Published online Jun 27, 2024. doi: 10.4254/wjh.v16.i6.883
Revised: April 26, 2024
Accepted: May 20, 2024
Published online: June 27, 2024
Processing time: 107 Days and 13.7 Hours
The standard approach to organ preservation in liver transplantation is by static cold storage and the time between the cross-clamping of a graft in a donor and its reperfusion in the recipient is defined as cold ischemia time (CIT). This simple definition reveals a multifactorial time frame that depends on donor hepatectomy time, transit time, and recipient surgery time, and is one of the most important donor-related risk factors which may influence the graft and recipient’s survival. Recently, the growing demand for the use of marginal liver grafts has prompted scientific exploration to analyze ischemia time factors and develop different organ preservation strategies. This review details the CIT definition and analyzes its different factors. It also explores the most recent strategies developed to implement each timestamp of CIT and to protect the graft from ischemic injury.
Core Tip: Many variables affect liver transplantation outcomes. Among these variables, cold ischemia time (CIT), defined as the time from the cold flushing of the donor organ until the graft is removed from ice to be implanted into the recipient, is the one of the most important and is incorporated into many predictive scoring systems. CIT is a multifactorial variable that depends on donor hepatectomy time, transit time and recipient surgery time and can only be calculated retrospectively.
- Citation: Cesaretti M, Izzo A, Pellegrino RA, Galli A, Mavrothalassitis O. Cold ischemia time in liver transplantation: An overview. World J Hepatol 2024; 16(6): 883-890
- URL: https://www.wjgnet.com/1948-5182/full/v16/i6/883.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i6.883
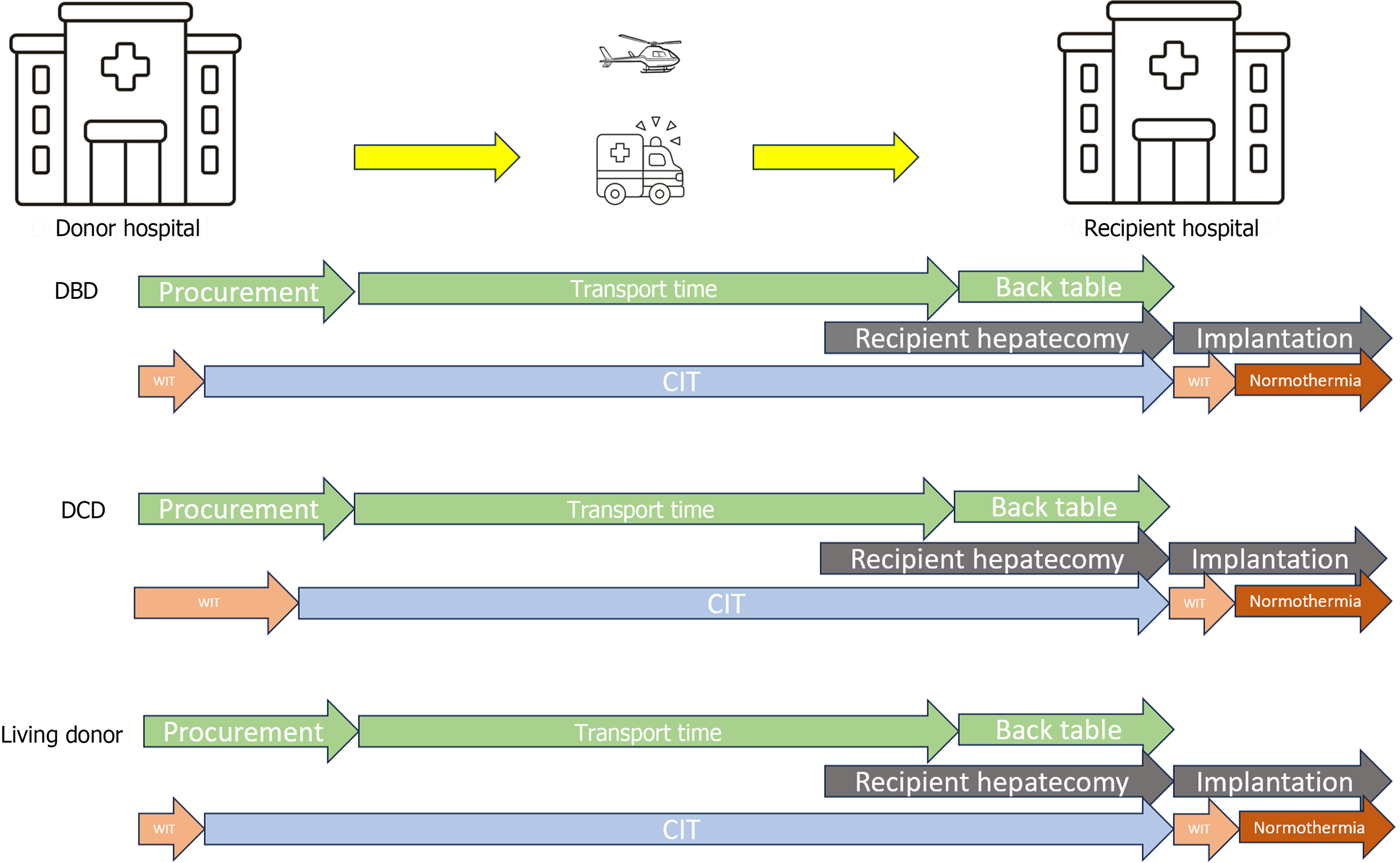
The outcome of liver transplantation (LT) has markedly improved in recent decades with patient survival rates of > 90%, 85% and 70% at 1-, 5-, and 10-years post-LT[1,2]. Such good results have been achieved thanks to improvements in the management of many variables that affect LT outcomes. Among these variables, cold ischemia time (CIT), defined as the time from the cold flushing of the donor organ until the graft is removed from ice to be implanted into the recipient, is the one of the most important factors that affects organ and patient survival. Indeed, several reports have documented that prolonged CIT is an independent risk factor for the development of delayed graft function and primary non-function[3]. Moreover, many of the predictive models proposed to estimate survival after LT incorporate CIT into their scoring systems[4-6]. However, CIT is a multifactorial variable that depends on donor hepatectomy time, transit time and recipient surgery time and can only be calculated retrospectively. Therefore, to reduce CIT and improve the results of transplantation, attention must be focused on each modifiable timestamp associated with CIT (Figure 1). In this editorial we analyze each step of CIT and describe ongoing research about this important LT variable.
Organ ischemia time is divided into cold and warm ischemia time (WIT). WIT is a term used to describe ischemia of cells and tissues under normothermic conditions[7]. In the transplant setting, WIT is used to describe two physiologically distinct periods of ischemia: (1) Ischemia during organ procurement, from the time of cross clamping (or of asystole in non-heart-beating donors), until cold perfusion or normothermic regional perfusion are commenced; and (2) Ischemia during implantation, from removal of the organ from ice until portal reperfusion[8]. CIT is defined as the time from cold perfusion of the graft until the removal of the graft from ice to be implanted. During this period, static cold storage lowers the temperature of the graft between 0 °C and 4 °C.
Hypothermia has been considered key to successful graft preservation since 1960 when Colins demonstrated that a kidney could be preserved for 30 h safely before transplantation[9]. During hypothermia, cellular metabolism is slowed (the coenzyme Q10 effect[10]), which limits the need for adenosine triphosphate (ATP). However, the beginning of CIT when organs are flushed with cold preservation solution causes inflammation and injury due to sodium-potassium membrane pump dysfunction, resulting in cellular edema with free calcium influx, and subsequent activation of enzyme cascades leading to cellular death. Once the graft is perfused by recipient blood at the end of WIT, there is a restoration of circulation and ATP breakdown with xanthine oxidase generation of free radicals. This causes lipid peroxidation with cellular destruction named ischemia-reperfusion (IR) injury[11,12]. This graft damage is exacerbated by prolonged CIT and it is responsible of primary non-function, arterial thrombosis, biliary complications and recipient mortality[13,14].
Therefore, organs with prolonged CIT are often deemed unsuitable for LT[15]. Many studies have attempted to define the ideal duration of CIT but there is no absolute consensus, and the only recommendation is that it should be as short as possible. Initially, some investigators considered acceptable a CIT of up to 18 h, while recipient survival was shown to be adversely affected by CIT over 12 h in a European survey and over 10 h in a United States survey[16,17].
Today, a CIT between 8 and 10 h is tolerable[18,19], demonstrating significant differences in complications compared with liver grafts implanted after this period. Recently Lozanovski et al[20], reported that each additional hour of CIT was associated with a 3.4% increase risk of liver graft loss and the extent of negative impact depended on underlying disease. Indeed, patients with hepatitis C virus (HCV)-cirrhosis demonstrated the highest risk of graft loss due to prolonged CIT, probably because during the hepatocyte proliferation in the IR injury, HCV infiltrates into the proliferating cells, leading to early HCV recurrence[21]. Additionally, Pan et al[22] demonstrated that risk of prolonged hospital stays (PLOS, > 30 d) steadily increased with increasing CIT, reaching the greatest odds ratio (OR) for PLOS with 13-14 h [odds ratio (OR) = 2.05; 95% confidence interval (CI): 1.57-2.67] and 15-16 h (OR = 2.06; 95%CI: 1.27-3.33) of CIT.
The impact of prolonged CIT varies between donation types, ages and graft steatosis severity. In donation after circulatory death (DCD) LT, successful outcomes hinge on events occurring during organ procurement and prolonged organ ischemia (donor WIT and CIT) dramatically impact LT results, so every step of procurement aims to minimize ischemic times. Paterno et al[23] reported that CIT cut-off > 4 h in DCD LT is associated with increased risk for graft loss, longer post-transplant hospital stays, higher rate of primary non-function, and hyperbilirubinemia. The elderly liver graft has a diminished regenerative response to partial resection and a reduction in the capacity to generate an acute phase protein response[24,25] so prolonged CIT results in an increase of substrate for reactive oxygen species potentiating the effects of IR injury[26]. It is unclear why steatotic livers demonstrate increased susceptibility to IR injury. Proposed mechanisms include impaired hepatic microcirculation[27,28] and mitochondrial dysfunction[29]. Macrovesicular steatosis leads to increased hepatocyte volume causing obstruction of the adjacent sinusoid space and increased hepatic microcirculation vascular resistance[30,31]. This can impair oxygen and nutrient delivery following reperfusion to an already susceptible organ. Increased lipid levels in steatotic livers result in the formation of reactive oxygen species which may lead to mitochondrial dysfunction[32]. The interruption of crucial mitochondrial processes disrupts normal cellular bioenergetics, impairs cellular function, and leads to cell necrosis or apoptosis[33]. Kupffer cell dysfunction and impaired leukocyte adhesion may also increase steatotic liver susceptibility to IR injury[34].
Donor CIT starts when organs are flushed with cold preservation solution. This is the donor cross-clamp timestamp. In the case of multiorgan donors, liver procurement begins after heart and lung retrieval. The surgical technique[35] is divided into warm and cold dissections according to the tissue perfusion time. Warm dissection has the advantage of perfusion after confirming the vascular structures and it is mandatory in case of living donor and recommended in case of in situ split LT[36,37] where hepatic vessels and parenchymal dissection should be performed before cross clamp to reduce CIT. Contrarily, cold dissection can reduce operative donor time and organ damage by rapid organ procurement. Both warm and cold dissection end with donor hepatectomy, with duration influencing early outcomes after LT. There are likely independent factors influencing donor hepatectomy time, as surgical technique and the surgeon’s experience, but duration > 60 min is associated with early allograft dysfunction[38]. European studies have demonstrated that donor hepatectomy time is an independent risk factor for graft loss and development of ischemic cholangiopathy[39-41]. Moreover, every 10-min increase in donor hepatectomy time has a detrimental effect on early allograft dysfunction similar to a 1-h increase in CIT[42]. Conversely, no significant interaction between donor hepatectomy time and donor type (donation after brain death vs DCD) has been observed, indicating that DCD is equally susceptible to the effect of donor hepatectomy time. A particular type of procurement is the super-rapid technique[43]. Utilized in DCD donors in case of absence of normothermic regional perfusion, it initiates in less than 4 min after skin incision to reduce the WIT. The supraceliac aorta is cross-clamped and the intrapericardial inferior vena cava is vented to avoid organ engorgement. The inferior mesenteric vein is then cannulated to perfuse the portal system. Once the liver becomes cold and free of blood, en bloc hepatectomy is performed expeditiously. However, it is strongly recommended that this technique be performed by experienced donor surgeons.
To avoid graft ischemia entirely, Gül et al[44] and He et al[45] proposed the “ischemia-free” LT techniques. The first technique proposed to perform a liver graft procurement without cold preservation, based on the setting of an in-house donor and a consecutive in situ preparation of the liver to be able to perform an immediate warm-ischemia-only LT without cold preservation. In the second method liver grafts are procured, preserved, and implanted under continuous normothermic machine perfusion. Both studies reported good results in 6 and 38 cases respectively. However, the risk of extrahepatic organ loss, technical difficulties, the need of an in-house donor and the cost of machine perfusion limited the consideration of such novel techniques as the gold standard in liver procurement.
Once procured, the donor liver graft is preserved for transport, either by static cold storage on ice or recently in machine perfusion. Transportation of the graft requires seamless coordination between the donor hospital, the national transplant organization or the Organ Procurement Organization, and the recipient’s transplant surgeons. Ambulances, helicopters, and airplanes could be utilized depending on the distance involved and the national allocation policy. Allocation policies have evolved in the last few decades from prioritizing local centers first to a priority national assignment of model for end-stage liver disease-sodium score[46] that resulted in reduced waitlist mortality and increased graft utilization in the United States. However, the allocation process is complex[47], and CIT can be managed by optimizing internal organization and regional allocation when estimated CIT exceeds acceptable limits. Moreover, it is universally accepted that there is no difference in clinical transplant outcomes for local and imported liver allografts[48] and with the ready availability of modern private jet travel and careful coordination, CIT could be minimized. To date, there are only two studies about the relationship between transport time and CIT in the LT setting. In 2002, Totsuka et al[49] reported that increased CIT decreases liver graft survival rate in case of organ transportation for a long distance (> 200 m) so they recommended avoiding long-distance graft transportation. Conversely, during the World Transplant Congress in 2014, Gentry et al[50] reported in a larger cohort that: (1) Graft survival rate is not affected by distance; (2) The transport time explains < 15% of variation in CIT; and (3) As for kidney transplantation[51] CIT is not dominated by transport factors. More specifically, these results indicated for the first time that CIT is the amount of time which accumulates between organ recovery and organ transplantation and is not only impacted by transit time. Although further investigations are required to clarify the detailed factors involved in CIT, literature reports new transport technology that make organs able to be transported thousands of miles from donor to recipient. In 2019, Scalea et al[52] tested an innovated unmanned aircraft system, or drone, in kidney and liver graft transport from the donor hospital to the recipient hospital. This modern technology which would not rely on inconvenient commercial flight times or prohibitively expensive charter flights could allow organs to be transplanted more expeditiously. Moreover, given the background of the era of COVID-19 when travel of in-person procurement surgeons has been discouraged and the 27 documented fatalities in 7 aircraft crashes while traveling on an organ procurement flight[53], real improvements in organ transportation with clinicians and pilot safety are needed. Alternatively, organs can be transported using a normothermic perfusion option. Indeed, portable normothermic perfusion enabling initiation of perfusion from the donor hospital until the recipient hospital allows maximal reduction of CIT and extended periods of preservation and observation relative to cold storage. With normothermic perfusion, organs are maintained under close-to-physiologic conditions. This technology also allows transplant centers to monitor organ function for an extended period and lowers the rate of IR injury, an approach that is particularly advantageous for marginal and older donor organs[54]. In the PROTECT trial, the use of normothermic machine perfusion resulted in a significant reduction in CIT (175.4 min compared with 338.8 min) and led to superior short-term and midterm clinical outcomes, with significant reduction of early allograft dysfunction[55]. However, this approach has not been widely implemented due to complexity and the cost.
Back table preparation and recipient hepatectomy are the last timestamp of CIT in LT. Theoretically, in the ideal logistical strategy both procedures should be performed simultaneously and synchronized. However recipient hepatectomy can be challenging due to local conditions such as portal hypertension and perihilar inflammation so it can delay the liver graft reperfusion in the recipient. Median recipient hepatectomy times reported vary widely from 45 min[56,57] to 131 min[58] but previous decompensated cirrhosis with variceal bleeding and/or ascites, higher body mass index, previous abdominal surgery, and surgeon experience are independently associated with prolonged recipient hepatectomy (> 131 min) which is associated significantly with CIT. Back table preparation can be performed in the recipient hospital or in the donor hospital immediately after the removal of the graft to avoid any additional CIT. It consists in checking for organ or vessel injuries and in a very thorough and meticulous vessel dissection to minimize the risk of allograft congestion and bleeding during implantation. However, surgical procedure could be modified and may prolong CIT. For example, in case of split LT or living donor, vascular conduits could be lengthened by patch, venoplasty or arterial graft interposition, to prevent vessel narrowing and optimize arterial supply or venous drainage. The procedure needs two surgeons and duration varies depending on the procurement technique: Extensive dissection[59] or the en bloc procurement technique[60]. In 2022, Song et al[61] proposed a magnetic anchoring traction assisted system able to assist the surgeon in the vascular exposure and dissection of liver graft back table. This system replaced the assistant surgeon in the back table preparation and reduced significantly (P = 0.019) the time taken for the procedure (55 min vs 85 min). However, the study did not report if this system impacted CIT but only the feasibility, safety, and effectiveness of the traction system. To our knowledge, none of the large transplant registries collect information on back-table time, although this might be a valuable variable to investigate[62].
The impacts of CIT could be also influenced by preservation solution. The most widely used preservation solutions have been Euro Collins[63], University of Wisconsin Solution (UW)[64], Institut Georges Lopez-1 (IGL-1), Celsior solution[65] and histidine-tryptophan-ketoglutarate (HTK) preservation solution. The “optimal” preservation solution should prevent graft damage by minimizing cellular changes during CIT and decrease IR injury to the organ after restoration of the blood supply in the recipient. A recent meta-analysis from the European Liver Transplant Registry compared results of LT in relation to liver preservation using four different preservation solutions (UW, IGL-1, Celsior solution, and HTK) and concluded that better results in LT could be achieved using UW and IGL-1, especially in the setting of prolonged CIT[66]. Other studies comparing HTK with UW, report no difference in the occurrence of common complications or necessity of operative revisions after LT, confirmed also in subgroup analyses for living donor and paediatric transplantation and cases with prolonged CIT[67,68]. Moreover, HTK could have an economically superior profile due to low cost. Thus, it remains unclear whether one of the solutions is preferable in situations with extensively prolonged CIT.
Dynamic preservation should be included among the prospects to minimize CIT and improve graft outcomes. This innovative strategy could not only replace static cold storage preservation and avoid CIT, but also offering a platform for viability assessment[69]. Liver allografts can be preserved through hypothermic machine perfusion, normothermic machine perfusion, and subnormothermic machine perfusion. Their use has the potential advantage of improving clinical results in LT especially in extended criteria donor allografts[70]. Although associated with increased costs, techniques employing machine perfusion of liver allografts have been considered clinically feasible but not yet well defined. Thus, hypothermic and normothermic approaches may have complementary applications depending on the clinical situation[71]. With the aim of reducing CIT, portable normothermic machine perfusion seems to be more effective since it allows ischemia free procurement (as mentioned above), supports safe organ transportation, extends preservation times, enables graft viability testing and improves recovery of injured liver graft[71].
In conclusion, CIT is one of the most important factors impacting graft and recipient survival and it is the result of the accumulated time between organ procurement and organ transplantation. Because of the complicated network involved, each step (donor, transit, recipient) should be considered to minimize CIT duration. Reducing donor hepatectomy, recipient hepatectomy and back table time by novel procurement techniques and adequate fellow training, and improving transportation with modern technologies maintaining organ (and surgeon) safety are the keys for a successful reduction of CIT. Portable donor liver machine perfusion offers an effective method to reduce CIT and mitigate the effects of CIT on graft injury, thereby expanding the liver donor pool and reducing waiting list mortality.
| 1. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 2. | Watt KD. Keys to long-term care of the liver transplant recipient. Nat Rev Gastroenterol Hepatol. 2015;12:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1488] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 5. | Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, Perera T, Isaac J, Dutkowski P, Muiesan P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplant Proc. 2007;39:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Al-Kurd A, Kitajima T, Delvecchio K, Tayseer Shamaa M, Ivanics T, Yeddula S, Yoshida A, Rizzari M, Collins K, Abouljoud M, Nagai S. Short recipient warm ischemia time improves outcomes in deceased donor liver transplantation. Transpl Int. 2021;34:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 952] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Yu JH, Lim SW, Luo K, Cui S, Quan Y, Shin YJ, Lee KE, Kim HL, Ko EJ, Chung BH, Kim JH, Chung SJ, Yang CW. Coenzyme Q(10) alleviates tacrolimus-induced mitochondrial dysfunction in kidney. FASEB J. 2019;33:12288-12298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 633] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Jassem W, Fuggle S, Thompson R, Arno M, Taylor J, Byrne J, Heaton N, Rela M. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Rauen U, de Groot H. New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med. 2004;52:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother. 2011;38:125-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Lozanovski VJ, Khajeh E, Fonouni H, Pfeiffenberger J, von Haken R, Brenner T, Mieth M, Schirmacher P, Michalski CW, Weiss KH, Büchler MW, Mehrabi A. The impact of major extended donor criteria on graft failure and patient mortality after liver transplantation. Langenbecks Arch Surg. 2018;403:719-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Adam R, Cailliez V, Majno P, Karam V, McMaster P, Caine RY, O'Grady J, Pichlmayr R, Neuhaus P, Otte JB, Hoeckerstedt K, Bismuth H. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, Zimmerman M, Hong J, Collins TE, Gornbein J, Amersi F, Weaver M, Cao C, Chen T, Hiatt JR, Busuttil RW. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243:748-53; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Sirivatanauksorn Y, Taweerutchana V, Limsrichamrern S, Kositamongkol P, Mahawithitwong P, Asavakarn S, Tovikkai C, Sanphasitvong V. Analysis of donor risk factors associated with graft outcomes in orthotopic liver transplantation. Transplant Proc. 2012;44:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Johnson SR, Alexopoulos S, Curry M, Hanto DW. Primary nonfunction (PNF) in the MELD Era: An SRTR database analysis. Am J Transplant. 2007;7:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Lozanovski VJ, Döhler B, Weiss KH, Mehrabi A, Süsal C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications-Who Is at Risk? A Collaborative Transplant Study Report. Front Immunol. 2020;11:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C posttransplant: early preservation injury may predict poor outcome. Liver Transpl. 2006;12:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Pan ET, Yoeli D, Galvan NTN, Kueht ML, Cotton RT, O'Mahony CA, Goss JA, Rana A. Cold ischemia time is an important risk factor for post-liver transplant prolonged length of stay. Liver Transpl. 2018;24:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Paterno F, Guarrera JV, Wima K, Diwan T, Cuffy MC, Anwar N, Woodle ES, Shah S. Clinical Implications of Donor Warm and Cold Ischemia Time in Donor After Circulatory Death Liver Transplantation. Liver Transpl. 2019;25:1342-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Suttner SW, Sürder C, Lang K, Piper SN, Kumle B, Boldt J. Retraction Note to: Does age affect liver function and the hepatic acute phase response after major abdominal surgery? Intensive Care Med. 2023;49:1045. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 27. | Seifalian AM, Chidambaram V, Rolles K, Davidson BR. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg. 1998;4:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. Why is fatty liver unsuitable for transplantation? Deterioration of mitochondrial ATP synthesis and sinusoidal structure during cold preservation of a liver with steatosis. Transplant Proc. 1997;29:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken). 2008;291:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 33. | Hand SC, Menze MA. Mitochondria in energy-limited states: mechanisms that blunt the signaling of cell death. J Exp Biol. 2008;211:1829-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Zhong Z, Connor H, Stachlewitz RF, Frankenberg M, Mason RP, Lemasters JJ, Thurman RG. Role of free radicals in primary nonfunction of marginal fatty grafts from rats treated acutely with ethanol. Mol Pharmacol. 1997;52:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Hwang HP, Kim JM, Shin S, Ahn HJ, Lee S, Joo DJ, Han SY, Haam SJ, Hwang JK, Yu HC. Organ procurement in a deceased donor. Korean J Transplant. 2020;34:134-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Angelico R, Nardi A, Adam R, Nadalin S, Polak WG, Karam V, Troisi RI, Muiesan P; European Liver and Intestine Transplant Association (ELITA). Outcomes of left split graft transplantation in Europe: report from the European Liver Transplant Registry. Transpl Int. 2018;31:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol. 2018;24:5312-5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Brustia R, Boleslawski E, Danion J, Savier E, Barrou B, Scatton O. The Impact of Deceased Donor Liver Extraction Time on Early Allograft Function in Adult Liver Transplant Recipients. "Vite fait, bien fait.". Transplantation. 2019;103:e218-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Jochmans I, Fieuws S, Tieken I, Samuel U, Pirenne J. The Impact of Hepatectomy Time of the Liver Graft on Post-transplant Outcome: A Eurotransplant Cohort Study. Ann Surg. 2019;269:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Farid SG, Attia MS, Vijayanand D, Upasani V, Barlow AD, Willis S, Hidalgo E, Ahmad N. Impact of Donor Hepatectomy Time During Organ Procurement in Donation After Circulatory Death Liver Transplantation: The United Kingdom Experience. Transplantation. 2019;103:e79-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | van Leeuwen OB, van Reeven M, van der Helm D, IJzermans JNM, de Meijer VE, van den Berg AP, Darwish Murad S, van Hoek B, Alwayn IPJ, Porte RJ, Polak WG. Donor hepatectomy time influences ischemia-reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery. 2020;168:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Gilbo N, Fieuws S, Meurisse N, Nevens F, van der Merwe S, Laleman W, Verslype C, Cassiman D, van Malenstein H, Roskams T, Sainz-Barriga M, Pirenne J, Jochmans I, Monbaliu D. Donor Hepatectomy and Implantation Time Are Associated With Early Complications After Liver Transplantation: A Single-center Retrospective Study. Transplantation. 2021;105:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Ayllón-Terán MD, Gómez-Luque I, Ciria-Bru R, Luque-Molina A, López-Cillero P, Rufián-Peña S, Briceño-Delgado J. "Super-rapid" Technique in Donation After Circulatory Death Liver Donors: Advantages and Disadvantages. Transplant Proc. 2019;51:25-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Gül S, Klein F, Puhl G, Neuhaus P. Technical feasibility of liver transplantation without cold storage. Langenbecks Arch Surg. 2014;399:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | He X, Guo Z, Zhao Q, Ju W, Wang D, Wu L, Yang L, Ji F, Tang Y, Zhang Z, Huang S, Wang L, Zhu Z, Liu K, Zhu Y, Gao Y, Xiong W, Han M, Liao B, Chen M, Ma Y, Zhu X, Huang W, Cai C, Guan X, Li XC, Huang J. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am J Transplant. 2018;18:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 46. | Latt NL, Niazi M, Pyrsopoulos NT. Liver transplant allocation policies and outcomes in United States: A comprehensive review. World J Methodol. 2022;12:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 47. | Mossdorf A, Kalverkamp S, Langenbrinck L, Ulmer TF, Temizel I, Neumann U, Heidenhain C. Allocation procedure has no impact on patient and graft outcome after liver transplantation. Transpl Int. 2013;26:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Mangus RS, Fridell JA, Vianna RM, Kwo PY, Chestovich P, Milgrom ML, Kazimi M, Hollinger EF, Read JT, Tector AJ. No difference in clinical transplant outcomes for local and imported liver allografts. Liver Transpl. 2009;15:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Totsuka E, Fung JJ, Lee MC, Ishii T, Umehara M, Makino Y, Chang TH, Toyoki Y, Narumi S, Hakamada K, Sasaki M. Influence of cold ischemia time and graft transport distance on postoperative outcome in human liver transplantation. Surg Today. 2002;32:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Gentry SE, Chow EK, Wickliffe CE, Massie AB, Leighton T, Segev DL. Impact of broader sharing on the transport time for deceased donor livers. Liver Transpl. 2014;20:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Placona AM, Humphries C, Curran C, Ambroise W, Orlowski JP, Gauntt K, Wainright J. Association of Transit Time With Cold Ischemic Time in Kidney Transplantation. JAMA Netw Open. 2021;4:e2141108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Scalea JR, Restaino S, Scassero M, Bartlett ST, Wereley N. The final frontier? Exploring organ transportation by drone. Am J Transplant. 2019;19:962-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Garcia VD, Vasconcelos L, Abbud-Filho M. The riskiest job in medicine: transplant surgeons and organ procurement travel. Am J Transplant. 2010;10:1334; author reply 1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Fodor M, Cardini B, Peter W, Weissenbacher A, Oberhuber R, Hautz T, Otarashvili G, Margreiter C, Maglione M, Resch T, Krendl F, Meszaros AT, Bogensperger C, Gasteiger S, Messner F, Henninger B, Zoller H, Tilg H, Öfner D, Schneeberger S. Static cold storage compared with normothermic machine perfusion of the liver and effect on ischaemic-type biliary lesions after transplantation: a propensity score-matched study. Br J Surg. 2021;108:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 247] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 56. | Heylen L, Pirenne J, Naesens M, Sprangers B, Jochmans I. "Time is tissue"-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am J Transplant. 2021;21:2653-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Chen M, Ju W, Lin X, Chen Y, Zhao Q, Guo Z, He X, Wang D. An Alternative Surgical Technique of Native Hepatectomy in Liver Transplantation. Ann Transplant. 2021;26:e929259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 58. | Ausania F, Al Shwely F, Farguell J, Beltrán J, Calatayud D, Sánchez-Cabús S, Ferrer J, Rull R, Fuster J, García-Valdecasas JC, Martínez-Palli G, Fondevila C. Factors Associated with Prolonged Recipient Hepatectomy Time During Liver Transplantation: A Single-Centre Experience. World J Surg. 2020;44:3486-3490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Rastogi AN, Yadav SK, Soin AS. Organ Procurement in the Brain Dead Donors Without In Vivo Cold Perfusion: A Novel Technique. J Clin Exp Hepatol. 2020;10:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Fernandez B, Marichez A, Savier E. Backtable preparation after liver harvesting with "en bloc" technique with video. Updates Surg. 2022;74:779-782. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Song J, Wang Y, Cai X, Shi J, Hu L, Chang P, Zhang W, Tang B, Lv Y, Zhang X. The application of magnetic anchoring traction device in assisting donor liver bench surgery in classic orthotopic liver transplantation. BMC Gastroenterol. 2022;22:462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Heylen L, Jochmans I. Author response to: Comment on: Effect of donor nephrectomy time during circulatory-dead donor kidney retrieval on transplant graft failure. Br J Surg. 2020;107:e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours' ice storage. Lancet. 1969;2:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 479] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 64. | Kawamura T, Kuroda Y, Suzuki Y, Fujiwara H, Fujino Y, Yamamoto K, Saitoh Y. Seventy-two-hour preservation of the canine pancreas by the two-layer (Euro-Collins' solution/perfluorochemical) cold storage method. Transplantation. 1989;47:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Menasché P, Termignon JL, Pradier F, Grousset C, Mouas C, Alberici G, Weiss M, Piwnica A, Bloch G. Experimental evaluation of Celsior, a new heart preservation solution. Eur J Cardiothorac Surg. 1994;8:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, Belghiti J, Pezet D, Castaing D, Le Treut YP, Gugenheim J, Bachellier P, Pirenne J, Muiesan P; ELTR contributing centres, the European Liver, Intestine Transplant Association (ELITA). Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Kaltenborn A, Gwiasda J, Amelung V, Krauth C, Lehner F, Braun F, Klempnauer J, Reichert B, Schrem H. Comparable outcome of liver transplantation with histidine-tryptophan-ketoglutarate vs. University of Wisconsin preservation solution: a retrospective observational double-center trial. BMC Gastroenterol. 2014;14:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Liu Q, Bruns H, Schultze D, Xue Y, Zorn M, Flechtenmacher C, Straub BK, Rauen U, Schemmer P. HTK-N, a modified HTK solution, decreases preservation injury in a model of microsteatotic rat liver transplantation. Langenbecks Arch Surg. 2012;397:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Liu Q, Nassar A, Buccini L, Grady P, Soliman B, Hassan A, Pezzati D, Iuppa G, Diago Uso T, Miller C, Quintini C. Ex situ 86-hour liver perfusion: Pushing the boundary of organ preservation. Liver Transpl. 2018;24:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Watson CJE, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K, Gimson AE, Allison M, Upponi S, Brais R, Jochmans I, Butler AJ. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 71. | Panconesi R, Flores Carvalho M, Mueller M, Meierhofer D, Dutkowski P, Muiesan P, Schlegel A. Viability Assessment in Liver Transplantation-What Is the Impact of Dynamic Organ Preservation? Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |