Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1348
Revised: August 18, 2024
Accepted: September 14, 2024
Published online: November 27, 2024
Processing time: 167 Days and 17.8 Hours
Pituitary stalk interruption syndrome (PSIS) is a rare disorder, often characterized by delayed growth and development, short stature, and hypogonadism as the main clinical manifestations. It is not clear whether PSIS can lead to liver cirrhosis.
This paper reported a case of liver cirrhosis of unknown origin. The patient was admitted to Beijing Ditan Hospital Affiliated to Capital Medical University in November 2023. The diagnosis of PSIS complicated with liver cirrhosis was established after a series of blood tests and pituitary magnetic resonance imaging examination.
We also reviewed the literature from both domestic and international sources to deepen the clinical understanding of PSIS in conjunction with liver cirrhosis among medical practitioners.
Core Tip: Our patient with pituitary stalk interruption syndrome (PSIS) showed a general decrease of anterior pituitary hormones and typical imaging findings. We excluded liver cirrhosis by other causes. The patient did not receive any treatment for many years, which may represent the natural progression of this disease and included rare cases of osteoporosis and cirrhosis. Liver cirrhosis caused by PSIS is very rare in clinical practice, and hepatologists should be alert to PSIS when encountering patients with liver cirrhosis complicated with abnormal growth and development.
- Citation: Chang M, Wang SY, Zhang ZY, Hao HX, Li XG, Li JJ, Xie Y, Li MH. Pituitary stalk interruption syndrome complicated with liver cirrhosis: A case report. World J Hepatol 2024; 16(11): 1348-1355
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1348.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1348
Pituitary stalk interruption syndrome (PSIS) is a clinical syndrome characterized by a slender or missing pituitary stalk, adenohypophysis hypoplasia, and ectopic neurohypophysis. PSIS is rare in clinical settings, and the precise incidence is unclear. With the development of imaging and the deepening understanding of the disease, the detection rate of PSIS has increased gradually. It was found that 6.8% of nonacquired growth hormone (GH) deficiency cases were due to PSIS[1].
At present, few cases of liver cirrhosis caused by PSIS have been reported[2-7]. Here, we reported a case of PSIS complicated with liver cirrhosis diagnosed by pancytopenia for more than 6 years and liver cirrhosis for 20 days. Physical examination showed that the secondary sexual characteristics were undeveloped, and splenomegaly combined with blood tests and pituitary magnetic resonance imaging (MRI) examinations, increased the understanding of PSIS complicated with liver cirrhosis.
A 37-year-old male patient was admitted to our hospital on November 13, 2023 because of pancytopenia for more than 6 years and liver cirrhosis for 20 days.
The patient was found to have pancytopenia by physical examination more than 6 years prior to admission. The details were unknown, and there was no further diagnosis and treatment. On October 17, 2023, the patient had no obvious cause of fever. The highest body temperature was 40.5 °C, accompanied by cough and a small amount of yellow sputum. He was admitted to the Department of Hematology of another hospital.
A blood routine examination showed that the white blood cell count was 1.46 × 109/L, neutrophil percentage (NEU%) was 56.80, NEU count was 0.89 × 109/L, lymphocyte count was 0.41 × 109/L, red blood cell count was 3.60 × 1012/L, hemoglobin was 89.00 g/L, and platelets (PLTs) were 44.00 × 109/L. Reticulocyte count was normal, prothrombin time was 14.50 seconds, prothrombin activity was 67.00%, and liver function was normal. Thyroid function showed that free thyroid hormone was 0.53 ng/dL, and other indicators were normal. Serum iron was 6.70 µmol/L, transferrin was 5.17 g/L, and total ferritin binding capacity was 129.30 µmol/L.
Five items of hepatitis B, hepatitis C antibodies, hepatitis A and E series, autoimmune liver disease antibodies, antinuclear antibodies, ceruloplasmin, and tuberculosis T cells were normal. Six sex hormones were as follows: Prolactin was increased; luteinizing hormone, estradiol, follicle-stimulating hormone, progesterone, and testosterone were decreased; and GH was 0.02 ng/mL. Cortisol and adrenocorticotropic hormone (ACTH) were normal. Bone mineral density showed osteoporosis.
Thoracic and abdominal computed tomography (CT) showed liver cirrhosis, portal hypertension, secondary splenomegaly, a small amount of fluid in the abdominal cavity, normal cholangiopancreas, increased blood vessels in the lesser curvature of the stomach, low density of liver parenchyma, a small prostate, no seminal vesicle gland, and a tiny nodule of the superior lingual segment of the upper lobe of the left lung. The body temperature was normal after anti-infective (unknown) and symptomatic treatment, but the cause of liver cirrhosis was unknown.
The patient had no special history.
Personal history: The patient denied a long-term history of heavy drinking. He was unmarried and childless.
History of growth and development: The patient was the first birth of the first fetus and was a full-term breech delivery. He had normal language, motor and intellectual development, and moderate grades. There was underdevelopment of secondary sexual characteristics, short height in childhood, and rapid growth to 180 cm from 23-years-old (less than 150 cm) to 27-years-old.
Family history: The patient’s father died of aortic dissection rupture 20 years prior to the patient’s admission. His father’s height was 167 cm. The patient’s mother was alive, and her height was 161 cm. The mother reported height and foot length increased after adulthood.
The patient’s vital signs were as follows: Body temperature 36.8 °C; pulse 80 beats per minute; respiration rate 19 breaths per minute; and blood pressure 132/77 mmHg. The patient’s height was 180 cm, weight was 78 kg, and body mass index was 24.07 kg/m². The patient was alert and oriented, with a youthful appearance, a round, slightly fatty face, and smooth skin. The voice was high-pitched, and there was no facial hair, armpit hair, or pubic hair. There was no evidence of thyroid enlargement, and mild bilateral breast development was noted. On pulmonary examination, breath sounds were clear bilaterally, with no dry or wet rales, and no signs of arrhythmia. There were no pathological murmurs detected on auscultation of the heart valves. The abdomen was flat, with no tenderness, rebound tenderness, or muscle guarding. The liver was not palpable, and the spleen was felt at 3 subcostal finger breadths, with a medium texture, smooth surface, and negative for palpable splenic friction rub. The extremities were characterized by slender fingers and toes. The upper-to-lower limb ratio was < 1, and the fingertip-to-floor distance was 183 cm, which was greater than the patient’s height. There was no edema in the lower limbs. Genital examination revealed underdeveloped penis and testes.
Blood routine examination revealed that: White blood cell count was 1.11 × 109/L; NEU% was 56.80; NEU count was 0.63 × 109/L; red blood cell count was 3.24 × 1012/L; hemoglobin was 83.00g/L; and PLTs were 38.00 × 109/L. Urine routine was normal, and fecal occult blood was negative. Blood coagulation function revealed that: Prothrombin time was 14.10 seconds; prothrombin activity was 69.00%; and international normalized ratio was 1.31.
Liver function revealed that: Alanine aminotransferase was 33.70 U/L; aspartate aminotransferase was 46.40 U/L; total bilirubin was 13.20 µmol/L; direct bilirubin was 5.30 µmol/L; albumin was 39.70 g/L; γ-glutamyltransferase was 48.10 U/L; and alkaline phosphatase was 82.60 U/L. Renal function, electrolysate, blood glucose, tumor series, ceruloplasmin, IgG, IgA, and IgM were normal, while hepatitis B, hepatitis B core antibody IgM, hepatitis C antibody, hepatitis A antibody IgM, hepatitis E antibody IgM, treponema pallidum antibody, anti-human immunodeficiency virus antibody, antinuclear antibody, and anti-mitochondrial antibody M2 were all negative.
Blood lipids revealed that: Total cholesterol was 3.15 mmol/L; triglyceride was 1.19 mmol/L; high density lipoprotein cholesterol was 1.07 mmol/L; low density lipoprotein cholesterol was 1.58 mmol/L; apolipoprotein A1 was 1.38 g/L; and apolipoprotein B was 0.50 g/L. Fasting C peptide was 5.96 ng/mL, and fasting insulin was 23.33 μg/mL. Iron metabolism revealed that: iron was 4.60 µmol/L; unsaturated iron-binding capacity was 77.90 µmol/L; and total iron-binding capacity was 82.50 µmol/L. Three items of anemia were tested and revealed that: Folic acid was 14.15 ng/mL; vitamin B12 was 703.00 pg/mL; and ferritin was 36.40 ng/mL.
Six items of reproduction were observed and revealed that: Prolactin was 35.10 ng/mL; testosterone was 0.02 ng/mL; estradiol was 5.38 pg/mL; and others were normal. Prostate specific antigen combination was not detected. All items of thyroid function revealed that: Free thyroxine was 0.52 ng/dL; and others were normal. ACTH (8 am) was 18.00 ng/L (7.20-63.30 ng/L), blood cortisol (8 am) was 54.60 nmol/L (166.00-507.00 nmol/L), serum GH was 0.04 ng/mL (0.03-2.47 ng/mL), and insulin-like growth factor-1 (IGF-1) was < 15.00 ng/mL (111.00-284.00 ng/mL).
Electrocardiogram (ECG) revealed: Sinus rhythm; ST-T changes; and abnormal ECG. Echocardiography revealed: Aortic regurgitation (mild); mitral regurgitation (mild); and tricuspid regurgitation (mild). Abdominal ultrasound revealed: Hepatosclerosis; splenomegaly (intercostal thickness 76 mm, length greater than 200 mm); splenic vein enlargement (inner diameter 18 mm); very small amount of ascites; rough gallbladder wall; portal hypertension (portal trunk 17 mm); and collateral circulation opening.
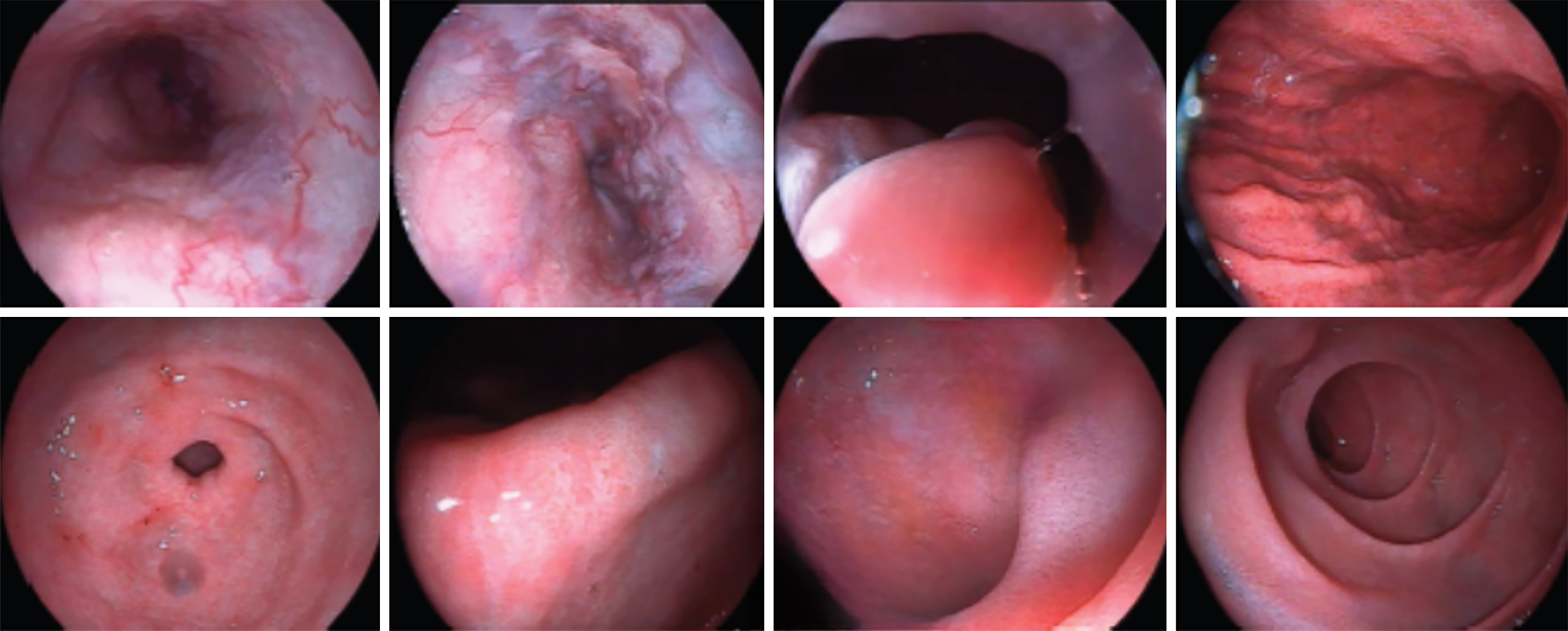
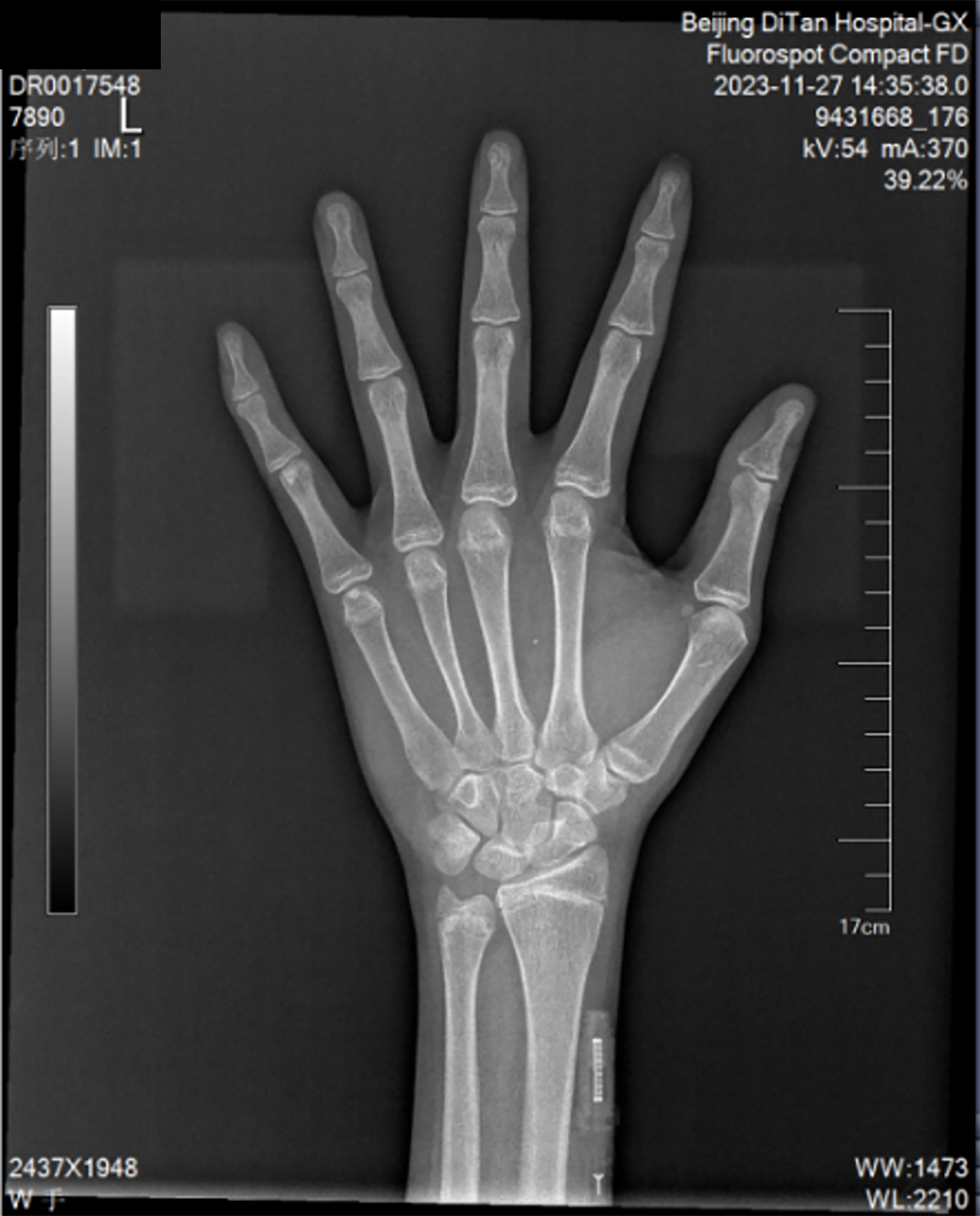
The liver elasticity was E 51.40 kPa and CAP 233 dB/m. Ultrasound of prostate revealed a small prostate volume (19 mm × 17 mm × 12 mm). Electronic gastroscope revealed severe esophageal and gastric varices (positive red sign) and portal hypertensive gastropathy with erosion (Figure 1). Osteoporosis of the spine and osteopenia of the left and right hip joint were observed. Bone age film revealed the recent closure of epiphysis (Figure 2).
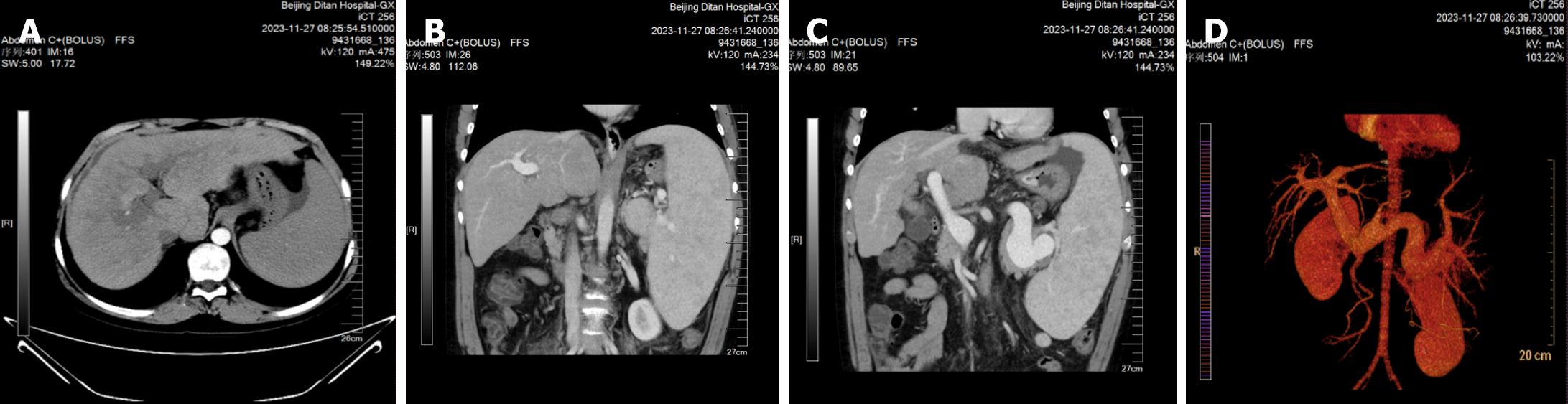
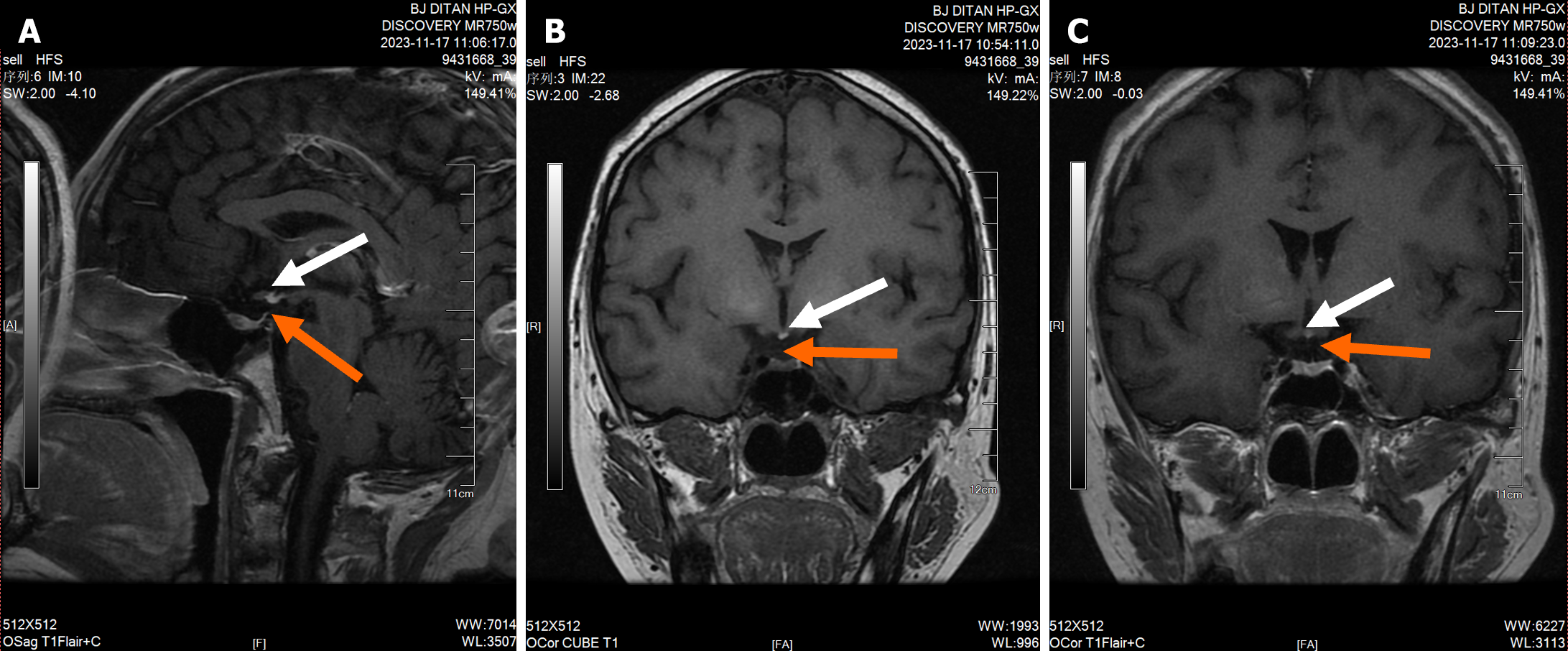
An abdominal CT scan + enhancement + portal vein reconstruction revealed liver cirrhosis, splenomegaly, mild esophagogastric varices, a small amount of ascites, minimal subsplenic nodules, and an accessory spleen (Figure 3). The pituitary stalk was not clearly displayed on pituitary-enhanced MRI. The gray nodule showed high signal on T1, and the ectopic posterior pituitary was considered, which was consistent with PSIS (Figure 4).
The final diagnosis was PSIS.
After consultation with the endocrinology department, it was recommended to initiate alternative therapy, including hydrocortisone 5 mg once daily, levothyroxine 50 µg once daily, testosterone undecanoate capsule 80 mg twice daily, and recombinant human GH (rhGH) 1 U once daily via subcutaneous injection. Unfortunately, the patient was unable to proceed with the recommended treatment due to financial constraints.
The patient’s condition was stable, and he will be followed up every 6 months.
PSIS is a rare developmental defect with an annual incidence of 5 cases per million people[8]. It was first reported by Fujisawa et al[9] in 1987. The ratio of male to female is 2.3-6.9:1. Previous studies have suggested that babies born in a breech position are mostly associated with pituitary dysplasia. Mechanical pituitary stalk injury and ischemia and hypoxia caused by breech delivery have been considered to be the main causes of PSIS. While most cases of PSIS are sporadic, instances of familial aggregation have been documented, suggesting a potential genetic component. Molecular defects during embryonic development may also lead to PSIS. Mutations in genes such as HESX1, LHX4, OTX2, SOX3, and PROP1 have been reported in patients with PSIS and are involved in pituitary growth and differentiation.
The main clinical manifestations of PSIS include the following aspects: (1) Anterior pituitary hormone deficiencies, which vary depending on the extent of anterior pituitary dysfunction. GH deficiency is the most prevalent. Guo et al[10] summarized 55 Chinese patients with PSIS. The results showed that the deficiency rates of GH, luteinizing hormone/follicle-stimulating hormone, ACTH, and thyroid stimulating hormone were 100%, 95.8%, 81.8% and 76.3%, respectively. The deficiency rate of 3 or 4 anterior pituitary hormones was as high as 92.7%; (2) The posterior pituitary gland, also known as the neuropituitary gland, generally has normal function, and diabetes insipidus is rare; and (3) Some patients may be complicated with midline structural abnormalities and other malformations, such as atrophy of corpus callosum, dysplasia of septum pellucidum, cleft lip, etc.
MRI is a reliable imaging method for the diagnosis of PSIS. The typical triad signs of MRI in PSIS are ectopic posterior pituitary, interruption of pituitary stalk, and adenohypophysis dysplasia[11]. The ectopic posterior pituitary is a characteristic sign of PSIS, and the most common ectopic position is median carina. The MRI of the patient’s head in this case conforms to the typical imaging features of PSIS, supporting the diagnosis of PSIS.
In this case report, the patient exhibited abnormalities in growth and development since childhood. However, due to feelings of inferiority regarding these physical abnormalities, the patient was reluctant to seek medical attention prior to the onset of cirrhosis symptoms. Pancytopenia was detected 6 years prior to admission, but no further diagnosis or treatment was undertaken. The patient presented to an external hospital for a respiratory infection and was found to have cirrhosis, which led to a referral to our hospital for further evaluation. Upon admission, the patient’s clinical manifestations of delayed growth and development and the absence of secondary sexual characteristics, along with laboratory blood results indicating hypofunction of the anterior pituitary, were noted. Gastroscopy revealed esophageal and gastric fundus varices, and an abdominal CT scan indicated liver cirrhosis, splenomegaly, and esophagogastric varices. Cranial MRI was consistent with the typical presentation of PSIS. After ruling out common causes of cirrhosis, such as viral hepatitis, drug-induced hepatitis, alcoholic liver disease, autoimmune liver disease, Wilson's disease, and Budd-Chiari syndrome, the patient was ultimately diagnosed with PSIS. The patient had a long course of disease, concealed onset, and relatively slow development. He had never been able to receive formal endocrine therapy, which indicates that this patient’s experience may represent the natural disease course of PSIS.
Several studies have explored the relationship between pituitary hypofunction (including PSIS) and non-alcoholic fatty liver disease (NAFLD). Both PSIS and pituitary surgery may lead to pituitary dysfunction, potentially resulting in metabolic syndrome, which encompasses conditions such as NAFLD, hyperlipidemia, and insulin resistance, with NAFLD serving as the foundation for liver fibrosis and cirrhosis. Typically, the evolution from NAFLD to cirrhosis requires a duration of approximately 30 years[12].
Yang et al[13] conducted a retrospective analysis of 5 young patients with rapidly progressive NAFLD complicated by pituitary hypofunction. The results indicated that all patients had decompensated cirrhosis, with an average time of 6.9 years from the onset of liver function abnormalities to the decompensated phase of cirrhosis. Fatty liver was present in all patients, leading to the hypothesis that pituitary hypofunction may be a rare etiology of rapidly progressive NAFLD.
Tian et al[3] reported the liver biopsy pathology of a patient with PSIS combined with cirrhosis, and the results showed structural disorder of liver lobules, formation of pseudolobular structures, diffuse swelling of liver cells, and steatosis. Therefore, it is speculated that this patient with PSIS may have developed cirrhosis due to NAFLD caused by GH deficiency. Previous literature reports suggested that the absence of a pituitary stalk indicated a severe degree of pituitary hormone deficiency in the patient[14]. However, in this case, the pituitary stalk was not shown, and no treatment was received. The condition continued to progress to the decompensated stage of liver cirrhosis.
In this case, the patient’s GH levels were near the lower limit of normal, while IGF-1 levels were significantly reduced, suggesting a GH deficiency. The deficiency in both GH and IGF-1 may contribute to the worsening of liver damage. Low serum IGF-1 levels are associated with increased histological severity of NAFLD[15]. Huang et al[16] conducted a retrospective analysis of 93 patients with PSIS and reported the incidence rates of NAFLD and advanced fibrosis/cirrhosis to be 50.5% and 4.3%, respectively. They assessed the independent impact of IGF-1 on NAFLD using logistic regression and concluded that NAFLD is a common comorbidity among adult Chinese patients with PSIS, which is closely associated with lower levels of IGF-1. However, the potential mechanisms underlying this association remain unclear.
PSIS should follow the principles of early detection, early diagnosis, and early treatment. The primary treatment approach involves anterior pituitary hormone replacement therapy, with recombinant rhGH being initiated as early as possible. In patients with concurrent adrenal insufficiency and thyroid dysfunction, cortisol should be administered prior to or concurrently with thyroid hormone therapy. Ji et al[17] presented a compelling case of an individual diagnosed with PSIS who also experienced hepatopulmonary syndrome and cirrhosis. The patient avoided liver transplantation following a treatment regimen that included rhGH and testosterone supplementation. Additionally, studies have demonstrated that rhGH therapy can improve or even reverse symptoms and prognosis in patients with cirrhosis and liver failure[18]. Delaying the timing of diagnosis and treatment not only affects the growth and development of patients but also has significant negative impacts on their personality and social psychology. The patient in this case had never received endocrine therapy. After confirming the diagnosis upon admission, it was recommended that the patient receive hydrocortisone, levothyroxine, undecanoate testosterone capsules, and GH replacement therapy. However, due to financial constraints, the patient declined these treatments.
There were several limitations in this case report. First, due to personal reasons, the patient did not undergo external genital examination and testicular ultrasound examination. Second, the patient was contraindicated for percutaneous liver biopsy due to a significant decrease in PLTs. Atorvatrippa and PLT transfusion were once added for treatment, but the PLT level could not reach 50 × 109/L. Therefore, the liver histopathological diagnosis could not be obtained. Finally, although the patient had a clear history of perinatal injury of gluteal presentation dystocia, financial constraints precluded the use of whole-exome sequencing to investigate possible pathogenic genetic mutations. Ultimately, the patient did not receive hormone replacement therapy, making it difficult to determine through follow-up whether hormone replacement therapy can reverse cirrhosis in the patient.
Our patient with PSIS showed a general decrease of anterior pituitary hormones and characteristic imaging findings. We excluded liver cirrhosis caused by other causes. The patient had never received any treatment for many years, which may indicate the natural progression of this disease that included rare cases of osteoporosis and cirrhosis. Liver cirrhosis caused by PSIS is very rare in clinical practice, and hepatologists should consider the possibility of PSIS in patients with liver cirrhosis accompanied by abnormal growth and development.
| 1. | Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB. Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol. 2013;168:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Gonzalez Rozas M, Hernanz Roman L, Gonzalez DG, Pérez-Castrillón JL. Panhypopituitarism due to Absence of the Pituitary Stalk: A Rare Aetiology of Liver Cirrhosis. Case Rep Endocrinol. 2016;2016:9071097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Tian H, Xu TJ, Zhu B, You SL, Lv S. [Pituitary stalk occlusion syndrome complicated with liver cirrhosis: a case report]. Linchunag Gandan Zazhi. 2018;34:1289-1291. [DOI] [Full Text] |
| 4. | Wu ZY, Li YL, Chang B. Pituitary stalk interruption syndrome and liver changes: From clinical features to mechanisms. World J Gastroenterol. 2020;26:6909-6922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 5. | Dawadi K, Dahal P, Poudyal B. Pituitary stalk interruption syndrome: A case report. Radiol Case Rep. 2023;18:4363-4365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | He H, Li DM. One Case of Pituitary Stalk Interruption Syndrome Associated with Liver Cirrhosis. Endocr Metab Immune Disord Drug Targets. 2023;23:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Liu Z, Zhao W, Cao C, Wang Y, Xiao L, Wang X, Jin C, Xiao J. Pituitary stalk interruption syndrome and liver cirrhosis associated with diabetes and an inactivating KCNJ11 gene mutation: a case report and literature review. Front Endocrinol (Lausanne). 2023;14:1297146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Smith-Bindman R, Chu P, Miglioretti DL, Quale C, Rosenberg RD, Cutter G, Geller B, Bacchetti P, Sickles EA, Kerlikowske K. Physician predictors of mammographic accuracy. J Natl Cancer Inst. 2005;97:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Guo Q, Yang Y, Mu Y, Lu J, Pan C, Dou J, Lv Z, Ba J, Wang B, Zou X, Yang L, Ouyang J, Yang G, Wang X, Du J, Gu W, Jin N, Chen K, Zang L, Erickson BJ. Pituitary stalk interruption syndrome in Chinese people: clinical characteristic analysis of 55 cases. PLoS One. 2013;8:e53579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Qi Wei P, Hui Juan Z, Feng Ying G, Nai Shi L, Tao Z, Gang B, Hui P, Xian-wei Z. Magnetic resonance image of sellar region in pituitary stalk interruption syndrome in children and adolescents. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1240] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Qi ZR, Zhang TT, Kang YJ, Wang X. Rapidly progressive non-alcoholic fatty liver disease due to hypopituitarism. Report of 5 cases. Neuro Endocrinol Lett. 2018;39:99-104. [PubMed] |
| 14. | Wang W, Wang S, Jiang Y, Yan F, Su T, Zhou W, Jiang L, Zhang Y, Ning G. Relationship between pituitary stalk (PS) visibility and the severity of hormone deficiencies: PS interruption syndrome revisited. Clin Endocrinol (Oxf). 2015;83:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Dichtel LE, Corey KE, Misdraji J, Bredella MA, Schorr M, Osganian SA, Young BJ, Sung JC, Miller KK. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8:e217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Huang Q, Xu H, Wang X, Mao J, Yu B, Zhu Y, Zhang R, Sun B, Zhang J, Ji W, Ma W, Nie M, Wu X. Relationship between growth hormone deficiency and nonalcoholic fatty liver disease in patients with pituitary stalk interruption syndrome. Clin Endocrinol (Oxf). 2022;97:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Ji W, Nie M, Mao JF, Zhang HB, Wang X, Wu XY. Growth hormone cocktail improves hepatopulmonary syndrome secondary to hypopituitarism: A case report. World J Clin Cases. 2021;9:4852-4858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Fujio A, Kawagishi N, Echizenya T, Tokodai K, Nakanishi C, Miyagi S, Sato K, Fujimori K, Ohuchi N. Long-term survival with growth hormone replacement after liver transplantation of pediatric nonalcoholic steatohepatitis complicating acquired hypopituitarism. Tohoku J Exp Med. 2015;235:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |