Published online Jan 27, 2024. doi: 10.4254/wjh.v16.i1.41
Peer-review started: November 21, 2023
First decision: December 5, 2023
Revised: December 18, 2023
Accepted: January 9, 2024
Article in press: January 9, 2024
Published online: January 27, 2024
Processing time: 63 Days and 0.2 Hours
Direct-acting antivirals (DAAs) revolutionized the treatment of chronic hepatitis C virus (HCV)-associated disease achieving high rates of sustained virological response (SVR). However, whether DAAs can reduce the occurrence of hepatocellular carcinoma (HCC) in patients with HCV-associated cirrhosis who are at high risk have not been concluded.
To investigate the effect of DAAs on the occurrence of HCC in patients with HCV-associated cirrhosis after achieving SVR.
Of 427 inpatients with HCV-associated cirrhosis were enrolled in Tianjin Second People's Hospital from January 2014 to April 2020. 118 patients weren’t received antiviral treatment with any reasons named non-antiviral treatment group, and 236 patients obtained from the 309 DAAs treatment patients according to the propensity score matching named DAAs treatment group. Demographic information and laboratory data were collected from baseline and the following up. Kaplan-Meier curve and Log-Rank test were used to compare the incidence and cumulative incidence of HCC between the two groups. Cox proportional risk regression was used to re-evaluate the risk factors for HCC.
HCC incidence was 4.68/100PY (95%CI, 3.09-6.81) in the DAAs treatment group, while it was 3.00/100PY (95%CI, 1.50-5.37) in the non-antiviral treatment group, and the relative risk was 1.82 (95%CI, 0.93-3.53, P > 0.05). The incidence of HCC at 12, 24, 36 and 48 months was 3.39%, 6.36%, 8.47% and 10.17% in the DAAs treatment group, and it was 0%, 0%, 3.39% and 9.32% in the non-antiviral treatment group, respectively. Age > 58 [hazard ratio (HR) = 1.089; 95%CI, 1.033-1.147; P = 0.002] and liver stiffness measurement > 27.85 kPa (HR = 1.043; 95%CI, 1.022-1.065; P = 0.000) were risk factors for HCC in all patients (n = 427), and DAAs treatment didn’t show protective efficacy.
DAAs treatment seems failed to reduce the incidence of HCC occurrence in HCV-associated cirrhosis in 48 months, and even increased the incidence of HCC in 36 months.
Core Tip: We evaluated the effect of direct-acting antivirals (DAAs) on the development of hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV)-associated cirrhosis during long-term follow-up. We performed propensity score matching, Kaplan-Meier curve and Log-Rank test, the incidence and cumulative incidence of HCC in DAAs treatment group (n = 236) and non-antiviral treatment group (n = 118) were retrospectively evaluated, and the risk factors for HCC were evaluated by Cox regression. We found that DAAs treatment of HCV-associated cirrhosis failed to reduce the incidence of HCC over 48 mo. Age and liver stiffness measurement were risk factors for developing HCC in all patients (n = 427), and DAAs treatment showed no protective effect.
- Citation: Tao XM, Zeng MH, Zhao YF, Han JX, Mi YQ, Xu L. Direct-acting antivirals failed to reduce the incidence of hepatocellular carcinoma occurrence in hepatitis C virus associated cirrhosis: A real-world study. World J Hepatol 2024; 16(1): 41-53
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/41.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.41
According to an estimate by the World Health Organization[1], more than 185 million people worldwide have been infected with hepatitis C virus (HCV), of which 350000 people died from HCV infection each year. China is a region with high incidence of HCV infection. It has been estimated[2] that the prevalence of HCV in China is between 0.4% and 2.0%, with over 14 million infected persons. HCV infection has been identified as an independent risk factor for hepatocellular carcinoma (HCC) development, especially in patients with cirrhosis[3]. In patients with HCV-associated cirrhosis, the risk of developing HCC is estimated at 3% to 8% per year[4]. Multiple studies have shown[5,6] all-cause mortality and the risk of HCC were reduced among chronic hepatitis C (CHC) patients who achieved sustained virological response (SVR) with IFN-based antiviral therapy. A large number of studies[7,8], however, have indicated that IFN therapy can only bring a low SVR rate, in addition, the therapeutic indications for IFN are limited, which effectiveness varies with the degree of fibrosis, stage of liver disease, viral genotype, and presence of comorbidities[9]. New regimens with direct-acting antiviral agents (DAAs) have not only changed the scope and spectrum of treatment, but also had high efficacy, sufficient safety and few contraindications to be used in patients with advanced liver disease who are not recommended to be treated with interferon[4]. Moreover, through different combined treatment solutions, more than 95% of SVR can be achieved, regardless of HCV genotype or degree of fibrosis[10,11]. Kanwal et al[12], through a large retrospective cohort study, found that DAAs treatment can reduce the occurrence of HCC in patients with HCV, and Calvaruso et al[10] discovered that DAAs treatment can reduce the occurrence of HCC in patients with HCV-associated cirrhosis. However, some studies[13,14] have shown that DAAs can increase the occurrence of HCC[15]. Hence, in this study, we evaluated the efficacy of DAA on prevention HCC in HCV-associated cirrhosis patients who were at high risk after achieving SVR, in the real-world. The Kaplan-Meier curve and log-rank test were used to compare the incidence of HCC development in our hospital with or without DAAs treatment. Cox regression was used to retrospectively study the risk factors of HCC in HCV-associated cirrhosis patients after achieving SVR with DAAs.
This was an institutional review board-approved retrospective clinical cohort study conducted at a large hepatology hospital in China. Patients with HCV-related cirrhosis were enrolled from January 2014 through April 2020. DAA or liver-protective therapy was prescribed by experienced specialists according to the patient's condition. Informed consent was obtained from all enrolled patients. Enrolled patients were followed and reviewed by specialists during follow-up. Prior to implementation, education was provided to all hepatologists who would manage or verify orders for HCV patients. Physician leadership and health care teams also participated in education about the protocol and its implementation.
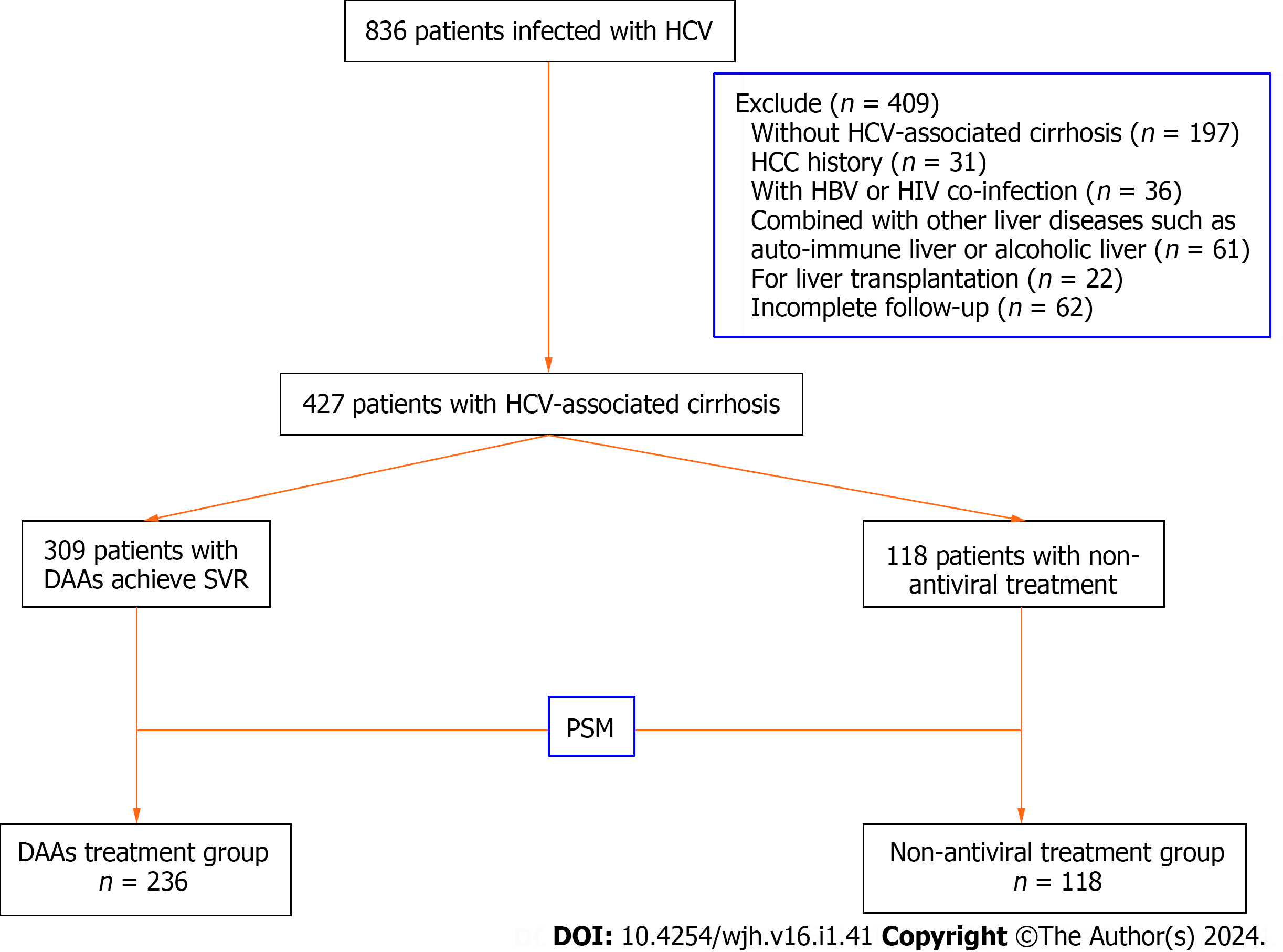
A total of 427 consecutive inpatients with HCV-associated cirrhosis were enrolled in Tianjin Second People's Hospital from January 2014 to April 2020. Of these, 309 patients received DAA treatment and the remaining 118 patients did not receive antiviral therapy.
Inclusion criteria: (1) Age ≥ 18, no gender limitation; (2) the serum anti-HCV and HCV RNA of all patients were positive, and the diagnosis was in line with the diagnostic criteria of hepatitis C associated cirrhosis in China's Hepatitis C Prevention and Treatment Guidelines 2019 Edition[16]; and (3) obtaining informed consent from all patients. Exclusion criteria: (1) Patients successfully treated with interferon combined with ribavirin; (2) patients who received direct antiviral therapy but did not achieve SVR; (3) For patients clinically diagnosed with HCC or previously diagnosed with HCC, the diagnostic criteria for HCC should be based on the 2018 European Association for the Study of the Liver (EASL) guidelines[4] and American Association for the Study of Liver Diseases guidance of HCC[17] and evaluated by imaging or pathological examination; (4) Patients with previous history of extrahepatic tumor; (5) patients who have received or are awaiting liver transplantation; (6) patients combined with HIV, HAV, HBV, HEV infection; (7) patients with alcoholic liver disease, autoimmune hepatitis, drug-induced liver injury, genetic metabolic liver disease and other liver diseases; and (8) patients with no follow-up records or incomplete follow-up data. A total of 836 inpatients with chronic HCV infection in our hospital were evaluated, and 409 patients were excluded from this study, as shown in Figure 1.
The Medical Ethics Committee of Tianjin Second People's Hospital approved the study protocol, which conformed to the ethical guidelines of the Declaration of Helsinki amended in 2008.
Antivirus solution: According to the guidelines[16,18], the 309 patients were treated by experienced clinicians at or above the attending level with the following regimen: (1) Sofosbuvir 400 mg/d + daclatasvir 60 mg/d + ribavirin 1000 mg/d (12 wk) regimen (95 cases); (2) sofosbuvir 400 mg/d+ Velpatasvir 100 mg/d (12 wk) (60 cases); (3) sofosbuvir 400 mg/d+ ribavirin 1000 mg/d (12 wk) (57 cases); (4) Ombitasvir 300 mg/d+ dasabuvir 500 mg/d (12 wk) (44 cases); (5) sofosbuvir 400 mg/d+ ledipasvir 90 mg/d+ ribavirin 1000 mg/d (12 wk) regimen (22 cases); (6) Elbasvir and Grazoprevir 50 mg/d (12 wk) (17 cases); and (7) Dasabuvir 60 mg+ Asunaprevir 100 mg (24 wk) regimen (14 cases).
Non-antiviral treatment: Inclusion reasons: (1) Because some patients could not choose pegylated interferon (peg-IFN) + ribavirin due to decompensation of cirrhosis, and DAA drugs were not released in China before 2017; and (2) Some patients cannot choose the treatment due to the economic burden.
Patients without antiviral treatment were received the corresponding hepatoprotective treatment and symptomatic treatment.
We recorded baseline data including gender, age, weight, height, body mass index, compensatory/decompensated cirrhosis, Child-Pugh score, nonspecific liver nodules, hypertension, diabetes, fatty liver, HCV genotype, HCV RNA (viral load was quantified by direct-PCR, Roche Diagnostics, 1080 US Highway 202 South, Branchburg, NJ 08876, BMI), Protein Induced by Vitamin K Absence or Antagonist-II (PIVKA-II), carcinoembryonic antigen (Cobas E601 electrochemiluminescence analyzer, Basel, Switzerland), alpha fetoprotein (AFP) (Cobas E601 electrochemiluminescence analyzer, Basel, Switzerland); serum biochemical indicators: alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-alanine transferase (γ-GT), total bilirubin (TBIL), total protein (TP), albumin (ALB), renal function, creatinine, uric acid, glomerular filtration rate, blood glucose, triglyceride, total cholesterol, low density lipoprotein, high density lipoprotein, all above assays were carried out in Hitachi 7180, automatic biochemical analyzer, Japan. coagulation function: prothrombin time (PT), international standardized ratio of prothrombin time (INR); blood routine: red blood cell, hemoglobin, white blood cell (WBC), platelet (PLT); liver stiffness measurement [liver stiffness measurement (LSM), in kPa] and controlled attenuation parameter (in dB/m) values were obtained by FibroScan (Echosens, Paris, France).
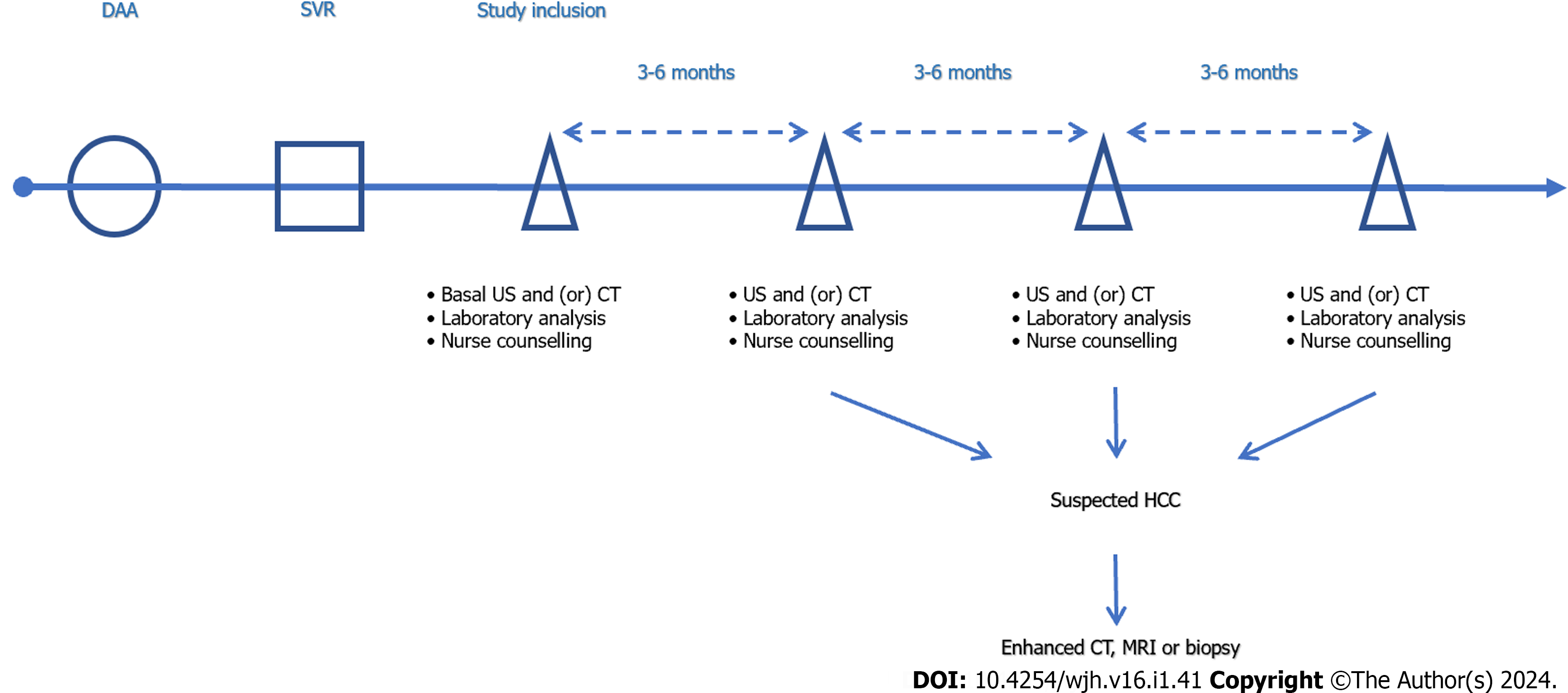
The end point of this study was the first occurrence of HCC or death among the enrolled subjects by the end of April 2020, and all patients received nurse counseling, clinical visit and laboratory assessment (biochemistry, blood routine test, bio-markers for HCC, etc.) at baseline and every 3-6 mo. According to the diagnostic criteria for HCC of EASL guidelines[4], patients were screened for HCC by ultrasound (US, Philips, No. IU22, 22100 Bothell Everett Highway Bothell, WA, United States) or AFP every 3-6 mo. When HCC was suspected, further examination such as enhanced computed tomography (CT) (Philips Row 64, Haifa, Israel), Gd-EOB-DTPA enhanced magnetic resonance imaging (MRI) (Siemens Skyra3.0T, Germany), hepatic digital subtraction angiography (Artis Zee 3, Fochheim County, Bavaria, Germany), or pathological examination were taken. The duration of follow-up was calculated from reaching SVR after DAA treatment to the diagnosis of HCC, death, or the end of follow-up (Figure 2).
SVR definition: according to EASL guidelines[18], SVR is defined as 12 wk after the end of treatment (SVR 12), and HCV RNA is not detected in serum or plasma, evaluated by highly sensitive molecular methods, additionally, the detection limit is 15 IU/mL.
Nonspecific liver nodules were defined[19] as ≤ 10 mm or nodules > 10 mm but in which HCC diagnosis was ruled out before starting DAA by contrast enhanced US (CEUS), CT, or MRI.
Data conforming to normal distribution were represented by (mean ± SD), and HCV RNA was calculated by denary logarithm. Independent sample t test was used to analyze and compare the two groups. The skewness distribution of measurement data was represented by M (P25, P75). The comparison between the two groups was analyzed and compared by Mann-Whitney U test, and the paired samples were analyzed and compared by Wilcoxon signed rank test. The statistical data were expressed as percentages, and analyzed by Chi-square test or Fisher's exact probability method. Kaplan-Meier curve and log-rank test were used to compare the difference in the cumulative incidence of HCC between the two groups. All clinical data were included in binary logistic regression analysis for univariate and multivariate analysis to evaluate the influencing factors of HCC occurrence and obtain a regression equation. Cox proportional risk regression was used to re-evaluate the risk factors for HCC before and after DAA treatment, and the independent predictors of HCC were obtained by incorporating the indicators with statistical differences in univariate analysis into multivariate analysis. Meanwhile, risk ratio and 95% confidence interval (CI) were calculated. P < 0.05 was considered to indicate that all analyses were statistically significant. Incidences, expressed as 100 patient-years (100PY), relative risks (RR) and their 95%CI were estimated utilizing Poisson regression models, using as offset the logarithm of radiological follow-up.
Statistical analyses were performed using SPSS version 26 (SPSS, Inc., Chicago, IL, United States), GraphPad Prism Version 9.0H (GRAPH PAD Software, Inc., La Jolla, CA, United States) and R Version 3.1.2 (R Core Development Team, 2010).
A total of 427 patients were included in this study from January 2014 to April 2020 (409 patients were not included according to the exclusion criteria) and completed follow-up. Among them, 309 patients were treated with DAAs and 118 patients were not treated with antiviral therapy. Considering the influence of gender and age on the results of this study, we used the propensity score matching (R Version 3.1.2) to adjust for age and sex, and divided patients into DAAs treatment group (n = 236) and non-antiviral treatment group (n = 118) (Figure 1). The baseline characteristics of the two groups of patients were introduced in Table 1.
| Variables | DAA treatment(n = 236) | Non-antiviral (n = 118) | t/Z/χ2 | P value |
| Ages (yr) | 55.01 ± 9.52 | 53.69 ± 10.07 | 1.208 | 0.228 |
| Male | 120 (50.85) | 68 (57.63) | 1.452 | 0.228 |
| Cirrhosis (compensate) | 187 (79.24) | 90 (76.27) | 0.407 | 0.524 |
| Nonspecific liver nodules | 191 (80.25) | 100/18 (15.13) | 0.782 | 0.377 |
| Child-Pugh (A/B/C) | 177/51/8 | 83/30/5 | 0.887 | 0.642 |
| Genotype (1a/1b/2a/3a/3b/6a) | 1/156/48/11/11/9 | 0/73/21/7/7/10 | 4.663 | 0.458 |
| lg (HCV RNA) IU/mL | 6 (5.6) | 5 (4.6) | -2.191 | 0.028 |
| Along with the disease | ||||
| Diabetes | 51 (21.61) | 30 (25.42) | 0.648 | 0.421 |
| Fatty liver | 52 (22.03) | 13 (11.02) | 6.370 | 0.012 |
| Hypertension | 50 (21.19) | 23 (19.49) | 0.138 | 0.710 |
| FIB-4 | 5.27 (3.51, 8.16) | 4.94 (2.30, 6.38) | -0.032 | 0.975 |
| CEA (ng/mL) | 3.27 (2.22, 4.86) | 2.76 (1.45, 4.70) | -1.015 | 0.310 |
| AFP (ng/mL) | 9,28 (5.15, 19.60) | 9.58 (4.27, 17.89) | -2.919 | 0.004 |
| PIVKA-II (mAU/mL) | 24 (18, 32) | 24 (21, 35.5) | -1.190 | 0.234 |
| PLT (109/L) | 108.02 ± 61.66 | 110.66 ± 65.68 | -0.342 | 0.733 |
| CAP (dB/m) | 232.76 ± 45.35 | 241.15 ± 53.59 | -0.986 | 0.325 |
| LSM (kPa) | 26.15 ± 16.90 | 29.50 ± 16.61 | -1.269 | 0.206 |
After DAAs treatment, all patients in the DAAs treatment group achieved SVR (patients who did not achieve SVR were not included in this study). After achieving SVR, the LSM value (20.55 ± 16.95 kPa) was significantly lower than baseline (26.15 ± 16.90 kPa) (t = 3.499, P = 0.001), and the values of ALT, AST, γ-GT, ALP and TP were significantly decreased compared with those at baseline (P < 0.05), and WBC and PLT were significantly increased (P < 0.05), as shown in Table 2.
| Variables | Before DAA | After DAA | t/Z/χ2 | P value |
| ALT (U/L) | 52 (34, 84.25) | 20.5 (16, 32) | 12.962 | 0 |
| AST (U/L) | 59.5 (40, 83.5) | 26 (20.2, 39) | 14.351 | 0 |
| γ-GT (U/L) | 58 (34.75, 108.5) | 34 (23, 54) | 8.691 | 0 |
| ALP (U/L) | 86 (65.75, 113.25) | 83.9 (64, 115) | 2.236 | 0.025 |
| TP (g/L) | 71.93 ± 8.23 | 73.62 ± 7.88 | -2.267 | 0.024 |
| ALB (g/L) | 38.78 ± 6.22 | 42.93 ± 7.08 | -6.755 | 0 |
| TBIL (μmol/L) | 18.6 (14.5, 27.5) | 23.7 (13.3, 41) | -1.452 | 0.146 |
| BUN (mmol/L) | 4.98 ± 2.24 | 5.83 ± 3.70 | -2.969 | 0.003 |
| GLU (mmol/L) | 6.34 ± 1.98 | 6.93 ± 2.44 | -2.717 | 0.007 |
| PT (s) | 14.41 ± 2.16 | 14.30 ± 6.35 | 0.16 | 0.873 |
| INR | 1.27 ± 1.22 | 1.21 ± 0.56 | 0.444 | 0.557 |
| WBC (109/L) | 4.39 ± 1.69 | 4.93 ± 2.13 | -3.066 | 0.002 |
| RBC (1012/L) | 4.14 ± 0.75 | 4.27 ± 0.79 | -1.803 | 0.072 |
| HGB (g/L) | 130.33 ± 23.05 | 131.91 ± 25.07 | -0.706 | 0.481 |
| PLT (109/L) | 108.20 ± 61.66 | 123.45 ± 65.98 | -2.57 | 0.01 |
| CEA (ng/mL) | 3.3 (2.22, 4.89) | 3.34 (2.3, 5.27) | -0.111 | 0.911 |
| AFP (ng/mL) | 9.38 (5.31, 19.67) | 5.81 (3.6, 9.02) | 9.683 | 0 |
| PIVKA-II (mAU/mL) | 24 (18,32) | 28 (20,41) | -1.958 | 0.05 |
| CAP (dB/m) | 232.76 ± 45.35 | 239.56 ± 45.35 | -2.062 | 0.04 |
| LSM (kPa) | 26.15 ± 16.90 | 20.55 ± 16.95 | 3.499 | 0.001 |
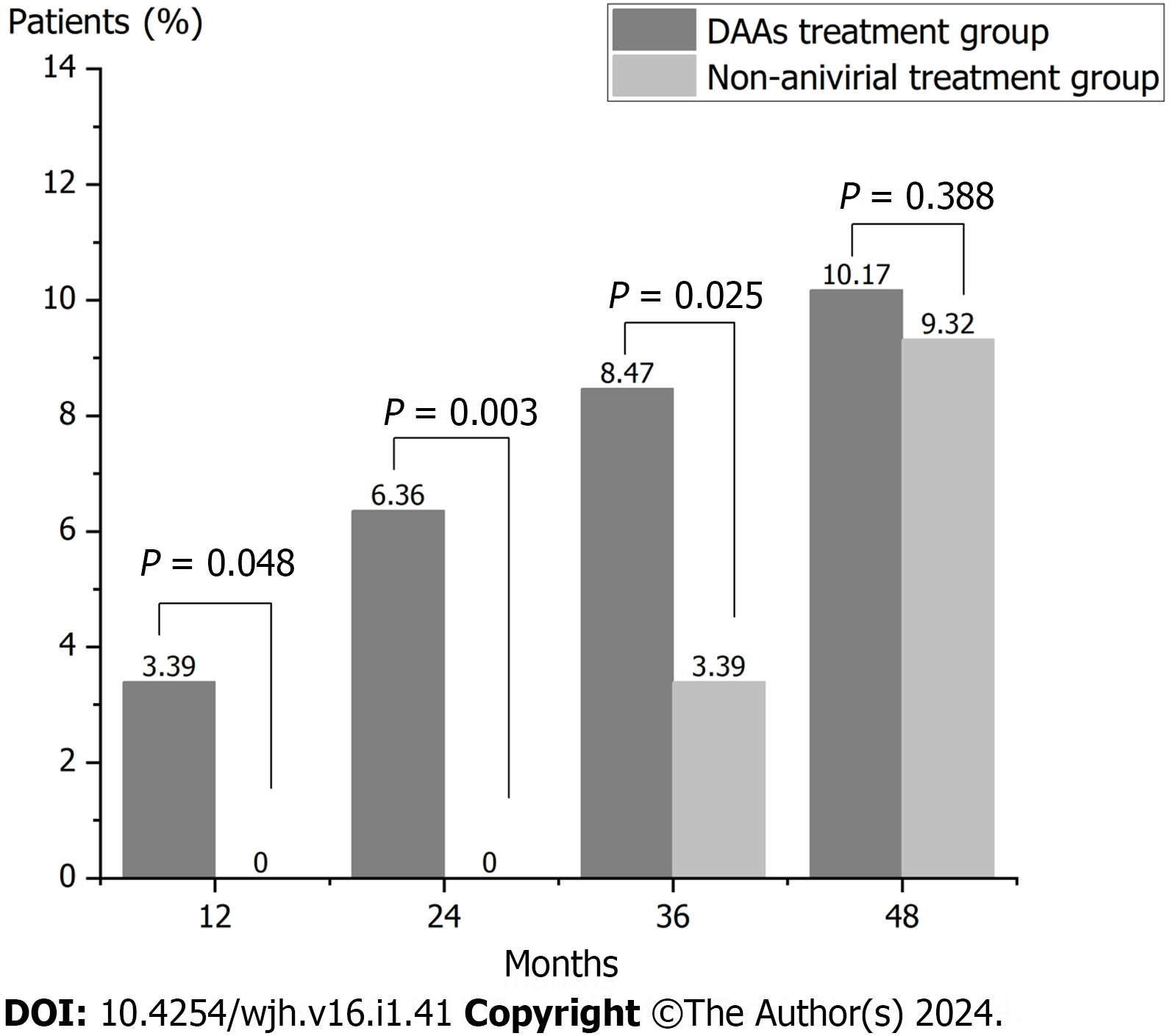
During the follow-up, 27 cases of HCC occurred in the DAAs treatment group (236 cases), while 11 cases of HCC occurred in the non-antiviral treatment group (118 cases), and there was no significant difference in the total incidence of HCC between the two groups (χ2 = 0.369, P = 0.544). In the DAAs treatment group, HCC incidence was 4.68/100PY (95%CI, 3.09-6.81), while it was 3.00/100PY (95%CI, 1.50-5.37) in the non-antiviral treatment group. Indeed, its RR was 1.82 (95%CI, 0.93-3.53, P > 0.05). The duration of follow-up in the DAAs treatment group was 1-84 mo (29.33 ± 16.20), the median follow-up time was 27 months and the time of HCC occurrence in the DAAs treatment group was 5-66 mo. The cumulative incidence of HCC at 12, 24, 36 and 48 mo was 3.39%, 6.36%, 8.47% and 10.17%, respectively. The duration of follow-up in the non-antiviral treatment group was 1-84 mo (37.25 ± 15.94), the median follow-up time was 41 months (t = -4.359, P = 0.000) and the time of HCC occurrence in the DAAs treatment group was 26-48 mo. The incidence of HCC at 12, 24 mo, 36 mo and 48 mo was 0%, 0%, 3.39% and 9.32%, respectively. There was significant difference in the incidence of HCC at 12, 24, and 36 mo between the two groups (P = 0.048, P = 0.003, and P = 0.025), while there was no significant difference in the cumulative incidence of HCC at 48 mo between the two groups (P = 0.388) (Figure 3).
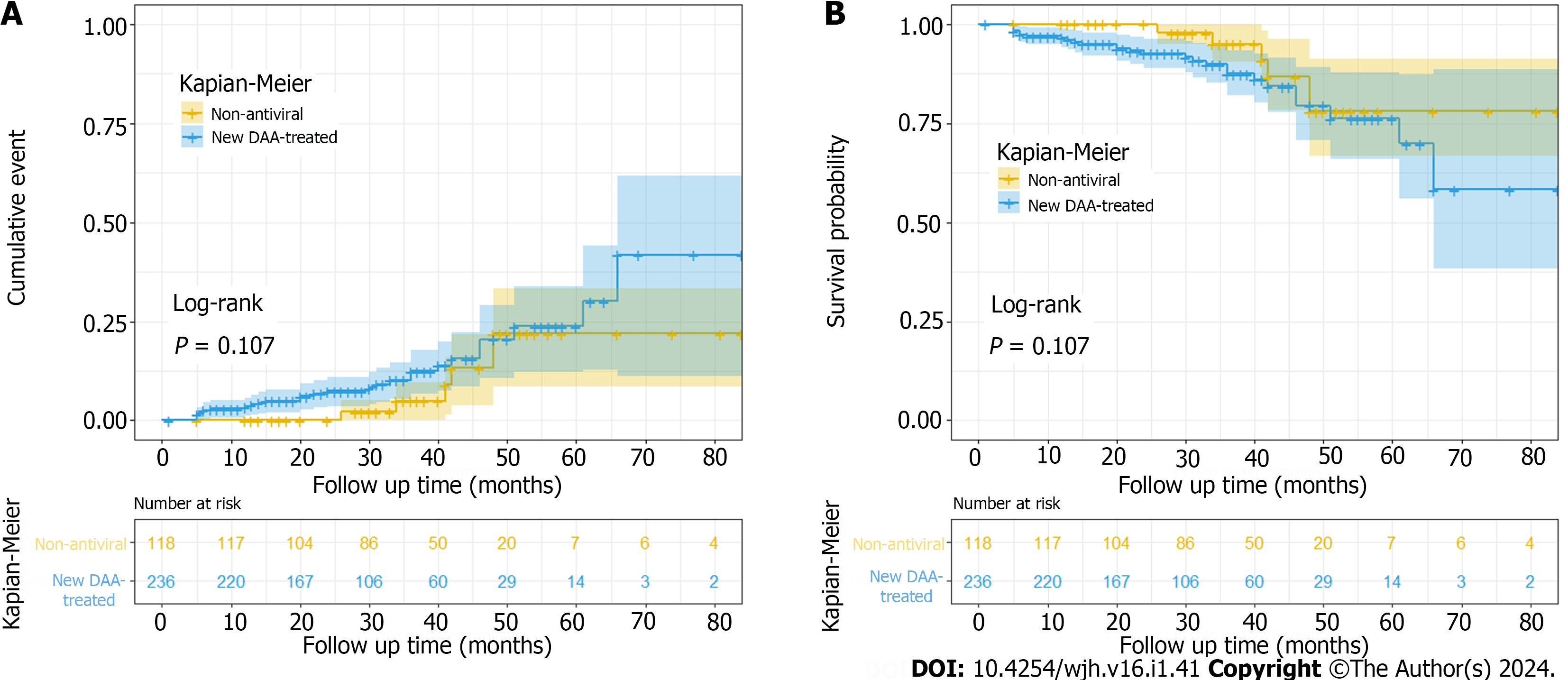
Log-rank test was used to compare and analyze the cumulative incidence of HCC between the two groups (log-rank test, P = 0.107). Kaplan-Meier curve was used to show the cumulative incidence of HCC between the two groups after adjusting for age and gender factors in Figure 4.
Table 3 shows the risk factors associated with the development of HCC in all patients (n = 426). Univariate analysis identified DAAs treatment, their age, cirrhosis (compensate/decompensate), nonspecific liver nodules, Child-Pugh (A/B/C), FIB-4 index, ALB, TBIL, PT, PLT and LSM as factors significantly associated with HCC. According to the multivariate analysis, age [hazard ratio (HR) = 1.089; 95%CI, 1.033-1.147; P = 0.002] and LSM (HR = 1.043; 95%CI, 1.022-1.065; P = 0.000) were independent factors significantly associated with HCC. The optimal cut-off value of age was 0.249, and the value was 58 years. And for LSM, the optimal cut-off value was 0.466, and the value was 27.85 kPa.
| Variables | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| DAA treated | 0.478 | 0.245-0.933 | 0.03 | |||
| Ages (yr) | 1.052 | 1.019-1.087 | 0.002 | 1.089 | 1.033-1.147 | 0.002 |
| Gender (M/F) | 1.297 | 0.750-2.242 | 0.352 | |||
| Cirrhosis (compensate/de) | 2.312 | 1.271-4.207 | 0.006 | |||
| nonspecific nodules (Y/N) | 3.112 | 1.734-5.586 | 0 | |||
| Child-Pugh (A/B/C) | 2.184 | 1.416-3.367 | 0 | |||
| Fatty liver | 0.686 | 0.271-1.738 | 0.427 | |||
| FIB-4 | 1.063 | 1.026-1.101 | 0.001 | |||
| AFP (ng/mL) | 0.994 | 0.981-1.008 | 0.419 | |||
| AST (U/L) | 1.005 | 0.999-1.010 | 0.126 | |||
| ALB (g/L) | 0.922 | 0.885-0.961 | 0 | |||
| TIBL (μmol/L) | 1.015 | 1.007-1.022 | 0 | |||
| PT | 1.23 | 1.083-1.398 | 0.001 | |||
| PLT | 0.992 | 0.986-0.997 | 0.005 | |||
| LSM | 1.038 | 1.018-1.058 | 0 | 1.043 | 1.022-1.065 | 0 |
A model-based study conducted in 2015 found that about 9.8 million people in China are chronic HCV infection, ranking first in the world[20]. HCV infection is one of the major risk factors for HCC occurrence, and data analysis in 2018 showed that 21% of new HCC cases and deaths were attributed to HCV infection[21]. The relative risk of HCV-infected patients developing HCC is 15-20 times larger than that of uninfected persons[22,23]. The incidence of HCV-associated HCC is mostly based on cirrhosis, and the annual incidence of HCC in non-sclerotic patients (pre-sclerotic) is only 0.68%[24], while the annual average incidence of HCC in patients with HCV-associated cirrhosis is 1%-4%, and even 7% in the Asia-Pacific region[25]. With the introduction of various DAAs, their superior antiviral efficacy, low adverse reactions, and the increasing availability of drugs driven by our medical insurance policy, the HCV antiviral treatment strategy has changed completely, and IFN is no longer the first-line treatment for HCV infection[16]. However, HCV clearance does not mean a decrease in HCV-associated HCC. The current literature mainly studied the influence of DAAs on HCC occurrence in patients with hepatitis C. In this study, we focused on patients with HCV-associated cirrhosis because they are in higher risk of progression to HCC than chronic hepatitis C.
In this study, liver function indicators and LSM became significantly better after DAA treatment in the DAAs treatment group (P < 0.05), indicating that DAAs can effectively improve liver function and alleviate liver fibrosis in patients with HCV-associated cirrhosis. Deterding et al[26], in a single-center study, showed that interferon-free DAAs treatment effectively improved liver function in patients with HCV-associated cirrhosis, and Quaranta et al[27] observed improvement in liver function after HCV eradication in most patients with cirrhosis. Gentile et al[28] and Flisiak et al[29] confirmed this trend by observing similar changes in their study. Chan et al[30] enrolled a total of 70 CHC patients treated with DAAs, and results showed that 34 patients (48.6%) were worthy of significant improvement in LSM value at the end of treatment (relative to the baseline LSM value improvement > 30%). In another study, Curry et al[31] also found that at least 85% of patients with liver cirrhosis had a 40% reduction in LSM after HCV elimination by DAAs treatment, although 10% of patients still had an increase. Hence, DAAs treatment should be used as early as possible in CHC patients. In general, the elimination of HCV by DAAs improved the degree of liver fibrosis in most patients, and even reversed cirrhosis in a few patients[32]. In a 5-years follow-up study of CHC patients, Flisiak et al[33] found that DAAs treatment could alleviate liver inflammation and fibrosis after HCV eradication, and suggested that the improvement in LSM might be related to the reduction of liver inflammation, which is consistent with our results. It is well known that hypersplenism as a complication happens in patients with cirrhosis, especially in decompensated cirrhosis, then the three systems of blood cells will decrease, and PLT and WBC are laboratory parameter closely related to the development of hypersplenism[34]. This study implyed that HCV eradication could alleviate hypersplenism.
The effect of interferon-free DAAs treatment on the occurrence of HCC is controversial, and some recent studies have explored the topic[10,12-15,19,35]. A study[36] from Egypt showed that the incidence of HCC was significantly lower in patients with HCV-associated advanced fibrosis and cirrhosis treated with DAAs than in a historical cohort of untreated patients. A long-term follow-up study from Poland[33] showed that DAAs treatment reduced the risk of HCC, whereas a Spanish study[37] included data from approximately 4000 DAA-treated patients and reported an annual HCC incidence of 0.93% within 18 months of initiation of DAAs treatment. They found that the incidence of HCC in patients with cirrhosis was higher regardless of their response to DAAs. A cohort of studies[38] from France revealed that the apparent increase in HCC incidence observed in patients with cirrhosis treated with DAAs compared with patients who achieved SVR following an IFN therapy could be explained by patient characteristics (age, diabetes, reduced liver function) and lower screening intensity. The results of this study indicating that DAAs treatment seems unable to reduce the risk of HCC in patients with HCV-associated cirrhosis. Furthermore, we calculated the HCC incidence of DAAs treatment group and non-antiviral treatment group at 12 mo, 24 mo and 36 mo. During this period, the incidence of HCC in the DAAs treatment group was higher than that in the non-antiviral treatment group (P < 0.05), which indicated that DAAs treatment may lead to an increased risk of short-term HCC occurrence, and this was consistent with the results of Mettke et al[35]. The reason might be that DAA treatment weakens the body’s ability to immune surveillance and control tumors. Due to the existence of an effective immune system, the tiny primary tumor has been under the strong surveillance of the immune system. However, the rapid eradication of HCV may lead to the sudden weakening or withdrawal of immune surveillance, which is conducive to the proliferation and growth of isolated tumor cells. Serti et al[39] have also reported that DAA therapy can affect the composition of the innate immune system. Furthermore, Faillaci et al[40] showed that DAAs-mediated increase of vascular endothelial growth factor favors HCC recurrence/occurrence in susceptible patients, i.e. those with more severe fibrosis and splanchnic collateralization, who already have abnormal activation in liver tissues of neo-angiogenetic pathways, as shown by increased Angiopoietin-2. However, there was no significant difference in HCC incidence between the two groups at 48 months, which may be due to the fact that DAAs treatment can reduce the long-term incidence of HCC, but it is also likely to be limited by the follow-up period of this study. Therefore, a large sample with long-term follow-up should attract the attention of researchers.
In this study, we found old age and high LSM value were factors of HCC in all patients with HCV-associated cirrhosis, and the results shew that patients with HCV-associated cirrhosis had a higher risk for HCC with age ≥ 58 years and baseline LSM ≥ 27.85 kPa. Research by Asahina et al[41] also showed that elderly people have a higher risk of HCC. The reason may be that the older the patient is, the worse the physical function is, the more the relative underlying diseases are, and of course, the incidence of HCC is also higher. The best cut-off value of age obtained in this study is 58 years old which we should pay more attention to screen HCC and carry out some drug interventions to minimize the occurrence of clinical HCC when older than that. Hepatic decompensation, liver failure and HCC are more likely to occur in patients with HCV-associated cirrhosis with high LSM. Morisco et al[42] also concluded that baseline LSM ≥ 20 kPa identifies HCV cirrhotic subjects at higher risk of liver-related events after SVR. In clinical practice, we usually perform liver function and ultrasound examinations on patients, which suggests that we also should pay more attention to LSM. It is worth mentioning that DAAs treatment showed a statistical difference in univariate analysis, while there was no statistical difference in multivariate analysis. In this study, after adjusting for age and sex, Kaplan-Meier curve and Cox analysis showed no statistical difference in cumulative HCC incidence between DAAs treatment group and non-antiviral treatment group (P =0.107). This result proved that DAAs therapy didn’t reduce the occurrence of HCC in patient with HCV-associated cirrhosis in a median 4 years. So, combining immunopotentiator agents or optimizing better DAAs might be considered.
Despite the important findings of this study, there are also limitations: First, this study was a single-center study. Second, the results of this study only reflect the events during the follow-up period of 1-84 mo, extending the follow-up time may have different results. Third, the non-antiviral treatment group is higher than the average follow-up time DAA treatment group, which may affect the results. Last, the DAA treatment regimen in this study is not uniform, so the influence of DAAs factors on the results cannot be excluded.
Our study shows that DAAs improved liver function, alleviated hepatic fibrosis and hypersplenism in patients with HCV-associated cirrhosis. This study found that DAAs did not reduce the incidence of HCC in HCV-associated cirrhosis compared without antiviral therapy, suggesting that the priority of DAAs for HCV patients in the clinic is reasonable. However, we should explore solutions to optimize DAAs treatment to reduce the occurrence of HCC in HCV-associated cirrhosis patients, and continued careful follow-up is necessary.
Direct-acting antivirals (DAAs) revolutionized the treatment of chronic hepatitis C virus (HCV)-associated disease achieving high rates of sustained virological response (SVR). However, whether DAAs can reduce the occurrence of hepatocellular carcinoma (HCC) in patients with HCV-associated cirrhosis who are at high risk have not been concluded.
The key to the retrospective cohort study is to explore DAA treatment in HCV-associated cirrhosis patients with HCC. Solutions to optimize DAAs treatment are explored to reduce the occurrence of HCC in patients with HCV-associated cirrhosis, and careful follow-up is needed.
To investigate the effect of DAAs on the occurrence of HCC in patients with HCV-associated cirrhosis after achieving SVR.
427 inpatients with HCV-associated cirrhosis were enrolled in Tianjin Second People's Hospital from January 2014 to April 2020. 118 patients weren’t received antiviral treatment with any reasons named non-antiviral treatment group, and 236 patients obtained from the 309 DAAs treatment patients according to the propensity score matching named DAAs treatment group. Demographic information and laboratory data were collected from baseline and the following up. Kaplan-Meier curve and Log-Rank test were used to compare the incidence and cumulative incidence of HCC between the two groups. Cox proportional risk regression was used to re-evaluate the risk factors for HCC.
The DAA treatment group was followed up for 1-84 mo, with a median follow-up of 28 mo, while the non-antiviral treatment group was followed up for 5-84 mo, with a median follow-up of 37 mo. Age > 58 [hazard ratio (HR) = 1.089; 95% confidence interval (CI), 1.033-1.147; P = 0.002] and liver stiffness measurement > 27.85 kPa (HR = 1.043; 95%CI, 1.022-1.065; P = 0.000) were risk factors for HCC in all patients (n = 427), and DAA treatment didn’t show protective efficacy. After adjusting for confounding factors (age and sex), 27 cases of HCC occurred in the new DAA treatment group (236 cases), and there was no significant difference in the total incidence of HCC between the two groups (χ2 = 0.369, P = 0.544). In the new DAA treatment group, HCC incidence was 4.68/100PY (95%CI, 3.09-6.81), while it was 3.00/100PY (95%CI, 1.50-5.37) in the non-antiviral treatment group. The follow-up time of the new DAA treatment group was 1-84 mo (29.33 ± 16.20), the median follow-up time was 27 mo and the time of HCC occurrence in the new DAA treatment group was 5-66 mo. The incidence of HCC at 12, 24, 36 and 48 mo was 3.39%, 6.36%, 8.47% and 10.17% in the new DAA treatment group, and it was 0%, 0%, 3.39% and 9.32% in the non-antiviral treatment group, respectively.
This is a novel assessment that provides theoretical insight into the impact of achieving SVR after DAA on HCC development in patients with HCV-associated cirrhosis. This study found that DAAs did not reduce the incidence of HCC in HCV-associated cirrhosis compared with no antiviral therapy, suggesting that the clinical priority of DAAs for patients with HCV is justified. We should also explore solutions to optimize DAAs therapy to reduce the occurrence of HCC in patients with HCV-associated cirrhosis.
In future study, we should be focused on the research of the multicenter, large data, in order to more accurately assess DAAs influence on HCV-associated liver diseases in patients with HCC, thereby reducing the occurrence of HCC, and it can from common biochemical indicator, liquid biopsy, multiple sets of multi-angle discussion such as HCV-associated liver disease risk factors in patients with HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R, India S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 2. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 3. | Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 5. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 6. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 653] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 7. | Janjua NZ, Chong M, Kuo M, Woods R, Wong J, Yoshida EM, Sherman M, Butt ZA, Samji H, Cook D, Yu A, Alvarez M, Tyndall M, Krajden M. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J Hepatol. 2017;66:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Bruno S, Di Marco V, Iavarone M, Roffi L, Crosignani A, Calvaruso V, Aghemo A, Cabibbo G, Viganò M, Boccaccio V, Craxí A, Colombo M, Maisonneuve P. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | van der Meer AJ, Wedemeyer H, Feld JJ, Hansen BE, Manns MP, Zeuzem S, Janssen HL. Is there sufficient evidence to recommend antiviral therapy in hepatitis C? J Hepatol. 2014;60:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V; Rete Sicilia Selezione Terapia–HCV (RESIST-HCV). Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411-421.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 11. | Sanduzzi-Zamparelli M, Mariño Z, Lens S, Sapena V, Iserte G, Pla A, Granel N, Bartres C, Llarch N, Vilana R, Nuñez I, Darnell A, Belmonte E, García-Criado A, Díaz A, Muñoz-Martinez S, Ayuso C, Bianchi L, Fuster-Anglada C, Rimola J, Forner A, Torres F, Bruix J, Forns X, Reig M. Liver cancer risk after HCV cure in patients with advanced liver disease without non-characterized nodules. J Hepatol. 2022;76:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 13. | Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, Stättermayer AF, Beinhardt S, Graziadei I, Freissmuth C, Maieron A, Gschwantler M, Strasser M, Peck-Radosalvjevic M, Trauner M, Hofer H, Ferenci P. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, Lopes S, Silva M, Andrade P, Morais R, Coelho R, Macedo G. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65:1070-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Hepatology; Chinese Medical Association Chinese Society of Infectious Diseases; Chinese Medical Association. Hepatitis c prevention guide (2019 edition), liver 2019; 1357-1373 + 1386. [DOI] [Full Text] |
| 17. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 736] [Article Influence: 368.0] [Reference Citation Analysis (23)] |
| 18. | European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series(☆). J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 778] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 19. | Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 21. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1496] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 22. | Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: A prospective cohort study in Korea. Cancer. 2018;124:2748-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Harnois DM. Hepatitis C virus infection and the rising incidence of hepatocellular carcinoma. Mayo Clin Proc. 2012;87:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, Taguri M, Tanaka K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | A Special Meeting Review Edition: Advances in the Treatment of Hepatitis C Virus Infection From EASL 2015: The 50th Annual Meeting of the European Association for the Study of the Liver • April 22-26, 2015 • Vienna, AustriaSpecial Reporting on:• Daclatasvir, Sofosbuvir, and Ribavirin Combination for HCV Patients With Advanced Cirrhosis or Posttransplant Recurrence: Phase 3 ALLY-1 Study• Efficacy and Safety of Grazoprevir and Elbasvir in Hepatitis C Genotype 1-Infected Patients With Child-Pugh Class B Cirrhosis (C-SALT Part A)• Ledipasvir/Sofosbuvir With Ribavirin Is Safe and Efficacious in Decompensated and Post Liver Transplantation Patients With HCV Infection: Preliminary Results of the Prospective SOLAR 2 Trial• Retreatment of Patients Who Failed 8 or 12 Weeks of Ledipasvir/Sofosbuvir-Based Regimens With Ledipasvir/Sofosbuvir for 24 Weeks• Sofosbuvir + Peginterferon/Ribavirin for 12 Weeks Vs Sofosbuvir + Ribavirin for 16 or 24 Weeks in Genotype 3 HCV Infected Patients and Treatment-Experienced Cirrhotic Patients With Genotype 2 HCV: The BOSON Study• Safety and Efficacy of the Combination Daclatasvir-Sofosbuvir in HCV Genotype 1-Mono-Infected Patients From the French Observational Cohort ANRS CO22 HEPATHER• C-SWIFT: Grazoprevir/Elbasvir + Sofosbuvir in Cirrhotic and Noncirrhotic, Treatment-Naive Patients With Hepatitis C Virus Genotype 1 Infection for Durations of 4, 6 or 8 Weeks and Genotype 3 Infection for Durations of 8 or 12 WeeksPLUS Meeting Abstract Summaries With Expert Commentary by: Steven L. Flamm, MD Chief, Liver Transplantation ProgramProfessor of Medicine and SurgeryNorthwestern University Feinberg School of MedicineChicago, Illinois. Gastroenterol Hepatol (N Y). 2015;11:1-23. [PubMed] |
| 26. | Deterding K, Höner Zu Siederdissen C, Port K, Solbach P, Sollik L, Kirschner J, Mix C, Cornberg J, Worzala D, Mix H, Manns MP, Cornberg M, Wedemeyer H. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Quaranta MG, Ferrigno L, Tata X, D'Angelo F, Coppola C, Ciancio A, Bruno SR, Loi M, Giorgini A, Margotti M, Cossiga V, Brancaccio G, Dallio M, De Siena M, Cannizzaro M, Cavalletto L, Massari M, Mazzitelli M, De Leo P, Laccabue D, Baiocchi L, Kondili LA. Liver function following hepatitis C virus eradication by direct acting antivirals in patients with liver cirrhosis: data from the PITER cohort. BMC Infect Dis. 2021;21:413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Gentile I, Scotto R, Coppola C, Staiano L, Amoruso DC, De Simone T, Portunato F, De Pascalis S, Martini S, Macera M, Viceconte G, Tosone G, Buonomo AR, Borgia G, Coppola N. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity-LINA cohort). Hepatol Int. 2019;13:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Flisiak R, Janczewska E, Łucejko M, Karpińska E, Zarębska-Michaluk D, Nazzal K, Bolewska B, Białkowska J, Berak H, Fleischer-Stępniewska K, Tomasiewicz K, Karwowska K, Simon K, Piekarska A, Tronina O, Tuchendler E, Garlicki A. Durability of virologic response, risk of de novo hepatocellular carcinoma, liver function and stiffness 2 years after treatment with ombitasvir/paritaprevir/ritonavir±dasabuvir±ribavirin in the AMBER, real-world experience study. J Viral Hepat. 2018;25:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Chan J, Gogela N, Zheng H, Lammert S, Ajayi T, Fricker Z, Kim AY, Robbins GK, Chung RT. Direct-Acting Antiviral Therapy for Chronic HCV Infection Results in Liver Stiffness Regression Over 12 Months Post-treatment. Dig Dis Sci. 2018;63:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton M; ASTRAL-4 Investigators. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 32. | Rockey DC. Fibrosis reversal after hepatitis C virus elimination. Curr Opin Gastroenterol. 2019;35:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Flisiak R, Zarębska-Michaluk D, Janczewska E, Łapiński T, Rogalska M, Karpińska E, Mikuła T, Bolewska B, Białkowska J, Flejscher-Stępniewska K, Tomasiewicz K, Karwowska K, Pazgan-Simon M, Piekarska A, Berak H, Tronina O, Garlicki A, Jaroszewicz J. Five-Year Follow-Up of Cured HCV Patients under Real-World Interferon-Free Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, Mo S, Sypsa V, Guha IN, Kumada T, Niu J, Dalekos G, Yasuda S, Barnes E, Lian J, Suri V, Idilman R, Barclay ST, Dou X, Berg T, Hayes PC, Flaherty JF, Zhou Y, Zhang Z, Buti M, Hutchinson SJ, Guo Y, Calleja JL, Lin L, Zhao L, Chen Y, Janssen HLA, Zhu C, Shi L, Tang X, Gaggar A, Wei L, Jia J, Irving WL, Johnson PJ, Lampertico P, Hou J. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 35. | Mettke F, Schlevogt B, Deterding K, Wranke A, Smith A, Port K, Manns MP, Vogel A, Cornberg M, Wedemeyer H. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol Ther. 2018;47:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Kilany S, Ata L, Gomaa A, Sabry A, Nada A, Tharwa ES, Badra G, Abogabal A, Elwaraky M, Moaz E, Ezzat S, Elsharawy A, Waked I. Decreased Incidence of Hepatocellular Carcinoma after Directly Acting Antiviral Therapy in Patients with Hepatitis C-Related Advanced Fibrosis and Cirrhosis. J Hepatocell Carcinoma. 2021;8:925-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, Gea F, Lens S, García-Samaniego J, Sacristán B, García-Eliz M, Llerena S, Pascasio JM, Turnes J, Torras X, Morillas RM, Llaneras J, Serra MA, Diago M, Rodriguez CF, Ampuero J, Jorquera F, Simon MA, Arenas J, Navascues CA, Bañares R, Muñoz R, Albillos A, Mariño Z; Spanish Group for the Study of the Use of Direct-acting Drugs Hepatitis C Collaborating Group. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017;66:1138-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Roudot-Thoraval F, Audureau E; ANRS CO12 CirVir Group. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology. 2018;155:1436-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 39. | Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149:190-200.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E, Andreani S, Vandelli G, Bernabucci V, Lei B, D'Ambrosio F, Bristot L, Cavalletto L, Chemello L, Sighinolfi P, Manni P, Maiorana A, Caporali C, Bianchini M, Marsico M, Turco L, de Maria N, Del Buono M, Todesca P, di Lena L, Romagnoli D, Magistri P, di Benedetto F, Bruno S, Taliani G, Giannelli G, Martinez-Chantar ML, Villa E. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology. 2018;68:1010-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 41. | Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Enomoto N, Izumi N. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 42. | Morisco F, Federico A, Marignani M, Cannavò M, Pontillo G, Guarino M, Dallio M, Begini P, Benigno RG, Lombardo FL, Stroffolini T. Risk Factors for Liver Decompensation and HCC in HCV-Cirrhotic Patients after DAAs: A Multicenter Prospective Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |