Published online Sep 27, 2024. doi: 10.4254/wjh.v16.i9.1278
Revised: August 10, 2024
Accepted: August 21, 2024
Published online: September 27, 2024
Processing time: 138 Days and 18.3 Hours
C23, an oligo-peptide derived from cold-inducible RNA-binding protein (CIRP), has been reported to inhibit tissue inflammation, apoptosis and fibrosis by binding to the CIRP receptor; however, there are few reports on its role in liver fibrosis and the underlying mechanism is unknown.
To explore whether C23 plays a significant role in carbon tetrachloride (CCl4)-induced liver fibrosis.
CCl4 was injected for 6 weeks to induce liver fibrosis and C23 was used beginning in the second week. Masson and Sirius red staining were used to examine changes in fiber levels. Inflammatory factors in the liver were detected and changes in α-smooth muscle actin (α-SMA) and collagen I expression were detected via immu
CCl4 successfully induced liver fibrosis in mice, while tumor necrosis factor-alpha (TNF-α), IL (interleukin)-1β, and IL-6 levels increased significantly and the IL-10 level decreased significantly. Interestingly, C23 inhibited this process. On the other hand, C23 significantly inhibited the activation of HSCs induced by CCl4, which inhibited the expression of α-SMA and the synthesis of collagen I. In terms of mechanism, C23 can block Smad3 phosphorylation significantly and inhibits TGF-β/Smad3 pathway activation, thereby improving liver injury caused by CCl4.
C23 may block TGF-β/Smad3 axis activation, inhibit the expression of inflammatory factors, and inhibit the activation of HSCs induced by CCl4, alleviating liver fibrosis.
Core Tip: C23, an oligo-peptide derived from cold-inducible RNA-binding protein inhibits the activation of hepatic stellate cells induced with carbon tetrachloride and the expression of collagen I and α-smooth muscle actin. C23 inhibits the expression of liver inflammatory factors and downregulates transforming growth factor-beta/Smad3 pathway activation, thereby alleviating liver fibrosis.
- Citation: Tang RX, Xie XJ, Xiong Y, Li S, Luo C, Wang YG. C23 ameliorates carbon tetrachloride-induced liver fibrosis in mice. World J Hepatol 2024; 16(9): 1278-1288
- URL: https://www.wjgnet.com/1948-5182/full/v16/i9/1278.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i9.1278
At present there is no specific and effective drug for treating liver fibrosis caused by acute or chronic injury. Although preclinical research has made breakthroughs, their suitability as clinical treatments is still unknown. The activation of hepatic stellate cells (HSCs) caused by chronic inflammation is a key process in the development of liver fibrosis and activated HSCs express α-smooth muscle actin (α-SMA) and transdifferentiate into myofibroblasts with proliferation, migration and secretion abilities, synthesizing the extracellular matrix to deposit in the hepatocyte space and subse
Extracellular cold inducible RNA binding protein (CIRP) has been shown to play a role in various acute and chronic inflammatory diseases by promoting tissue inflammation and apoptosis and inducing fibrosis through its receptor Toll-like receptor 4 (TLR4)[3]. C23 is a recognized competitive inhibitor of CIRP that can competitively bind to CIRP receptors and reduce tissue damage in inflammatory diseases[4]. C23 has been shown to significantly reduce serum tumor necrosis factor-alpha (TNF-α), IL (interleukin)-6 and IL-1β levels. In addition, it can reduce tissue TLR4, TNF-α, IL-6 and IL-1β levels and inhibit the colocalization of CIRP and TLR4, which plays a significant role in systemic inflammation[5]. Re
These animal experiments were approved by the Ethics Committee of the General Hospital of Western Theater Command (protocol code 2023EC004) and comply with the Guidelines for Animal Experiments on Laboratory Animals. Thirty-two male C57BL/6J mice (20 ± 2 g) were purchased from Chengdu Dashuo Experimental Animal Company and adaptively fed for 1 week at 22 °C with 50% humidity and a 12-hour day/night cycle. The animals were randomly divided into four groups: a control group, C23 group, CCl4 group, and a CCl4 + C23 group. In the CCl4 and CCl4 + C23 groups, CCl4 (0.5 μL/g) (Meryer, m81121, China) was intraperitoneally injected twice a week for 6 weeks to induce liver fibrosis. C23 (Peptide sequence: GRGFSRGGGDRGYGG) was synthesized by Tgpeptide, Inc. (China). In the CCl4 + C23 group C23 (8 mg/kg) was injected for 5 weeks. In the sham group, an equal volume of solvent was injected and in the C23 group C23 (8 mg/kg) was injected for 5 weeks. After the experiment, livers were removed for fixation and freezing.
The liver tissues were removed, trimmed to a size of 1 cm × 1 cm, fixed in 4% paraformaldehyde (Biosharp, BL539A, China), routinely dehydrated, paraffin embedded, sectioned at 3 μM, pasted on adhesive glass slides, and stored at room temperature after being baked in paraffin[9]. The samples were subjected to conventional dewaxing and rehydration, hematoxylin staining for 3 minutes, washing with running water for 3 minutes, differentiation with 1% hydrochloric acid (concentrated hydrochloric acid:75% alcohol = 1:99) for 5 s, washing with running water for 10 minutes, eosin staining for 5 s, washing with running water without bound eosin, and sealing with neutral gum after drying naturally. After the neutral gum was completely solidified, photos were taken under a light microscope.
Sirius red staining was performed on the paraffin sections. The procedure used before dewaxing and rehydration of the sections was the same as that used for hematoxylin and eosin (HE) staining. After dewaxing and rehydration the sections were stained with iron hematoxylin for 5 minutes, washed twice with distilled water for 3 minutes each time, stained with Sirius red dye solution (Solarbio, G1472, China) for 15 minutes, washed twice with distilled water for 3 minutes each time, dehydrated, cleared routinely, and sealed with medium gum. Finally, images were taken under a light microscope and the fibrosis ratio was determined using image-pro plus (IPP).
Masson (Solarbio, G1340, China) staining was performed on the paraffin sections. The procedure used before dewaxing and rehydration of the sections was the same as that used for HE staining. After dewaxing and rehydration, mordant staining was performed in a 60 °C incubator for 1 h and the sections were rinsed with running water for 10 min, stained with azurol blue for 3 minutes, washed with distilled water twice for 10 s each time, subjected to Mayer hematoxylin stain
Immunohistochemical staining was performed on paraffin-embedded sections. The procedure used before dewaxing and rehydration of the sections was the same as that used for HE staining. The sections were boiled in acid repair solution for 10 minutes to expose the antigen, blocked with 5% goat serum (ZSGB-BIO, ZLI-9056, China), incubated with primary antibodies against α-SMA (Abcam, ab7817, United Kingdom) and collagen I (Abcam, ab270993, United Kingdom) at a dilution ratio of 1:100, and incubated overnight at 4 °C. According to the manufacturer’s instructions for the secondary antibody (ZSGB-BIO, PV9000, China), the secondary antibody was added dropwise and incubated at room temperature for 30 minutes. Diaminobenzidine (ZSGB-BIO, ZLI-9018, China) was used for color development followed by hemato
At 5 weeks after the procedure the mice were euthanized and their livers were isolated and stored in RNA later at 80 °C. Total RNA was transcribed into cDNA, synthesized into cRNA and labeled with cyanine-3-CTP. A mouse microarray was generated and a heatmap was plotted at https://www.bioinformatics.com.cn (last accessed on 20 Feb 2024), an on
The frozen liver was added to phosphate buffered solution, homogenized, and centrifuged at 10000 rpm for 20 minutes, after which the supernatant was removed. Liver TNF-α (Nanjing Jiancheng BIO, H052-1-2, China), IL-1β (Nanjing Jiancheng BIO, H002-1-2, China), IL-6 (Nanjing Jiancheng BIO, H007-1-2, China) and IL-10 (Nanjing Jiancheng BIO, H009-1-2, China) levels were detected according to the kit instructions.
Total protein was extracted from 100 mg of frozen liver tissue according to the instructions of the protein extraction kit (KeyGEN, KGB5303, China) and the protein content was calculated with a BCA kit (KeyGEN, KGB2101, China). Proteins were separated with SDS-PAGE electrophoresis, transferred to PVDF membranes (Millipore, IPFL00010, United States), blocked with 5% nonfat dry milk for 30 minutes, and incubated with primary antibodies against Smad3 (CST, 9523, United States) (dilution ratio, 1:1000), phosphorylated Smad3 (CST, 9520, United States) (dilution ratio, 1:500), TGF-β (Abcam, ab179695, United Kingdom) (dilution ratio, 1:1000) and β-actin (CST, 4967, United States) (dilution ratio, 1:4000) overnight at 4 °C. Goat anti-rabbit antibody (dilution ratio, 1:2000) was added dropwise and the samples were incubated at room temperature for 30 minutes. The gray value of each group was calculated after chemiluminescence.
Statistical analysis was performed using IBM SPSS statistics software V19.0 (IBM, United States). The data are expressed as means ± SDs. The normality of the data distribution was tested using the Shapiro-Wilk test. Unpaired Student's t tests (two-tailed) were used to compare two groups and one-way ANOVA and Tukey's post hoc test were used to compare the mean values of each group with those of the other group. A P value < 0.05 was considered statistically significant. The statistical methods were reviewed by Yao-Lei Zhang from the General Hospital of Western Theater Command.
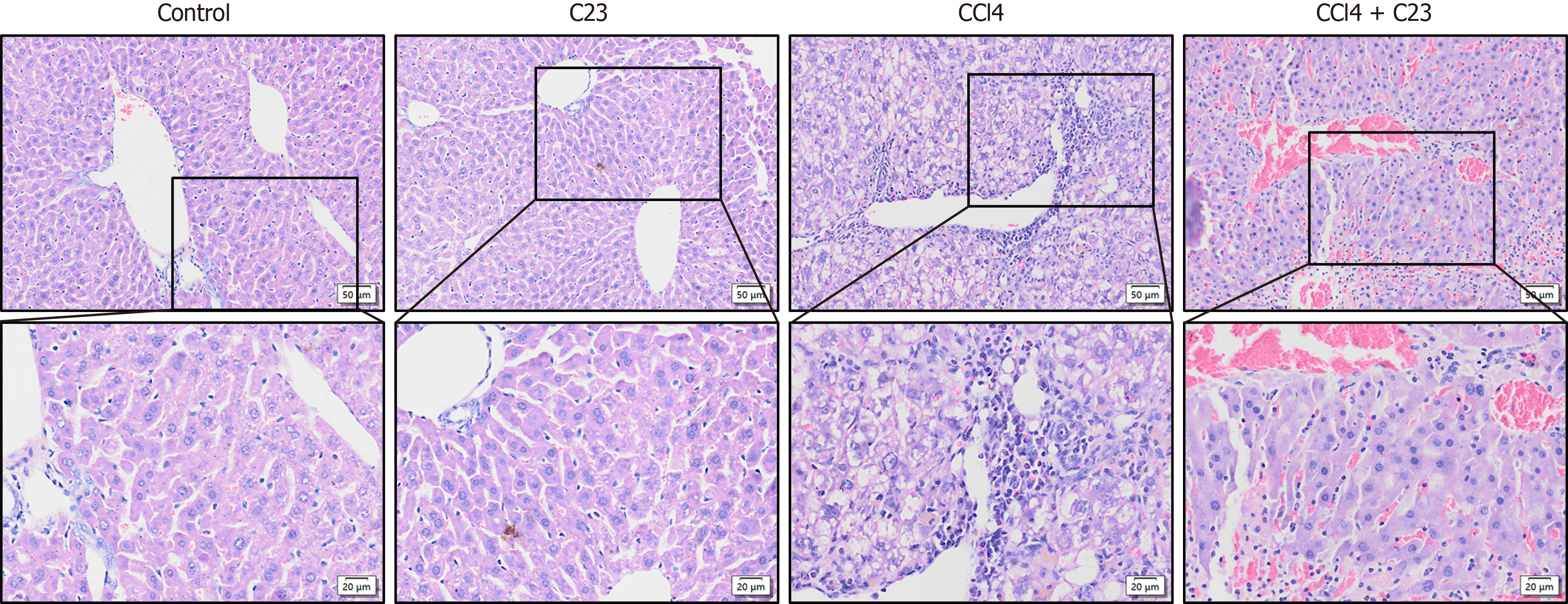
HE staining was used to evaluate the liver pathological changes in each group. Compared with those in the control group CCl4-induced liver diffuse inflammatory cell infiltration, vacuolar fat changes and fibrosis were not caused by C23 injection alone. Compared with those in the CCl4 group diffuse inflammatory cell infiltration and the areas of fibrosis and vacuolar fat were significantly lower in the CCl4+C23 group (Figure 1). These results suggest that C23 can significantly ameliorate CCl4-induced liver fibrosis and hepatocyte inflammation.
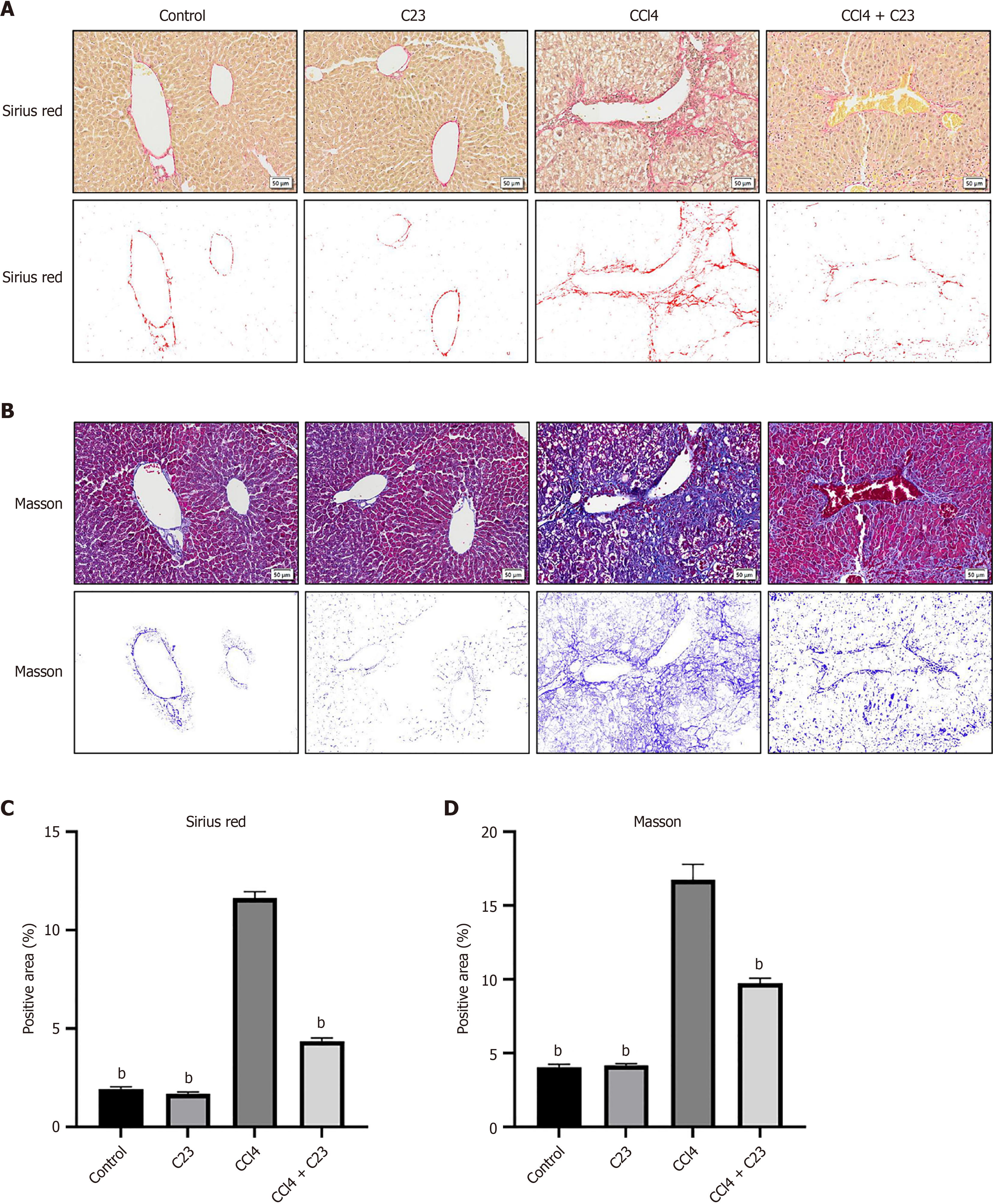
To further investigate the effect of C23 on collagen synthesis in the liver, changes in collagen expression in each group were detected with Sirius red and Masson staining (Figure 2A and B). Compared with the control C23 injection alone did not significantly change the collagen content (P > 0.05), and CCl4 significantly promoted the expression of type I and type III collagen (P < 0.05) (Figure 2C). Compared with CCl4, C23 significantly inhibited the production of type I and type III collagen (Figure 2C). These results were consistent with the above results (Figure 2B and D). These data further suggest that C23 could significantly improve CCl4-induced liver fibrosis.
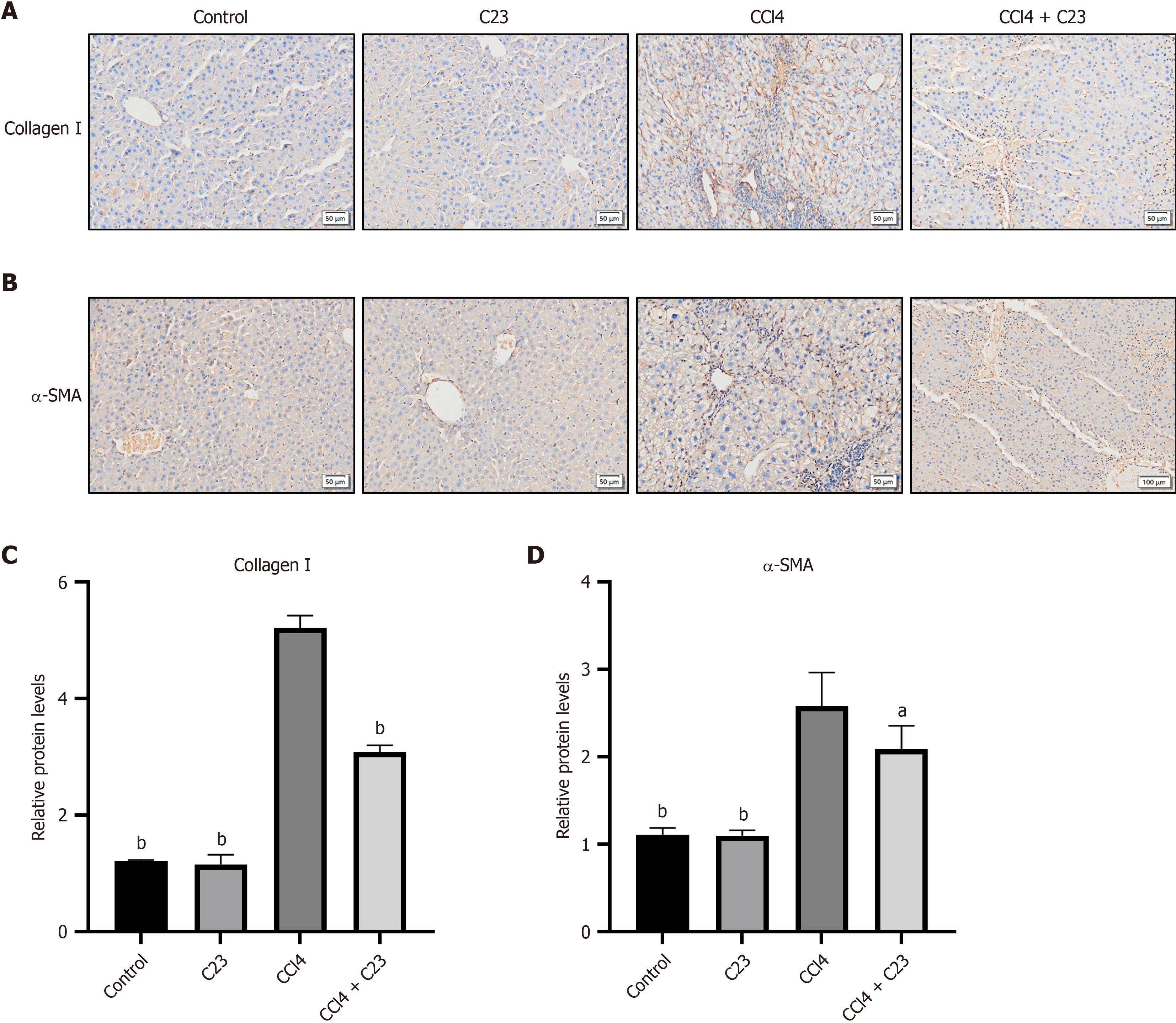
α-SMA is an important marker of HSC proliferation and activation. Activated HSCs express a large amount of collagen I, which leads to liver fibrosis. According to the immunohistochemical results (Figure 3A and B), compared with the control C23 injection alone did not affect α-SMA or collagen I expression (P > 0.05). Compared with the control CCl4 significantly promoted the expression of α-SMA and collagen I (P < 0.05), and C23 inhibited the expression of α-SMA and collagen I (P < 0.05) (Figure 3C and D). These results suggest that C23 could inhibit the activation of HSCs induced by CCl4 and alleviate liver fibrosis.
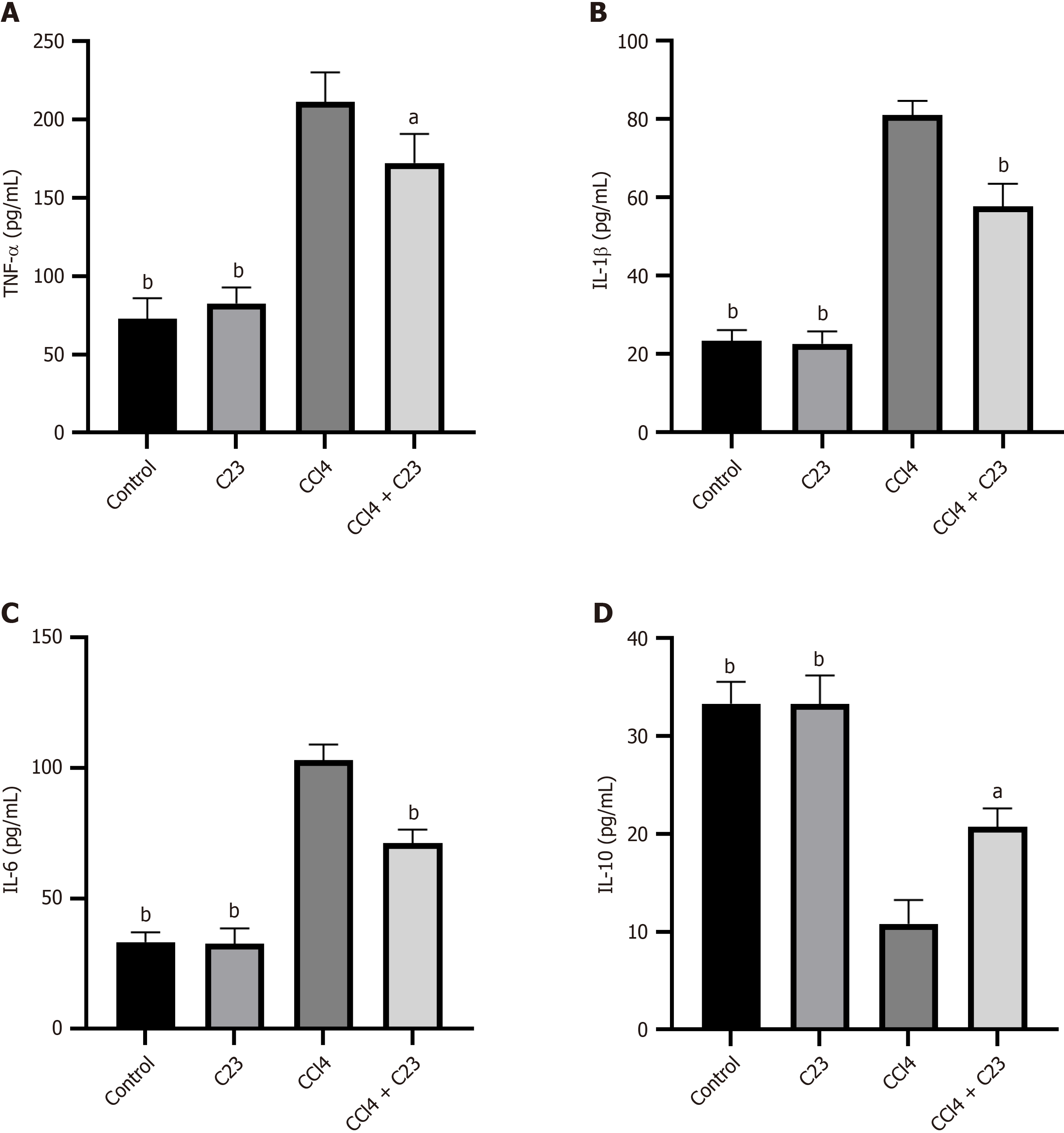
Inflammation is an important part of CCl4-induced fibrosis. Changes in the expression of inflammatory factors in the liver were detected using enzyme-linked immunosorbent assays. Compared with the control, CCl4 promoted the expression of inflammatory factors (TNF-α, IL-1β, and IL-6) (P < 0.05) (Figure 4A-C) and the expression of the anti-inflammatory factor IL-10 was downregulated (P < 0.05) (Figure 4D). Compared with CCl4, C23 significantly inhibited TNF-α, IL-1β, and IL-6 expression (P < 0.05) (Figure 4A-C) and promoted IL-10 expression (P < 0.05) (Figure 4D). These results suggest that C23 could significantly alleviate CCl4-induced liver inflammation.
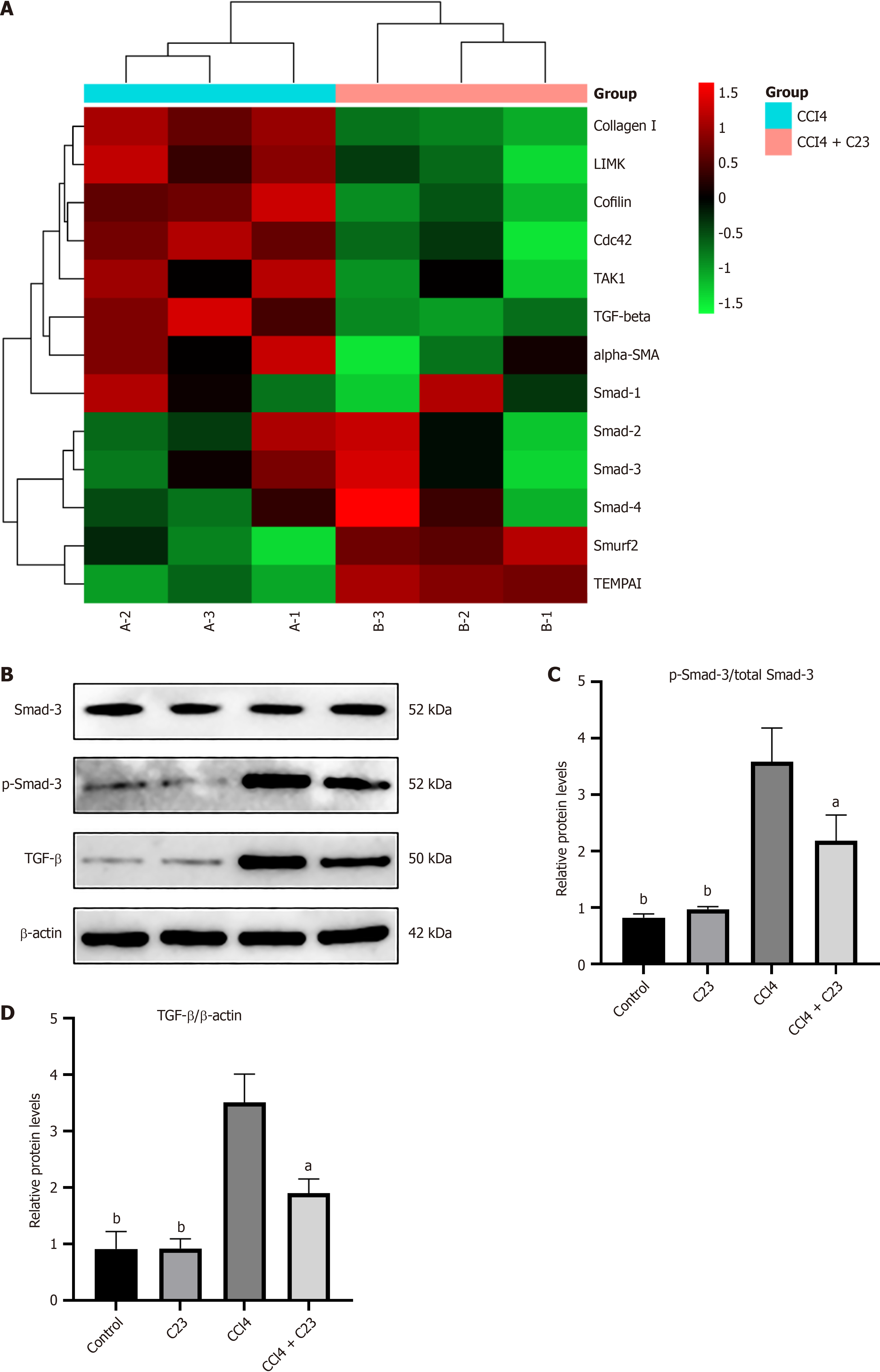
Five weeks after the procedure the livers were harvested for transcriptome array analysis. Among the 30 genes involved in the regulation of fibrosis function, 5 were significantly upregulated while 8 genes were downregulated in the CCl4+C23 group compared with the CCl4 group (Figure 5A). Notably, TGF-β was the gene whose expression was most significantly decreased on Day 30 (Figure 5A). To confirm the change in TGF-β expression in the liver, we measured the protein level using western blotting (Figure 5B). The TGF-β/Smad3 pathway is an important regulatory pathway in
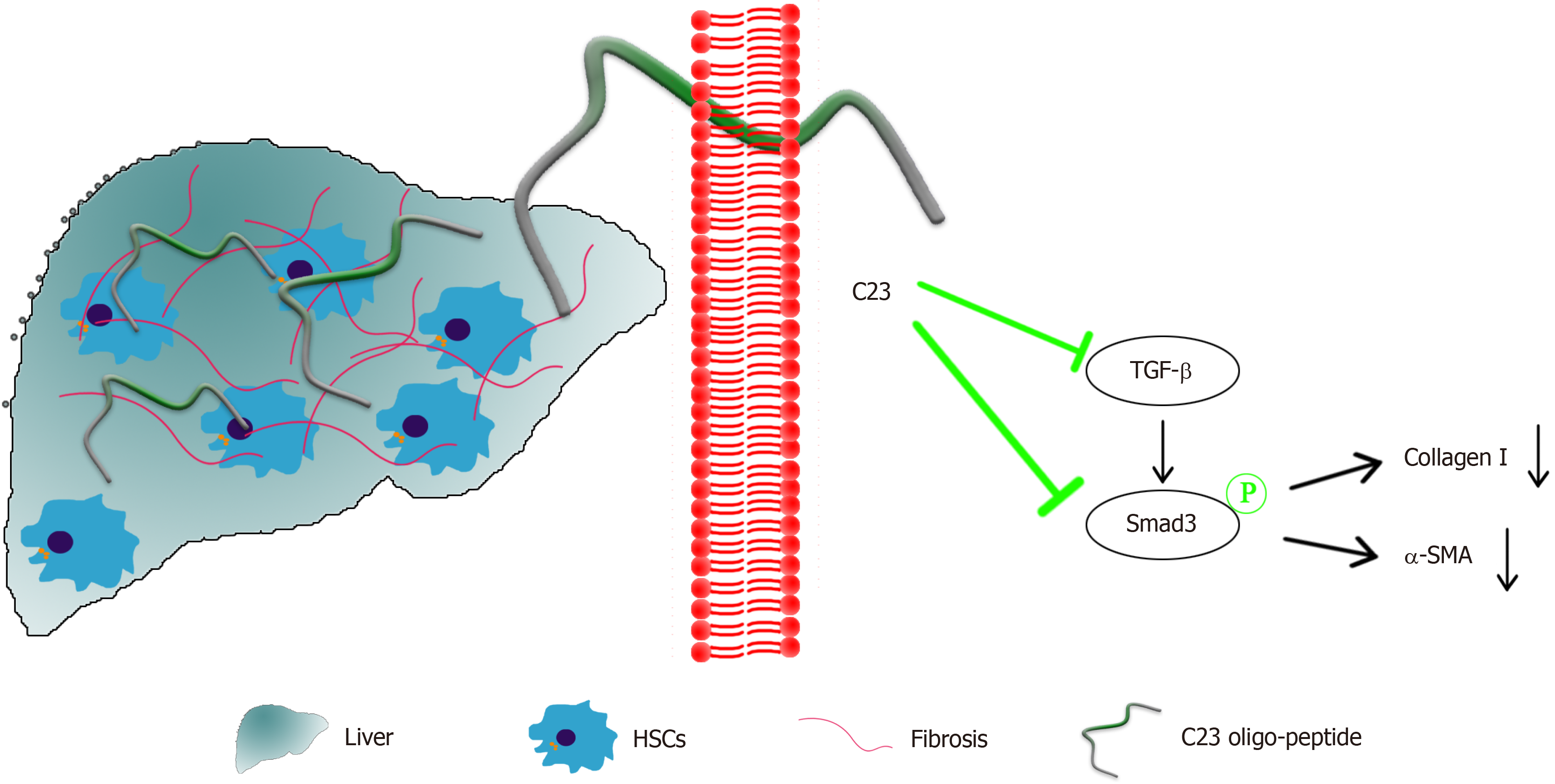
This study revealed for the first time the inhibitory effect of C23 on liver fibrosis and the underlying mechanism involved. C23 was first shown to inhibit the activation of HSCs induced by CCl4 and to inhibit the expression of collagen I and α-SMA. C23 inhibits the expression of liver inflammatory factors and downregulates TGF-β/Smad3 pathway activation, thereby alleviating liver fibrosis (Figure 6).
Liver fibrosis is a progressive pathological process involving the failure of the regenerative capacity of hepatocytes eventually leading to the development of liver cirrhosis and even hepatocellular carcinoma[10]. Liver fibrosis is a mu
Some studies have investigated the use of other molecules for reducing liver fibrosis. Sappanone A alleviates liver fibrosis by regulating inflammation and macrophage polarization. Oxytrimene alleviates fibrosis by regulating inflammation and the TGF signaling pathway. However, these small molecules have demonstrated varying levels of patho
In preclinical models of inflammatory diseases, CIRP-/- mice exhibited significant inhibition of inflammation and alleviation of tissue damage and the levels of IL-6, TNF-α, IL-1β and other inflammatory factors were attenuated. Mo
TGF-β is a recognized profibrotic cytokine that exerts its biological and pathological effects through the Smad signaling pathway. A variety of inhibitors that inhibit the TGF signaling pathway have been found to block liver fibrosis[21,22]. To clarify the relationship between the TGF-β/Smad3 signaling pathway and C23 we explored the molecular mechanism by which C23 alleviates liver fibrosis. Experiments showed that C23 significantly inhibits TGF-β expression and Smad3 phosphorylation. These findings suggest that C23 may inhibit the proliferation and activation of HSCs through the TGF-β/Smad3 signaling pathway. Whether C23 can also affect liver fibrosis through other pathways needs further in-depth study.
Our investigation demonstrated that C23 could act as a negative regulator to inhibit inflammation and alleviate liver fibrosis by blocking the activation of the TGF-β/Smad3 signaling pathway. These findings provide theoretical evidence that C23 is a molecular target for the treatment of liver fibrosis.
| 1. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 1059] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 2. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 259] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 3. | Liao Y, Tong L, Tang L, Wu S. The role of cold-inducible RNA binding protein in cell stress response. Int J Cancer. 2017;141:2164-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Wang S, Wang W, Song L, Feng S, Wang J, Kang T, Yang P, Wang N, Yang P, Bai R, Shao Y, Zheng Y. Extracellular CIRP Upregulates Proinflammatory Cytokine Expression via the NF-kappaB and ERK1/2 Signaling Pathways in Psoriatic Keratinocytes. Mediators Inflamm. 2022;2022:5978271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Gao Y, Liu H, Zhou J, Guo M, Sun J, Duan M. THE PROTECTIVE EFFECT OF C23 IN A RAT MODEL OF CARDIAC ARREST AND RESUSCITATION. Shock. 2023;59:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bolourani S, Sari E, Brenner M, Wang P. Extracellular CIRP Induces an Inflammatory Phenotype in Pulmonary Fibroblasts via TLR4. Front Immunol. 2021;12:721970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Bolourani S, Sari E, Brenner M, Wang P. The role of eCIRP in bleomycin-induced pulmonary fibrosis in mice. PLoS One. 2022;17:e0266163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Zhang F, Brenner M, Yang WL, Wang P. A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice. Sci Rep. 2018;8:3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Guo X, Li T, Feng Y, Li W, Zhu X, Gu R, Zhou L. Uncoupling protein 2 prevents ischaemia reperfusion injury through the regulation ROS/NF-κB signalling in mice. Mol Membr Biol. 2019;35:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1166] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 11. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 12. | Trauner M, Fuchs CD. Novel therapeutic targets for cholestatic and fatty liver disease. Gut. 2022;71:194-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 13. | Qi J, Li L, Yan X, Hua W, Zhou Z. Sappanone A Alleviates the Severity of Carbon Tetrachloride-Induced Liver Fibrosis in Mice. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 14. | Zhao HW, Zhang ZF, Chai X, Li GQ, Cui HR, Wang HB, Meng YK, Liu HM, Wang JB, Li RS, Bai ZF, Xiao XH. Oxymatrine attenuates CCl4-induced hepatic fibrosis via modulation of TLR4-dependent inflammatory and TGF-β1 signaling pathways. Int Immunopharmacol. 2016;36:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Sharma A, Brenner M, Jacob A, Marambaud P, Wang P. Extracellular CIRP Activates the IL-6Rα/STAT3/Cdk5 Pathway in Neurons. Mol Neurobiol. 2021;58:3628-3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Geng H, Zhang H, Cheng L, Dong S. Corrigendum to "Sivelestat ameliorates sepsis-induced myocardial dysfunction by activating the PI3K/AKT/mTOR signaling pathway" [Int. Immunopharmacol. 128 (2024) https://doi.org/10.1016/j.intimp.2023.111466]. Int Immunopharmacol. 2024;131:111873. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Lujan DA, Ochoa JL, Hartley RS. Cold-inducible RNA binding protein in cancer and inflammation. Wiley Interdiscip Rev RNA. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Shimizu J, Murao A, Nofi C, Wang P, Aziz M. Extracellular CIRP Promotes GPX4-Mediated Ferroptosis in Sepsis. Front Immunol. 2022;13:903859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 19. | Zhou K, Cui S, Duan W, Zhang J, Huang J, Wang L, Gong Z, Zhou Y. Cold-inducible RNA-binding protein contributes to intracerebral hemorrhage-induced brain injury via TLR4 signaling. Brain Behav. 2020;10:e01618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Park HS, Song JW, Park JH, Lim BK, Moon OS, Son HY, Lee JH, Gao B, Won YS, Kwon HJ. TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy. 2021;17:2549-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 21. | Györfi AH, Matei AE, Distler JHW. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018;68-69:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 22. | Yang JH, Ku SK, Cho ILJ, Lee JH, Na CS, Ki SH. Neoagarooligosaccharide Protects against Hepatic Fibrosis via Inhibition of TGF-β/Smad Signaling Pathway. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |