Published online Aug 27, 2024. doi: 10.4254/wjh.v16.i8.1111
Revised: May 28, 2024
Accepted: June 18, 2024
Published online: August 27, 2024
Processing time: 116 Days and 7.3 Hours
Acute liver failure (ALF) may be the first and most dramatic presentation of Wilson’s disease (WD). ALF due to WD (WD-ALF) is difficult to distinguish from other causes of liver disease and is a clear indication for liver transplantation. There is no firm recommendation on specific and supportive medical treatment for this condition.
To critically evaluate the diagnostic and therapeutic management of WD-ALF patients in order to improve their survival with native liver.
A retrospective analysis of patients with WD-ALF was conducted in two pediatric liver units from 2018 to 2023.
During the study period, 16 children (9 males) received a diagnosis of WD and 2 of them presented with ALF. The first was successfully treated with an unconventional combination of low doses of D-penicillamine and zinc plus steroids, and survived without liver transplant. The second, exclusively treated with supportive therapy, needed a hepatotransplant to overcome ALF.
Successful treatment of 1 WD-ALF patient with low-dose D-penicillamine and zinc plus steroids may provide new perspectives for management of this condition, which is currently only treated with liver transplantation.
Core Tip: There is no firm recommendation on the management of acute liver failure due to Wilson's disease, for which liver transplantation is the only reliable treatment option. The retrospective study highlights the successful treatment of a patient with an unconventional low-dose therapy of D-penicillamine and zinc plus steroids with the aim of opening new perspectives in the management of the condition, stimulating a critical evaluation on the topic and thus promoting the use of all possible therapeutic opportunities to increase survival with the native liver.
- Citation: Delle Cave V, Di Dato F, Calvo PL, Spagnuolo MI, Iorio R. Successful treatment of acute liver failure due to Wilson’s disease: Serendipity or fortuity? World J Hepatol 2024; 16(8): 1111-1119
- URL: https://www.wjgnet.com/1948-5182/full/v16/i8/1111.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i8.1111
Wilson’s disease (WD) is an autosomal recessive disorder of copper metabolism, caused by mutations in the ATP7B gene[1]. The estimated global prevalence of WD is 1:30000 to 1:50000 cases in the United States, Europe, and Asia[2], with a carrier frequency of a single mutation of 1 in 90 individuals[3]. In some areas, such as Croatia, Costa Rica, and the Italian island of Sardinia, a higher frequency of WD has been reported, probably related to inbreeding or higher mutation frequencies[2].
Clinical presentation can vary widely, but the key features are liver disease and neurological and psychiatric disorders. Hepatic manifestations may range from asymptomatic conditions with increased liver enzymes or hepatomegaly[4], to acute or chronic liver disease. In some cases, acute liver failure (ALF) is the initial and most dramatic presentation of WD[5-7]. Fulminant WD can be associated with Coombs-negative hemolytic anemia and quickly progresses to hepatic and renal failure leading to 95% mortality if untreated[8]. An urgent liver transplant is often the only chance for survival.
ALF due to WD (WD-ALF) is a diagnostic challenge due to the lack of sensitive and specific diagnostic criteria[9]. The overall therapeutic goal of WD is to block or reverse organ damage related to copper accumulation, which can be achieved through medical therapy, or in the case of non-response, through liver transplantation. The treatment for WD patients includes copper chelators (D-penicillamine or trientine) or zinc salts according to available guidelines[10-12]. It has been reported that the early diagnosis and treatment of WD prevents the onset of neurologic damage, even at the subclinical level[13]. For WD patients, lifelong medical therapy is required. The choice of drug depends on the clinical phenotype, and treatment should be individualized based on the severity of symptoms. Unfortunately, no consensus is available on the pharmacological strategies to use for WD-ALF. Therefore, given the scarcity of literature to date, the management of WD-ALF patients remains one of the greatest challenges for pediatric and adult hepatologists.
The purpose of this article is to focus on the diagnostic and therapeutic management of WD-ALF and stimulate critical evaluation of the possible treatments that can be used to promote survival with native liver.
A retrospective analysis was conducted on patients with WD diagnosed at the Pediatric Liver Unit of the University of Naples Federico II (Naples, Italy) and the Pediatric Gastroenterology Unit at Regina Margherita Pediatric Hospital (Turin, Italy), from 2018 to 2023. Among these patients, those with WD-ALF were selected. The diagnosis of WD-ALF was performed according to current guidelines[10-12]. The clinical and laboratory characteristics, as well as the therapeutic strategies used, were recorded and compared with the literature. New Wilson Index[14] and Nazer score[15] were applied for prognostic evaluation of the disease. The study was conducted in accordance with the Declaration of Helsinki for Human Research.
During the study period, 16 patients (9 males) were diagnosed with WD at a median age of 9 years (range: 5-18 years). Among these were 7 patients with hepatic presentation characterized by hepatomegaly and/or increased liver enzymes, 4 patients identified following molecular screening in absence of clinical manifestations, 2 patients with ALF onset, 1 patient with neurologic symptoms (tremors, dysarthria, gait disturbance), 1 patient with mixed hepatic and neuropsychiatric manifestations, and 1 patient with tubular nephropathy and concomitant signs of chronic liver disease. The 2 cases with WD-ALF underwent two different therapeutic strategies with a different outcome, as reported below.
An 11-year-old Caucasian male was admitted to a local hospital in Eastern Europe due to sudden onset of jaundice and melena. There were no previous problems in his medical history. Physical examination revealed severe jaundice, hepatomegaly, and mild ascites. His vital signs were normal. Laboratory examination showed the following: Hyperbilirubinemia [total bilirubin (TB) 14.5 mg/dL, normal range: < 1.20 mg/dL, 70% unconjugated]; increased levels of alanine aminotransferase (ALT) (264 IU/L, normal range: < 40 IU/L), aspartate aminotransferase (AST) (120 IU/L, normal range: < 40 IU/L), and gamma-glutamyl transferase (GGT) (170 IU/L, normal range: < 70 IU/L); and low alkaline phosphatase (ALP) serum levels (69 IU/L, normal range: 142-335 IU/L). Coagulation parameters were impaired, with a strikingly abnormal international normalized ratio (INR) (4.48, normal range: 0.8-1.2) that was unresponsive to vitamin K infusion. Renal function was normal.
The diagnosis of ALF was performed according to the criteria of the Pediatric ALF Study Group[16]. There were no signs of hepatic encephalopathy. Neuropsychiatric symptoms were absent. Higher mental function, central nervous system examination, and electroencephalography were normal. The blood count showed low hemoglobin (9.1 g/dL, normal range: 11.0-16.0 g/dL) and platelets (22.000/μL, normal range: 150.000-450.000/μL). Unfortunately, the Coombs test and reticulocyte count were not tested. The patient denied taking alcohol, drugs, or any medication. Ultrasound investigation showed hepatosplenomegaly and a moderate amount of ascitic fluid. Abdominal computed tomography was suggestive of liver cirrhosis with ascites.
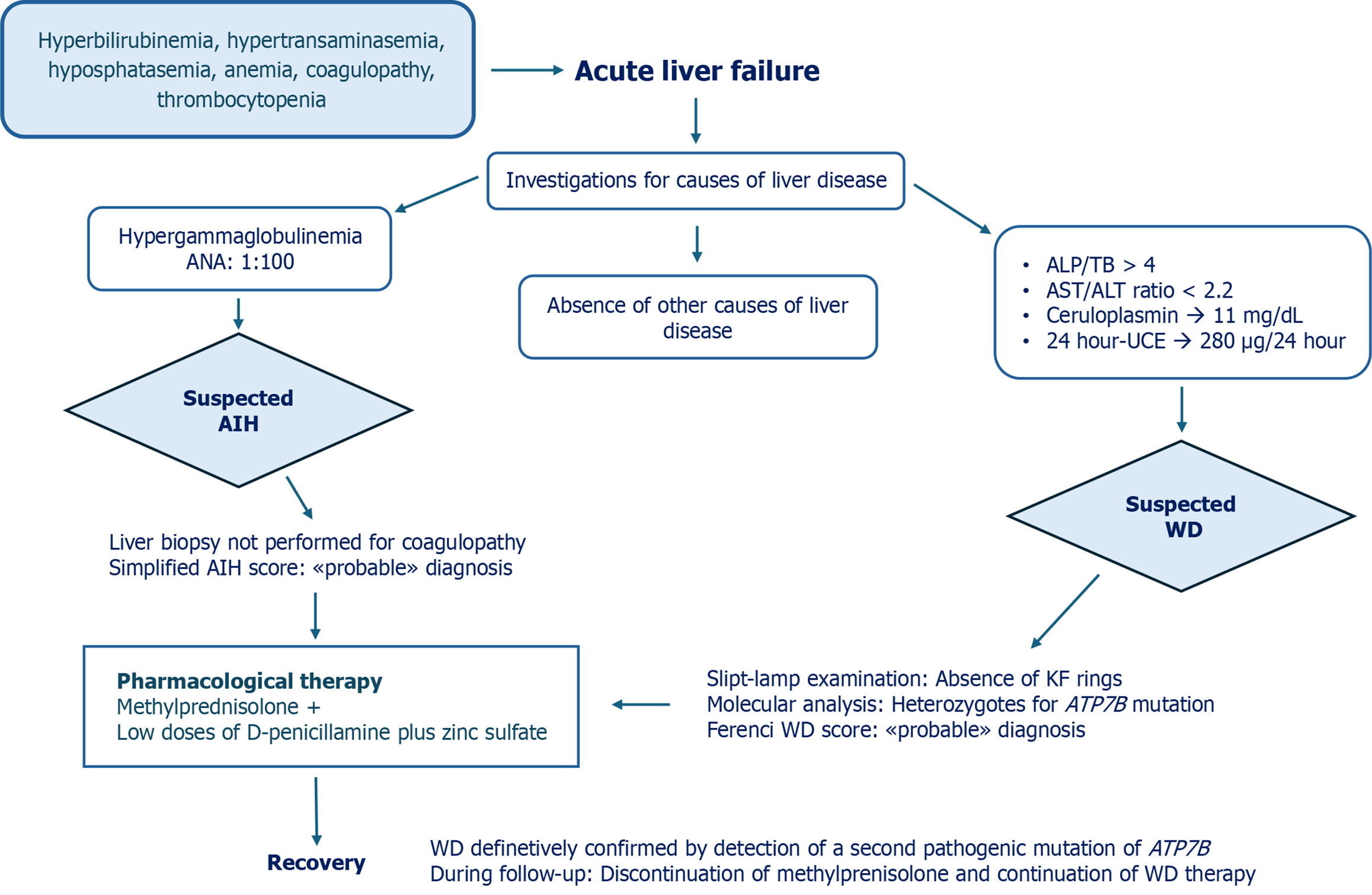
Further investigations showed positive antinuclear antibody (ANA) (titer 1:100) as well as an increase in gamma globulin serum levels. To rule out WD, ceruloplasmin and 24-hour urinary copper excretion (UCE) were tested and yielded the following results: 11 mg/dL (normal range: 20-60 mg/dL) and 280 µg/24 hour (normal range: < 40 µg/24 hour), respectively.
ALF associated with the presence of hemolysis (anemia and unconjugated hyperbilirubinemia), low levels of ceruloplasmin, increased 24-hour UCE, and low serum ALP levels raised the suspicion of WD. Slit-lamp examination revealed no evidence of Kayser-Fleischer rings in the cornea. Liver biopsy was not performed due to the high risk of bleeding.
To define the diagnosis of WD, molecular analysis of the ATP7B gene was performed and the H1069Q mutation heterozygosity was detected. Applying the Ferenci score, the patient obtained a score of 3, suggestive of “probable” diagnosis of WD[17]. The diagnostic tools for rapid diagnosis of WD in presence of ALF-the ALP/TB ratio and AST/ALT ratio-yielded findings of 4.75 and 0.45, respectively. These results, according to the criteria of Korman et al[5], were not suggestive of WD-ALF. Given the ALF setting, the New Wilson Index and Nazer score were 13 and 8, respectively, both indicative of poor survival without liver transplantation (Table 1)[14,15].
| Parameter | Value | New Wilson Index | Nazer score |
| Total bilirubin in mg/dL | 14.7 | 3 | 3 |
| INR | 4.48 | 4 | 4 |
| AST in IU/L | 139 | 1 | 1 |
| WBC as × 109/L | 14.9 | 3 | NA |
| Alb in g/dL | 2.77 | 2 | NA |
| Total score | NA | 13 | 8 |
Due to the undefined WD diagnosis and given the presence of elements suggestive of autoimmune hepatitis (AIH) such as hypergammaglobulinemia, positive ANA, absence of infectious diseases, and no exposure to alcohol or drugs, the simplified criteria for the diagnosis of AIH were applied, yielding a score of 6 indicative of “probable” AIH diagnosis[18].
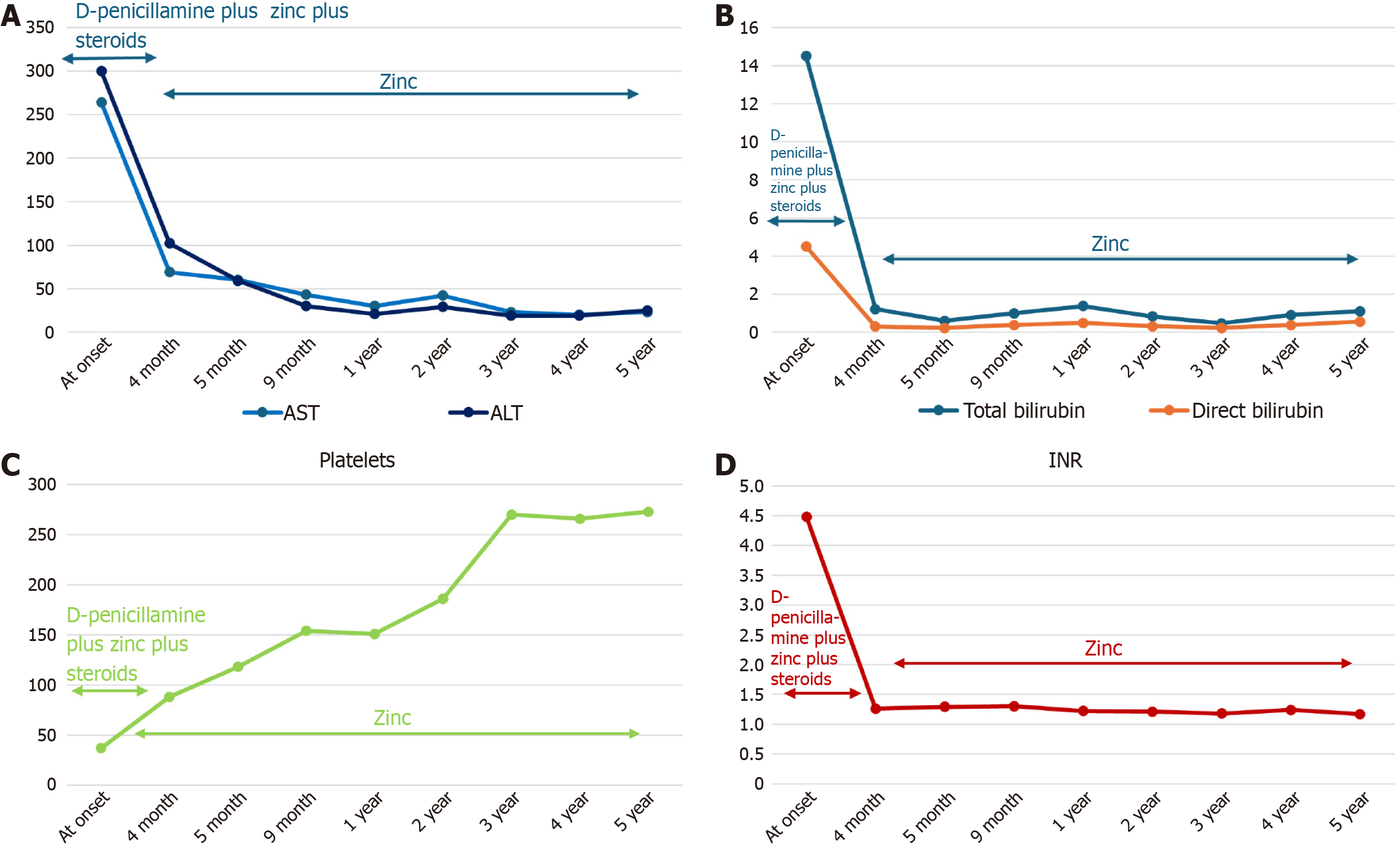
Given the probable diagnosis of both AIH and WD, the patient was treated at the local hospital with methylprednisolone at a dosage of 1 mg/kg/d and low doses of D-penicillamine (1.4 mg/kg/day) and zinc sulfate (124 mg/day). Vitamin K and ursodeoxycholic acid were also prescribed and an intravenous plasma infusion was administered. Because of progressive improvement in clinical conditions and laboratory tests, liver transplantation was not performed. After 4 months from onset, the patient was referred to the Federico II Pediatric Liver Unit for a better diagnostic definition. At the first clinical evaluation, he was anicteric, in good clinical condition, and had a normal INR. He no longer had anemia, hypergammaglobulinemia, or ANA positivity, and the platelets had increased to 88.000/μL. His 24-hour UCE was 60 μg while on combined therapy with D-penicillamine and zinc, and ceruloplasmin serum levels were confirmed to be 11 mg/dL. Abdominal ultrasound showed hepatomegaly with mild hepatic steatosis. Esophagogastroduodenoscopy did not detect esophageal varices.
Given the clinical suspicion of WD, the finding of ANA in the serum only once, the early normalization of serum immunoglobulins, and the absence of relapse during steroid decalage (the patient was taking 5 mg/day methylprednisolone), it was hypothesized that the patient’s ALF was due to WD rather than AIH. Therefore, the molecular analysis was re-evaluated with a more sensitive method and a liver biopsy was proposed but not performed due to parental refusal. The finding of two mutations-H1069Q/c.3556 + 281_4001del- in the ATP7B gene allowed us to make a definitive diagnosis of WD.
Consequentially, methylprednisolone treatment was interrupted. Considering that the patient now had mild liver disease, we decided to suspend D-penicillamine and continue zinc acetate at the appropriate dosage for age (25 mg elemental zinc three times a day)[11]. In Figure 1, we summarize the diagnostic process, therapeutic interventions, and outcome of this case.
After steroid discontinuation, the patient remained in good clinical condition without biochemical relapse and without new ANA positivity. During follow-up, the patient did not develop any clinical neurological signs. Brain magnetic resonance imaging did not show significant abnormal findings, and Kayser-Fleischer’s ring was not detected. After 5 years, he was still being treated for WD with zinc acetate and maintained a complete response with normal liver function and normalization of platelet levels (Table 2 and Figure 2).
| Parameter | At onset | 4 months | 5 months | 9 months | 1 year | 2 years | 3 years | 4 years | 5 years |
| AST in IU/L | 264 | 69 | 60 | 43 | 30 | 42 | 23 | 20 | 23 |
| ALT in IU/L | 300 | 102 | 59 | 30 | 21 | 29 | 19 | 19 | 25 |
| Total bilirubin in mg/dL | 14.5 | 1.21 | 0.59 | 0.99 | 1.37 | 0.82 | 0.46 | 0.90 | 1.10 |
| Direct bilirubin in mg/dL | 4.5 | ND | 0.22 | 0.38 | 0.49 | 0.31 | 0.22 | 0.37 | 0.55 |
| GGT in IU/L | ND | 38 | 31 | 29 | 16 | 16 | 17 | 19 | 16 |
| ALP in IU/L | ND | 344 | 717 | 750 | 413 | 413 | 429 | 430 | 190 |
| CHE in IU/L | ND | 3424 | 3955 | 5362 | 7890 | 7890 | 8357 | 7912 | 6054 |
| Gamma globulin, % | 34 | 17.9 | 13.2 | 14 | 12.6 | 15.5 | 14.9 | 12 | 13.2 |
| INR | 4.48 | 1.26 | 1.29 | 1.3 | 1.22 | 1.21 | 1.18 | 1.24 | 1.17 |
| Platelets as × 103/μL | 37 | 88 | 118 | 154 | 151 | 186 | 270 | 266 | 273 |
| Ceruloplasmin in mg/dL | 11 | 11 | ND | ND | ND | 9 | 5 | 6 | 0 |
| 24-hour UCE in µg | 200 | 60 | 69 | 61 | 40.4 | 40.4 | 38.7 | 23.9 | 62.4 |
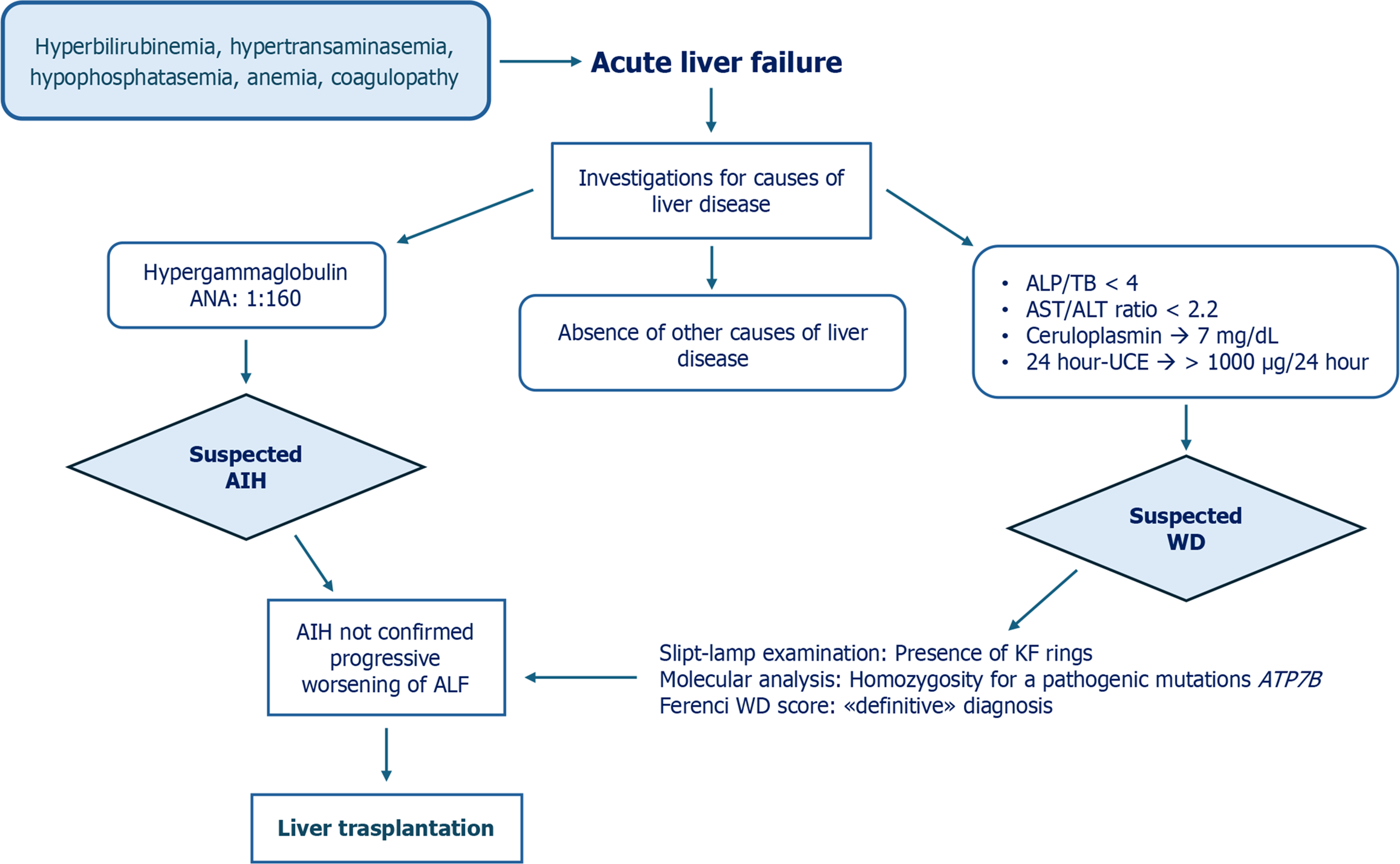
A 15-year-old Chinese girl was admitted to an Italian hospital following a 2-week history of jaundice. On physical examination, she presented with hepatomegaly, jaundice, and mild ascites. No clinical problems were reported in her medical history. Laboratory findings showed TB at 14.5 mg/dL (normal range: < 1.2 mg/dL), conjugated bilirubin at 11.6 mg/dL (normal range: < 0.3 mg/dL), AST at 50 IU/L (normal range: < 40 IU/L), ALT at 30 IU/L (normal range: < 40 IU/L), GGT within reference limits (normal range: < 70 IU/L), and low ALP (24 IU/L, normal range: 142-335 IU/L). Cell blood count showed normal leucocytes and platelets, but a slight decrease in hemoglobin value (11.6 g/dL, normal range: 11.0-16.0 g/dL) with a high reticulocyte count of 586 × 109/L (25-90 × 109/L). Coombs test was negative as well as the inflammation indices. Renal function was normal. Further investigations revealed ANA positivity (titer 1:160; normal range: < 1:40) and an increase in gamma globulin serum levels (42%; normal range: < 18%). Abdominal ultrasound showed hepatomegaly, spleen of normal size, and mild ascites. Infectious diseases were excluded. The patient reported not taking alcohol, drugs, or any medication. Coagulation parameters were abnormal with prolonged prothrombin time (INR 3.8, normal range: 0.8-1.2) unresponsive to vitamin K infusion.
The diagnosis of ALF was made according to Pediatric ALF Study Group criteria, and the patient was transferred to the Pediatric Liver Transplant Reference Centre in Turin[16]. A second autoantibody evaluation showed negative ANA and positive anti-liver cytosol type 1 and anti-mitochondrial antibody. A reduced ceruloplasmin level (7 mg/dL, normal range: 20-60 mg/dL) was found with an extremely high 24-hour UCE (> 1000 µg/24 hour; normal range: < 40 µg/24 hour). The ALP/TB ratio was 1.65; lower than 4 meets the screening criteria for WD in patients with ALF[5]. The AST/ALT ratio did not meet this criterion because it was not higher than 2.2 (AST/ALT = 1.6). Slit-lamp examination revealed evidence of the Kayser-Fleischer ring in the cornea. Neurologic and psychiatric symptoms were absent. WD was strongly suspected based on the presence of Coombs-negative hemolytic anemia, Kayser-Fleischer ring on slit-lamp examination, low serum levels of ceruloplasmin and ALP, and elevated 24-hour UCE. The New Wilson Index and Nazer score were 13 and 7, respectively, indicative of poor survival without liver transplantation (Table 3)[14,15].
| Parameter | Value | New Wilson Index | Nazer score |
| Total bilirubin in mg/dL | 14.5 | 3 | 3 |
| INR | 3.8 | 4 | 4 |
| AST in UI/L | 50 | 0 | 0 |
| WBC as × 109/L | 14.7 | 3 | NA |
| Alb in g/dL | 2.4 | 3 | NA |
| Total score | NA | 13 | 7 |
Treatment included antibiotic therapy, albumin infusion combined with furosemide to reduce the ascites, and intravenous plasma infusion. Specific therapy for WD was not started. As liver function worsened (INR 6), the New Wilson Index became 14. The patient had an urgent liver transplantation at 6 d after admission to the Pediatric Liver Transplant Center. MELD score at the time of transplant was 35. The histopathology of the explanted liver showed necrosis, cirrhosis, macro-nodularity, steatosis, ballooning of hepatocytes, and Mallory hyaline bodies. These findings were consistent with WD-ALF. DNA sequence analysis of the ATP7B gene detected a mutation in homozygosity c.2975C > T (p.Pro992 Leu) at exon 13, which allowed for a definitive diagnosis of WD. In Figure 3, we summarize the diagnostic process, therapeutic interventions, and outcome of this case.
WD presenting as ALF is a significant challenge, because differential diagnosis with other etiologies is difficult due to the lack of sensitive and specific diagnostic tests for WD. Even from a management point of view, this condition is demanding because, once the diagnostic suspicion has been established, it is necessary to choose the most appropriate pharmacological and supportive therapy and quickly decide whether or not to perform liver transplant as a life-saving procedure. In the described cases, the diagnosis of WD was made difficult by the presence of hypergammaglobulinemia and positive autoantibodies, both indicative of AIH which, like WD, can have an acute onset with ALF. Furthermore, WD-ALF diagnosis is complicated because markers of copper metabolism (ceruloplasmin serum levels and 24-hour UCE) are of limited diagnostic value. In fact, they can give both false-positive and false-negative results. Serum concentration of ceruloplasmin may be falsely normal under the influence of inflammation, while 24-hour UCE may be above the normal range; this may also occur in non-WD ALF due to the massive necrosis of hepatocytes[9]. An example is the ceruloplasmin value at onset in Case 1 which, although lower than the cutoff for the diagnostic suspicion of WD, was clearly higher than that observed during follow-up. On the other hand, patients with ALF due to causes other than WD may show low levels of ceruloplasmin due to reduced protein synthetic capacity of the liver[9]. In the 2 cases described, ceruloplasmin and 24-hour UCE were indicative of WD, although in the first case, the levels of ceruloplasmin and 24-hour UCE were not as severely altered as would be expected.
The diagnostic difficulties are then amplified by the fact that liver biopsy and molecular analysis are not feasible in the context of ALF. Indeed, liver biopsy usually cannot be performed due to the ALF-associated coagulopathy, and molecular analysis takes no less than 1 month to generate results[19]. Regarding the meaning of autoantibodies in WD patients, a low titer of autoantibodies has been reported in patients with WD without AIH[20]. Overall, there are few cases in the literature of coexistence of WD and AIH in the same patient[21,22]. It is likely that the abnormal immune response seen in WD with severe liver disease may depend by the impaired degradation of circulating antigens which in turn causes an increase in the humoral immune response. Furthermore, a direct influence of free copper on lymphocytes and the metabolic activity of leukocytes cannot be excluded[23].
Regarding the diagnostic role of other biochemical parameters, an ALP/TB ratio < 4 and AST/ALT ratio > 2.2 reportedly have acceptable sensitivity and specificity for the early diagnosis of WD-ALF[19]. However, the accuracy of ALP/TB is limited in children who usually have higher ALP levels due to the bone growth. The limited reliability of these parameters is confirmed by the fact that only in the second case described, one of these ratios was positive.
Once the diagnosis of WD-ALF is established, it is critical to stage the severity of the liver disease through prognostic scores, in order to evaluate the need for liver transplantation[14,15].
In our 2 cases, although both scores showed a low probability of surviving without a liver transplant, the first patient was able to survive with native liver following a combined treatment with low doses of D-penicillamine and zinc plus steroids. At this point, it is interesting to ask whether the chance of surviving with a native liver is related to the type of treatment received. Most patients who survive without transplant, despite a score above the cutoff, are treated with chelators[11,12]. However, some patients have been successfully treated with zinc monotherapy or with the combination of chelator and zinc or sometimes with supportive therapies alone[24-26]. The dosages of the drugs used are not always available in the literature, nor is it possible to make a reliable comparison of the basic clinical conditions of these patients. It is generally believed that specific and supportive therapies have little impact in modifying the outcome of patients with WD-ALF[10,11]. This is probably true, and it cannot be ruled out that a favorable outcome may occur due to factors other than treatment for WD. For example, in Case 1, it could be hypothesized that concomitant AIH contributed to ALF and that steroids acted on the autoimmune comorbidity. Likewise, the role of intercurrent infections could be invoked in other cases[27]. In Case 1, however, the hypothesis of AIH was not confirmed in the following years during which no relapse of hypergammaglobulinemia or positivity of autoantibodies nor an increase in liver enzymes was ever observed despite steroids withdrawal. The idea that in Case 1 steroid therapy could have contributed to the improvement of the ALF picture, even in the absence of AIH, needs to be tested in further studies, but may open new perspectives in the management of WD-ALF.
There are no clear indications in the literature on how to use specific drugs (chelators and zinc) and supportive therapies in patients with WD-ALF. In clinical practice, when the diagnosis of WD-ALF is made, there is general agreement that it is advisable to promptly start symptomatic supportive therapies, while no recommendation is available about therapy with chelators or zinc or both. Case 1 seems to support the use of combined therapy. Since the patient initially received an uncertain diagnosis between AIH and WD, he was treated with a combined strategy including D-penicillamine, zinc salts, and methylprednisolone, with a good clinical response. Some experts believe that chelators should be initially used at low doses due to the risk of paradoxical neurologic deterioration and/or severe cutaneous reactions. In addition, in the setting of WD-ALF, zinc may be administered, but since it presents a slow onset of action, it is not generally recommended[11]. When Case 1 came to our attention, he had a stabilized clinical situation with only a mild liver disease; therefore, D-penicillamine therapy was discontinued and monotherapy with zinc acetate continued, according to the observation that zinc monotherapy is effective in WD children with mild liver disease[10]. In the second patient, ALF progressed so rapidly that a liver transplant was immediately performed, and no specific therapy was started. Unlike the first case, there were no doubts about the diagnosis of WD-ALF; the positivity of autoantibodies was considered non-specific.
On the basis of these cases and what has been reported in literature, liver transplant remains a reliable option for WD-ALF patients, but should there be no available donor and pending liver transplant procedure, maximum efforts should be made to optimize medical therapy. Surely, it is desirable to be very critical in evaluating the efficacy of different therapies especially when the number of cases analyzed is small, but from an ethical point of view we must not deny each patient every possible support. The presumed efficacy of the unusual cocktail of D-penicillamine, zinc, and steroids used in Case 1, could be an example of serendipity. Although a causal link cannot be proven, it also cannot be ruled out. As aforementioned, it is likely that even in ALF patients, therapy with D-penicillamine should be started at a low dose to avoid the rapid mobilization of copper deposits. Less easily explained is the rationale for a low initial dosage of zinc, which acts by inhibiting the intestinal absorption of copper but also by inducing hepatic metallothioneins which consequently bind copper in a non-toxic form[11,28]. Another interesting point in Case 1 was the progressive resolution of the severe thrombocytopenia presented at onset. Considering that normalization occurred in a few months and the absence of splenomegaly and esophageal varices, the thrombocytopenia could not be explained by portal hypertension but rather by the systemic inflammation associated with ALF, and in this context steroids might have played a role[29].
In light of the cases presented, greater attention to the medical management of WD-ALF would be desirable through surveys and multicenter retrospective and/or prospective studies in order to produce stronger evidence on the best therapeutic management of patients with WD-ALF. Even sporadic cases should probably not be neglected, and maximum efforts should be made to promote the survival of WD-ALF patients with native liver.
| 1. | Roberts EA, Schilsky ML; Division of Gastroenterology and Nutrition, Hospital for Sick Children, Toronto, Ontario, Canada. A practice guideline on Wilson disease. Hepatology. 2003;37:1475-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The Prevalence of Wilson's Disease: An Update. Hepatology. 2020;71:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 3. | Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 545] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 4. | Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010;24:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Korman JD, Volenberg I, Balko J, Webster J, Schiodt FV, Squires RH Jr, Fontana RJ, Lee WM, Schilsky ML; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol. 2012;56:671-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 781] [Article Influence: 60.1] [Reference Citation Analysis (1)] |
| 7. | Ferenci P, Członkowska A, Merle U, Ferenc S, Gromadzka G, Yurdaydin C, Vogel W, Bruha R, Schmidt HT, Stremmel W. Late-onset Wilson's disease. Gastroenterology. 2007;132:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Pan JJ, Fontana RJ. CAQ Corner: Acute liver failure management and liver transplantation. Liver Transpl. 2022;28:1664-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Delle Cave V, Di Dato F, Iorio R. Wilson's Disease with Acute Hepatic Onset: How to Diagnose and Treat It. Children (Basel). 2024;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 10. | Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, Washington MK, Weiss KH, Zimbrean PC. A multidisciplinary approach to the diagnosis and management of Wilson disease: Executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2023;77:1428-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 11. | Socha P, Janczyk W, Dhawan A, Baumann U, D'Antiga L, Tanner S, Iorio R, Vajro P, Houwen R, Fischler B, Dezsofi A, Hadzic N, Hierro L, Jahnel J, McLin V, Nobili V, Smets F, Verkade HJ, Debray D. Wilson's Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 12. | Nagral A, Sarma MS, Matthai J, Kukkle PL, Devarbhavi H, Sinha S, Alam S, Bavdekar A, Dhiman RK, Eapen CE, Goyal V, Mohan N, Kandadai RM, Sathiyasekaran M, Poddar U, Sibal A, Sankaranarayanan S, Srivastava A, Thapa BR, Wadia PM, Yachha SK, Dhawan A. Wilson's Disease: Clinical Practice Guidelines of the Indian National Association for Study of the Liver, the Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition, and the Movement Disorders Society of India. J Clin Exp Hepatol. 2019;9:74-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Dubbioso R, Ranucci G, Esposito M, Di Dato F, Topa A, Quarantelli M, Matarazzo M, Santoro L, Manganelli F, Iorio R. Subclinical neurological involvement does not develop if Wilson's disease is treated early. Parkinsonism Relat Disord. 2016;24:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Dhawan A, Taylor RM, Cheeseman P, De Silva P, Katsiyiannakis L, Mieli-Vergani G. Wilson's disease in children: 37-year experience and revised King's score for liver transplantation. Liver Transpl. 2005;11:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Nazer H, Ede RJ, Mowat AP, Williams R. Wilson's disease: clinical presentation and use of prognostic index. Gut. 1986;27:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 156] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, Rosenthal P, Rodriguez-Baez N, Murray KF, Horslen S, Martin MG, Lopez MJ, Soriano H, McGuire BM, Jonas MM, Yazigi N, Shepherd RW, Schwarz K, Lobritto S, Thomas DW, Lavine JE, Karpen S, Ng V, Kelly D, Simonds N, Hynan LS. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 569] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 17. | Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, Schilsky M, Cox D, Berr F. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 603] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 18. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 19. | Dhawan A. Acute liver failure in children and adolescents. Clin Res Hepatol Gastroenterol. 2012;36:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Naorniakowska M, Woźniak M, Pronicki M, Grajkowska W, Kamińska D, Jańczyk W, Dądalski M, Cukrowska B, Socha P. Autoimmune hepatitis, Wilson’s disease, or both? An analysis of challenging cases. Pediatria polska. 2020;95:18-24. [DOI] [Full Text] |
| 21. | Milkiewicz P, Saksena S, Hubscher SG, Elias E. Wilson's disease with superimposed autoimmune features: report of two cases and review. J Gastroenterol Hepatol. 2000;15:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Dara N, Imanzadeh F, Sayyari AA, Nasri P, Hosseini AH. Simultaneous Presentation of Wilson's Disease and Autoimmune Hepatitis; A Case Report and Review of Literature. Hepat Mon. 2015;15:e29043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Czlonkowska A, Milewski B. Immunological observations on patients with Wilson's disease. J Neurol Sci. 1976;29:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Sallie R, Katsiyiannakis L, Baldwin D, Davies S, O'Grady J, Mowat A, Mieli-Vergani G, Williams R. Failure of simple biochemical indexes to reliably differentiate fulminant Wilson's disease from other causes of fulminant liver failure. Hepatology. 1992;16:1206-1211. [PubMed] |
| 25. | Esezobor CI, Banjoko N, Rotimi-Samuel A, Lesi FE. Wilson disease in a Nigerian child: a case report. J Med Case Rep. 2012;6:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lee VD, Northup PG, Berg CL. Resolution of decompensated cirrhosis from Wilson's disease with zinc monotherapy: a potential therapeutic option? Clin Gastroenterol Hepatol. 2006;4:1069-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Wendon J, Cordoba J, Dhawan A, Larsen FS, Manns M, Nevens F, Samuel D, Simpson KJ, Yaron I; Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 617] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 28. | Wiggelinkhuizen M, Tilanus ME, Bollen CW, Houwen RH. Systematic review: clinical efficacy of chelator agents and zinc in the initial treatment of Wilson disease. Aliment Pharmacol Ther. 2009;29:947-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Stravitz RT, Ellerbe C, Durkalski V, Reuben A, Lisman T, Lee WM; Acute Liver Failure Study Group. Thrombocytopenia Is Associated With Multi-organ System Failure in Patients With Acute Liver Failure. Clin Gastroenterol Hepatol. 2016;14:613-620.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |