Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.101704
Revised: November 8, 2024
Accepted: December 2, 2024
Published online: January 27, 2025
Processing time: 104 Days and 11.2 Hours
The treatment of metabolic dysfunction-associated steatotic liver disease (MA
To evaluate whether the SM-ALA formulation (LUDLEV®), in combination with the Mediterranean diet (MD), could improve MASLD-related liver injury.
A randomized, double-blind clinical trial was conducted on patients with MA
Fifty patients aged 54 ± 10 years were included, and the majority (74%) were female. Reduced visceral fat and umbilical circumference were reported in both groups, with significance in group A (P = 0.045 and 0.003, respectively). The de
SM-ALA (LUDLEV®) combined with the MD can promote the improvement of metabolic parameters, reducing visceral fat and hepatic steatosis in Mexican patients with MASLD.
Core Tip: Administration of Silybum marianum and alpha-lipoic acid in conjunction with a Mediterranean-style diet demonstrated a decrease in hepatic steatosis as measured by controlled attenuation parameter, improved biochemical parameters, and a reduction in the percentage of visceral fat. In addition to these metabolic benefits, the combination of Silybum marianum and alpha-lipoic acid is considered safe and well-tolerated.
- Citation: Cano Contreras AD, Del Rocío Francisco M, Vargas Basurto JL, Gonzalez-Gomez KD, Amieva-Balmori M, Roesch Dietlen F, Remes-Troche JM. Effect of alpha-lipoic acid and Silybum marianum supplementation with a Mediterranean diet on metabolic dysfunction-associated steatosis. World J Hepatol 2025; 17(1): 101704
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/101704.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.101704
Fatty liver associated with metabolic dysfunction, now called metabolic dysfunction-associated steatotic liver disease (MASLD) by international consensus, is currently the most frequent chronic liver disease worldwide. Presenting in 25%-30% of the general population, it is considered a global public health problem[1,2]. Pharmacologic treatment has focused on the control of comorbidities, and currently only one drug, not yet available in Mexico, has been approved by the United States Food and Drug Administration for the treatment of patients with MASLD and fibrosis.
The implementation of a low-calorie Mediterranean diet (MD), characterized by foods low in saturated fats and high in vegetable oils and green vegetables, fruits, cereals, nuts, legumes, fish, dairy products, and red wine, has been shown to improve fatty acid content and liver inflammation. This is reflected in a decrease in transaminases when a weight loss of 5% to 7% is achieved. Additionally, the MD enhances insulin sensitivity and lipid profiles, aids in the prevention of MASLD-related diseases (such as diabetes and high blood pressure), and improves fibrosis and inflammation when weight loss exceeds 10%, regardless of physical activity regimens[3,4].
Silybum marianum (SM), a milk thistle seed extract, has demonstrated antioxidant, anti-inflammatory, and antifibrotic effects and has been used for centuries for treating liver diseases. Its therapeutic actions are related to silymarin, the active component of the seeds. In preclinical trials, silybin, the main active constituent of silymarin, was found to inhibit oxidative stress in the endoplasmic reticulum of hepatocytes and the NLRP3 inflammasome assembly through the NAD+/SIRT2 pathway. It eliminates reactive oxygen species and increases glutathione production through the bioavailability of cysteine, thus exerting its antioxidant effect. At the same time, it lowers elevated glucose and hemoglobin A1c levels, which is why it has been considered for treating MASLD[5].
Alpha-lipoic acid (ALA) is a cofactor of mitochondrial enzymes and is considered an antioxidant because its reduced form, dihydrolipoate, reacts with reactive oxygen species, thus protecting the cell membrane[6]. The administration of ALA has shown beneficial effects on adipokine levels and metabolic disorders, such as diabetes and obesity, which are clinical entities found in patients with MASLD[7]. Given this evidence, in addition to the improvement of metabolic syndrome components, the combination of these compounds is posited to have positive effects for treating MASLD. The aim of the present clinical trial was to evaluate whether the SM-ALA formulation (LUDLEV®) combined with the MD could improve liver injury related to MASLD in Mexican patients.
A double-blind, randomized controlled clinical trial was conducted on patients above 18 years of age who had so
The patients included in the study were randomized through 1:1 systematic sampling to receive SM-ALA (LUDLEV® 300 mg/46.2 mg) twice a day in association with the MD vs placebo plus the MD for 24 weeks. The sample size was calculated based on the study by Martínez-Rodríguez et al[8], in which 40 patients with nonalcoholic fatty liver disease were ran
Medical and nutritional evaluations, biochemical testing, and transient liver elastography with FibroScan were carried out at baseline and at follow-up weeks 12 and 24. Medical evaluation included a complete clinical history, anthropometric measurements, adverse event documentation, and weekly follow-up via telephone to assess drug treatment adherence.
Biochemical testing included complete blood count, blood chemistry, liver function tests, lipid profile, C-reactive protein, and serum insulin. Dietary plans were established by nutritional clinic personnel according to nutritional dia
For the result analysis, the numerical variables were expressed as measures of central tendency and dispersion, according to their distribution. The categorical variables were expressed as frequency and percentage. Data distribution was evaluated through the Kolmogorov-Smirnov test, and the Levene’s test was applied to examine homogeneity of variance in the numerical variables. Numerical variable means were compared using the Student’s t-test or the Wilcoxon test, according to the nature of the data and their distribution. On the other hand, the categorical variables were compared using the χ2 test or the Fisher’s exact test. Correlations were evaluated through the Pearson or Spearman coefficients, according to the nature of the variables and data distribution. Statistical significance was set at a P < 0.05. SPSS version 22.0 statistical software (IBM Corp., Armonk, NY, United States) was utilized to perform the statistical analysis and create the figures and tables.
The study was reviewed and authorized by the research and ethics committee of the Instituto de Investigaciones Médico-Biológicas of the Universidad Veracruzana, folio IIMB-010-2023. All participants signed letters of informed consent that explained the procedure, risks, and benefits of the study as well as the use and protection of their personal information. The study was carried out according to the ethics regulations established by the Declaration of Helsinki of the World Medical Association (64th general assembly, Fortaleza, Brazil, October 2013) and the general health law: Fifth title; health research: Single chapter; the general health law in research: Second title; ethical aspects of research on humans: Chapter one; and articles 16 and 23 of the federal law for the protection of personal data. The study was registered as a clinical trial, identification number NCT05913986 (ClinicalTrials.gov).
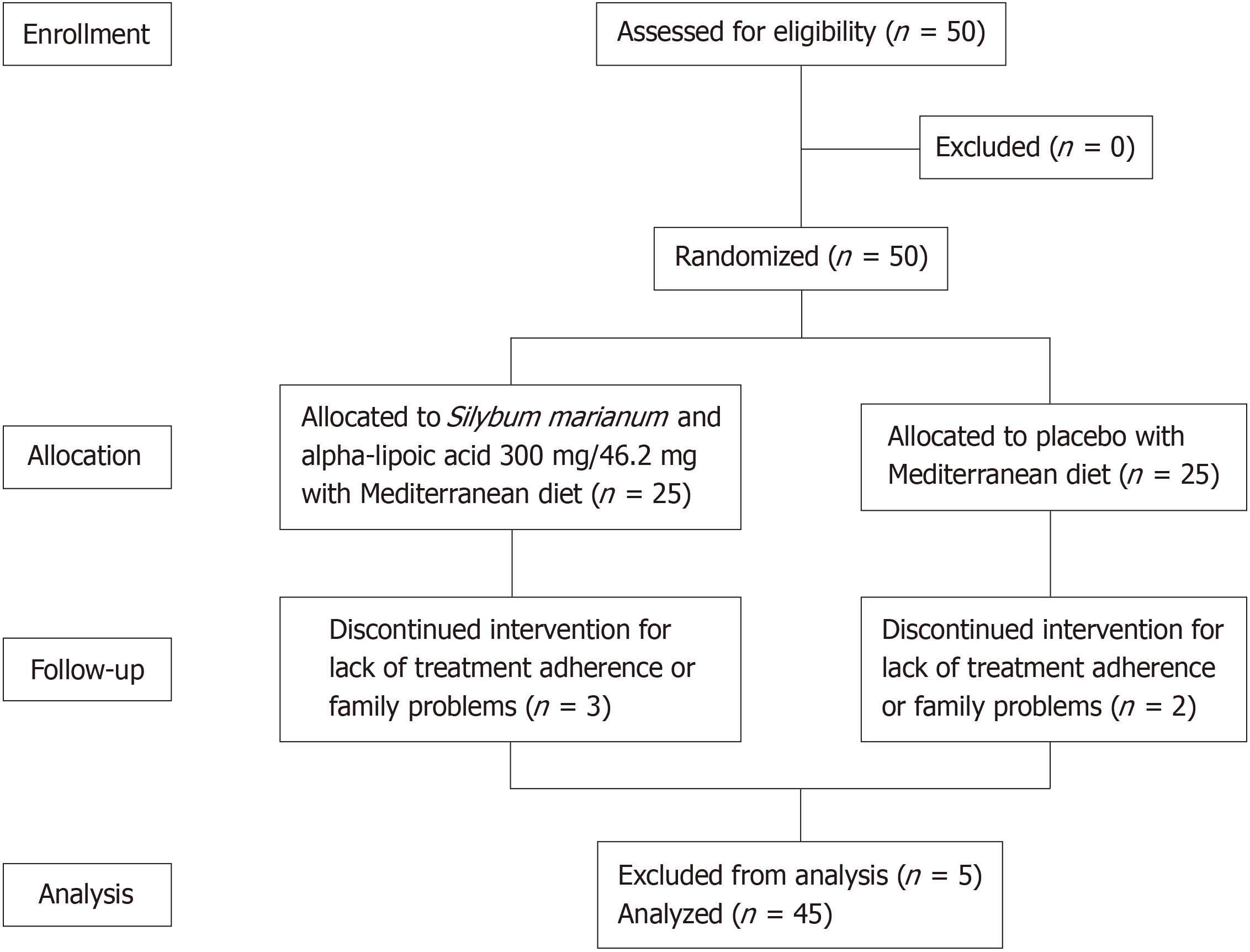
Fifty patients with MASLD were included in the study. Their mean age was 54 ± 10 years, mean body mass index (BMI) was 33.0 ± 5.8 kg/m2, and the majority of patients were female (74%). They were randomized 1:1, with 25 patients (50%) in group A (ALA and SM) and 25 patients (50%) in group B (placebo). There were no differences in sex, age, weight, BMI, or comorbidities between the two groups. Two patients (4%) were lost due to lack of treatment adherence or family problems at follow-up week 12 and 3 patients (6%) at week 24 (Figure 1). Table 1 describes the population characteristics.
| Parameter | Group A, n = 25 | Group B, n = 25 | P value |
| Sex | 0.333 | ||
| Male | 5 (20) | 8 (32) | |
| Female | 20 (80) | 17 (68) | |
| Age in years | 54.7 + 10.5 | 54.3 + 11.5 | 0.229 |
| Weight | 82.2 + 16.4 | 82.1 + 14.2 | 0.473 |
| BMI | 32.8 + 6.9 | 33.3 + 4.7 | 0.394 |
| Normal weight | - | - | |
| Overweight | 10 (40) | 5 (20) | |
| Obesity I | 5 (20) | 10 (40) | |
| Obesity II | 6 (24) | 9 (36) | |
| Obesity III | 4 (16) | 1 (4) | |
| Comorbidities | |||
| Diabetes | 6 (24) | 6 (24) | 0.629 |
| High blood pressure | 7 (28) | 8 (32) | 0.758 |
| Hypercholesterolemia | 8 (32) | 5 (20) | 0.333 |
| Hypertriglyceridemia | 6 (24) | 4 (16) | 0.480 |
| Hypothyroidism | 2 (8) | 2 (8) | 0.695 |
| Others | - | - |
In group A, the mean age was 54.7 ± 10.5 years, the majority (80%) were female, weight was 82.2 ± 16.4 kg, and BMI was 32.8 ± 6.9 kg/m2. The main comorbidities were dyslipidemia, which was present in 15 patients (56%), followed by high blood pressure (28%) and diabetes (24%). At 12 weeks of treatment, body weight and visceral fat tended to decrease (P = 0.256 and 0.125, respectively), achieving a statistically significant loss in the visceral fat percentage at week 24 (P = 0.045). The total fat percentage gradually decreased at weeks 12 and 24 but not significantly (P = 0.543 and 0.744, respectively), and muscle mass remained unchanged (P = 0.554). In general, change was achieved in body composition, with a decrease in visceral fat percentage, with an average loss of -0.95% at week 24 (Table 2). There were no statistically significant changes in transaminase levels (P = 0.201 and 0.267), and they were within the normal range in the baseline measurement. There was a significant decrease in gamma-glutamyl transferase and C-reactive protein at treatment weeks 12 and 24 (P = 0.033 and 0.035, respectively). The lipid profile showed a tendency to decrease, especially low-density lipoprotein cho
| SM-ALA + MD | Baseline | 12 weeks | 24 weeks | P value |
| Somatometry | ||||
| Weight in kg | 82.2 + 16.4 | 80.1 + 16.2 | 78.8 + 16.7 | 0.393 |
| ∆weight | - | -1.7 + 2.9 | -2.5 + 3.5 | 0.388 |
| BMI | 32.8 + 6.9 | 32.5 + 6.8 | 32.4 + 7.05 | 0.57 |
| Visceral fat as % | 12.4 + 3.4 | 11.9 + 3.3 | 11.5 + 3.5 | 0.045 |
| ∆visceral fat | - | -0.54 + 1.6 | -0.95 + 2.1 | 0.268 |
| Total fat as % | 41.9 + 10.8 | 42.0 + 10.5 | 41.0 + 11.5 | 0.744 |
| Muscle as % | 25.5 + 5.4 | 26.5 + 22.5 | 26.6 + 8.6 | 0.554 |
| Circumference in cm | ||||
| Umbilical | 101.8 + 14.7 | 95.4 + 22.5 | 97.4 + 14.5 | 0.003 |
| Hip | 112.8 + 16.2 | 110.2 + 14.0 | 109.9 + 14.4 | 0.248 |
| Waist | 99.9 + 15.2 | 96.3 + 13.4 | 95.9 + 15.0 | 0.117 |
| Waist-to-hip ratio | 0.88 + 0.8 | 0.87 + 0.06 | 0.87 + 0.06 | 0.206 |
| Elastography | ||||
| kPa1 | 6.2 + 2.6 | 5.9 + 2.3 | 5.4 + 2.03 | 0.083 |
| ∆kPa | - | -0.29 + 2.9 | -0.71 + 1.89 | 0.33 |
| Biochemical parameters | Baseline | 12 weeks | 24 weeks | P value |
| Leukocytes in thousands/μL1 | 7.1 (5.3-13.7) | 5.7 (4.5-10) | 7.0 (4.7-12) | 0.876 |
| Hb in g/dL2 | 13.4 + 1.3 | 13.1 + 1.3 | 13.3 + 1.2 | 0.101 |
| Platelets in miles/μL2 | 278 + 52 | 259 + 59 | 256 + 57 | 0.014 |
| TB in mg/dL2 | 0.54 + 0.24 | 0.60 + 0.18 | 0.72 + 0.20 | 0.002 |
| AST in U/L1 | 27 (11-49) | 25 (20-63) | 24 (15-45) | 0.201 |
| ALT in U/L1 | 32 (4-102) | 30 (17-81) | 25 (17-69) | 0.267 |
| ALP in IU/L2 | 99 + 49 | 92 + 24 | 81 + 16 | 0.205 |
| GGT in U/L1 | 51 (18-370) | 25 (10-130) | 28 (12-142) | 0.033 |
| TP in g/dL1 | 7.4 (6.4-8.3) | 7.4 (6.6-8.1) | 7.4 (7.0-8.3) | 0.125 |
| Albumin in g/dL2 | 4.3 + 0.56 | 4.3 + 0.28 | 4.4 + 0.3 | 0.443 |
| Cholesterol in mg/dL2 | 202 + 45 | 191 + 52 | 184 + 49 | 0.073 |
| HDL in mg/dL1 | 44 (23-66) | 40 (31-61) | 46 (29-168) | 0.850 |
| LDL in mg/dL2 | 123 + 41 | 113 + 39 | 110 + 37 | 0.056 |
| VLDL in mg/dL2 | 31 + 11 | 41 + 43 | 32 + 15 | 0.663 |
| Triglycerides in mg/dL2 | 165 + 11 | 163 + 55 | 32 + 76 | 0.987 |
| Glucose in mg/dL1 | 110 (69-316) | 101 (83-200) | 115 (91-242) | 0.616 |
| Creatinine in mg/dL2 | 0.77 + 0.16 | 0.67 + 0.14 | 0.66 + 0.25 | 0.223 |
| CRP in mg/L1 | 4.60 (0.50-1.03) | 3.90 (1.80-6.00) | 0.51 (0.03-1.40) | 0.035 |
| Insulin in mg/L2 | 16.0 + 9.8 | 20.0 + 11.0 | 16.0 + 5.0 | 0.580 |
| HOMA2 | 4.8 + 3.0 | 5.7 + 3.3 | 5.3 + 2.8 | 0.230 |
In group B, the mean age was 54.3+11.5 years, the majority (68%) were female, weight was 82.1 ± 14.2 kg, and BMI was 33.3 ± 4.7 kg/m2. The main comorbidities were dyslipidemia, which was present in 15 patients (36%), followed by high blood pressure (32%) and diabetes (24%). At treatment week 12, there was a statistically significant decrease in body weight (P = 0.053), averaging -1.6 kg, but at follow-up week 24 there was less total body weight loss compared with week 12 (-1.0 vs -1.6 kg) not achieving significant weight loss (P = 0.165). The percentages of total fat, visceral fat, and muscle mass had no significant changes at treatment weeks 12 and 24 (P = 0.873, 0.341, and 0.730, respectively; Table 4). There were no statistically significant changes in transaminase levels (P = 0.185 and 0.644) and their baseline measurements were within the normal range, as in group A. There was a significant decrease in alkaline phosphatase, very LDL-C, C-reactive protein, and insulin at treatment weeks 12 and 24 (P = 0.011, 0.041, 0.018, and 0.015, respectively). There were no significant differences in the remaining biochemical parameters (Table 5).
| Placebo + MD | Baseline | 12 weeks | 24 weeks | P value |
| Somatometry | ||||
| Weight in kg | 82.1 + 14.1 | 79.3 + 12.6 | 79.2 + 13.3 | 0.165 |
| ∆weight | - | -1.6 + 3.7 | -1.0 + 4.6 | 0.206 |
| BMI | 33.3 + 4.7 | 32.1 + 4.3 | 32.2 + 5.0 | 0.16 |
| Visceral fat as % | 11.8 + 2.9 | 12.1 + 2.9 | 12.8 + 4.3 | 0.341 |
| ∆visceral fat | - | 0.33 + 1.6 | 0.05 + 1.6 | 0.624 |
| Total fat as % | 44.5 + 7.3 | 43.0 + 9.6 | 43.7 + 9.1 | 0.873 |
| Muscle as % | 23.9 + 4.2 | 25.0 + 6.2 | 24.0 + 4.1 | 0.73 |
| Circumference in cm | ||||
| Umbilical | 101.2 + 13.2 | 98.8 + 11.8 | 94.1 + 18.7 | 0.07 |
| Hip | 113.0 + 11.3 | 110.3 + 10.2 | 109.3 + 11.3 | 0.182 |
| Waist | 98.4 + 12.6 | 96.4 + 11.8 | 95.7 + 12.3 | 0.056 |
| Waist-to-hip ratio | 0.87 + 0.7 | 0.87 + 0.08 | 0.87 + 0.08 | 0.876 |
| Elastography | ||||
| kPa | 6.9 + 2.3 | 6.5 + 2.8 | 5.9 + 3.1 | 0.132 |
| ∆kPa | - | -0.27 + 2.8 | 1.13 + 3.4 | 0.032 |
| Biochemical parameters | Baseline | 12 weeks | 24 weeks | P value |
| Leukocytes in thousands/μL1 | 6.6 (4.1-9.5) | 6.3 (4-9.6) | 7.7 (4.2-9.9) | 0.050 |
| Hb in g/dL2 | 13.1 + 1.4 | 13.0 + 1.3 | 12.9 + 2.3 | 0.071 |
| Platelets in miles/μL2 | 278 + 73 | 254 + 59 | 251 + 50 | 0.107 |
| TB in mg/dL2 | 0.52 + 0.32 | 0.65 + 0.40 | 0.72 + 0.33 | 0.001 |
| AST in U/L1 | 24 (12-148) | 29 (18-110) | 25 (17-75) | 0.185 |
| ALT in U/L1 | 30 (10-137) | 30 (15-97) | 28 (18-86) | 0.644 |
| ALP in IU/L2 | 101 + 41 | 86 + 30 | 38 + 18 | 0.011 |
| GGT in U/L1 | 36 (19-198) | 26 (11-152) | 72 (28-98) | 0.160 |
| TP in g/dL1 | 7.3 (6.3-8.8) | 7.4 (6.6-8.8) | 7.7 (6.7-8.9) | 0.661 |
| Albumin in g/dL2 | 4.00 + 0.57 | 4.20 + 0.59 | 7.50 + 0.52 | 0.056 |
| Cholesterol in mg/dL2 | 195 + 51 | 203 + 39 | 185 + 39 | 0.457 |
| HDL in mg/dL1 | 44 (23-66) | 44 (32-70) | 48 (27-95) | 0.213 |
| LDL in mg/dL2 | 125 + 53 | 128 + 37 | 110 + 35 | 0.425 |
| VLDL in mg/dL2 | 31.0 + 14.0 | 29.0 + 10.0 | 27.0 + 9.6 | 0.041 |
| Triglycerides in mg/dL2 | 151 + 67 | 145 + 51 | 138 + 48 | 0.116 |
| Glucose in mg/dL1 | 104 (71-175) | 91 (81-148) | 108 (90-177) | 0.332 |
| Creatinine in mg/dL2 | 0.76 + 0.21 | 0.66 + 0.13 | 0.69 + 0.20 | 0.233 |
| CRP in mg/L1 | 2.70 (0.40-1.45) | 0.80 (0.70-0.90) | 0.27 (0.03-3.10) | 0.018 |
| Insulin in mg/L2 | 8.8 + 6.7 | 20.0 + 10.0 | 15.9 + 6.2 | 0.015 |
| HOMA2 | 5.6 + 4.6 | 5.1 + 3.0 | 4.1 + 1.9 | 0.497 |
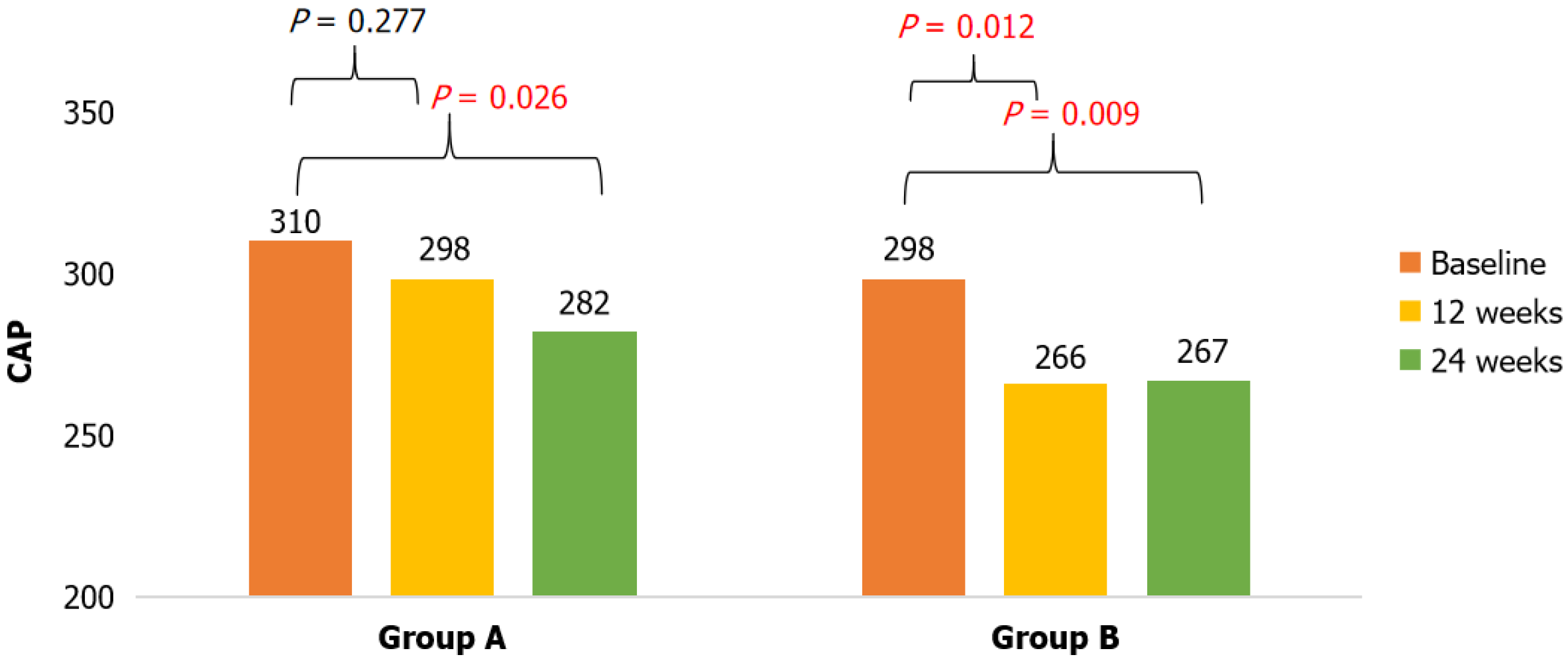
Even though there was no statistically significant decrease in body weight or BMI at treatment weeks 12 and 24 in the two groups, there were greater changes in body composition in the group A patients, with a decrease in the percentage of visceral fat (∆-0.95 kg) and umbilical circumference. The two groups showed a significant decrease in the mean CAP values. In group A, the decrease was gradual and maintained at weeks 12 and 24 (P = 0.026), whereas a greater decrease was seen in group B at 12 weeks and remained unchanged at 24 weeks (∆CAP -27 dB/m). In group A, steatosis deter
Mild adverse effects were reported in 4 patients (16%) in group A and 4 patients (16%) in group B, with no significant differences between the groups (P = 0.641). The most frequently reported symptom was heartburn (3 patients, 6%), followed by nausea, constipation, and diarrhea. No patient suspended treatment, and no severe adverse effects were reported during the follow-up period.
Our results showed that the SM-ALA formulation (LUDLEV®) reduced liver steatosis demonstrated by CAP and improved body composition after 24 weeks of treatment, together with a Mediterranean-style dietary regimen. The implementation of the Mediterranean-style diet and physical activity was the therapeutic intervention demonstrating that the greatest benefit in improving MASLD was weight loss through lifestyle change. The MD has been shown to reduce insulin levels and the Homeostasis Model Assessment of Insulin Resistance index while maintaining body weight stability. Despite these positive results, there are a limited number of studies validating its efficacy, and they point out variations due to geographical regions that may limit its effectiveness[14].
Nehmi-Filho et al[15] evaluated the response to silymarin, without a nutritional intervention or changes in physical activity for 180 days in 133 patients with obesity (BMI ≤ 34.9 kg/m2). They demonstrated a trend toward reduced waist circumference and waist-to-hip ratio in the silymarin group, with no changes in transaminases or the lipid profile. This was similar to results in our patients, in which the group with SM-ALA achieved a decrease in hip and waist circumference, with no statistical significance.
Regarding body composition, a decrease in the percentage of visceral fat was significant but not in the group with placebo. Chiurazzi et al[16] analyzed patients with obesity and MASLD that received a low-calorie MD plus nutraceutical supplementation (vitamin E, L-glutathione, and silymarin) compared with a low-calorie MD and placebo for 3 months. Nutraceutical supplementation significantly improved the anthropometric parameters (weight, waist and hip circumference) compared with the placebo group. Federico et al[17] evaluated the effect at 6 months from having administered the combination of silybin and vitamin D and E in patients with MASLD, showing improvement in the metabolic parameters, oxidative stress markers, and endothelial dysfunction in the treatment group vs the placebo group. Addi
Biochemical parameters showed a trend toward reduced transaminases, total cholesterol, LDL-C, and triglycerides. Changes in metabolic parameters resulting from the administration of SM-ALA have been shown in preclinical trials, especially with silymarin supplements. In our patients, there was a decrease in total cholesterol, LDL-C, and triglycerides at follow-up weeks 12 and 24[18,19].
Transaminases [aspartate aminotransferase and alanine aminotransferase (ALT)], gamma-glutamyl transpeptidase, and alkaline phosphatase are serum markers for liver injury, the most specific of which is ALT[20]. Despite the fact that baseline transaminase levels were within normal parameters, there was a trend toward a decrease in ALT in the SM-ALA group of patients. These results show the hepatoprotective effects of SM-ALA as well as their contribution to improving the biochemical parameters of patients with MASLD.
Reinforcing the hepatoprotective role of silymarin, Moezian et al[21] showed that oral silymarin in patients with nonmetastatic breast cancer significantly reduced liver involvement after chemotherapy with the doxorubicin/cyclophosphamide-paclitaxel regimen. However, there were no differences with the placebo in liver elastography and liver function tests. The benefits to the liver of ALA were described by Tutunchi et al[22] in their comparison of the metabolic effects of ALA, myoinositol, and propolis in obese patients with MASLD. Their clinical trial showed that ALA could improve anthropometric measurements and serum levels of cholesterol, LDLs, triglycerides, and aminotransferases after 8 weeks of use.
No severe adverse effects were reported during the follow-up period. The adverse events that were reported were minimal and mild, and the supplement did not need to be suspended. Thus, the combination of SM and ALA is con
Of our study’s limitations, the first was that the evaluation of steatosis and fibrosis was carried out using noninvasive methods, although the gold standard is the evaluation of histologic improvement. Liver biopsy was not performed because it is an invasive procedure with complication risks. Second, is the fact that the majority of the patients had normal levels of aspartate aminotransferase and ALT, meaning that the response in steatohepatitis could not be evaluated. Importantly, our study excluded patients with cirrhosis of the liver because they did not have the typical clinical progression of steatosis and presented with other complications characteristic of portal hypertension. Therefore, the results could not be generalized to that population.
In conclusion, the SM-ALA formulation (LUDLEV®) combined with the MD may favor the improvement of metabolic parameters as well as the decrease in visceral fat and hepatic steatosis in Mexican patients with MASLD. These data represent an initial study; more studies with larger sample sizes are needed for our results to be generalizable.
The authors kindly acknowledge Productos Medix SA de CV for the investigational drug provided for this study.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7541] [Article Influence: 837.9] [Reference Citation Analysis (0)] |
| 2. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1563] [Cited by in RCA: 1320] [Article Influence: 660.0] [Reference Citation Analysis (0)] |
| 3. | Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Nutr. 2021;8:716783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Hohenester S, Christiansen S, Nagel J, Wimmer R, Artmann R, Denk G, Bischoff M, Bischoff G, Rust C. Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G329-G338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Gillessen A, Schmidt HH. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv Ther. 2020;37:1279-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 6. | Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1358] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 7. | Najafi N, Mehri S, Ghasemzadeh Rahbardar M, Hosseinzadeh H. Effects of alpha lipoic acid on metabolic syndrome: A comprehensive review. Phytother Res. 2022;36:2300-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Martínez-Rodríguez LA, Rojas Serrano J, Torre A. Siliphos Selenium Methionine Alpha Lipoic Acid for Non Alcoholic Fatty Liver Disease: Results of a Pilot Study. Clin Exp Pharmacol. 5:1000167. [DOI] [Full Text] |
| 9. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 10. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 11. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2285] [Article Influence: 126.9] [Reference Citation Analysis (1)] |
| 12. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 840] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 13. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 14. | Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37:936-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 15. | Nehmi-Filho V, Santamarina AB, de Freitas JA, Trarbach EB, de Oliveira DR, Palace-Berl F, de Souza E, de Miranda DA, Escamilla-Garcia A, Otoch JP, Pessoa AFM. Novel nutraceutical supplements with yeast β-glucan, prebiotics, minerals, and Silybum marianum (silymarin) ameliorate obesity-related metabolic and clinical parameters: A double-blind randomized trial. Front Endocrinol (Lausanne). 2022;13:1089938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Chiurazzi M, Cacciapuoti N, Di Lauro M, Nasti G, Ceparano M, Salomone E, Guida B, Lonardo MS. The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Federico A, Dallio M, Masarone M, Gravina AG, Di Sarno R, Tuccillo C, Cossiga V, Lama S, Stiuso P, Morisco F, Persico M, Loguercio C. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxid Med Cell Longev. 2019;2019:8742075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Nehmi VA, Murata GM, Moraes RCM, Lima GCA, De Miranda DA, Radloff K, Costa RGF, Jesus JCR, De Freitas JA, Viana NI, Pimenta R, Leite KRM, Otoch JP, Pessoa AFM. A novel supplement with yeast β-glucan, prebiotic, minerals and Silybum marianum synergistically modulates metabolic and inflammatory pathways and improves steatosis in obese mice. J Integr Med. 2021;19:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Santamarina AB, Moraes RCM, Nehmi Filho V, Murata GM, de Freitas JA, de Miranda DA, Cerqueira ARA, Costa SKP, Ferreira AFF, Britto LR, de Camargo JA, Rodrigues de Oliveira D, de Jesus FN, Otoch JP, Pessoa AFM. The Symbiotic Effect of a New Nutraceutical with Yeast β-Glucan, Prebiotics, Minerals, and Silybum marianum (Silymarin) for Recovering Metabolic Homeostasis via Pgc-1α, Il-6, and Il-10 Gene Expression in a Type-2 Diabetes Obesity Model. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 21. | Moezian GSA, Javadinia SA, Sales SS, Fanipakdel A, Elyasi S, Karimi G. Oral silymarin formulation efficacy in management of AC-T protocol induced hepatotoxicity in breast cancer patients: A randomized, triple blind, placebo-controlled clinical trial. J Oncol Pharm Pract. 2022;28:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Tutunchi H, Arefhosseini S, Ebrahimi-Mameghani M. Clinical effectiveness of α-lipoic acid, myo-inositol and propolis supplementation on metabolic profiles and liver function in obese patients with NAFLD: A randomized controlled clinical trial. Clin Nutr ESPEN. 2023;54:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |