INTRODUCTION
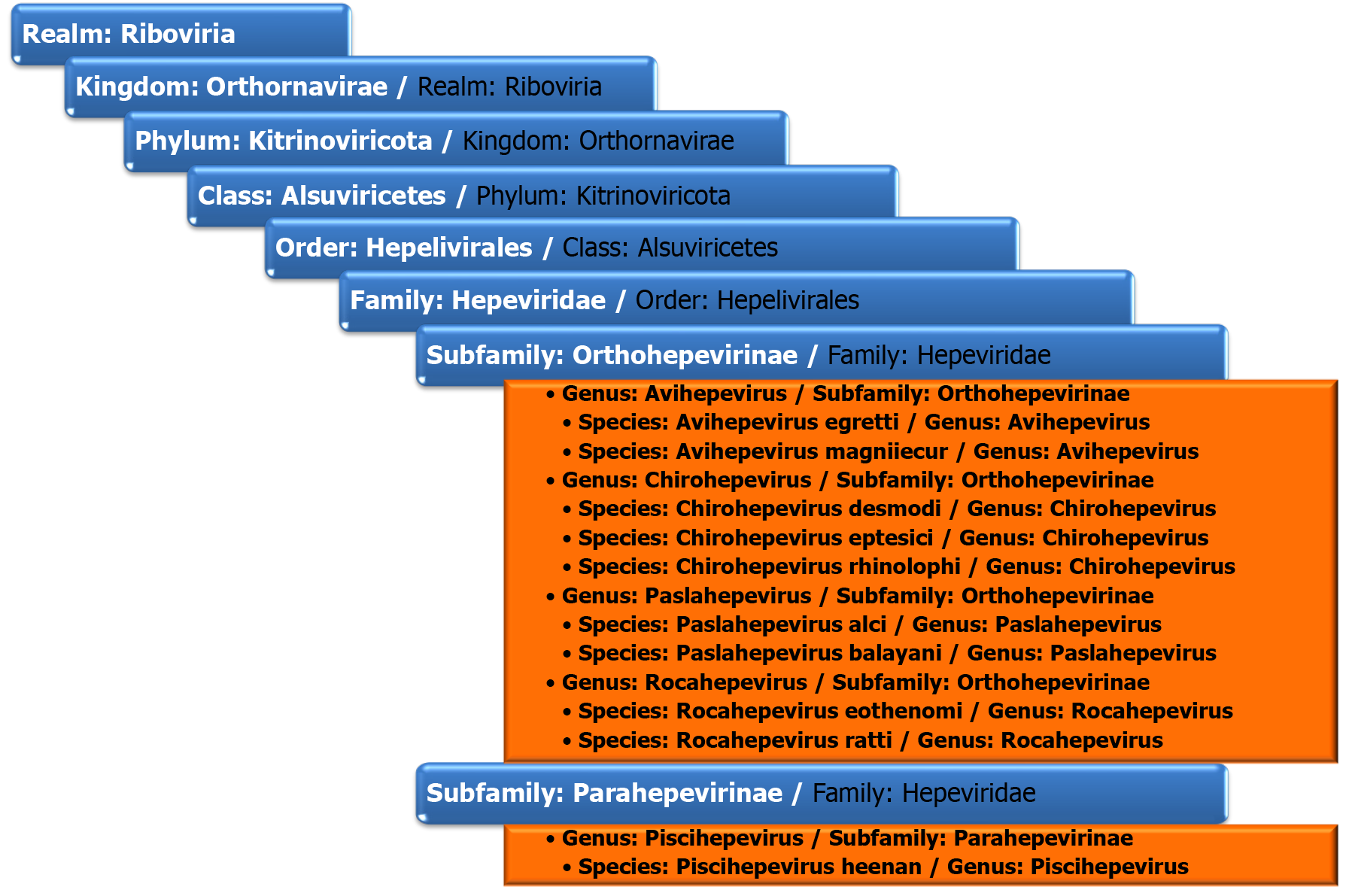
Hepatitis E virus (HEV) is a causative agent of viral hepatitis that is more and more recognised as an important emergent public health problem. With an annual estimate of 20 million new HEV infections globally, which leads to approximately 3.3 million symptomatic cases, some studies consider this virus to be the leading cause of viral hepatitis worldwide[1-3]. A recent meta-analysis pointed out that 12.47% of the global population (approximately 939 million people) had already had contact with HEV. HEV is a single-stranded positive-sense RNA virus in the Hepeviridae family. According to the last data from the International Committee on the Taxonomy of Viruses, this family belongs to the realm of Riboviria, kingdom Orthornavirinae, phylum Kitrinoviricota, order Hepelivirales[4]. The Hepeviridae family comprises two subfamilies: Orthohepevirinae and Parahepevirinae. The viruses that can infect humans are part of Orthohepevirinae, genus Paslahepevirus and Rocahepevirus. The other two genera of Orthohepevirinae are Avihepevirus and Chirohepevirus, and they seem to be restricted to birds (avian HEV) and, respectively, bats (bat HEV)[5]. The taxonomy of the Hepeviridae family is shown in Figure 1. Members of the Paslahepevirus balayani species are the “classical” agents of hepatitis E (HEV-A); they are assigned to 8 different genotypes, HEV-1 to HEV-8. HEV-1, HEV-2, HEV-3 and HEV-4 are associated with HEV infection in humans. Genotypes HEV-1 and HEV-2 are restricted to humans, whereas genotypes HEV-3 and HEV-4 also have an animal reservoir and are zoonotic. Genotype HEV-3 has been documented in humans, pigs, deer[6], goats[7], rats[8], bottlenose dolphins[9] and recently in cats[10]. One variant of HEV-3 with specific mutations in the ORF-1 region has been described in rabbits and humans[11,12]. Genotype HEV-4 was found in cattle and sheep[13,14]. HEV-5 and HEV-6 have only been described in wild boar in Japan[15]. HEV-7 is found in dromedary camels, and only one case of human infection with this genotype has been described[16,17]. HEV-8 has only been detected in Bactrian camels in China[18]. Paslahepevirus alci, the other species of the Paslahepevirus genus, is the etiologic agent of moose E hepatitis[4]. Members of the genus Rocahepevirus are phylogenetically distinct from other viruses in the Orhtohepevirinae subfamily and have a different host range, being found in rodents, shrews and carnivores. They are divided into two species, Rocahepevirus eothenomi and Rocahepevirus Ratti[19]. Rocahepevirus eothenomi has two genotypes, HEV-C3 and HEV-C4, that have not been described in humans and were found in vole species[20]. Rocahepevirus ratti (rat HEV virus – RHEV) comprises two genotypes: HEV-C1 and HEV-C2. Members of the species Rocahepevirus ratti of genotype C1 can infect humans and have been demonstrated in 21 cases of human infections. Other families infected by Rocahepevirus ratti are Muridae, Soricidae, Hominidae, Mustelidae and Cricetidae. Within the family Muridae, the genus Rattus represented the majority (88%), and all the strains isolated from this family belonged to the HEV-C1 genotype, while genotype C2 has been detected mostly in mustelids (ferret and mink)[21]. The name Rocahepevirus derives from Rodentia & Carnivora, the known hosts of members of the genus.
Figure 1
The taxonomy of the Hepeviridae family[4].
HEV was first described in 1983[22]. Evidence of anti-HEV antibodies in rats in the United States dates back to 1999[23]. The first isolation of a member of the Rocahepevirus genus was made in 2010 in Germany[24]. This strain was initially classified as Ortohepevirus C, sharing only 50%-60% of the genome with Paslahepevirus balayani (previously known as Ortohepevirus A). The first case of RHEV infection in humans was documented in 2017 in Hong Kong in an immune-compromised patient after receiving a liver transplant[25].
GLOBAL EPIDEMIOLOGY OF HEV-C1 IN ANIMALS AND HUMANS
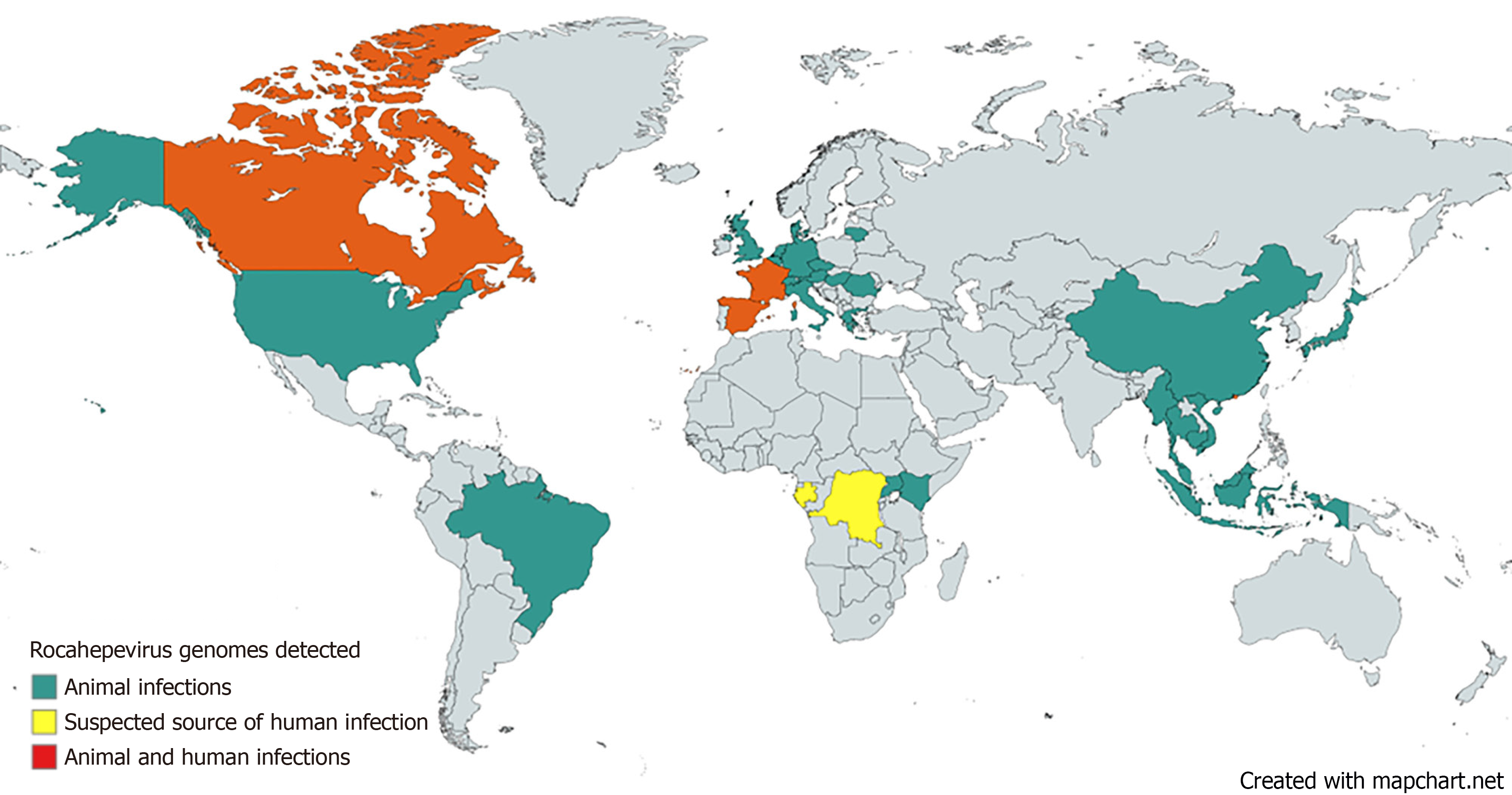
As of October 2023, 817 Rocahepevirus sequences have been identified and listed in the United States National Institute of Health’s GenBank® database[21]. Of the 817 identified genotypes, 631 were HEV-C1, 70 were HEV-C2, and the rest of 116 were HEV-C3 and C4 and a new candidate for HEV-C5[21]. Most of the cases are found in Asia (586 of 817), in Indonesia (129 HEV-C1), Vietnam (28 HEV-C1), Myanmar (21 HEV-C1), Malaysia (14 HEV-C1), Thailand (1 HEV-C1), Cambodia (1 HEV-C1), Japan (66 HEV-C1 and 15 HEV-C2) and China (182 HEV-C1, 58 HEV-C2 and 71 HEV-C3/C4/novel). In Europe, Rocahepevirus sequences were identified in Germany (45 HEV-C1 and 6 HEV- novel), Czech Republic (5 HEV-C1 and 8 HEV- novel), the United Kingdom (7 HEV-C1), Hungary (6 HEV-C1 and 11 HEV- novel), Italy (70 HEV-C1), Denmark (3 HEV-C1 and 4 HEV-C2), Austria (8 HEV-C1), Switzerland (4 HEV-C1), France (6 HEV-C1), Greece (2 HEV-C1), Belgium (6 HEV-C1), Spain (7 HEV-C1), Lithuania (6 HEV-C1), Netherlands (1 HEV-C1 and 2 HEV-C2) and Romania (9HEV-C1), a total of 216 cases. Figure 2 depicts the countries where various Rocahepevirus genomes have been documented in both animal and human infections. 21 cases of human infection have been described until now, most of them in Hong Kong (16), 3 in Spain, one in France and one in Central Africa (D.R. Congo or Gabon – the source being unclear in this last case).
Figure 2 Countries where Rocahepevirus genomes have been detected in human and animal infections.
Created with mapchart.net.
While the transmission of HEV to humans is relatively well understood, the transmission of HEV-C1 is not completely clarified. HEV-1 and HEV-2 are mainly transmitted by fecal-oral route, and epidemics are most frequently waterborne. HEV-3 and HEV-4 are most frequently associated with foodborne transmission, mainly from the consumption of undercooked pork, boar, or deer meat or other products (like contaminated milk from cows, goats or sheep) but also from various marine living forms. For HEV-C1, the following means of transmission have been proposed: Exposure to contaminated environments, direct contact with infected source animals, and consumption of contaminated water or food[26]. The risk of transmission through blood transfusion or organ transplantation has not been established[26].
CLINICAL MANIFESTATIONS OF HEV-C1 INFECTION IN HUMANS
In humans, most cases of Rocahepevirus ratti infections were identified in immunocompromised hosts[25,27-31]. Only 5 of the 21 described cases had no evident cause of immune suppression. The forms of hepatitis ranged from subclinical (one case), mild acute hepatitis (11 cases), to severe acute hepatitis (one case). 7 patients developed persistent hepatitis (defined as viral load detectable for more than 3 months) and one cirrhosis. The patient who developed severe acute hepatitis is a 49-year-old immune-competent patient from Canada who travelled in D.R. Congo, Gabon and Caiman Island. His clinical manifestations began with urticarial rash, jaundice, steatorrhea, nausea and loss of appetite. Liver biopsy showed severe acute hepatitis with inflammation, interface activity, and significant hepatocyte damage. His clinical outcome was good, with complete spontaneous resolution of clinical manifestations and normalisation of blood tests[31]. All 7 patients who developed persistent forms of hepatitis were under immune-suppressed therapies for various indications, mainly transplantation of solid organs. The patient who developed cirrhosis is a 61-year-old who received renal transplantation in 2006 and has been on immune-suppressant treatment with corticosteroids, tacrolimus and mycophenolate ever since. In 2008, he had a reactivation of HBV for which he was on entecavir. In 2016, after a trip to India, he developed liver dysfunction, which evolved into cirrhosis and led to a double liver-kidney transplantation in 2017 without any identifiable cause. Since the second transplantation, the patient has continuously unexplained modified liver function tests. In 2022, he was again diagnosed with cirrhosis, and this time, HEV-C RNA was detected in his blood tests. Retrospectively, all available samples since 2017 were positive with HEV-C RNA[27]. The patient who developed subclinical HEV-C infection had metastatic cancer. He had neither clinical symptoms nor biochemical hepatitis[29]. The small number of patients does not allow us to draw any statistical conclusions, but we can make a descriptive analysis of the described cases: Most of them were mild, the one severe case occurred in an immune-competent patient, all patients that developed persistent hepatitis were immune-suppressed by specific therapies. Immunocompromised people might be more vulnerable to HEV-C1 infections[32]. Additionally, the possibility of subclinical infections, which may not cause considerable liver enzyme changes, poses a potential risk for blood transfusion safety, especially given the lack of effective screening tests for HEV-C1.
DIAGNOSIS OF HEV-C1 INFECTION
Markers for HEV-A diagnosis have been developed and are reliable for diagnosing acute Hepatitis E infection: HEV-A IgM and HEV-A RNA. Because of the significant divergence between HEV-A and HEV-C1, serological methods based on antibodies against HEV-A may lead to false negative results when diagnosing Rocahepevirus infections[33]. In the study by Sridhar et al[33], the identity between HEV-A and HEV-C1 immunodominant E2 peptide sequences was only 48%. Anti-HEV-A monoclonal antibodies bound HEV-C1 poorly in homology modelling and antigen enzyme-linked immunoassays. The sensitivity of the commercial HEV-A antigen method was 0%, and that of the antibody was 10%-70% for HEV-C1 diagnosis. HEV-A4 p239 and HEV-C1 p241 are two peptides developed based on HEV-A (genotype 4 )and HEV-C1 that have been tested for diagnosing HEV-A and HEV-C infections. Immunoblots based on the above-mentioned peptides succeeded in accurately differentiating HEV-A and HEV-C1 serological profiles. Commercial assays for detecting HEV-C1 antigens or antibodies are not yet available. Currently, rat hepatitis E infection can only be confirmed by detecting HEV-C1 RNA in blood by reverse transcriptase-polymerase chain reaction (RT-PCR). Several in-house reverse transcription PCRs have been imagined and used in different research settings that showed good performance in identifying HEV-C1 RNA, but no commercial kits have been available so far[25,34].
TREATMENT AND PREVENTION
The disease is usually self-limiting in immunocompetent patients; therefore, in most cases, only supportive care is needed. Some patients may need hospitalisation: Immune-compromised patients, patients that develop severe forms of hepatitis or persistent forms, and, based on previous experience with HEV-A infections, most probably pregnant women. Ribavirin has been shown to be efficient in both severe forms of human hepatitis for reducing the severity of the disease[25] and persistent forms of HEV-C1 infections for eliminating the virus[28]. Reducing the immune suppression in patients on immune-suppressant medication is also efficient for managing persistent infections with Rocahepevirus ratti[28].
A vaccine for hepatitis E prevention, Hecolin, was developed and licensed in China in 2011. It is available for use in China and Pakistan. The vaccine was also used with success in an outbreak in South Sudan in March 2022. World Health Organization does not recommend the routine use of this vaccine because there is no sufficient data[35]. There are no data also on the efficiency of Hecolin in preventing HEV-C1 infections in humans, but a study on rats showed that HEV-A vaccination is not protective for HEV-C1 infection[33]. This study suggests the p241 peptide of Rocahepevirus ratti as an antigenic structure for a future vaccine against HEV-C1.
FUTURE PUBLIC HEALTH MEASURES TO PREVENT HEV-C1 SPREAD
The following directions should be considered as measures of prevention: Public health surveillance measures and epidemiological investigations. In areas where cases of acute hepatitis of undetermined origin appear, testing for HEV-C1 by RT-PCR should be carried out, and then epidemiological investigations should be performed. Investigations should include the patients as well as their close contacts, and a thorough history of food, travel, contact with rodents or other factors that may lead to the identification of the means of transmission has to be collected.
Rodent control
Rodent control is a crucial measure in preventing rodent-borne zoonoses. Public authorities need to be actively involved because such efforts must be implemented on a large scale.
Environmental and personal hygiene
Rigorous food, personal and environmental hygiene are essential in the prevention of any zoonose. Extra caution should be carried out in persons who are at a higher risk of developing more severe forms of HEV-C1 hepatitis: Immune-compromised patients of any cause and pregnant women, but not limited to them. Environmental control should include simple measures such as limiting the food, harbourage and passage of rats. Avoiding contact is the easiest way to avoid zoonotic diseases[36].
Public health education
Multiple channels should be used to raise awareness regarding the risk of rodent-borne zoonoses, such as press releases, social media, health education materials and campaigns, public interest announcements, etc.
CONCLUSION
Zoonoses are part of our existence; they have accompanied humankind since the dawns of civilisation, and they are here to stay. Rocahepevirus ratti is one agent that could become a regular cause of hepatitis in the future, and raising awareness regarding this possibility is important. Understanding its provenience and the means of transmission helps to identify newly emerged cases. Clinical manifestations need more attention as there might be some particular features in the disease caused by this agent related to the HEV-A virus. Serological and molecular techniques for the diagnosis of HEV-C1 infection need to be developed and standardised. Data regarding the management of diagnosed cases should be prospectively collected, and a standardised treatment must be established. Personal and public health measures must be implemented to prevent the infection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Clinical Microbiology and Infectious Diseases, 57056.
Specialty type: Infectious diseases
Country of origin: Romania
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: McClure CP S-Editor: Liu JH L-Editor: A P-Editor: Zhang L