Published online Nov 27, 2024. doi: 10.4254/wjh.v16.i11.1331
Revised: September 1, 2024
Accepted: October 8, 2024
Published online: November 27, 2024
Processing time: 100 Days and 18 Hours
Acute kidney injury (AKI) in cirrhosis is common. The diagnosis of AKI in cirrhosis patients depends on clinical presentation and laboratory tests like serum creatinine. However, urine biomarkers could also be used to assess the type of AKI and the severity of the disease. We performed a systematic review with meta-analysis to evaluate the association with urine neutrophil gelatinase-associated lipocalin (NGAL) marker in identifying acute tubular necrosis (ATN) in patients with cirrhosis.
To assess the reliability of urine NGAL in the detection of ATN in patients with cirrhosis.
We systematically searched MEDLINE and PubMed using keywords including “urine biomarkers”, “NGAL”, “kidney dysfunction”, and “cirrhosis” to identify relevant studies. Data was screened and extracted. Included studies assessed hospitalized cirrhosis patients with AKI using the urine NGAL biomarker. We synthesized the data using diagnostic odds ratio (DOR), comparative and descriptive analyses, and Cochran Mantel-Haenszel (CMH) statistics to evaluate heterogeneity.
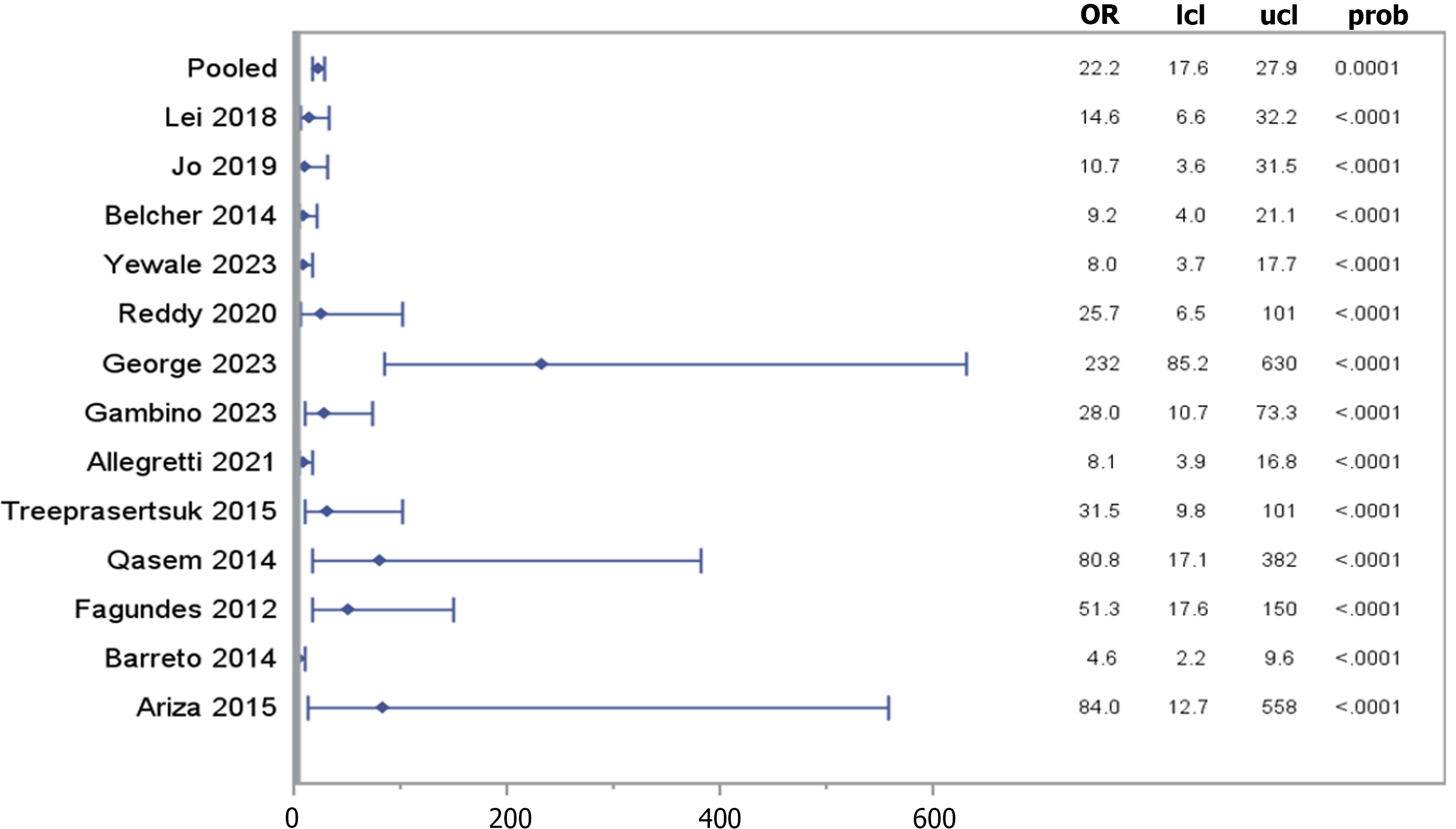
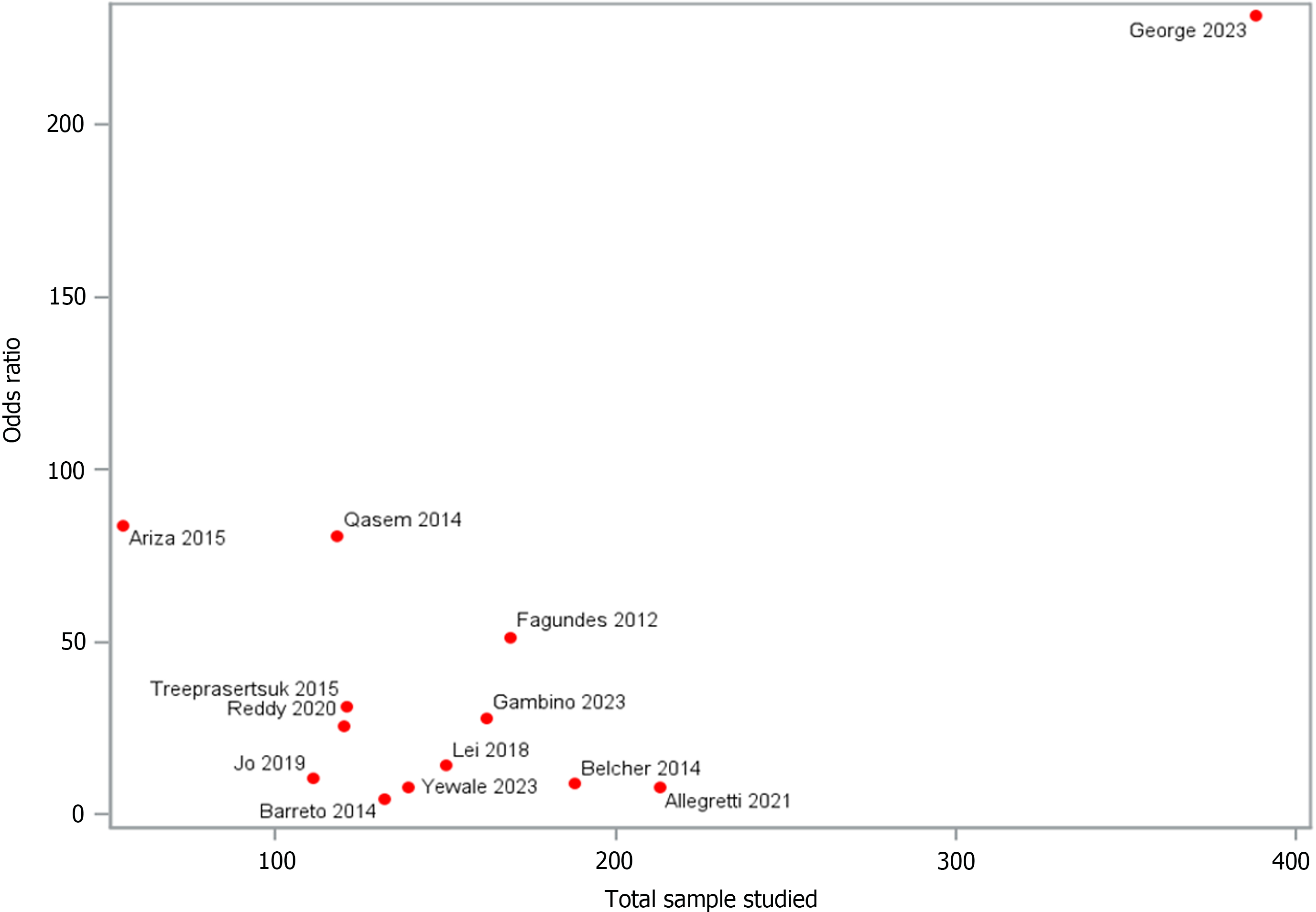
Three thousand seven hundred and one patients with cirrhosis were analyzed from a total of 21 cohort studies. The DOR of 14 of those studies [pooled DOR: 22.150, (95%CI: 17.58-27.89), P < 0.0001] demonstrated a significant association between urine NGAL levels and its identification of ATN. Following stratification by cirrhosis status, heterogeneity was analyzed and showed a significant non-zero correlation between NGAL and AKI (CMH statistic = 702.19, P < 0.0001).
In patients with cirrhosis, the use of urine NGAL is a reliable biomarker for detecting ATN and identifying the etiology of AKI.
Core Tip: The findings of this systematic review and meta-analysis supports that neutrophil gelatinase-associated lipocalin (NGAL) can reliably predict acute tubular necrosis (ATN) among patients with decompensated cirrhosis. 2. The pooled diagnostic odds ratio of 22.15 (95%CI: 17.58-27.89) from 14 studies indicates a strong correlation between high urinary NGAL levels and the presence of ATN in cirrhosis patients with acute kidney injury.
- Citation: Agrawal N, Louis-Jean S, Ladiwala Z, Adnani H, Kamal A, Karpman M, Fleisher AS, Singh S. Reliability of neutrophil gelatinase-associated lipocalin in detecting acute tubular necrosis in decompensated cirrhosis: Systematic review and meta-analysis. World J Hepatol 2024; 16(11): 1331-1338
- URL: https://www.wjgnet.com/1948-5182/full/v16/i11/1331.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i11.1331
Renal dysfunction in cirrhosis is associated with higher morbidity and mortality rates, which has led to the need for early and reliable identification. The causes of renal dysfunction in cirrhosis is multifactorial, often propagated by processes such as hypovolemia, nephrotoxins, endotoxins, and hepatorenal syndrome (HRS)[1,2]. In patients with cirrhosis there are different classifications of renal dysfunction, which are categorized based on (1) acuity and (2) cause of the dysfunction, such as prerenal azotemia, HRS-acute kidney injury (AKI), intrinsic renal/parenchymal disease, and post-renal disease[3].
Regardless of the precipitant, patients who do not have any improvement in renal dysfunction progress to HRS, which has a higher rate of mortality with reversibility only achieved through liver transplant[1,3]. Due to the tenuous nature of HRS, a stringent set of criteria, which has been reflected in the Model for End-Stage Liver Disease (MELD) score, has included serum creatinine[1]. Studies have shown that the sensitivity of serum creatinine levels in patients with cirrhosis were low[4]. Additionally, serum creatinine has not been shown to be a reliable identifier in tubulointerstitial or vascular damage, as eGFR, which it calculates, remains preserved in these pathological states. AKI in cirrhosis, due to its acuity, can be sans renal structural changes and consequently not associated with serum creatinine elevations[3,5]. Thus, serum creatinine is not a reliable biomarker to determine renal dysfunction in cirrhotic patients.
An emergence of new biomarkers is being used to differentiate structural and functional AKI in patients with cirrhosis[3]. NGAL has been shown to be a highly sensitive biomarker for ischemic AKI and is noted to be a good predictor of mortality in declining renal function[6]. NGAL is released in the setting of renal regeneration following tubular damage, caused by prerenal azotemia, ischemia, nephrotoxic exposure, and sepsis. NGAL levels can be detected in plasma or urine within two hours of AKI onset with a peak concentration occurring within 6 hours. The degree of NGAL elevation is often reflective of the severity and duration of ischemia, which makes it a reliable marker for the detection of acute tubular necrosis (ATN)[6]. In this systematic review, we explore its utility in identifying ATN in patients with cirrhosis.
A comprehensive search strategy was designed to identify relevant studies in the PubMed and MEDLINE databases. The following keywords were utilized to retrieve potentially eligible articles: ("Biomarker" OR "biomarkers" OR "biological marker" OR "biological markers") AND ("acute kidney injury" OR "renal injury" OR "kidney damage" OR "kidney dysfunction" OR "nephropathy" OR "kidney impairment") AND ("decompensated cirrhosis" OR "advanced cirrhosis" OR "end-stage liver disease" OR "cirrhotic decompensation" OR "decompensated liver disease" OR "severe liver dysfunction"). The search was limited to articles written in English. Supplementary Table 1 discusses the PICO formulated for this study.
The study selection involved a two-step screening process using the Covidence platform. Two independent reviewers were assigned to screen titles and abstracts of the identified studies based on the inclusion and exclusion criteria. The inclusion criteria encompassed studies involving patients aged 18 years and older with decompensated cirrhosis, and different types of biomarkers. The exclusion criteria involved animal studies and studies related to pregnancy.
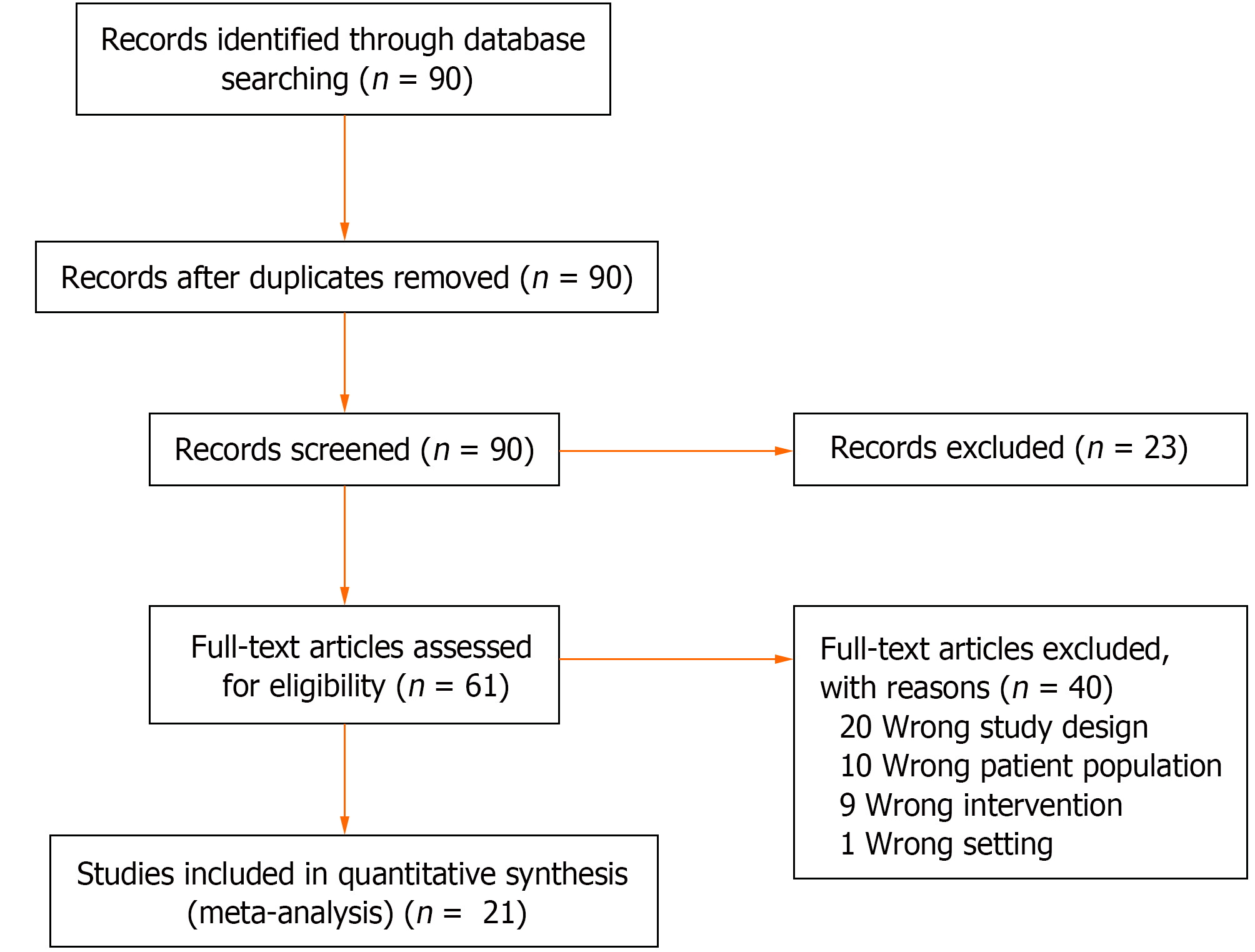
In situations where conflicts in decision-making arose, a third reviewer was consulted to provide a consensus on the inclusion of the study. The final set of included studies underwent a full-text review to ensure alignment with the research objectives. This rigorous screening process aimed to minimize bias and ensure that only studies meeting the predefined criteria were included in the systematic review. Figure 1 shows the PRISMA flowchart depicting the process of study inclusion and exclusion.
Data were extracted from the selected studies using a standardized data extraction method. Two independent reviewers performed the data extraction process, and any discrepancies were resolved through discussion and consultation with a third reviewer when necessary. The extracted data were as follows; author(s) and year, study design, sample size, sex, biomarkers, and outcomes.
In the process of data synthesis, we employed a multifaceted approach to comprehensively analyze the relationship between NGAL biomarker and kidney function in patients with decompensated cirrhosis. Descriptive analyses were conducted to provide a comprehensive overview of key study characteristics. Our findings were illustrated using forest plots to demonstrate effect sizes and confidence intervals from individual studies in the meta-analysis. Funnel plots were utilized to assess potential publication bias. This comprehensive synthesis was used to elucidate the intricate interplay of biomarkers and kidney function in this complex clinical context.
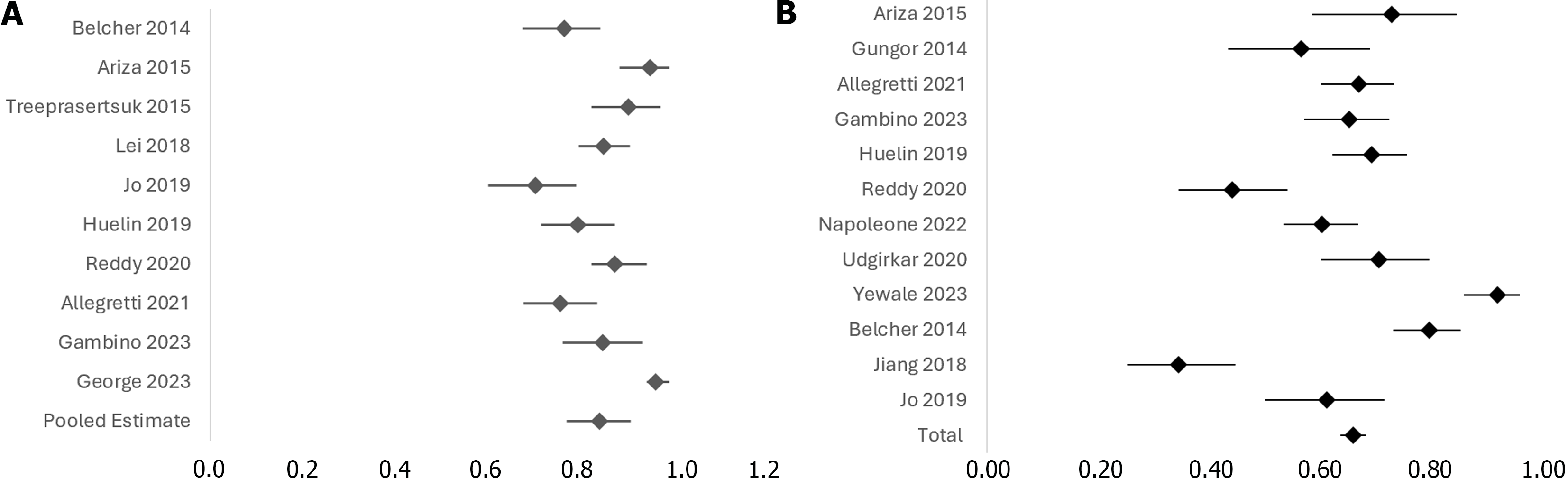
Twenty-one cohort studies were included in the analysis. Of which, the data points of 3701 patients with cirrhosis were analyzed. The mean age of the participants was 54.49 ± 8.34 years. The majority of the participants, 2591 (76%), are male (3 studies excluded). The mean serum creatinine level is 1.45 ± 0.65 mg/dL (7 studies excluded). The mean international normalized ratio (INR) is 1.46 ± 0.41, (11 studies excluded). The mean MELD score is 19.04 ± 5.05, (1 study excluded; Supplementary Table 2). The diagnostic odds ratio (DOR) of 14 studies [pooled DOR: 22.150, (95%CI: 17.58-27.89), P < 0.0001] demonstrated a significant association between urine NGAL levels and ATN, in comparison to other types of AKI (Figure 2). Following stratification by cirrhosis status, heterogeneity was analyzed and showed a significant non-zero correlation between NGAL and AKI (CMH statistic = 702.19, P < 0.0001), which is presented by a funnel plot in Figure 3. Furthermore, a forest plot demonstrating the area under the receiver operating characteristic curve (AUROC) for the urine NGAL marker predicting the diagnosis of AKI was performed (Figure 4A). The pooled estimate for AUROC was 0.85 (95%CI: 0.78-0.92), 11 studies excluded. Table 1 presents data from 13 different studies, each reporting the event rate or sample size and the corresponding proportion (%) with 95%CI. The total combined event rate across all studies is 34.38% (95%CI: 32.07%-36.75%), with a total sample size of 1623 participants. The forest plot (Figure 4B) shows the illustrative form of mortality in decompensated cirrhosis patients with AKI.
| Studies | Event/ sample size | Proportion (%) (95%CI) |
| Ariza et al[11], 2015 | 14/51 | 27.45 (15.89%-41.74%) |
| Gungor et al[16], 2014 | 28/64 | 43.75 (31.37%-56.72%) |
| Allegretti et al[17], 2021 | 71/213 | 33.33 (27.04%-40.10%) |
| Gambino et al[12], 2023 | 57/162 | 35.19 (27.86%-43.07%) |
| Huelin et al[18], 2019 | 62/199 | 31.16 (24.79%-38.09%) |
| Krishna Reddy et al[19], 2020 | 60/107 | 56.07 (46.15%-65.66%) |
| Napoleone et al[20], 2022 | 88/220 | 40.00 (33.47%-46.80%) |
| Udgirkar et al[13], 2020 | 28/94 | 29.79 (20.79%-40.10%) |
| Yewale et al[14], 2023 | 12/139 | 8.63 (4.54%-14.59%) |
| Belcher et al[21], 2014 | 39/188 | 20.74 (15.19%-27.25%) |
| Jiang et al[22], 2018 | 65/99 | 65.66 (55.44%-74.91%) |
| Jo et al[23], 2019 | 34/87 | 39.08 (28.79%-50.13%) |
| Total | 558/1623 | 34.38 (32.07%-36.75%) |
Renal dysfunction is a common phenomenon affecting approximately 30%-50% of patients with advanced cirrhosis, with the poorest outcomes observed among those with HRS and ATN[7]. In a multi-center case series conducted in the United States, of 2063 patients included in the analysis, 53% of individuals with ATN experienced mortality within 90 days, whereas those with HRS and pre-renal AKI had an associated 90-day mortality of 49% and 22%, respectively[7]. Considering the higher mortality rates in ATN, early identification of this process is crucial.
NGAL, a secretory protein derived from secondary granules of human neutrophils has been shown to predict outcome and severity of AKI with a high degree of accuracy and has notably had significant predictive and prognostic value in several disease processes[8,9]. The synthesis of NGAL is upregulated rapidly in ischemia-reperfusion-induced AKI, and its role is both renoprotective and regenerative[9]. It is completely reabsorbed by the proximal tubules, leading to reduced urinary levels. Its uptake by proximal tubular cells leads to reduced tubular damage, apoptosis, and increased cellular proliferation[9]. NGAL is also known to increase in cardiovascular diseases, cardiac surgery, sepsis, acute exacerbation of obstructive pulmonary diseases, and acute and chronic inflammatory states, such as sepsis, acute on chronic liver disease, and CKD[9,10]. These pathological states lead to the unpredictable release of NGAL from both hematopoietic and non-hematopoietic stem cells, including the colon, trachea, lung, and renal epithelium.
NGAL is synthesized in three different molecular forms, a 25k-Da monomer, 45-kDa disulfide-linked homodimer, and a 135-kDa heterodimer[9]. The kidney is known to secrete monomeric NGAL during states of stress, such as urinary tract infections and AKI, with the heterodimeric form having low concentrations in AKI. Additionally, performance in critically ill or septic patients showed low overall diagnostic accuracy[9]. The liver has also been associated with elevations in blood NGAL levels with patients with hepatocellular carcinoma and chronic liver disease demonstrating greater elevations and higher mortality rates. Conversely, the presence or absence of cirrhosis did not indicate a difference in NGAL levels[10]. Nevertheless, the performance of NGAL has been shown to increase with the severity of AKI, although recent studies demonstrate an AUROC of < 0.70 in predicting progression to severe AKI[9].
Of the 21 studies included in this analysis, 14 showed NGAL was statistically significant in the identification of ATN over other types of AKI. The mean NGAL value for detecting ATN was 672.12 ug/g (95%CI: 636.22-708.02). The mean cutoff NGAL value was 279.11 ug/g (95%CI: 260.60-297.61). However, there weren’t enough data points among all the studies to calculate a true positive or negative predictive value. Additionally, the bivariate nature of the conventional expressions of the test performances, threshold differences, and heterogeneity among the studies made simple pooling of the data inappropriate for an overall analysis. Thus, a pooled DOR was conducted, indicating a non-zero slope of 22.15, (95%CI: 17.58-27.89, P < 0.0001), which suggests NGAL had a high ATN predictability.
The mean AUROC for 13 of the studies that assessed AKI predictability of NGAL was 0.85 (95%CI: 0.83-0.86), with a mean of 0.88 (95%CI: 0.86-0.90) seen among 7 studies that assessed the ability of NGAL to detect ATN. Two of the studies did not assess AUROC, and one study analyzed the role of NGAL in HRS. Analysis of the progression to severe AKI could not be determined from the available data points, although Ariza et al[11] and Gambino et al[12], reported NGAL values of 437 ug/g (range 92–2515) and 1269 (range 282–2782) in AKI stage 3, respectively, suggesting higher levels of NGAL in these two studies were associated with higher AKI severity.
There was a 34.38% mortality rate among the total of 1623 participants included in the overall analysis. The NGAL values associated with mortality ranged from 159 to 717.17 ug/g, whereas the proportion of living participants had a range of 38 to 331.65 ug/g. This indicated that higher levels of NGAL were associated with a greater likelihood of mortality, although only four studies assessed 90-day mortality rates, whereas two focused on 30-day mortality. The highest mean NGAL value associated with 30-day mortality was 717.17 ± 494.26 ug/g, with a 6.5 positive likelihood ratio and zero negative likelihood ratio, as reported by Udgirkar et al[13]. There were limited studies evaluating the role of NGAL in HRS. In our analysis, Yewale et al[14] was the only study that assessed this relationship. The authors reported an AUROC of 0.74, with a statistically non-significant sensitivity of 77.8% and specificity of 68.7% (P = 0.016).
The mean MELD score was 19.04 ± 5.05, with highly variable scores appreciated among the included studies. MELD score is an independent predictor of mortality, that is calculated by using the following biomarkers: serum bilirubin
In this systematic review and meta-analysis, the study purpose was to identify the reliability of NGAL in recognizing renal dysfunction in patients with decompensated cirrhosis, and its role as a predictor for mortality within the same subset of patients. Through our literature review, the majority of studies that involved NGAL in renal dysfunction focused mainly on AKI or ATN. Due to the higher mortality outcomes in ATN and larger abundance of data, analysis of the predictability and reliability of NGAL in ATN was pursued as the primary focus. Based on the study inclusion criteria, 7 studies were excluded due to lack of data availability to do the analysis, which in turn reduced confounding variables in the analysis of NGAL. Overall, this limited the evaluation of the predictability and performance of the biomarker in patients with comorbidities associated with NGAL elevation.
Considering the assays needed to differentiate between monomeric and heterodimeric forms of NGAL might differ depending on institutional resources, interpretation of NGAL may be variable and difficult to interpret. Its overall utility may be questionable in septic or critically ill patients. Additionally, the data on HRS also remains to be further elucidated, but this may prove to be difficult as the management standard of the condition follows an algorithm that seeks HRS identification as a diagnosis of exclusion. Further exploration in understanding NGAL in HRS-AKI and HRS-CKD is needed. Nevertheless, a significant association exists with urine NGAL levels and the presence of ATN in patients with cirrhosis.
Our analysis revealed significant heterogeneity among the included studies, particularly in terms of NGAL assay techniques, patient populations, and definitions of AKI and ATN. Various methods were used to measure urine NGAL levels, including enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassay, and other immunoassay techniques. These differences in assay methods can lead to variability in NGAL measurements, potentially affecting the interpretation and comparability of results across studies.
To address this limitation, future research should focus on standardizing NGAL detection techniques. We recommend utilizing consistent methods, such as a specific type of ELISA or chemiluminescent immunoassay, to reduce variability between studies. Additionally, adopting universally recognized standards for defining AKI and ATN, such as the Kidney Disease Improving Global Outcomes criteria, would enhance the comparability and clinical applicability of findings.
Due to the increased morbidity and mortality associated with renal dysfunction in patients with decompensated cirrhosis, early identification is crucial in mobilizing early management strategies. The standard use of serum creatinine underpredicts renal dysfunction in cirrhosis due to preserved eGFR and parenchymal structure, as is the case with ATN and HRS. The findings of this systematic review and meta-analysis supports that NGAL can reliably predict ATN among patients with decompensated cirrhosis.
We are grateful for Ms. Joyce Miller, a librarian who helped with the literature search and building the search strategy.
| 1. | Adebayo D, Morabito V, Davenport A, Jalan R. Renal dysfunction in cirrhosis is not just a vasomotor nephropathy. Kidney Int. 2015;87:509-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Peng JL, Techasatian W, Hato T, Liangpunsakul S. Role of endotoxemia in causing renal dysfunction in cirrhosis. J Investig Med. 2020;68:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Schoenen J. [Spinal neurotransmitters: chemical neuroanatomy of the human spinal cord and the cultured sensory neurons of the adult rat]. Bull Mem Acad R Med Belg. 1989;144:407-19; discussion 419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201-205. [PubMed] |
| 5. | Thomas D, Zachariah S, Elamin AEE, Hashim ALO. Limitations of serum creatinine as a marker of renal function. Sch Acad J Pharm. 2017;6:168-170. [DOI] [Full Text] |
| 6. | Romejko K, Markowska M, Niemczyk S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 7. | Attieh RM, Wadei HM. Acute Kidney Injury in Liver Cirrhosis. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Marakala V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury - A systematic review. Clin Chim Acta. 2022;536:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 9. | Mårtensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014;37:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Yoshikawa K, Iwasa M, Eguchi A, Kojima S, Yoshizawa N, Tempaku M, Sugimoto R, Yamamoto N, Sugimoto K, Kobayashi Y, Hasegawa H, Takei Y. Neutrophil gelatinase-associated lipocalin level is a prognostic factor for survival in rat and human chronic liver diseases. Hepatol Commun. 2017;1:946-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodríguez E, Huelin P, Solé C, Fernández J, Jiménez W, Arroyo V, Ginès P. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. [PubMed] [DOI] [Full Text] |
| 12. | Gambino C, Piano S, Stenico M, Tonon M, Brocca A, Calvino V, Incicco S, Zeni N, Gagliardi R, Cosma C, Zaninotto M, Burra P, Cillo U, Basso D, Angeli P. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology. 2023;77:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Udgirkar S, Rathi P, Sonthalia N, Chandnani S, Contractor Q, Thanage R, Jain S. Urinary neutrophil gelatinase-associated lipocalin determines short-term mortality and type of acute kidney injury in cirrhosis. JGH Open. 2020;4:970-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yewale RV, Ramakrishna BS, Venugopal G, Doraiswami BV, Rajini K. Urine neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury and prognosis in decompensated chronic liver disease: A prospective study. Indian J Gastroenterol. 2023;42:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Emenena I, Emenena B, Kweki AG, Aiwuyo HO, Osarenkhoe JO, Iloeje UN, Ilerhunmwuwa N, Torere BE, Akinti O, Akere A, Casimir OE. Model for End Stage Liver Disease (MELD) Score: A Tool for Prognosis and Prediction of Mortality in Patients With Decompensated Liver Cirrhosis. Cureus. 2023;15:e39267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Gungor G, Ataseven H, Demir A, Solak Y, Gaipov A, Biyik M, Ozturk B, Polat I, Kiyici A, Cakir OO, Polat H. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int. 2014;34:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Allegretti AS, Parada XV, Endres P, Zhao S, Krinsky S, St Hillien SA, Kalim S, Nigwekar SU, Flood JG, Nixon A, Simonetto DA, Juncos LA, Karakala N, Wadei HM, Regner KR, Belcher JM, Nadim MK, Garcia-Tsao G, Velez JCQ, Parikh SM, Chung RT; HRS-HARMONY study investigators. Urinary NGAL as a Diagnostic and Prognostic Marker for Acute Kidney Injury in Cirrhosis: A Prospective Study. Clin Transl Gastroenterol. 2021;12:e00359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Huelin P, Solà E, Elia C, Solé C, Risso A, Moreira R, Carol M, Fabrellas N, Bassegoda O, Juanola A, de Prada G, Albertos S, Piano S, Graupera I, Ariza X, Napoleone L, Pose E, Filella X, Morales-Ruiz M, Rios J, Fernández J, Jiménez W, Poch E, Torres F, Ginès P. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology. 2019;70:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Krishna Reddy SS, Wyawahare M, Priyamvada PS, Rajendiran S. Utility of Urinary Neutrophil Gelatinase Associated Lipocalin (NGAL) in Decompensated Cirrhosis. Indian J Nephrol. 2020;30:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Napoleone L, Solé C, Juanola A, Ma AT, Carol M, Pérez-Guasch M, Rubio AB, Cervera M, Avitabile E, Bassegoda O, Gratacós-Ginès J, Morales-Ruiz M, Fabrellas N, Graupera I, Pose E, Crespo G, Solà E, Ginès P. Patterns of kidney dysfunction in acute-on-chronic liver failure: Relationship with kidney and patients' outcome. Hepatol Commun. 2022;6:2121-2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, Lim J, Coca SG, Parikh CR; TRIBE-AKI Consortium. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol. 2014;9:1857-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Jiang QQ, Han MF, Ma K, Chen G, Wan XY, Kilonzo SB, Wu WY, Wang YL, You J, Ning Q. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol. 2018;24:2300-2310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Jo SK, Yang J, Hwang SM, Lee MS, Park SH. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci Rep. 2019;9:14508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |