Published online Aug 27, 2024. doi: 10.4254/wjh.v16.i8.1099
Revised: April 27, 2024
Accepted: May 17, 2024
Published online: August 27, 2024
Processing time: 224 Days and 10.2 Hours
Alpha-1 antitrypsin deficiency (AATD) is a codominant autosomal hereditary condition that predisposes patients to the development of lung and/or liver disease, and Pi*Z allele is the most clinically relevant mutation.
To evaluate the impact of clinical parameters and AATD phenotypes, particularly the Pi*Z allele, in liver fibrosis.
Cross-sectional cohort study including consecutive patients with AATD followed in Pulmonology or Hepatology consultation.
Included 69 patients, 49.3% had Pi*MZ phenotype and 10.1% Pi*ZZ. An age ≥ 55 years, age at diagnosis ≥ 41 years and AAT at diagnosis < 77 mg/dL predicted a nonalcoholic fatty liver disease fibrosis score (NFS) not excluding advanced fibrosis [area under the curve (AUC) = 0.840, P < 0.001; AUC = 0.836, P < 0.001; AUC = 0.681, P = 0.025]. An age ≥ 50 years and age at diagnosis ≥ 41 years predicted a fibrosis-4 index of moderate to advanced fibrosis (AUC = 0.831, P < 0.001; AUC = 0.795, P < 0.001). Patients with hypertension, type 2 diabetes mellitus (DM), dyslipidaemia, metabolic syndrome, and regular alcohol consumption were more likely to have a NFS not excluding advanced fibrosis (P < 0.001, P = 0.002, P = 0.008, P < 0.001, P = 0.033). Patients with at least one Pi*Z allele and type 2 DM were 8 times more likely to have liver stiffness measurement ≥ 7.1 kPa (P = 0.040).
Risk factors for liver disease in AATD included an age ≥ 50 years, age at diagnosis ≥ 41 years, metabolic risk factors, regular alcohol consumption, at least one Pi*Z allele, and AAT value at diagnosis < 77 mg/dL. We created an algorithm for liver disease screening in AATD patients to use in primary care, selecting those to be referred to Hepatology consultation.
Core Tip: Patients with alpha-1 antitrypsin deficiency (AATD) who have more than 50 years of age and had a diagnosis after 41 years of age are at increased risk of developing liver fibrosis, as well as those who have at least one Pi*Z allele. In patients with AATD, the presence of metabolic risk factors, such as hypertension, type 2 diabetes mellitus, and dyslipidaemia, and regular alcohol consumption, should be closely monitored and controlled, since these are important risk factors for the development of liver fibrosis.
- Citation: Ferreira AI, Guimarães C, Macedo Silva V, Xavier S, Magalhães J, Cotter J. Alpha-1 antitrypsin deficiency and Pi*Z allele as important co-factors in the development of liver fibrosis. World J Hepatol 2024; 16(8): 1099-1110
- URL: https://www.wjgnet.com/1948-5182/full/v16/i8/1099.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i8.1099
Alpha-1 antitrypsin deficiency (AATD) is one of the most common genetic diseases, a codominant autosomal hereditary condition, caused by pathogenic mutations in SERPINA1[1,2]. This condition results in decreased circulating levels of AAT and predisposes patients to the development of lung and/or liver disease[1,2].
AAT is mainly synthesized in the hepatocytes, within the endoplasmic reticulum and secreted through the Golgi apparatus into the circulation[1]. In patients with severe deficiency alleles, approximately 70% of the mutated AAT is degraded in the endoplasmic reticulum, 15% is secreted, and 15% persists within the endoplasmic reticulum as inclusions, which are positive on periodic acid-Schiff staining and are the histologic hallmark of the disease[3,4]. Thus, the liver retention of misfolded AAT leads to endoplasmic reticulum stress and mitochondrial dysfunction, which results in liver disease in a “gain of function” mechanism[1,5,6].
AAT is an important circulating proteinase inhibitor, protecting lung tissue against attack by the enzyme neutrophil elastase, which degrades elastin and connective tissue in the lungs[7]. Low plasma AAT levels, seen in severe deficiencies, are associated with a markedly increased risk of early-onset emphysema, through a “loss of function” mechanism, especially when associated with exposure to cigarette smoke, environment irritants, or infections[1,2].
The wild-type allele of SERPINA1 which encodes AAT is the Pi*M[1]. Although more than 100 SERPINA1 variants have been identified, the Pi*Z allele being the most clinically relevant mutation[8]. Pi*Z homozygotes have the highest risk of prominent disease and heterozygotes have a clear predisposition but low absolute risk for the development of relevant lung or liver disease[8,9]. The Pi*S allele is also clinically significant, although patients with Pi*SZ are less likely to develop lung disease than Pi*Z homozygotes[10] and the Pi*S allele confers only a weak risk of liver disease in patients with excessive alcohol intake[11].
The Pi*ZZ phenotype although rare, is sufficient to cause end-stage liver disease, while the Pi*MZ phenotype is more common and considered a disease modifying factor in patients with other comorbidities, such as cystic fibrosis, alcoholic liver disease, and metabolic associated fatty liver disease[1,11,12]. Therefore, patients with AATD should be monitored for liver disease, using non-invasive methods[1]. Serum liver scores, such as nonalcoholic fatty liver disease fibrosis score (NFS) and fibrosis-4 index (FIB-4), are clinical scores validated for excluding advanced liver fibrosis[13-15], with FIB-4 index having better diagnostic accuracy for estimation of liver fibrosis among various serum markers[16]. Transient elastography (TE) is also useful for excluding significant liver fibrosis. The use of non-invasive tests for monitoring has been evaluated in various liver diseases, including alcoholic liver disease, non-alcoholic liver disease and infectious hepatitis[17]. However, studies about the non-invasive assessment and surveillance of AATD are lacking. Abbas et al[18] established a weak but statistically significant correlation between the FIB-4 index and liver stiffness measurement (LSM), with FIB-4 > 1.26 corresponding to LSM > 6 kilopascals (kPa). Generally, the LSM cut-offs used are > 7.1 kPa as suggestive of significant fibrosis, and > 10.0 kPa suggestive of advanced fibrosis[19,20]. One study compared LSM with histology in patients with AATD and established lower cut-offs, 5.45 kPa for significant fibrosis and 8.45 kPa for advanced fibrosis, both with a high negative predictive value, but low positive predictive value[5]. Therefore, the higher cut-offs had a higher positive predictive value compared to the lower cut-offs described in that study and are still the most frequently used[5,20]. The aim of this study was to evaluate the impact of demographic, anthropometric, and clinical parameters, including the patients’ comorbidities, and AATD phenotypes, particularly the Pi*Z allele, in liver fibrosis.
A cross-sectional cohort study was performed in a university affiliated hospital, including patients with AATD followed in Pulmonology or Hepatology consultation. Inclusion criteria were being an adult and having the diagnosis of AATD with known phenotype. Patients with a concomitant liver disease (positive serum hepatitis markers, autoimmune hepatitis, hereditary liver diseases), extra-hepatic cholestasis, right-sided heart failure, liver transplantation, hepatic surgery, ascites, or pregnancy were excluded, as well as patients with medications that may cause steatosis (amiodarone, methotrexate, tamoxifen, valproic acid, antimycotic medications or oral corticosteroids), and patients that refused to participate in the study. All patients gave informed, written consent prior to enrolling. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board (Approval number 15/2022).
Clinical, laboratory and imaging data were collected prospectively and registered anonymously in a database. Demographic, anthropometric, clinical, laboratory, and TE data were obtained. Patients’ demographics included age and sex, and anthropometric data included weight, height, and waist circumference. The body weight was measured in kg, using a weighing scale and the height in m was determined with measuring tape. The body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference was measured at a level midway between the lowest rib and the iliac crest, evaluated in cm with measuring tape.
The presence of comorbid conditions was reported, namely hypertension, type 2 diabetes mellitus (DM), dyslipidaemia, obesity, metabolic syndrome, medication use, alcohol consumption, and smoking habits. Obesity was regarded as a BMI equal or superior to 30.0 kg/m2. Metabolic syndrome was defined by the International Diabetes Federation criteria[21,22] as central obesity that corresponds to abdominal circumference ≥ 94 cm in men or ≥ 80 cm in women and at least 2 of the following: Triglycerides ≥ 150 mg/dL or specific treatment for hypertriglyceridemia; high-density lipoprotein cholesterol < 40 mg/dL in men or < 50 mg/dL in women or specific treatment for dyslipidaemia; systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or treatment for previously diagnosed arterial hypertension; and fasting glucose ≥ 100 mg/dL or type 2 DM previously diagnosed. Regular alcohol intake was considered as a consumption of 25 or more grams of alcohol per day, every day. The AAT phenotype and value at diagnosis was also reported, with the AAT value at diagnosis measured in our institution by immunoturbidimetry, with a protective threshold of 78 mg/dL. Finally, the forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLCO) values were collected from the pulmonary function tests performed in our institution.
The primary endpoints evaluated included the stage of liver fibrosis, which was assessed using non-invasive serum scores and TE. The evaluated fibrosis serum scores were NFS and FIB-4. The NFS was calculated according to the following formula: -1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/DM (yes = 1, no = 0) + 0.99 × aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio - 0.013 × platelet count (× 109/L) - 0.66 × albumin (g/dL)[13]. A value of less than -1.455 was regarded as excluding advanced fibrosis[13,23]. The FIB-4 index was calculated as: Age × AST (IU/L)/[platelet count (×109/L) × ALT (IU/L)0.5][13]. A score below 1.45 was indicative of mild fibrosis, while a score above 3.25 was indicative of advanced fibrosis; a score between 1.45 and 3.25 was regarded as moderate fibrosis[13]. The LSM was assessed through TE in kPa. This exam was performed with at least 3-h fasting, with the patient laying in dorsal decubitus and with maximal abduction of the right arm, and the probe being placed in the right medial-clavicular line, between the 9th and 11th intercostal space[24]. The probe M or XL was used according to the device’s suggestion, with the XL probe being used if the distance between the skin and the hepatic capsule is ≥ 25 mm[24]. A valid TE result was considered after 10 valid measurements and interquartile range ≤ 30%[25]. Since there are no established LSM cut-offs for AATD liver disease, a LSM > 7.1 kPa was used as suggestive of significant fibrosis and ≥ 10 kPa was suggestive of advanced fibrosis, according to previously established cut-offs in other liver diseases (mainly viral aetiologies and alcoholic liver disease)[19,20]. The presence of steatosis was assessed by the controlled attenuation parameter and results were expressed in decibel per meter (dB/m). The cut-off > 268 dB/m was used as an indicator of moderate steatosis, and > 280 dB/m for severe steatosis[18].
Categorical variables were described as frequencies and percentages, and continuous variables as mean and SD if normally distributed, or as median if not normally distributed. Comparison of categorical variables was performed using the χ2 test, which was also used to calculate the odds ratio (OR), as well as 95% confidence intervals (CI) for OR. Means and medians of continuous variables were compared using independent group t-tests or Mann-Whitney U test, respectively. Receiver operating characteristic (ROC) curves were obtained to illustrate the diagnostic ability of continuous variables to predict a NFS that does not exclude advanced fibrosis and a FIB-4 indicative of moderate to advanced fibrosis. A P value < 0.05 was considered statistically significant. Statistical analysis software IBM SPSS version 29.0 (IBM Corp., Armonk, NY, United States) was used for all tests performed.
A total of 69 patients were included. The details of the population’s baseline characteristics are present in Table 1. Metabolic syndrome was present in 22 patients (31.9%) and regular alcohol intake occurred in 29 patients (42.0%). The main clinical presentation of AATD was pulmonary disease, in 40 patients (58.0%); 17 patients had altered biochemical liver parameters (24.6%) and, in 12 patients, AATD was diagnosed through screening of affected family members (17.4%). The median value of AAT at diagnosis was 67 mg/dL and the presence of at least one Pi*Z allele occurred in 55 patients (79.7%). The most frequent phenotype was Pi*MZ, in 34 patients (49.3%). The Pi*ZZ genotype was present in 7 patients (10.1%). Regarding the study outcomes, the median LSM value was 5.0 kPa, the mean NFS value was -2.225 ± 1.392 and the median FIB-4 value was 1.05. Pi*ZZ patients had a median LSM value of 5.7 kPa, a mean NFS value of -1.779 ± 1.517 and a median FIB-4 value of 1.17. Pi*MZ patients had a median LSM value of 5.1 kPa, a mean NFS value of -2.295 ± 1.526 and a median FIB-4 value of 1.03.
| Variable | Study population’s characteristics |
| Sex, n (%) | |
| Female | 28 (40.6) |
| Male | 41 (59.4) |
| Age at diagnosis (yr), mean ± SD | 45 ± 17 |
| Age (yr), mean ± SD | 50 ± 17 |
| BMI (kg/m2), mean ± SD | 26.4 ± 4.5 |
| Waist circumference (cm), mean ± SD | 94 ± 13 |
| Arterial hypertension, n (%) | 24 (34.8) |
| Type 2 DM, n (%) | 14 (20.3) |
| Dyslipidaemia, n (%) | 27 (39.1) |
| Obesity, n (%) | 15 (21.7) |
| Metabolic syndrome, n (%) | 22 (31.9) |
| Regular alcohol intake, n (%) | 29 (42.0) |
| Smoking habits, n (%) | |
| Non-smoker | 46 (66.7) |
| Smoker | 7 (10.1) |
| Ex-smoker | 16 (23.2) |
| AAT clinical presentation, n (%) | |
| Respiratory | 40 (58.0) |
| Hepatic | 17 (24.6) |
| Screening | 12 (17.4) |
| AAT value at diagnosis (mg/dL), median | 67 |
| AAT phenotype, n (%) | |
| MZ | 34 (49.3) |
| SZ | 14 (20.3) |
| ZZ | 7 (10.1) |
| SS | 6 (8.7) |
| MS | 4 (5.8) |
| MMHeerlen | 2 (2.9) |
| SMHeerlen | 2 (2.9) |
| FEV1 (%), mean ± SD | 94 ± 29 |
| DLCO, mL of CO/s/mmHg, median | 92 |
NFS: A NFS value of more than -1.455 was considered as not excluding advanced fibrosis[13], with 27.5% of patients having a NFS value that does not exclude advanced fibrosis. Factors associated with a NFS not excluding advanced fibrosis are present in Table 2.
| Variable | Excluding advanced fibrosis (NFS < -1.455), n = 49 | Not excluding advanced fibrosis (NFS ≥ -1.455), n = 19 | P value | OR | 95%CI |
| Sex, n (%) | 0.232 | 0.523 | 0.179-1.526 | ||
| Female | 18 (36.7) | 10 (52.6) | |||
| Male | 31 (63.3) | 9 (47.4) | |||
| Age at diagnosis (yr), mean ± SD | 39 ± 16 | 57 ± 11 | < 0.001a | - | - |
| Age (yr), median | 46 | 64 | < 0.001a | - | - |
| BMI (kg/m2), mean ± SD | 26.1 ± 4.4 | 27.1 ± 4.9 | 0.409 | - | - |
| Waist circumference (cm), median | 94 | 99 | 0.235 | - | - |
| Arterial hypertension, n (%) | < 0.001a | 16.667 | 4.457-62.318 | ||
| Yes | 9 (18.4) | 15 (78.9) | |||
| No | 40 (81.6) | 4 (21.1) | |||
| Type 2 DM, n (%) | 0.002a | 7.920 | 2.178-28.799 | ||
| Yes | 5 (10.2) | 9 (47.4) | |||
| No | 44 (89.8) | 10 (52.6) | |||
| Dyslipidaemia, n (%) | 0.008a | 4.286 | 1.399-13.127 | ||
| Yes | 14 (28.6) | 12 (63.2) | |||
| No | 35 (71.4) | 7 (36.8) | |||
| Obesity, n (%) | 0.329 | 2.051 | 0.613-6.863 | ||
| Yes | 9 (18.4) | 6 (31.6) | |||
| No | 40 (81.6) | 13 (68.4) | |||
| Metabolic syndrome, n (%) | < 0.001a | 6.686 | 2.091-21.382 | ||
| Yes | 10 (20.4) | 12 (63.2) | |||
| No | 39 (79.6) | 7 (36.8) | |||
| Regular alcohol intake, n (%) | 0.033a | 3.227 | 1.072-9.716 | ||
| Yes | 17 (34.7) | 12 (63.2) | |||
| No | 32 (65.3) | 7 (36.8) | |||
| AAT value at diagnosis (mg/dL), median | 71 | 56 | 0.025a | - | - |
| FEV1 (%), mean ± SD | 95 ± 27 | 93 ± 34 | 0.801 | - | - |
| DLCO, mL of CO/s/mmHg, median | 95 | 88 | 0.225 | - | - |
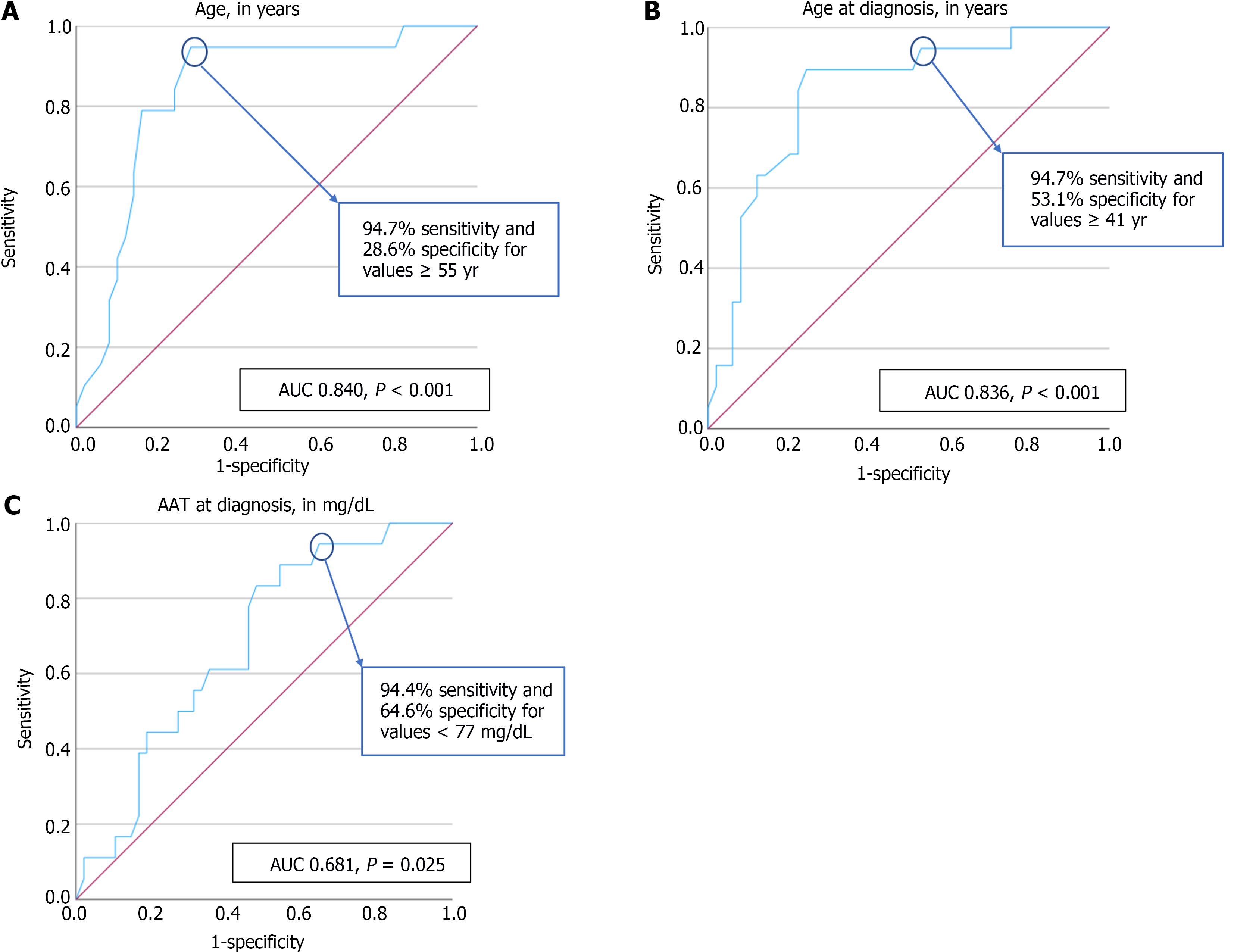
Patients with a NFS value that does not exclude advanced fibrosis had a statistically significant higher age (64 vs 46 years, P < 0.001) and higher age at diagnosis (57 ± 11 vs 39 ± 16 years, P < 0.001). A patients’ age equal or superior to 55 years had a very good discriminative ability in predicting a NFS not excluding advanced fibrosis, with a sensitivity of 94.7% and specificity of 28.6% [area under the curve (AUC) = 0.840, P < 0.001]. Additionally, a patients’ age at diagnosis equal or superior to 41 years also had a very good discriminative ability in predicting a NFS not excluding advanced fibrosis, with a sensitivity of 94.7% and specificity of 53.1% (AUC = 0.836, P < 0.001).
Patients with hypertension were 17 times more likely to have a NFS not excluding advanced fibrosis (78.9% vs 18.4%, P < 0.001), and those with type 2 DM were 8 times more likely to have a NFS not excluding advanced fibrosis (47.4% vs 10.2%, P = 0.002). Patients with dyslipidaemia were 4 times more likely to have a NFS not excluding advanced fibrosis (63.2% vs 28.6%, P = 0.008), and those with metabolic syndrome were 7 times more likely to have a NFS not excluding advanced fibrosis (63.2% vs 20.4%, P < 0.001). Patients with regular alcohol consumption were 3 times more likely to have a NFS not excluding advanced fibrosis (63.2% vs 34.7%, P = 0.033).
Patients with NFS that does not exclude advanced fibrosis had a statistically significant lower AAT at diagnosis (56 mg/dL vs 71 mg/dL, P = 0.025). AAT value at diagnosis inferior to 77 mg/dL showed a good discriminative capacity in predicting a NFS value considered as not excluding advanced fibrosis, with a sensitivity of 94.4% and specificity of 64.6% (AUC = 0.681, P = 0.025).
There was no statistically significant association between patients with or without Pi*ZZ phenotype and a NFS not excluding advanced fibrosis (P = 0.390). No statistically significant association was found between FEV1 and DLCO and a NFS not excluding advanced fibrosis (P = 0.801 and P = 0.225, respectively). The ROC curves illustrating the diagnostic ability of the patients’ age, age at diagnosis, and AAT at diagnosis in predicting a NFS value that does not exclude advanced fibrosis are present in Figure 1.
FIB-4: A FIB-4 score below 1.45 was regarded as indicative of mild fibrosis, while a score equal or superior to 1.45 was considered moderate to advanced fibrosis[13], which occurred in 27.5% of the patients in our cohort. Factors associated with FIB-4 scores indicative of mild and moderate to advanced fibrosis are present in Table 3.
| Variable | Mild fibrosis (FIB-4 < 1.45), n = 50 | Moderate to severe fibrosis (FIB-4 ≥ 1.45), n = 19 | P value | OR | 95%CI |
| Sex, n (%) | 0.479 | 0.681 | 0.234-1.978 | ||
| Female | 19 (38.0) | 9 (47.4) | |||
| Male | 31 (62.0) | 10 (52.6) | |||
| Age at diagnosis (yr), mean ± SD | 40 ± 16 | 56 ± 11 | < 0.001a | - | - |
| Age (yr), mean ± SD | 45 ± 16 | 63 ± 11 | < 0.001a | - | - |
| BMI (kg/m2), mean ± SD | 26.5 ± 4.4 | 26.2 ± 4.9 | 0.800 | - | - |
| Waist circumference (cm), mean ± SD | 94 ± 12 | 93 ± 14 | 0.898 | - | - |
| Arterial hypertension, n (%) | 0.002a | 5.429 | 1.743-16.903 | ||
| Yes | 12 (24.0) | 12 (63.2) | |||
| No | 38 (76.0) | 7 (36.8) | |||
| Type 2 DM, n (%) | 0.508 | 1.627 | 0.466-5.680 | ||
| Yes | 9 (18.0) | 5 (26.3) | |||
| No | 41 (82.0) | 14 (73.7) | |||
| Dyslipidaemia, n (%) | 0.387 | 1.600 | 0.549-4.664 | ||
| Yes | 18 (36.0) | 9 (47.4) | |||
| No | 32 (64.0) | 10 (52.6) | |||
| Obesity, n (%) | 1.000 | 0.945 | 0.260-3.435 | ||
| Yes | 11 (22.0) | 4 (21.1) | |||
| No | 39 (78.0) | 15 (78.9) | |||
| Metabolic syndrome, n (%) | 0.586 | 1.361 | 0.448-4.135 | ||
| Yes | 15 (30.0) | 7 (36.8) | |||
| No | 35 (70.0) | 12 (63.2) | |||
| Regular alcohol intake, n (%) | 0.028a | 3.328 | 1.107-10.003 | ||
| Yes | 17 (34.0) | 12 (63.2) | |||
| No | 33 (66.0) | 7 (36.8) | |||
| AAT value at diagnosis (mg/dL), median | 71 | 58 | 0.060 | - | - |
| FEV1 (%), mean ± SD | 94 ± 27 | 94 ± 36 | 0.944 | - | - |
| DLCO, mL of CO/s/mmHg, median | 94 | 88 | 0.325 | - | - |
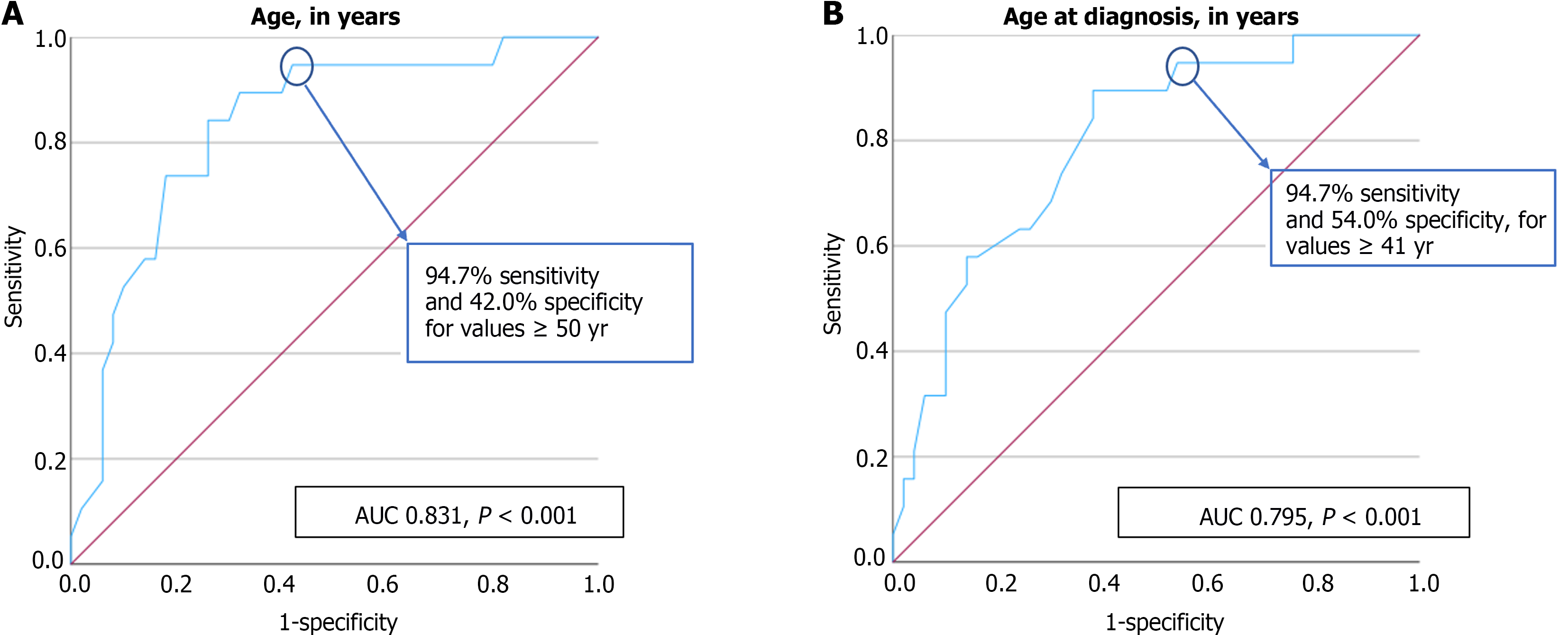
Patients with moderate to advanced fibrosis had a statistically significant higher age (63 ± 11 vs 45 ± 16 years, P < 0.001) and higher age at diagnosis (56 ± 11 vs 40 ± 16 years, P < 0.001). A patients’ age equal or superior to 50 years had a very good discriminative ability in predicting moderate to advanced fibrosis, with a sensitivity of 94.7% and specificity of 42.0% (AUC = 0.831, P < 0.001). Additionally, a patients’ age at diagnosis equal or superior to 41 years also had a very good discriminative ability in predicting moderate to advanced fibrosis, with a sensitivity of 94.7% and specificity of 54.0% (AUC = 0.795, P < 0.001). The ROC curves illustrating the diagnostic ability of the patients’ age and age at diagnosis in predicting a FIB-4 indicative of moderate to advanced fibrosis are present in Figure 2.
Patients with hypertension were 5 times more likely to have a FIB-4 score equal or superior to 1.45 (63.2% vs 24.0%, P = 0.002). Patients with regular alcohol consumption were 3 times more likely to have a FIB-4 score equal or superior to 1.45 (63.2% vs 34.0%, P = 0.028). Other patients’ comorbid conditions, namely type 2 DM, dyslipidaemia, obesity, and metabolic syndrome were not associated with FIB-4 values indicative of moderate to advanced fibrosis (P = 0.508, P = 0.387, P = 1.000 and P = 0.586, respectively).
There was no statistically significant association between patients with or without Pi*ZZ phenotype and a FIB-4 indicative of moderate to advanced fibrosis (P = 0.384). No statistically significant association was found between AAT values at diagnosis, FEV1 and DLCO and a FIB-4 indicative of moderate to advanced fibrosis (P = 0.060, P = 0.944 and P = 0.325).
LSM: Considering LSM cut-off for significant fibrosis (≥ F2) as 7.1 kPa, 10.8% of patients were included in this subgroup. The patients’ age, sex, and anthropometric values (BMI and waist circumference) were not associated with significant fibrosis (P = 0.553, P = 698, P = 0.787, and P = 0.207). No statistically significant association was found between patients’ comorbid conditions, namely hypertension, type 2 DM, dyslipidaemia, obesity, metabolic syndrome, and regular alcohol consumption, and significant fibrosis with LSM value equal or superior to 7.1 kPa (P = 0.215, P = 0.136, P = 0.703, P = 0.638, P = 0.667, and P = 1.000), as well as between AAT values at diagnosis, FEV1 and DLCO and significant fibrosis (P = 0.743, P = 0.404, and P = 0.235). There was no statistically significant association between AAT phenotypes and a LSM value equal or superior to 7.1 kPa (Pi*MZ vs Pi*SZ P = 0.256; Pi*MZ vs Pi*ZZ P = 0.533; Pi*SZ vs Pi*ZZ P = 0.243; Pi*SZ vs Pi*SS P = 0.386; Pi*ZZ vs Pi*SS P = 0.315).
Considering only patients with at least one Pi*Z allele, factors associated with a NFS value that does not exclude advanced fibrosis were similar to the general AATD population of our study, and included age, age at diagnosis, hypertension, type 2 DM, dyslipidaemia, and metabolic syndrome (P < 0.001, P < 0.001, P < 0.001, P = 0.014, P = 0.012, and P < 0.001). Factors associated with a FIB-4 score indicative of moderate to advanced fibrosis in patients with at least one Pi*Z allele were the same as in the general AATD population of our study, and included age, age at diagnosis and hypertension (P < 0.001, P < 0.001, and P = 0.005). In patients with at least one Pi*Z allele, the presence of DM was associated with 8 times higher likelihood of significant liver fibrosis with LSM equal or superior to 7.1 kPa (P = 0.040).
Our study identified 27.5% of patients with AATD, regardless of the phenotype, as having significant fibrosis through serum liver scores, namely NFS and FIB-4. In evaluation via TE, a LSM suggesting significant fibrosis occurred in 10.8% of our patients with AATD, regardless of the phenotype. In previous studies, clinically significant liver fibrosis, through non-invasive assessment, is present in 20%-35% of adults with AATD[5,20].
Patients with NFS that does not exclude advanced fibrosis and a FIB-4 indicative of moderate to advanced fibrosis had a significant higher age and a significant higher age at diagnosis, mainly in those with 41 or more years of age at diagnosis. Increasing age is associated with an increased risk of liver fibrosis, considering it is a genetic disease and the duration of the exposure of hepatocytes to the misfolded AAT increases with age[20], which is also associated with increased proteotoxic stress and decreased autophagic capacity[26,27]. Age younger than 50 years has been associated with lower rates of significant liver fibrosis, especially in males[12,20,28,29], although no major impact was found in females[20].
AATD patients with hypertension, type 2 DM, dyslipidaemia, metabolic syndrome, and regular alcohol intake were more likely to have liver serum tests not excluding advanced fibrosis. In patients with at least one Pi*Z allele, the presence of DM was associated with a higher likelihood of significant liver fibrosis (≥ F2), which is not surprising since these are the main risk factors for chronic liver disease and their association with AATD is an important contributor for liver fibrosis. Risk factors for advanced liver fibrosis in AATD patients include male sex, metabolic syndrome, obesity, and type 2 DM, especially in patients with Pi*ZZ[1,5,12,20,29,30]. In fact, obesity is associated with liver fibrogenesis[31], and insulin resistance leads to an increase in lipolysis and thus increasing the endoplasmic reticulum stress of patients with AATD[32]. Obesity, insulin resistance, and type 2 DM are risk factors associated with LSM equal or superior to 7.1 kPa in patients with Pi*MZ phenotype[20,33].
In our cohort, an AAT value at diagnosis inferior to 77 mg/dL, measure by immunoturbidimetry, was able to predict a NFS value considered as not excluding advanced fibrosis. It is important to consider the method used for AAT measurement, since different methods are available and have different ranges of normal values and protective thresholds[2]. For instance, in the method used in our institution, the normal values range between 150-200 and 350-400 mg/dL, with a protective threshold of 80 mg/dL, while, in nephelometry, the normal values range between 83-120 and 200-220 mg/dL, with a protective threshold of 57 mg/dL, and, in purified standard tests, the normal values are 20-53 μmol/L, with a protective threshold of 11 μmol/L[2]. In fact, previous studies have demonstrated that higher serum levels of AAT in PI*ZZ individuals are significantly and independently associated with better FEV1, with a strong relationship between lower levels of AAT and lung parenchymal disease[34].
There was no statistically significant association between AAT phenotypes and a NFS not excluding advanced fibrosis, a FIB-4 indicative of moderate to advanced fibrosis or a LSM value equal or superior to 7.1 kPa. It has been established that the Pi*MZ phenotype is disease-modifying, a risk factor for the development of liver disease, in the presence of other liver diseases[12,20,29]. In fact, Pi*ZZ patients are 20 times more likely to develop liver fibrosis and cirrhosis and Pi*SZ have a 3 times higher risk, compared to non-carriers, while Pi*MZ individuals have a lower increased odds ratio for liver fibrosis[33]. These differences between our cohort and previous studies can be explained by the disproportion of patients with Pi*MZ and Pi*ZZ phenotypes, and the small patient sample.
In our cohort, no statistically significant association was found between FEV1 and DLCO and a NFS not excluding advanced fibrosis, a FIB-4 indicative of moderate to advanced fibrosis or a LSM value equal or superior to 7.1 kPa. There is controversy regarding the correlation between the severity of lung and liver involvement, with several studies not identifying a statistically significant association between lung function parameters and liver disease, confirming the current model of two different and independent pathogenic mechanisms[5,20]. However, another study, with 10% of patients with chronic liver disease, 7% liver cirrhosis, and 2% hepatocellular carcinoma, has found that lung function is poorer in those with liver disease, and chronic obstructive pulmonary disease was more common in patients with liver disease[28]. The differences between this study and our results can be due to the low prevalence of liver fibrosis in our sample. More recently, it was reported that patients with predominant impairment in diffusion capacity also have signs of liver involvement, with higher FIB-4 values, lower platelet levels and higher percentage of individuals with diagnosed liver disease[34].
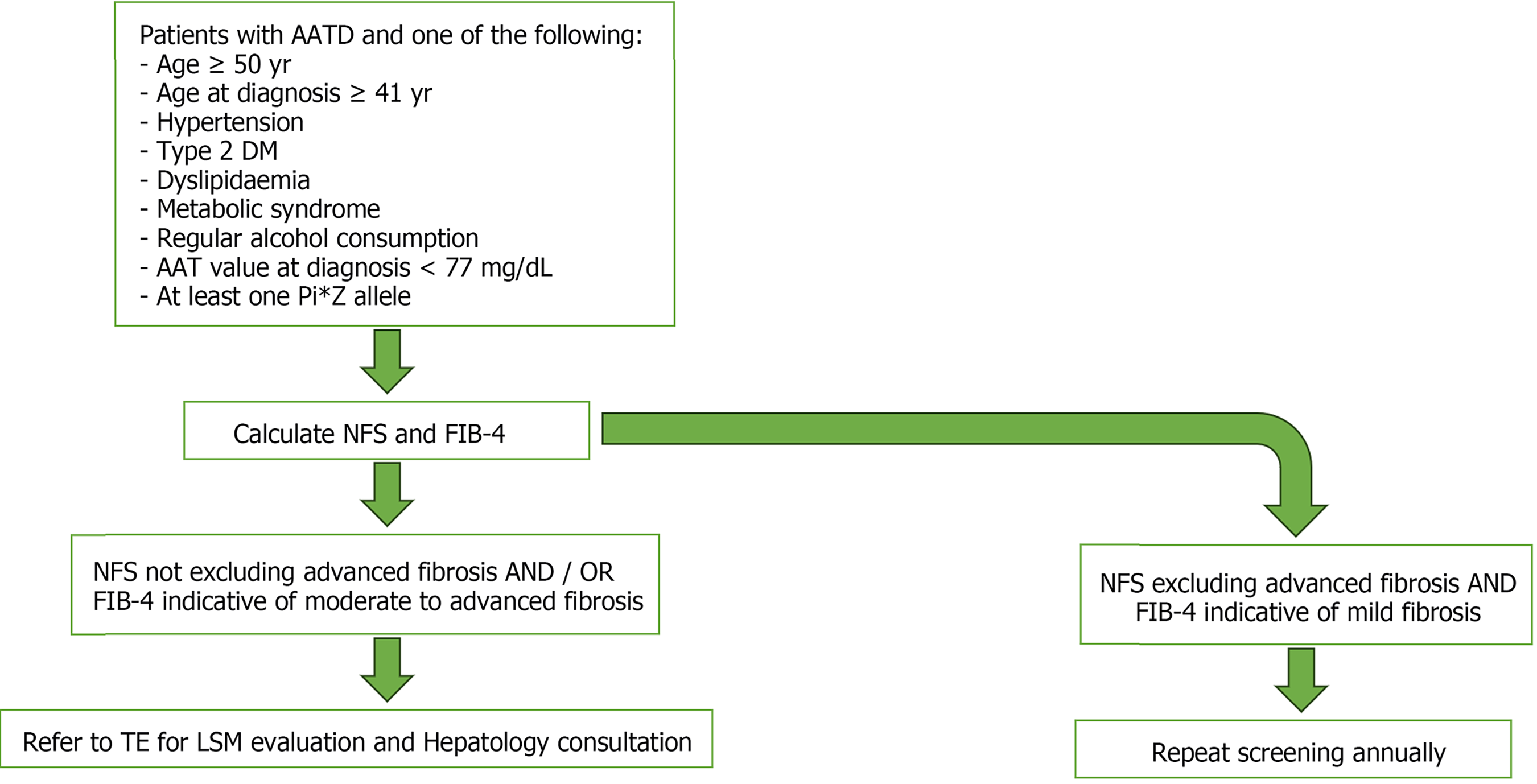
Using the associations found in our cohort, we developed an algorithm for the screening of liver disease in patients with AATD (Figure 3). AATD patients and one additional risk factor for liver fibrosis should be monitored and their NFS and FIB-4 calculated. Those with a NFS not excluding advanced fibrosis and/or a FIB-4 indicative of moderate to advanced fibrosis must be referred to TE for LSM evaluation and Hepatology consultation. This algorithm can be a useful tool in primary care and Pulmonology consultation, stratifying patients with AATD at increased risk of liver fibrosis, allowing patient selection for referral to Hepatology consultation and emerging clinical trials in AATD liver therapies.
Our study supports the increasing evidence that patients with AATD are at risk of developing liver disease in the presence of comorbid conditions, namely metabolic risk factors and regular alcohol consumption. Additional risk factors were identified, such as age equal or superior to 50 years, having at least one Pi*Z allele, an age at diagnosis equal or superior to 41 years, and an AAT value at diagnosis inferior to 77 mg/dL. Therefore, it is important to minimise coexisting factors in individuals with AATD phenotypes that confer an increased risk of liver fibrosis (Pi*MZ, Pi*ZZ and Pi*SZ). These patients should have a tight control of metabolic factors, with weight reduction, nutrition consultations, controlled blood pressure, dyslipidaemia and glycaemia, and reduction of alcohol consumption, be advised to lose weight and avoid alcohol use. Long-term, longitudinal, and prospective studies are crucial to monitor these patients and determine the rate of disease progression and the occurrence of complications of advanced chronic liver disease, such as cirrhosis and liver cancer, as well as the impact of the correction of metabolic factors in the progression of liver disease.
| 1. | Strnad P, McElvaney NG, Lomas DA. Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2020;382:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 2. | Lopes AP, Mineiro MA, Costa F, Gomes J, Santos C, Antunes C, Maia D, Melo R, Canotilho M, Magalhães E, Vicente I, Valente C, Gonçalves BG, Conde B, Guimarães C, Sousa C, Amado J, Brandão ME, Sucena M, Oliveira MJ, Seixas S, Teixeira V, Telo L. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology. 2018;24 Suppl 1:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Le A, Ferrell GA, Dishon DS, Le QQ, Sifers RN. Soluble aggregates of the human PiZ alpha 1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992;267:1072-1080. [PubMed] |
| 4. | Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 767] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Clark VC, Marek G, Liu C, Collinsworth A, Shuster J, Kurtz T, Nolte J, Brantly M. Clinical and histologic features of adults with alpha-1 antitrypsin deficiency in a non-cirrhotic cohort. J Hepatol. 2018;69:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Khodayari N, Wang RL, Oshins R, Lu Y, Millett M, Aranyos AM, Mostofizadeh S, Scindia Y, Flagg TO, Brantly M. The Mechanism of Mitochondrial Injury in Alpha-1 Antitrypsin Deficiency Mediated Liver Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Rodrigues JF, Mineiro A, Reis A, Ventura DG, Fernandez-Llimos F, Costa F, Gomes J, Silva JM, Lopes P, Cordeiro CR. Alpha-1 Antitrypsin Deficiency: Principles of Care. Acta Med Port. 2020;33:433-439. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Fromme M, Schneider CV, Trautwein C, Brunetti-Pierri N, Strnad P. Alpha-1 antitrypsin deficiency: A re-surfacing adult liver disorder. J Hepatol. 2022;76:946-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Foreman MG, Wilson C, DeMeo DL, Hersh CP, Beaty TH, Cho MH, Ziniti J, Curran-Everett D, Criner G, Hokanson JE, Brantly M, Rouhani FN, Sandhaus RA, Crapo JD, Silverman EK; Genetic Epidemiology of COPD (COPDGene) Investigators *. Alpha-1 Antitrypsin PiMZ Genotype Is Associated with Chronic Obstructive Pulmonary Disease in Two Racial Groups. Ann Am Thorac Soc. 2017;14:1280-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Lara B, Miravitlles M. Spanish Registry of Patients With Alpha-1 Antitrypsin Deficiency; Comparison of the Characteristics of PISZ and PIZZ Individuals. COPD. 2015;12 Suppl 1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Strnad P, Buch S, Hamesch K, Fischer J, Rosendahl J, Schmelz R, Brueckner S, Brosch M, Heimes CV, Woditsch V, Scholten D, Nischalke HD, Janciauskiene S, Mandorfer M, Trauner M, Way MJ, McQuillin A, Reichert MC, Krawczyk M, Casper M, Lammert F, Braun F, von Schönfels W, Hinz S, Burmeister G, Hellerbrand C, Teufel A, Feldman A, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Ridinger M, Wodarz N, Soyka M, Rietschel M, Kiefer F, Weber T, Marhenke S, Vogel A, Hinrichsen H, Canbay A, Schlattjan M, Sosnowsky K, Sarrazin C, von Felden J, Geier A, Deltenre P, Sipos B, Schafmayer C, Nothnagel M, Aigner E, Datz C, Stickel F, Morgan MY, Hampe J, Berg T, Trautwein C. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut. 2019;68:1099-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Hamesch K, Strnad P. Non-Invasive Assessment and Management of Liver Involvement in Adults With Alpha-1 Antitrypsin Deficiency. Chronic Obstr Pulm Dis. 2020;7:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1027] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 14. | Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Umbro I, Baratta F, Angelico F, Del Ben M. Nonalcoholic Fatty Liver Disease and the Kidney: A Review. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 17. | European Association for the Study of the Liver; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1042] [Article Influence: 260.5] [Reference Citation Analysis (0)] |
| 18. | Abbas SH, Pickett E, Lomas DA, Thorburn D, Gooptu B, Hurst JR, Marshall A. Non-invasive testing for liver pathology in alpha-1 antitrypsin deficiency. BMJ Open Respir Res. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Guillaud O, Dumortier J, Traclet J, Restier L, Joly P, Chapuis-Cellier C, Lachaux A, Mornex JF. Assessment of liver fibrosis by transient elastography (Fibroscan(®)) in patients with A1AT deficiency. Clin Res Hepatol Gastroenterol. 2019;43:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Hamesch K, Mandorfer M, Pereira VM, Moeller LS, Pons M, Dolman GE, Reichert MC, Schneider CV, Woditsch V, Voss J, Lindhauer C, Fromme M, Spivak I, Guldiken N, Zhou B, Arslanow A, Schaefer B, Zoller H, Aigner E, Reiberger T, Wetzel M, Siegmund B, Simões C, Gaspar R, Maia L, Costa D, Bento-Miranda M, van Helden J, Yagmur E, Bzdok D, Stolk J, Gleiber W, Knipel V, Windisch W, Mahadeva R, Bals R, Koczulla R, Barrecheguren M, Miravitlles M, Janciauskiene S, Stickel F, Lammert F, Liberal R, Genesca J, Griffiths WJ, Trauner M, Krag A, Trautwein C, Strnad P; European Alpha1-Liver Study Group. Liver Fibrosis and Metabolic Alterations in Adults With alpha-1-antitrypsin Deficiency Caused by the Pi*ZZ Mutation. Gastroenterology. 2019;157:705-719.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5314] [Article Influence: 265.7] [Reference Citation Analysis (0)] |
| 22. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10528] [Article Influence: 658.0] [Reference Citation Analysis (0)] |
| 23. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2278] [Article Influence: 126.6] [Reference Citation Analysis (1)] |
| 24. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 575] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 25. | Berger A, Shili S, Zuberbuhler F, Hiriart JB, Lannes A, Chermak F, Hunault G, Foucher J, Oberti F, Fouchard-Hubert I, Cales P, de Ledinghen V, Boursier J. Liver Stiffness Measurement With FibroScan: Use the Right Probe in the Right Conditions! Clin Transl Gastroenterol. 2019;10:e00023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 992] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 27. | Leidal AM, Levine B, Debnath J. Autophagy and the cell biology of age-related disease. Nat Cell Biol. 2018;20:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 28. | Tanash HA, Piitulainen E. Liver disease in adults with severe alpha-1-antitrypsin deficiency. J Gastroenterol. 2019;54:541-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Bouchecareilh M. Alpha-1 Antitrypsin Deficiency-Mediated Liver Toxicity: Why Do Some Patients Do Poorly? Chronic Obstr Pulm Dis. 2020;7:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Townsend SA, Edgar RG, Ellis PR, Kantas D, Newsome PN, Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Stender S, Kozlitina J, Nordestgaard BG, Tybjærg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 314] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 32. | Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1740] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 33. | Fromme M, Schneider CV, Pereira V, Hamesch K, Pons M, Reichert MC, Benini F, Ellis P, H Thorhauge K, Mandorfer M, Burbaum B, Woditsch V, Chorostowska-Wynimko J, Verbeek J, Nevens F, Genesca J, Miravitlles M, Nuñez A, Schaefer B, Zoller H, Janciauskiene S, Abreu N, Jasmins L, Gaspar R, Liberal R, Macedo G, Mahadeva R, Gomes C, Schneider KM, Trauner M, Krag A, Gooptu B, Thorburn D, Marshall A, Hurst JR, Lomas DA, Lammert F, Gaisa NT, Clark V, Griffiths W, Trautwein C, Turner AM, McElvaney NG, Strnad P. Hepatobiliary phenotypes of adults with alpha-1 antitrypsin deficiency. Gut. 2022;71:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 34. | Miravitlles M, Turner AM, Torres-Duran M, Tanash H, Rodríguez-García C, López-Campos JL, Chlumsky J, Guimaraes C, Rodríguez-Hermosa JL, Corsico A, Martinez-González C, Hernández-Pérez JM, Bustamante A, Parr DG, Casas-Maldonado F, Hecimovic A, Janssens W, Lara B, Barrecheguren M, González C, Stolk J, Esquinas C, Clarenbach CF. Clinical and functional characteristics of individuals with alpha-1 antitrypsin deficiency: EARCO international registry. Respir Res. 2022;23:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |