drugs and can be caused by the drug itself, its metabolites, hypersensitivity reactions, or decreased tolerance. The severity of DILI can range from mild, in which DILI resolves upon drug cessation, to severe, in which DILI leads to acute liver failure. The incidence of DILI in patients with NAFL is four times greater than that in healthy individuals[7]. However, the clinical characteristics and prognosis of DILI in these patients remain unclear. Therefore, this study aimed to investigate the clinical features and prognosis of NAFL patients with DILI.
Our study revealed that patients with DILI combined with NAFL had more pronounced abnormalities in metabolic parameters, including BMI; CAP score; and triglyceride, total cholesterol, low-density lipoprotein, and insulin levels. These abnormalities are closely related to the presence of NAFL in DILI patients, as previous studies have shown that 70% of obese and diabetic patients have simple hepatic steatosis, insulin resistance, and hyperlipidemia as major pathogenic factors of NAFL[8].
Complement proteins are a group of proteins with enzymatic activity that are activated in normal human and animal sera and tissue fluids. Physiologically, complement proteins participate in the anti-infection and expanded humoral immune responses of the body and promote tissue repair. Complement proteins are activated to mediate immune responses and inflammatory reactions. However, when the complement system is overactivated, these proteins can also mediate an inflammatory response, which can lead to tissue damage and even organ failure. C3 is a crucial protein that is necessary for all complement system functions. Complement proteins are produced mainly in the liver, and there may be a link between liver function and complement levels. As C3 is the central molecule of the complement system, its activation is essential for all functions performed by this system. In the present study, we found that DILI patients with combined NAFL had high levels of C3, which suggests possible immune-mediated hepatocellular injury.
We also found that IL-4 levels were higher in DILI patients with combined NAFL than in those with DILI alone. Cytokines are a class of small soluble proteins that are secreted by immune cells and play important roles in cell-to-cell regulation, such as regulating cell growth and differentiation and immune responses and participating in the development of inflammation. Arslan[8] reported higher IL-4 serum levels in patients who developed anesthetic DILI after inhalation of volatile anesthetics than in control individuals. In an earlier study by Njoku[9], IL-4 was found to play a key role in the initiation and pathogenesis of immune-mediated DILI. IL-4 inhibits the regulatory response of the drug metabolism CYP2E1 autoantigen and induces a proinflammatory response to drug hemiantigens. IL-4+ and CD4+ T cells can contribute to the development of allergic and autoimmune diseases by inducing B-cell proliferation and isotype switching from IgM to IgG4 and immunoglobulin E. In patients with immune-mediated DILI, IgG4 autoantibodies form circulating immune complexes that can damage hepatocytes, and IL-4 generates additional neoantigens by inducing the mRNA and protein expression of CYP2E1. IL-4 also inhibits IL-6 expression during the CYP2E1 autoantigen response, whereas IL-6 promotes hepatic regeneration, thereby protecting the liver from various forms of injury. In DILI, IL-4 plays an important proinflammatory role. In our study, IL-4 levels were found to be higher in DILI patients with comorbid NAFL than in those with DILI only, which suggests more pronounced expression of immune factors when pharmacological liver injury is combined with nonalcoholic steatosis.
Steatosis alone has been shown to have a sensitizing effect on cholestasis, which is associated with altered bile salt composition. The bile salt profile is dominated by TβMCA and TCA, and the combination of DILI with NAFL was shown to increase HepG2 cell death by decreasing the TβMCA/TCA ratio[10]. Our study also revealed that patients with DILI in the context of NAFL were more likely to develop cholestasis than those with DILI alone.
Exposure to certain drugs in the context of fatty liver can lead to more frequent and severe liver injury[11]. This susceptibility is believed to be linked to the metabolic environment found in patients with NAFLD. This environment includes factors such as increased levels of reactive oxygen species (ROS), reduced ATP synthesis, inflammation, excess CYP-generated metabolites of toxic drugs, and a decrease in the activity of other detoxifying CYPs[12]. These factors can cause drugs to become more toxic to the liver, even when they are taken at therapeutic doses[13], resulting in liver injury. NAFLD results in altered activity and expression of numerous metabolizing enzymes involved in drug disposal, which could have implications for the safety of xenobiotics in these patients[14]. CYPs are the primary enzymes involved in drug metabolism. The majority of chemical modifications catalyzed by CYPs inactivate drugs, thereby mitigating their potential toxicity. However, CYPs can also facilitate drug bioactivation by converting the drugs into active metabolites that can cause cellular damage[15]. CYP3A is the most abundant CYP isoenzyme in the liver and is involved in the metabolism of approximately 50% of clinical drugs. It has been demonstrated that a decrease in CYP3A activity is correlated with the severity of steatosis and the progression of NAFLD in humans[16]. An impairment in CYP3A activity due to reduced protein levels has been reported in diabetic patients, a factor likely associated with the occurrence of NAFLD in these individuals[17]. Downregulation of CYP3A4 by cytokine-mediated activation of the JAK/STAT signaling pathway during the inflammatory response[18] or by FGF21-mediated activation of the MAPK pathway[19] has been proposed.
CYP2E1 is a major source of ROS and its activity and expression are increased in NAFLD; these changes are believed to worsen the oxidative stress associated with NAFLD and promote the progression of NAFLD to nonalcoholic steatohepatitis NASH[20]. Acetaminophen can be catalyzed by CYP2E1 to produce the more toxic product NAPQI, and patients with NAFLD are more likely to experience DILI when taking acetaminophen[21]. Similarly, halothane, isoflurane, losartan, ticlopidine, and omeprazole more often lead to acute DILI in patients with NAFLD and obesity, most likely due to the induction and further formation of reactive intermediates by CYP2E1[12].
Glutathione S-transferases (GSTs) conjugate many electrophilic drugs and drug metabolites with the nucleophilic molecule glutathione. GST activity was reported to be decreased in human liver samples from NAFLD patients, a factor that may be aggravated by the depleted glutathione in these patients[22]. Our study also confirmed that the degree of pharmacological liver injury in the context of fatty liver is severe and that the recovery time is long.
In conclusion, in the context of NAFL, DILI is more likely to be cholestatic, with a greater degree of liver injury, a longer recovery time, and more pronounced expression of immune factors. Clinicians should be more cautious when prescribing drugs to NAFL patients and should closely monitor their liver function. More attention should be given to the selection of drugs for NAFL patients in clinical practice.
This study has several limitations. We compared DILI due to different classes of drugs between the DILI group and the DILI + NAFL group. However, some of the individual drugs in certain classes differed between the DILI group and the DILI+ NAFL group. For example, the anti-infective drug classes, including scefoperazone sodium sulbactam sodium, azithromycin, clindamycin, isoniazid, rifampicin, streptomycin, and bispyrazinamide, in the DILI group were compared with levofloxacin, furotoxin, amoxicillin, itraconazole, and roxithromycin in the DILI + NAFL group. Certain individual drugs, as anti-infective drugs, cause distinct patterns of liver injury, and the time taken for recovery also varies. The observed differences in the patterns of liver injury and time taken for recovery could also be due to the individual drugs themselves rather than the effects of NAFL. This study was only a single-center retrospective clinical study, which lacked the rigor of a randomized controlled trial and suffered from selection and recall bias. In addition, owing to many confounding factors, such as genetics, environmental factors, and lifestyle habits, this study has certain limitations, and it is necessary to expand the sample size to further explore the clinical characteristics of patients with DILI+NAFL and the factors that affect the prognosis.
Despite progress made in the field of DILI, there is still a large unmet clinical need. Future research should focus on the common problems of the pathogenesis of DILI on the basis of chronic liver disease and the individual problems of liver injury caused by specific drugs, as well as their underlying associations; the epidemiology, natural history, clinical characteristics and risk factors for liver injury caused by different specific drugs; translational research on biomarkers used for the diagnosis and prediction of prognosis; and the development of new drugs for drug therapy. To this end, pharmacoepidemiologic studies, prospective large-scale registries and cohort studies, and the establishment of a large DILI repository are the basis for translatable results.
In the context of NAFL, DILI is more likely to be cholestatic, with a greater degree of liver injury, a longer recovery time, and more pronounced expression of immune factors.
| 1. | Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156:2230-2241.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 2. | Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J; United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340-52.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 3. | Lammert C, Imler T, Teal E, Chalasani N. Patients With Chronic Liver Disease Suggestive of Nonalcoholic Fatty Liver Disease May Be at Higher Risk for Drug-Induced Liver Injury. Clin Gastroenterol Hepatol. 2019;17:2814-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, Ding Y, Duan ZP, Fu QC, Guo XY, Hu P, Hu XQ, Jia JD, Lai RT, Li DL, Liu YX, Lu LG, Ma SW, Ma X, Nan YM, Ren H, Shen T, Wang H, Wang JY, Wang TL, Wang XJ, Wei L, Xie Q, Xie W, Yang CQ, Yang DL, Yu YY, Zeng MD, Zhang L, Zhao XY, Zhuang H; Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 6. | Fan JG, Wei L, Zhuang H; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Liu YH, Guo Y, Xu H, Feng H, Chen DY. Impact of Non-Alcoholic Simple Fatty Liver Disease on Antituberculosis Drug-Induced Liver Injury. Infect Drug Resist. 2021;14:3667-3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20:16452-16463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Njoku DB. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury: a review. Expert Opin Drug Metab Toxicol. 2010;6:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lionarons DA, Heger M, van Golen RF, Alles LK, van der Mark VA, Kloek JJ, de Waart DR, Marsman HA, Rusch H, Verheij J, Beuers U, Paulusma CC, van Gulik TM. Simple steatosis sensitizes cholestatic rats to liver injury and dysregulates bile salt synthesis and transport. Sci Rep. 2016;6:31829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, Leo E. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther. 2010;332:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 450] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 15. | Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 934] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 16. | Kolwankar D, Vuppalanchi R, Ethell B, Jones DR, Wrighton SA, Hall SD, Chalasani N. Association between nonalcoholic hepatic steatosis and hepatic cytochrome P-450 3A activity. Clin Gastroenterol Hepatol. 2007;5:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Dostalek M, Court MH, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol. 2011;163:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Werk AN, Cascorbi I. Functional gene variants of CYP3A4. Clin Pharmacol Ther. 2014;96:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Woolsey SJ, Beaton MD, Mansell SE, Leon-Ponte M, Yu J, Pin CL, Adams PC, Kim RB, Tirona RG. A Fibroblast Growth Factor 21-Pregnane X Receptor Pathway Downregulates Hepatic CYP3A4 in Nonalcoholic Fatty Liver Disease. Mol Pharmacol. 2016;90:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 432] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |