Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.822
Revised: February 12, 2024
Accepted: April 12, 2024
Published online: May 27, 2024
Processing time: 129 Days and 0.8 Hours
The gut–liver axis and bacterial translocation are important in cirrhosis, but there is no available universal biomarker of cellular bacterial translocation, for which presepsin may be a candidate.
To evaluate the relationship of the blood presepsin levels with the state of the gut microbiota in cirrhosis in the absence of obvious infection.
This study included 48 patients with Child–Pugh cirrhosis classes B and C and 15 healthy controls. The fecal microbiome was assessed using 16S rRNA gene sequencing. Plasma levels of presepsin were measured. A total of 22 patients received a probiotic (Saccharomyces boulardii) for 3 months.
Presepsin levels were higher in patients with cirrhosis than in healthy individuals [342 (91-2875) vs 120 (102-141) pg/mL; P = 0.048]. Patients with elevated presepsin levels accounted for 56.3% of all included patients. They had lower levels of serum albumin and higher levels of serum total bilirubin and overall severity of cirrhosis as assessed using the Child–Pugh scale. Patients with elevated presepsin levels had an increased abundance of the main taxa responsible for bacterial translocation, namely Bacilli and Proteobacteria (including the main class Gammaproteobacteria and the minor taxa Xanthobacteraceae and Stenotrophomonas), and a low abundance of bacteria from the family Lachnospiraceae (including the minor genus Fusicatenibacter), which produce short-chain fatty acids that have a positive effect on intestinal barrier function. The presepsin level directly correlated with the relative abundance of Bacilli, Proteobacteria, and inversely correlated with the abundance of Lachnospiraceae and Propionibacteriaceae. After 3 months of taking the probiotic, the severity of cirrhosis on the Child–Pugh scale decreased significantly only in the group with elevated presepsin levels [from 9 (8-11) to 7 (6-9); P = 0.004], while there were no significant changes in the group with normal presepsin levels [from 8 (7-8) to 7 (6-8); P = 0.123]. A high level of presepsin before the prescription of the probiotic was an independent predictor of a greater decrease in Child–Pugh scores (P = 0.046), as well as a higher level of the Child–Pugh scale (P = 0.042), but not the C-reactive protein level (P = 0.679) according to multivariate linear regression analysis.
The level of presepsin directly correlates with the abundance in the gut microbiota of the main taxa that are substrates of bacterial translocation in cirrhosis. This biomarker, in the absence of obvious infection, seems important for assessing the state of the gut–liver axis in cirrhosis and deciding on therapy targeted at the gut microbiota in this disease.
Core Tip: The gut–liver axis and bacterial translocation are important in cirrhosis; however, there is no available universal biomarker for cellular bacterial translocation, although presepsin may be a candidate. The level of presepsin directly correlated with the abundance in the gut microbiota of the main taxa that are substrates of bacterial translocation in cirrhosis. This biomarker, in the absence of obvious infection, seems important for assessing the state of the gut–liver axis in cirrhosis to decide on therapy targeted at the gut microbiota in this disease.
- Citation: Efremova I, Maslennikov R, Poluektova E, Medvedev O, Kudryavtseva A, Krasnov G, Fedorova M, Romanikhin F, Zharkova M, Zolnikova O, Bagieva G, Ivashkin V. Presepsin as a biomarker of bacterial translocation and an indicator for the prescription of probiotics in cirrhosis. World J Hepatol 2024; 16(5): 822-831
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/822.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.822
Cirrhosis is the final stage of chronic liver disease, and its development makes the prognosis poor[1-4]. Currently, the gut–liver axis is of great importance in the pathogenesis of cirrhosis[5-9]. An increase in intestinal permeability and changes in the composition of the intestinal microbiota (gut dysbiosis) occur in cirrhosis. As a manifestation of gut dysbiosis, the number of facultative anaerobes, i.e., bacteria that can survive in oxygenated living human tissues, increases. The facultative anaerobes of the gut microbiota include most representatives of the class Bacilli and the phylum Proteobacteria that are minor taxa in the normal gut microbiota but become much more abundant in cirrhosis[5-9]. These changes lead to bacterial translocation represented by the penetration of bacteria and their components from the intestinal contents into the intestinal wall, lymph nodes, ascitic fluid, and portal and systemic blood flow[5,10-13]. Bacterial translocation triggers a pathological cascade, which results in the worsening of portal hypertension and decreased liver function[5,10-13]. Despite this important role of bacterial translocation, there are currently no adequate biomarkers to evaluate it in clinical practice[14]. Traditionally, lipopolysaccharide (LPS), which is an endotoxin of Gram-negative bacteria, was thought to play this role[14]. Its advantage is that its presence in the blood is a direct manifestation of bacterial translocation. However, its disadvantage is that it reflects only the translocation of Gram-negative bacteria, since Gram-positive bacteria do not contain LPS. In addition, the detection of LPS does not indicate the penetration of bacteria into the body, since this molecule can enter the bloodstream through damaged tight junctions of the intestinal epithelium separately from bacterial cells after their death and destruction. Other candidates for the role of biomarkers of bacterial translocation (LPS-binding protein, procalcitonin, CD14, and others), as a rule, represent various manifestations of the systemic inflammatory response, which causes by bacterial translocation[14]. Presepsin is interesting in this regard. This protein is a fragment of CD14, a molecule that is involved in the recognition of conserved molecular patterns of both Gram-positive and Gram-negative bacteria. CD14 participates in the phagocytosis of recognized bacterial cells, plunging with them into the phagosome. After fusion with lysosomes, their enzymes cleave off the CD14 fragment, which is ejected from the cell outwards. This ejected fragment is presepsin. Experiments have shown that the formation of presepsin is stimulated by living and dead cells of both Gram-positive and Gram-negative bacteria, while their noncellular components, such as LPS, do not have such properties. Therefore, presepsin can be considered a biomarker of phagocytosis of whole bacterial cells, both Gram-negative and Gram-positive[15-19]. Presepsin is successfully used in clinical practice as a biomarker of bacterial infection and sepsis[15-19], including in patients with cirrhosis[20-24]. Its blood level is higher in decompensated cirrhosis than in compensated cirrhosis[24-26]. Its high blood level determines the poor prognosis of patients with cirrhosis[23-27]. We suggest that in the absence of obvious infection, presepsin can also be used as a universal biomarker of cellular bacterial translocation in cirrhosis. The aim of this study was to evaluate the relationship of the blood presepsin levels with the state of the gut microbiota in cirrhosis in the absence of obvious infection.
Patients with cirrhosis classes B and C according to their Child–Pugh score who presented to our clinic for routine examination were screened for participation in this study. The study procedures were explained to the potential participants, and written informed consent was obtained before enrollment. The study was approved by the Ethics Committee of Sechenov University and performed in accordance with the Declaration of Helsinki.
The inclusion criteria were the presence of cirrhosis, the diagnosis of which was made based on histology or a combination of physical examination, laboratory and instrumental data, signed written informed consent, and an age of between 18 years and 70 years. The exclusion criteria were as follows: Use of drugs that could affect the composition of the gut microbiota (lactulose, lactitol, or other prebiotics, probiotics, antibiotics, and metformin) in the preceding six weeks; alcohol consumption in the preceding six weeks; current bacterial infection; inflammatory bowel disease, cancer, renal failure, or any other serious disease.
The patient was considered free of current bacterial infection if he had a normal body temperature, no open wounds, no complaints of respiratory (cough, shortness of breath), intestinal (diarrhea, abdominal pain) or urinary (lower back or abdominal pain, urinary disorder) infection, normal chest x-ray and routine urinalysis.
The control group consisted of 15 healthy individuals who visited the clinic for routine health examinations.
The day after the initial medical examination, fasting blood was collected from patients and immediately centrifuged. The plasma was separated, divided into several aliquots, and frozen. Once all patients were recruited, aliquots were thawed and the levels of presepsin were assessed by enzyme immunoassay. Assays were performed according to the manufacturers’ instructions. The blood plasma of healthy controls was examined in the same way.
All patients underwent a standard examination that included physical and neurological examination, abdominal ultrasound, complete blood count, blood chemistry, coagulation tests, and a number connection test for the diagnosis of covert hepatic encephalopathy.
On the same day as the blood collection, after voluntary defecation by patients, stool samples were collected in a special disposable sterile container and immediately frozen at -80 ℃ for 16S rRNA gene sequencing. Gut microbiota analysis was performed as previously described (Supplementary material)[28-32].
Statistical analysis was performed with Statistica 10 (StatSoft Inc., Tulsa, United States) software. The data are presented as medians (interquartile ranges). The Mann-Whitney method was used to assess the differences between continuous variables. The differences between categorical variables were assessed with Fisher’s exact test. Spearman’s rank test was used to assess the correlations between variables. The assessment of changes in the levels of variables was performed with the Wilcoxon test. Comparison of the composition of the gut microbiota between groups was carried out by linear discriminant analysis effect size (LEfSe) using the online resource. P value ≤ 0.05 were considered as statistically significant.
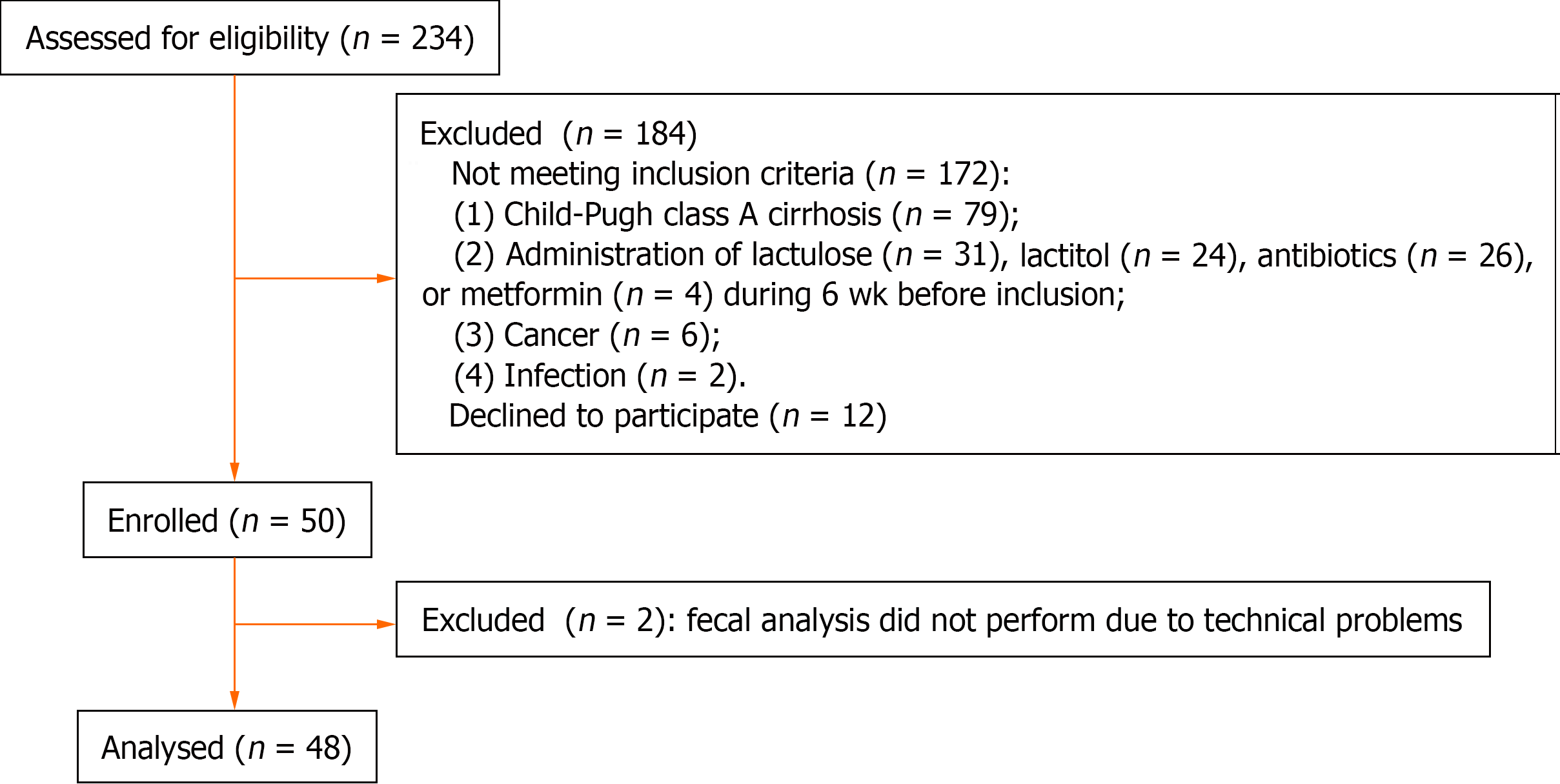
Fifty patients were included in the study. Fecal 16S rRNA gene sequencing was not possible in two patients due to technical problems; these patients were excluded from the study. In total, 48 cirrhosis patients and 15 healthy individuals were included (Figure 1). Patients and controls did not differ in age [50 (43–58) years vs 46 (39–54) years; P=0.194], body mass index [25.7 (23.1-28.9) kg/m2vs 25.0 (23.7–25.8) kg/m2; P = 0.294], and sex distribution (male/female: 19/29 vs 7/8; P = 0.423).
Presepsin levels were higher in patients with cirrhosis than in healthy individuals [342 (91-2875) pg/mL vs 120 (102-141) pg/mL; P = 0.048]. The mean and σ for presepsin in healthy individuals was 118 pg/mL and 27 pg/mL, which allowed us to use the m+-2σ rule to estimate the normal interval for presepsin in our study from 64 pg/mL to 172 pg/mL; this included all the results of healthy individuals, with the exception of one that was slightly below (61 pg/mL) this lower limit.
Based on this, cirrhotic patients were divided into a group with increased presepsin (> 172 pg/mL) and a group with normal presepsin (< 172 pg/L). Patients with elevated presepsin levels accounted for 56.3% of all included patients. They had lower levels of serum albumin and higher levels of serum total bilirubin and overall severity of cirrhosis as assessed using the Child–Pugh scale. Moreover, the C-reactive protein level, despite the obvious tendency to be higher in patients with increased presepsin levels, did not differ significantly between groups (Table 1).
| Patients with increased presepsin level | Patients with normal presepsin level | P value | |
| Age, yr | 49 (43-59) | 52 (42-56) | 0.868 |
| Body mass index, kg/m2 | 25.9 (24.7-30.1) | 25.3 (21.6-28.4) | 0.275 |
| Male/female | 12/15 | 7/14 | 0.316 |
| Etiology of cirrhosis: Alcohol | 13 (48.1) | 10 (47.6) | > 0.050 |
| HBV | 3 (11.1) | - | |
| HCV | 3 (11.1) | 3 (14.3) | |
| Metabolic dysfunction-associated steatotic liver disease | 2 (7.4) | 1 (4.8) | |
| Mixed | 4 (14.8) | 3 (14.3) | |
| Cryptogenic | 2 (7.4) | 4 (18.0) | |
| Child–Pugh score | 9 (8-10) | 8 (7-9) | 0.009 |
| Esophageal varices (Grade 1) | 9 (33.3) | 9 (42.9) | 0.353 |
| Esophageal varices (Grade 2-3) | 15 (55.5) | 10 (47.6) | 0.399 |
| Minimal hepatic encephalopathy | 19 (70.4) | 14 (66.7) | 0.588 |
| Overt hepatic encephalopathy | 1 (3.7) | 3 (14.3) | 0.215 |
| Ascites | 21 (77.7) | 16 (76.2) | 0.582 |
| Ascites (Grade 1) | 11 (40.7) | 12 (57.1) | 0.201 |
| Ascites (Grade 2-3) | 10 (37.0) | 4 (19.0) | 0.149 |
| Serum total protein, g/L | 73 (65-76) | 70 (61-74) | 0.344 |
| Serum albumin, g/L | 32 (30-34) | 35 (33-37) | 0.049 |
| Serum total bilirubin, μmol/L | 60 (36-78) | 37 (26-57) | 0.022 |
| International normalized ratio | 1.63 (1.39-1.75) | 1.48 (1.36-1.61) | 0.151 |
| Red blood cells, 1012 cell/L | 3.6 (3.1-4.2) | 3.6 (3.3-4.2) | 0.934 |
| White blood cells, 109 cell/L | 3.7 (2.6-5.2) | 3.3 (2.6-5.9) | 0.942 |
| Platelets, 109 cell/L | 94 (52-104) | 94 (66-105) | 0.64 |
| Serum creatinine, μmol/L | 77 (67-91) | 76 (66-107) | 0.625 |
| Serum sodium, mmol/L | 141 (139-142) | 141 (140-142) | 0.983 |
| Alanine aminotransferase, U/L | 26 (18-50) | 28 (20-45) | 0.723 |
| Aspartate aminotransferase, U/L | 52 (41-77) | 48 (28-51) | 0.151 |
| Gamma glutamyl transferase, U/L | 72 (44-114) | 68 (37-269) | 0.575 |
| Alkaline phosphatase, U/L | 261 (194-330) | 242 (215-388) | 0.506 |
| C-reactive protein, mg/L | 9 (5-15) | 7 (3-12) | 0.399 |
| Splenic length, cm | 15.0 (13.0-17.0) | 15.0 (13.2-18.4) | 0.708 |
Presepsin levels were directly correlated with Child–Pugh scores (r = 0.365, P = 0.011) and C-reactive protein levels (r = 0.483, P = 0.025 for the range of presepsin levels from 1 pg/mL to 1000 pg/mL) and inversely correlated with serum albumin levels (r=-0.340, P = 0.018).
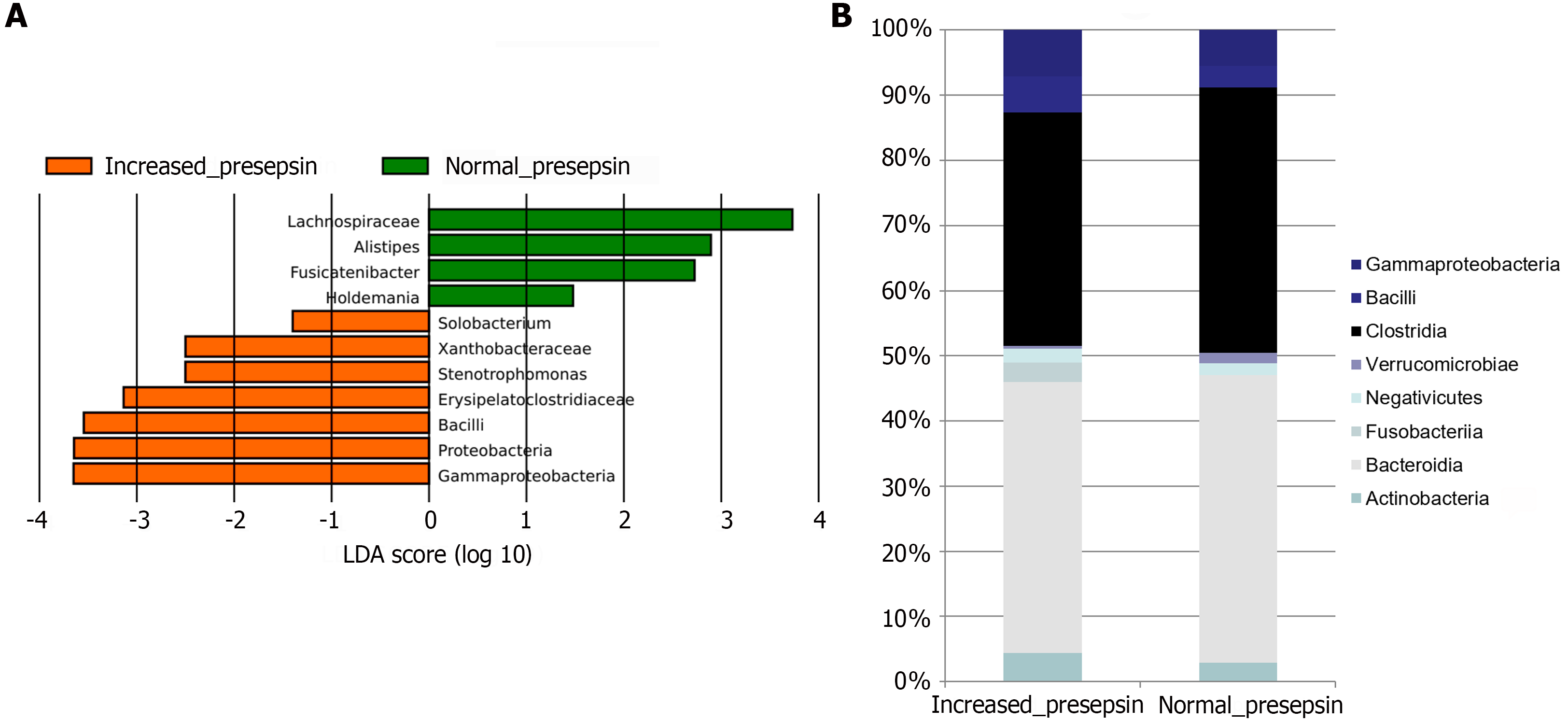
LEfSe showed that in the group of cirrhosis patients with elevated presepsin there was an increased abundance of the main taxa responsible for bacterial translocation, namely Bacilli and Proteobacteria (including the main class Gammaproteobacteria and the minor taxa Xanthobacteraceae and Stenotrophomonas), and a low abundance of bacteria from the family Lachnospiraceae (including the minor genus Fusicatenibacter), which produce short-chain fatty acids that have a positive effect on intestinal barrier function (Figure 2A). In addition, high levels of presepsin in cirrhosis were associated with higher levels of minor taxa Erysipelatoclostridiaceae and Solobacterium and lower levels of minor taxa Holdemania and Alistipes.
At the level of gut microbiota classes, cirrhosis patients with high presepsin levels had a higher proportion of Bacilli and Gammaproteobacteria (the main class of the Proteobacteria phylum in the human gut microbiota) and a lower proportion of the Clostridia class (containing the family Lachnospiraceae) (Figure 2B).
The presepsin level directly correlated with the relative abundance of Bacilli, Proteobacteria, taxa included in this phylum, and some minor taxa, and inversely correlated with the abundance of Lachnospiraceae, Propionibacteriaceae, and some minor taxa (Table 2).
| Direct correlations | Inverse correlations | ||||
| Taxon rank | Taxon | R, P value | Taxon rank | Taxon | R, P value |
| Phylum | Proteobacteria | 0.316, 0.029 | Family | Lachnospiraceae | 0.447, 0.0421 |
| Class | Bacilli | 0.470, 0.0321 | Propionibacteriaceae | -0.302, 0.038 | |
| Gammaproteobacteria | 0.351, 0.014 | Genus | Fusicatenibacter | -0.297, 0.040 | |
| Family | Erysipelatoclostridiaceae | 0.384, 0.007 | Holdemanella | -0.315, 0.029 | |
| Eubacteriaceae | 0.340, 0.018 | ||||
| Vibrionaceae | 0.366, 0.010 | ||||
| Xanthobacteraceae | 0.363, 0.011 | ||||
| Genus | Escherichia-Shigella | 0.338, 0.019 | |||
| Stenotrophomonas | 0.365, 0.011 | ||||
| Vibrio | 0.346, 0.016 | ||||
Some patients further participated in a study on the effect of the probiotic (Saccharomyces boulardii) on the course of cirrhosis[33]. We chose this probiotic because it has pleiotropic effects: It reestablishes the gut microbiome after dysbiosis[34], strengthens the intestinal immune barrier[35], improves the trophic function of gut microbiota[36], restores the impaired gut barrier, and protects against bacterial translocation[37] in experimental models and in patients with gut diseases. S. boulardii administration in an experimental mouse model of cirrhosis led to the correction of gut dysbiosis and decreased intestinal permeability, as well as the reduced severity of liver inflammation and fibrosis[38]. That study included 40 patients from this study, but 2 patients included in that study were excluded from this study because the gut microbiota analysis could not be completed due to technical problems, as described at the beginning of this section. We additionally enrolled 10 patients for this study after the closure of that study. Details of that study are described in the published article[33]. Among the patients receiving the probiotic, 13 patients had elevated presepsin levels, and 9 patients had normal levels. After 3 months of taking the probiotic, the severity of cirrhosis on the Child–Pugh scale decreased significantly only in the group with elevated presepsin levels, while there were no significant changes in the group with normal presepsin levels (Table 3). A significant reduction in the severity of cirrhosis (by more than 1 point on the Child–Pugh scale) after taking the probiotic was observed in 9 (69.2%) patients with elevated presepsin levels and only in 2 (22.2%) patients with normal presepsin levels (P = 0.040). In the placebo group (n = 16), such a significant reduction in the severity of cirrhosis was observed in 3 (18.8%) patients, which was not significantly different from the probiotic subgroup with normal presepsin levels (P = 0.610) and was below the probiotic subgroup with elevated presepsin levels (P = 0.008). A high level of presepsin before the prescription of the probiotic was an independent predictor of a greater decrease in Child–Pugh scores (P = 0.046), as well as a higher baseline level of the Child–Pugh scale (P = 0.042), but not the baseline C-reactive protein level (P = 0.679) according to multivariate linear regression analysis. In addition, in the group with elevated presepsin levels, there was a significant decrease in the levels of total bilirubin, aspartate aminotransferase, and alkaline phosphatase, as well as the incidence of ascites and minimal hepatic encephalopathy, as a result of taking the probiotic, in contrast to the group with normal presepsin levels (Table 3).
| Elevated baseline presensin level (n = 13) | Normal baseline presensin level (n = 9) | |||||
| At the beginning of the study | After the probiotic course | P value | At the beginning of the study | After the probiotic course | P value | |
| Child–Pugh score | 9 (8-11) | 7 (6-9) | 0.004 | 8 (7-8) | 7 (6-8) | 0.124 |
| Child–Pugh class, A/B+C | 0/13 | 5/8 | 0.020 | 0/9 | 2/7 | 0.235 |
| No hepatic encephalopathy | 2 (15.4) | 8 (61.5) | 0.021 | 2 (22.2) | 3 (33.3) | 0.500 |
| Ascites | 12 (92.3) | 5 (38.4) | 0.006 | 8 (88.9) | 5 (55.5) | 0.147 |
| Serum albumin, g/L | 32 (30-34) | 33 (32-39) | 0.033 | 35 (33-36) | 38 (37-39) | 0.008 |
| Serum total bilirubin, μmol/L | 52 (36-120) | 29 (26-40) | 0.016 | 34 (26-44) | 25 (22-35) | 0.066 |
| Alanine aminotransferase, U/L | 26 (18-47) | 26 (18-40) | 0.272 | 30 (21-41) | 33 (25-39) | 0.813 |
| Aspartate aminotransferase, U/L | 60 (37-75) | 39 (27-51) | 0.012 | 45 (28-51) | 36 (36-42) | 0.314 |
| Gamma glutamyl transferase, U/L | 92 (44-207) | 56 (40-89) | 0.196 | 37 (31-317) | 62 (30-115) | 0.193 |
| Alkaline phosphatase, U/L | 268 (245-355) | 210 (187-245) | 0.013 | 261 (227-446) | 215 (188-275) | 0.110 |
In this study, we found that the presepsin level directly correlated with the relative abundance of Bacilli and Proteobacteria. These bacteria are represented in the gut microbiota by facultative anaerobes, which are not afraid of oxygen and live normally in oxygenated human tissues[34]. An increase in their abundance is a manifestation of cirrhosis-specific gut dysbiosis[39-43]. They were substrates of bacterial translocation in animal models of cirrhosis[44,45], and their DNA dominated among bacterial DNA in the blood of patients with cirrhosis[46,47].
Moreover, we found that the presepsin level inversely correlated with the abundance of bacteria from the family Lachnospiraceae. These bacteria are strict anaerobes and do not contain LPS. They die at a minimum concentration of oxygen in the environment, and thus, their living cells are not able to penetrate living oxygenated tissues; that is, they are not capable of either cellular or molecular bacterial translocation[39]. The Lachnospiraceae abundance in the gut microbiota decreases in cirrhotic gut dysbiosis[42,43].
Although presepsin in cirrhosis has been studied in many publications, none of them have examined its blood level in combination with the composition of the gut microbiota[19-23], which is the novelty and strength of our study. We were able to confirm our hypothesis that in the stable course of cirrhosis without bacterial infection, the blood level of presepsin directly correlates with the abundance of substrates for bacterial translocation in the gut microbiota and inversely correlates with the abundance of bacteria not involved in it. Therefore, we believe that presepsin can be used as a universal biomarker of cellular bacterial translocation.
Our study, in accordance with previously published studies[24-26], has confirmed that the level of presepsin in the blood increases as cirrhosis worsens according to the Child–Pugh scale. Gut dysbiosis[40,48] and disruption of the intestinal barrier[49-53] also worsen as cirrhosis becomes more severe, which may explain this pattern.
We also studied how the level of presepsin before the therapy targeted at the gut microbiota affects its effectiveness. We recently published a study showing that a probiotic has a positive effect on the severity of cirrhosis[33]. In this study, we examined how this effect depended on presepsin levels before the start of the therapy. A total of 59% of patients in the probiotics group had elevated presepsin levels, suggesting they had significant bacterial translocation. The use of the probiotic in these patients had a significant positive effect, as a result of which the value of the Child–Pugh cirrhosis severity scale in them decreased by an average of 2 points. However, the effect of the same probiotic in patients with normal presepsin levels, which suggests minimal bacterial translocation, was insignificant. Multivariate regression analysis confirmed that the positive effect of the probiotic was determined by a higher level of presepsin, regardless of the severity of cirrhosis on the Child–Pugh scale and the level of C-reactive protein. Moreover, the level of the latter, unlike the level of presepsin, did not determine the effectiveness of this therapy.
A limitation of our study is that we did not have the technology to determine the bacterial DNA level in the blood and conduct a correlation analysis between this direct marker of bacterial translocation and presepsin. This is a task for future research. The second limitation of our study was the small number of participants; however, this did not prevent us from obtaining significant results. Larger studies are needed to confirm our findings.
New studies using other probiotics, antibiotics, prebiotics, etc., are also needed to further test our hypothesis that presepsin levels in cirrhosis can be used to guide decisions about gut microbiota-targeted therapies.
The level of presepsin, a proposed universal biomarker of cellular bacterial translocation, directly correlates with the abundance in the gut microbiota of the main taxa that are substrates of bacterial translocation in cirrhosis. This biomarker, in the absence of obvious infection, seems important for assessing the state of the gut–liver axis in cirrhosis and deciding on therapy targeted at the gut microbiota in this disease.
The authors are grateful to the staff of the Department of Hepatology: Alexei Lapshin, Shauki Ondos, Petr Tkachenko, Igor Tikhonov and others.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 2. | Jalan R, Szabo G. New concepts and perspectives in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Chandna S, Zarate ER, Gallegos-Orozco JF. Management of Decompensated Cirrhosis and Associated Syndromes. Surg Clin North Am. 2022;102:117-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol. 2014;20:5442-5460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (7)] |
| 5. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 6. | Singh TP, Kadyan S, Devi H, Park G, Nagpal R. Gut microbiome as a therapeutic target for liver diseases. Life Sci. 2023;322:121685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 7. | Lee NY, Suk KT. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Luo M, Xin RJ, Hu FR, Yao L, Hu SJ, Bai FH. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J Gastroenterol. 2023;29:144-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 9. | Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 331] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 10. | Simbrunner B, Caparrós E, Neuwirth T, Schwabl P, Königshofer P, Bauer D, Marculescu R, Trauner M, Scheiner B, Stary G, Mandorfer M, Reiberger T, Francés R. Bacterial translocation occurs early in cirrhosis and triggers a selective inflammatory response. Hepatol Int. 2023;17:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25:5897-5917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (4)] |
| 14. | Koutsounas I, Kaltsa G, Siakavellas SI, Bamias G. Markers of bacterial translocation in end-stage liver disease. World J Hepatol. 2015;7:2264-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Memar MY, Baghi HB. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed Pharmacother. 2019;111:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Yang HS, Hur M, Yi A, Kim H, Lee S, Kim SN. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS One. 2018;13:e0191486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | de Guadiana Romualdo LG, Torrella PE, Acebes SR, Otón MDA, Sánchez RJ, Holgado AH, Santos EJ, Freire AO. Diagnostic accuracy of presepsin (sCD14-ST) as a biomarker of infection and sepsis in the emergency department. Clin Chim Acta. 2017;464:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Papp M, Tornai T, Vitalis Z, Tornai I, Tornai D, Dinya T, Sumegi A, Antal-Szalmas P. Presepsin teardown - pitfalls of biomarkers in the diagnosis and prognosis of bacterial infection in cirrhosis. World J Gastroenterol. 2016;22:9172-9185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Novelli S, Morabito V, Ruberto F, Bini F, Marinozzi F, Pugliese F, Berloco P, Pretagostini R. Diagnostic Value of Presepsin for Bacterial Infection in Cirrhosis: A Pilot Study. Transplant Proc. 2020;52:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ferrarese A, Plebani M, Frigo AC, Burra P, Senzolo M. Presepsin as a biomarker of inflammation and prognosis in decompensated liver disease. J Hepatol. 2021;75:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ferrarese A, Frigo AC, Mion MM, Plebani M, Russo FP, Germani G, Gambato M, Cillo U, Cattelan A, Burra P, Senzolo M. Diagnostic and prognostic role of presepsin in patients with cirrhosis and bacterial infection. Clin Chem Lab Med. 2021;59:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Fischer P, Grigoras C, Bugariu A, Nicoara-Farcau O, Stefanescu H, Benea A, Hadade A, Margarit S, Sparchez Z, Tantau M, Ionescu D, Procopet B. Are presepsin and resistin better markers for bacterial infection in patients with decompensated liver cirrhosis? Dig Liver Dis. 2019;51:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Elefsiniotis I, Tsakiris SA, Barla G, Tasovasili A, Vrachatis D, Mavrogiannis C. Presepsin levels in cirrhotic patients with bacterial infections and/or portal hypertension-related bleeding, presenting with or without acute kidney injury. Ann Gastroenterol. 2018;31:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Igna R, Gîrleanu I, Cojocariu C, Muzîca C, Huiban L, Sfarti C, Cuciureanu T, Chiriac S, Sîngeap AM, Petrea OC, Stafie R, Zenovia S, Năstasă R, Stratina E, Rotaru A, Stanciu C, Trifan A, Blaj M. The Role of Presepsin in Diagnosing Infections in Patients with Liver Cirrhosis and Overt Hepatic Encephalopathy. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Igna R, Gîrleanu I, Cojocariu C, Huiban L, Muzîca C, Sîngeap AM, Sfarti C, Chiriac S, Petrea OC, Zenovia S, Nastasa R, Cuciureanu T, Stafie R, Stratina E, Rotaru A, Stanciu C, Blaj M, Trifan A. The Role of Presepsin and Procalcitonin in Early Diagnosis of Bacterial Infections in Cirrhotic Patients with Acute-on-Chronic Liver Failure. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Zanetto A, Pelizzaro F, Mion MM, Bucci M, Ferrarese A, Simioni P, Basso D, Burra P, Senzolo M. Toward a more precise prognostic stratification in acute decompensation of cirrhosis: The Padua model 2.0. United European Gastroenterol J. 2023;11:815-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Kovaleva A, Poluektova E, Maslennikov R, Karchevskaya A, Shifrin O, Kiryukhin A, Tertychnyy A, Kovalev L, Kovaleva M, Lobanova O, Kudryavtseva A, Krasnov G, Fedorova M, Ivashkin V. Effect of Rebamipide on the Intestinal Barrier, Gut Microbiota Structure and Function, and Symptom Severity Associated with Irritable Bowel Syndrome and Functional Dyspepsia Overlap: A Randomized Controlled Trial. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 29. | Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinformatics. 2016;17:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18515] [Cited by in RCA: 17756] [Article Influence: 1972.9] [Reference Citation Analysis (0)] |
| 31. | Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261-5267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12842] [Cited by in RCA: 13188] [Article Influence: 732.7] [Reference Citation Analysis (0)] |
| 32. | Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590-D596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15104] [Cited by in RCA: 18176] [Article Influence: 1514.7] [Reference Citation Analysis (0)] |
| 33. | Maslennikov R, Efremova I, Ivashkin V, Zharkova M, Poluektova E, Shirokova E, Ivashkin K. Effect of probiotics on hemodynamic changes and complications associated with cirrhosis: A pilot randomized controlled trial. World J Hepatol. 2022;14:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 34. | McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014;4:e005047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Terciolo C, Dobric A, Ouaissi M, Siret C, Breuzard G, Silvy F, Marchiori B, Germain S, Bonier R, Hama A, Owens R, Lombardo D, Rigot V, André F. Saccharomyces boulardii CNCM I-745 Restores intestinal Barrier Integrity by Regulation of E-cadherin Recycling. J Crohns Colitis. 2017;11:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, Pompei A, Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11:6165-6169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Generoso SV, Viana ML, Santos RG, Arantes RM, Martins FS, Nicoli JR, Machado JA, Correia MI, Cardoso VN. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur J Nutr. 2011;50:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Maslennikov R, Poluektova E, Zolnikova O, Sedova A, Kurbatova A, Shulpekova Y, Dzhakhaya N, Kardasheva S, Nadinskaia M, Bueverova E, Nechaev V, Karchevskaya A, Ivashkin V. Gut Microbiota and Bacterial Translocation in the Pathogenesis of Liver Fibrosis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 41. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 42. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 43. | Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol. 2021;13:557-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 542] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 45. | Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Bruns T, Reuken PA, Stengel S, Gerber L, Appenrodt B, Schade JH, Lammert F, Zeuzem S, Stallmach A. The prognostic significance of bacterial DNA in patients with decompensated cirrhosis and suspected infection. Liver Int. 2016;36:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Alvarez-Silva C, Schierwagen R, Pohlmann A, Magdaleno F, Uschner FE, Ryan P, Vehreschild MJGT, Claria J, Latz E, Lelouvier B, Arumugam M, Trebicka J. Compartmentalization of Immune Response and Microbial Translocation in Decompensated Cirrhosis. Front Immunol. 2019;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 48. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 49. | Wang Z, Wang A, Gong Z, Biviano I, Liu H, Hu J. Plasma claudin-3 is associated with tumor necrosis factor-alpha-induced intestinal endotoxemia in liver disease. Clin Res Hepatol Gastroenterol. 2019;43:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Lian XX, Sun YP, Guo XX. Correlation between intestinal mucosal permeability and prognosis in patients with liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2020;28:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Rainer F, Horvath A, Sandahl TD, Leber B, Schmerboeck B, Blesl A, Groselj-Strele A, Stauber RE, Fickert P, Stiegler P, Møller HJ, Grønbaek H, Stadlbauer V. Soluble CD163 and soluble mannose receptor predict survival and decompensation in patients with liver cirrhosis, and correlate with gut permeability and bacterial translocation. Aliment Pharmacol Ther. 2018;47:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 53. | Wang X, Li MM, Niu Y, Zhang X, Yin JB, Zhao CJ, Wang RT. Serum Zonulin in HBV-Associated Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma. Dis Markers. 2019;2019:5945721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |