Published online Aug 27, 2023. doi: 10.4254/wjh.v15.i8.985
Peer-review started: May 6, 2023
First decision: June 1, 2023
Revised: June 18, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 27, 2023
Processing time: 107 Days and 17.2 Hours
Recently, a group of hepatologists proposed to rename non-alcoholic fatty liver disease (NAFLD) as metabolic associated fatty liver disease (MAFLD) with modified diagnostic criteria. It is important to note, however, that there are some differences between the diagnostic criteria used for NAFLD and MAFLD. Since the research on MAFLD is just beginning, however, evidence on its incidence and prevalence in the general population and in specific subpopulations remains limited.
To assess epidemiology of fatty liver in new definition and compare MAFLD with NAFLD. Exploring risk factors of MAFLD individuals.
This was a retrospective, cross-sectional study. A total of 85242 adults were selected from the Chinese health management database in 2017–2022. The data of general information, laboratory indicators, lifestyle management and psychological status were obtained. MAFLD was diagnosed as ultrasound diagnosis of fatty liver and at least one between these three conditions: Overweight/obesity, type 2 diabetes mellitus (T2DM) or metabolic dysregulation. Metabolic factors were not considered in NAFLD diagnosis standard. The clinical characteristics of MAFLD and NAFLD were analysed using descriptive statistics. Continuous variables normally distributed were expressed as means ± SD. Categorical variables were expressed as frequencies and proportions. Binary logistic regression was used to determine risk factors of the MAFLD.
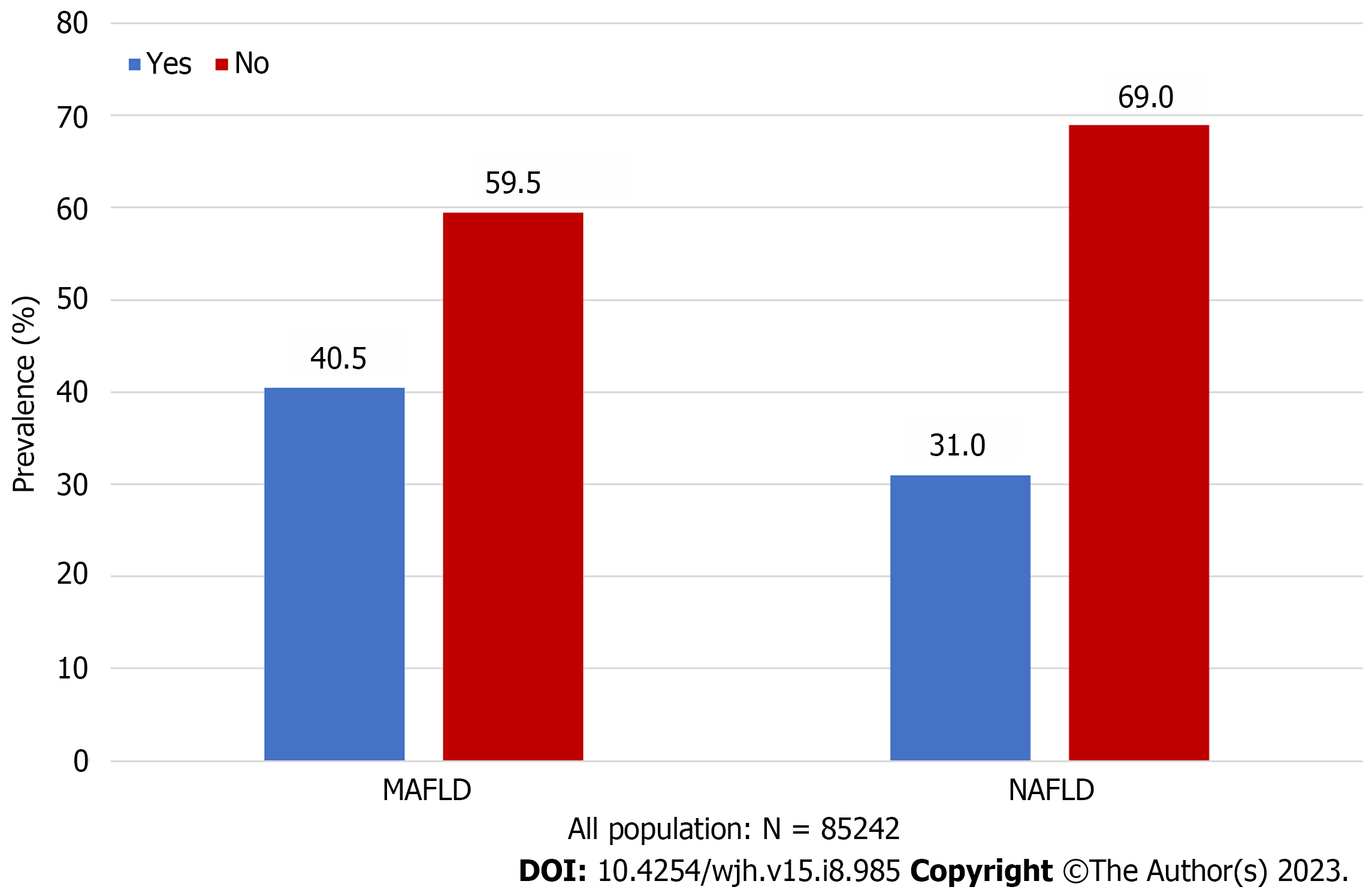
The prevalence of MAFLD and NAFLD was 40.5% and 31.0%, respectively. The MAFLD or NAFLD population is more likely to be older (M: 47.19 ± 10.82 vs 43.43 ± 11.96; N: 47.72 ± 11.17 vs 43.71 ± 11.66), male (M: 77.21% vs 44.43%; N: 67.90% vs 53.12%) and high body mass index (M: 26.79 ± 2.69 vs 22.44 ± 2.48; N: 26.29 ± 2.84 vs 23.29 ± 3.12) than the non-MAFLD or non-MAFLD population. In multivariate analysis, general information (e.g., ≥ 2 metabolic abnormalities OR = 3.38, (95%CI: 2.99-3.81), P < 0.001; diastolic blood pressure OR = 1.01, (95%CI: 1.00–1.01), P = 0.002), laboratory results [e.g.,total bilirubin (TBIL) OR = 0.98, (95%CI: 0.98-0.99), P < 0.001; serum uric acid(SUA) OR = 1.01, (95%CI: 1.01-1.01), P < 0.001], and lifestyle factors [e.g., drink beverage OR = 0.32, (95%CI: 0.17-0.63), P = 0.001] were influence factors for MAFLD. Our study results offer new insight into potential risk factors associated with fatty liver disease, including SUA, TBIL and creatinine, all of which are related to chronic renal disease (CKD).
MAFLD is more prevalent than NAFLD, with two-fifths of individuals meeting the diagnosis criteria. MAFLD and NAFLD populations have different clinical characteristics. CKD may be related with MAFLD.
Core Tip: This study explores the epidemiological characteristics, risk factors and draws reliable conclusions based on the new diagnostic criteria for metabolic associated fatty liver disease, using a large sample of data, and provides evidence for subsequent studies.
- Citation: Huang XJ, Yin M, Zhou BQ, Tan XY, Xia YQ, Qin CX. Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors. World J Hepatol 2023; 15(8): 985-1000
- URL: https://www.wjgnet.com/1948-5182/full/v15/i8/985.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i8.985
Non-alcoholic fatty liver disease (NAFLD) is associated with excessive lipid accumulation in the liver resulting from disordered hepatic lipid metabolism that is stimulated by non-alcohol-related factors[1]. In 2019, the global prevalence of NAFLD was approximately 30.6%[2]. In China, the prevalence is as high as 32.3%[3], making it the number one cause of chronic liver disease and abnormal liver biochemical indicators during routine physical examination. These findings indicate that NAFLD imposes a heavy disease burden on patients and society. As its disease mechanism has become better understood, the limitations of the NAFLD nomenclature have become more apparent. These include: (1) The lack of a uniform standard for calculating alcohol intake, which has led to an underestimation of the role of alcohol consumption in disease pathogenesis; and (2) a failure to recognize the influence of metabolic factors in disease etiology[4]. In 2020, Eslam et al[5] suggested renaming NAFLD as ‘metabolic (dysfunction) associated fatty liver disease’ (MAFLD). A diagnosis of MAFLD includes the presence of hepatic steatosis and one or more of the following features: (1) Overweight based on body mass index (BMI); (2) type 2 diabetes mellitus; or (3) lean or normal weight with evidence of metabolic dysregulation[6]. The new nomenclature aims to reflect the close relationship between fatty liver and overnutrition, sedentary lifestyle, and metabolic conditions such as type 2 diabetes, hypertension, dyslipidemia, and obesity[7]. Adopting a positive diagnosis like MAFLD recognizes the impact of metabolic conditions and fatty liver on the natural history of different liver diseases such as chronic viral hepatitis and alcohol-related liver disease[8].
It is important to note, however, that there are some differences between the diagnostic criteria used for NAFLD and MAFLD. Study indicates that[6] some NAFLD patients are excluded under the proposed MAFLD definition, based on disparate characteristics included in each definition. The rates of diabetes, hypertriglyceridemia, hypertension, and fibrosis risk are significantly higher among MAFLD than NAFLD patients. The proposed MAFLD definition challenges the current understanding of the prevalence and associated factors of fatty liver. Meanwhile, MAFLD is shown to be a better predictor of cardiovascular disease risk among asymptomatic individuals than NAFLD[9]. Since the research on MAFLD is just beginning, however, evidence on its incidence and prevalence in the general population and in specific subpopulations remains limited. The few studies are based on small sample sizes and do not directly compare the characteristics of NAFLD and MAFLD[10]. Thus, our study aims to conduct an updated analysis of the prevalence and factors associated with MAFLD. A more comparative analysis of the clinical characteristics of patients with NAFLD and MAFLD is also performed in order to identify MAFLD-specific risk factors.
A cross-sectional study was conducted by recruiting participants from the health management center of the general tertiary hospital of Southern China between August 1, 2017 and October 31, 2022. Patients who were ≥ 18 years of age, had received a fatty liver color Doppler ultrasonography result, blood lipid examination, exercise and dietary evaluation, and were voluntary participants in this study were included. Patients who lacked imaging or laboratory data for a MAFLD diagnosis, had incomplete Diet and Exercise Health Check survey responses, or were pregnant at the time of examination due to different waist circumference and BMI measurements caused by pregnancy, were excluded from the study. This study was reviewed and approved by the Central South University Ethics Review Board (IRB2022-S217). All patients provided their written informed consent to participate in the study.
Definition of hepatic steatosis: Hepatic steatosis was defined in NHANES III participants using the Hepatic Steatosis Ultrasound Examination. Adult patients received a hepatic ultrasound at a mobile examination center using a Toshiba Sonolayer SSA-90A ultrasound machine (Toshiba America Medical Systems, Inc., Tustin, CA, United States)[11]. Board-certified radiologists used five different parameters to assess hepatic steatosis: parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition. Ultrasonographic assessments were reported as normal, mild, moderate, or severe hepatic steatosis. Abiding by quality control procedures, reliability results (intra-rater and inter-rater) were calculated. The intra-rater and inter-rater reliabilities were 91.3% (kappa 0.77) and 88.7% (kappa 0.70), respectively[12].
Definition of MAFLD: MAFLD was defined[13] as the presence of hepatic steatosis by liver ultrasound plus one or more of the following conditions: (1) overweight/obesity (BMI>23 kg/m2); (2) type 2 diabetes mellitus (T2DM); (3) at least two metabolic risk abnormalities. Metabolic risk abnormalities included: (1) A waist circumference ≥ 90 cm in males or ≥ 80 cm in females; (2) a blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) plasma triglycerides ≥ 150 mg/dL (≥ 1.70 mmol/L) or specific drug treatment; (4) plasma high density lipoprotein cholesterol(HDL-C) < 40 mg/dL (< 1.0 mmol/L) for males and < 50 mg/dL (< 1.3 mmol/L) for females or specific drug treatment; (5) prediabetes [fasting glucose levels of 100–125 mg/dL (5.6–6.9 mmol/L) or HbA1c (5.7%–6.4%) 39–47 mmol/L]; (6) homeostasis model assessment of insulin resistance (HOMA-IR) score ≥ 2.5; (7) a plasma high sensitivity C-reactive protein level > 2 mg/L.
Definition of NAFLD: NAFLD was diagnosed according to the EASL-European Association for the Study of Diabetes-European Association for the Study of Obesity and American Association for the Study of Liver Diseases Clinical Practice Guidelines for the Management of NAFLD: (1) Fatty liver by abdominal ultrasonography; (2) alcohol consumption< £30 g/d for men and <£20 g/d for women; and (3) no competing etiologies for fatty liver or coexisting causes of chronic liver disease[14].
The following demographic variables were obtained from the patient electronic record database: age, gender, BMI, waist circumference, hip circumference, smoking, alcohol consumption, hypertension, diabetes, and actively acquisition of medical knowledge. BMI was calculated as the weight (in kilograms) divided by the square of the height (in meters). Overweight/obesity was defined as BMI > 23 kg/m2. Waist and hip circumferences were determined in centimeters using a tape measure. Blood pressure was recorded in the sitting position using standardized equipment. Hypertension was defined as a systolic blood pressure (SBP) ≥ 130 mmHg and a diastolic blood pressure ≥ 85 mmHg or the use of antihypertensive medications. A diagnosis of diabetes was based on a history of diabetes, use of antidiabetic medications, and/or a fasting plasma glucose ≥ 7.0 mmol/L. Information on lifestyle and psychological factors was acquired from the patient self-report questionnaires.
Laboratory measurements included total bilirubin (TBIL), aspartate aminotransferase (AST), alanine transaminase (ALT), albumin/globulin (A/G), fasting plasma glucose (FPG), glycated haemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), platelet, creatinine, ≥ 2 metabolic abnormalities, blood urea nitrogen (BUN) and serum uric acid (SUA). All biochemical assessments were performed using standard laboratory methods. HDL-C, LDL-C, FPG, BUN, TC and TG were reported in millimoles per liter (mmol/L). The units of TBIL, SUA, total bile acid and creatinine were micromoles per liter( umol/L), HbA1c is expressed in percentage terms, and liver enzymes(AST,ALT) were reported in units per liter (U/L).
Continuous variables normally distributed were expressed as means ± SD. Categorical variables were expressed as frequencies and proportions. The prevalence of MAFLD and NAFLD was determined as the number of subjects with the corresponding conditions divided by the total number of subjects. Univariable and multivariable binary logistic regression analyses were also performed to determine factors associated with MAFLD. The univariate and multivariate odd ratios (OR) were reported along with 95% confidence intervals (CI). All tests were two-tail and results with a P value < 0.05 were considered statistically significant. All analyses were conducted using SPSS 24 version.
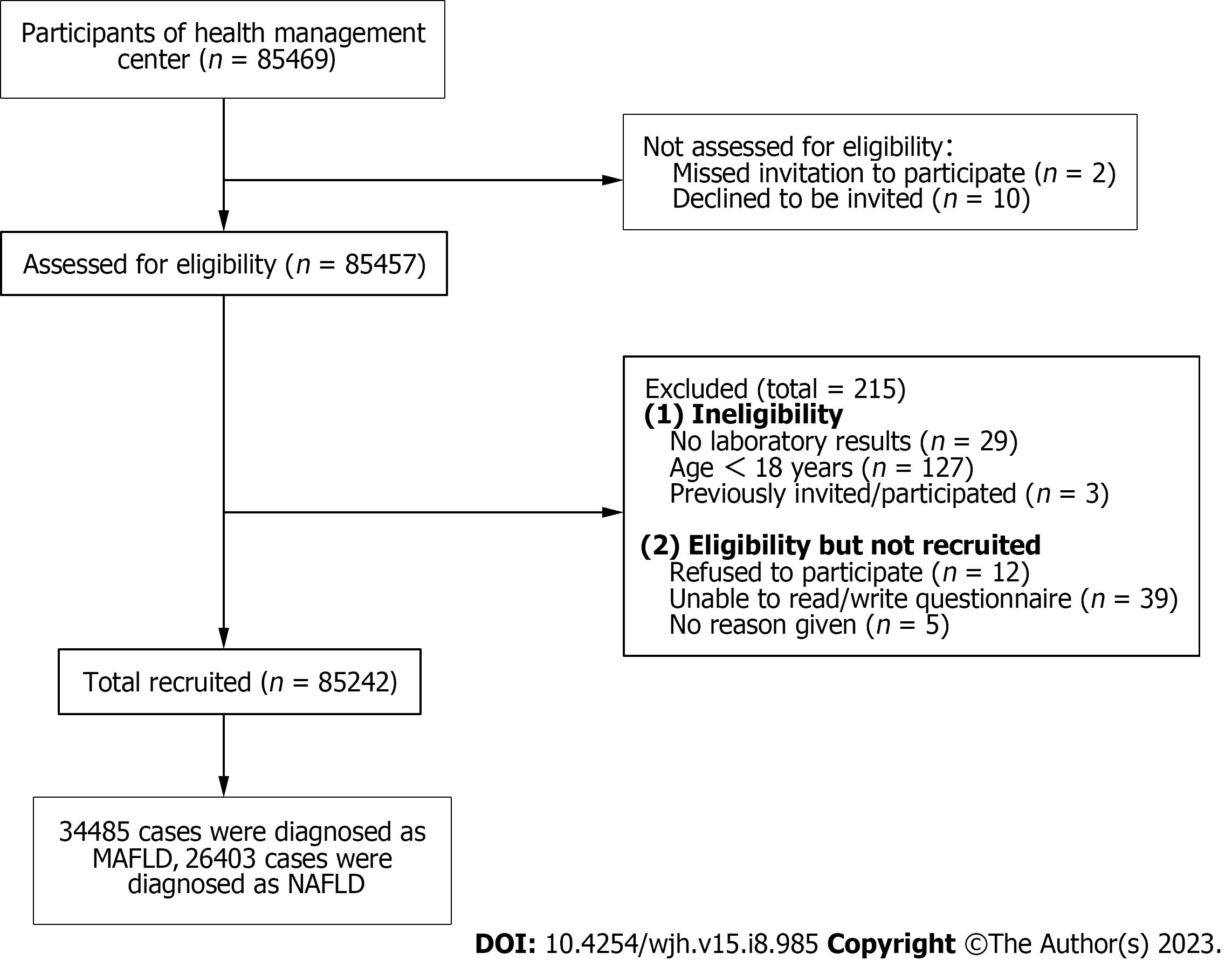
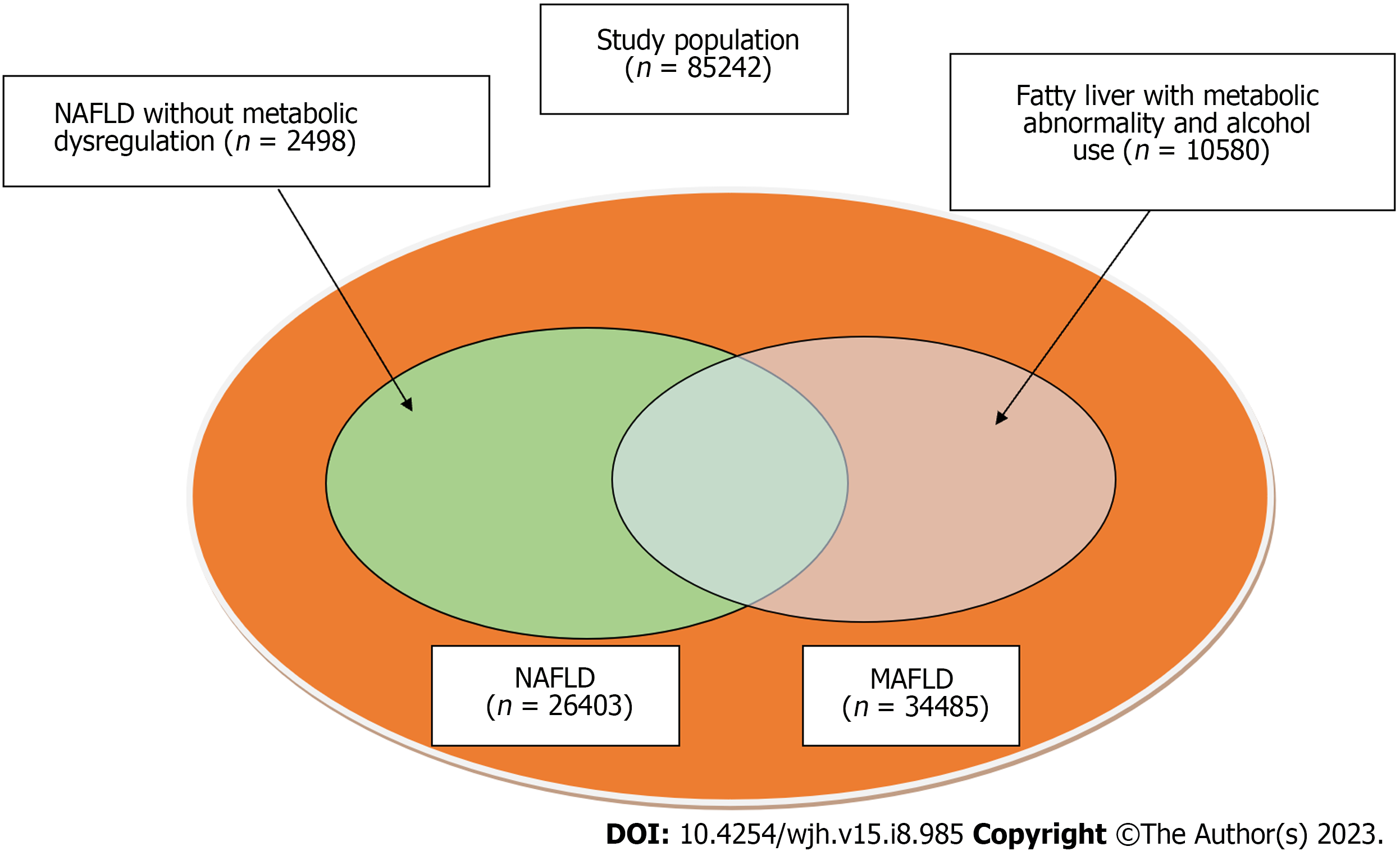
Of the 85242 recruited participants (Figure 1), 26403 (31.0%) had NAFLD [8476 (32.10%) women, median age 47.72 ± 11.17 years], and 34485 (40.5%) met the criteria for MAFLD [7858 (22.79%) women; median age 47.19 ± 10.82 years] (Figure 2, Table 1). Total 23905 (28.0%) participants diagnosed with both MAFLD and NAFLD [7555 (31.60%) women; median age 47.85 ± 11.18 years]. Patients with MAFLD had a higher BMI than those without [26.79 ± 2.69 kg/m2vs 22.44 ± 2.48 kg/m2, respectively]. 5.15% (1775/34485) of patients diagnosed with MAFLD have diabetes, and 79.85% (27536/34485) had two or more metabolic abnormalities. Meanwhile, 2498 patients met the definition of NAFLD but did not meet the MAFLD criteria (Figure 3). The general information , laboratory, lifestyle, and psychological characteristics of the study population are summarized in Table 1. All the patients were ethnic Chinese.
| Characteristics | All | MAFLD | Not MAFLD | NAFLD | Not NAFLD | MAFLD & NAFLD |
| N | 85242 | 34485 (40.5) | 50757 (59.5) | 26403 (31.0) | 58839 (69.0) | 23905 (28.0) |
| General information | ||||||
| Age, yr | 47.19 ± 10.82 | 43.43 ± 11.96 | 47.72 ± 11.17 | 43.71 ± 11.66 | 47.85 ± 11.18 | |
| Sex | ||||||
| Male | 49177 | 26627 (77.21) | 22550 (44.43) | 17927 (67.90) | 31259 (53.12) | 16350 (68.40) |
| Female | 36065 | 7858 (22.79) | 28207 (55.57) | 8476 (32.10) | 27589 (46.88) | 7555 (31.60) |
| BMI, kg/m2 | 26.79 ± 2.69 | 22.44 ± 2.48 | 26.29 ± 2.84 | 23.29 ± 3.12 | 26.64 ± 2.71 | |
| Systolic blood pressure, mmHg | 128.50 ± 15.42 | 118.67 ± 15.13 | 127.40 ± 15.67 | 120.52 ± 15.68 | 128.21 ± 15.69 | |
| Diastolic blood pressure, mmHg | 79.97 ± 11.06 | 72.17 ± 10.42 | 78.49 ± 10.96 | 73.91 ± 11.24 | 79.06 ± 10.96 | |
| Waist circumference, cm | 90.41 ± 7.68 | 76.78 ± 8.01 | 88.57±8.03 | 79.51 ± 10.03 | 89.43 ± 7.74 | |
| Hip circumference, cm | 97.81 ± 5.52 | 91.53 ± 5.03 | 96.92 ± 5.63 | 92.80 ± 5.83 | 97.42 ± 5.55 | |
| Smoke | ||||||
| Never | 57452 | 19713 (57.18) | 37739 (74.36) | 17589 (66.63) | 39863 (67.76) | 15824 (66.21) |
| Always | 20951 | 11443 (33.19) | 9508 (18.73) | 6663 (25.24) | 14288 (24.29) | 6127 (25.64) |
| Smoke in the past | 3106 | 1666 (4.83) | 1440 (2.84) | 1047 (3.97) | 2059 (3.50) | 956 (4.00) |
| Passive exposure to secondhand smoke | 3720 | 1655 (4.80) | 2065 (4.07) | 1098 (4.16) | 2622 (4.46) | 992 (4.15) |
| Alcohol consumption | ||||||
| Yes | 27567 | 14899 (44.10) | 12668 (25.31) | 5546 (21.31) | 22021 (38.09) | 5042 (21.41) |
| No | 56275 | 18882 (55.90) | 37393 (74.69) | 20478 (78.69) | 35797 (61.91) | 18506 (78.59) |
| Diabetes | ||||||
| Yes | 2596 | 1775 (5.15) | 821 (1.62) | 1287 (5.12) | 1309 (2.22) | 1287 (5.38) |
| No | 82645 | 32710 (94.85) | 49935 (98.38) | 25116 (94.88) | 57529 (97.78%) | 22618 (94.61) |
| Inappetence | ||||||
| Never | 57298 | 24017 (69.66) | 33281 (65.57) | 18671 (70.73) | 38627 (65.65) | 16988 (71.08) |
| Occasionally | 24989 | 9446 (27.40) | 15543 (30.62) | 6976 (26.43) | 18013 (30.62) | 6232 (26.08) |
| Often | 2947 | 1016 (2.95) | 1931 (3.80) | 751 (2.84) | 2196 (3.73) | 680 (2.85) |
| Take the initiative to acquire medical knowledge | ||||||
| Yes | 48284 | 19268 (55.88) | 29016 (57.17) | 14989 (56.78) | 33295 (56.59) | 13534 (56.63) |
| No | 36946 | 15210 (44.12) | 21736 (42.83) | 11409 (43.22) | 25537 (43.41) | 10366 (43.37) |
| Laboratory inspection | ||||||
| TG, mmol/L | 2.67 ± 2.40 | 1.32 ± 1.00 | 2.38 ± 2.07 | 1.63 ± 1.67 | 2.47 ± 2.14 | |
| TC, mmol/L | 5.24 ± 1.04 | 4.89 ± 0.92 | 5.19 ± 1.00 | 4.96 ± 0.97 | 5.19 ± 1.01 | |
| LDL-C, mmol/L | 2.88 ± 0.89 | 2.86 ± 0.78 | 2.94 ± 0.87 | 2.84 ± 0.80 | 2.92 ± 0.88 | |
| HDL-C, mmol/L | 1.18 ± 0.24 | 1.42 ± 0.30 | 1.19 ± 0.24 | 1.38 ± 0.31 | 1.18 ± 0.23 | |
| TBIL, μmol/L | 13.38 ± 5.15 | 13.52 ± 5.28 | 13.24 ± 5.15 | 13.56 ± 5.26 | 13.21 ± 5.16 | |
| AST, U/L | 26.83 ± 18.10 | 22.55 ± 17.75 | 25.76 ± 13.67 | 23.74 ± 19.70 | 26.00 ± 14.07 | |
| ALT, U/L | 35.73 ± 27.93 | 21.28 ± 21.60 | 33.86 ± 25.81 | 24.09 ± 24.57 | 34.59 ± 26.44 | |
| A/G | 1.76 ± 0.29 | 1.76 ± 0.31 | 1.75 ± 0.28 | 1.77 ± 0.31 | 1.74 ± 0.28 | |
| FPG, mmol/L | 5.96 ± 1.68 | 5.32 ± 1.77 | 5.88 ± 1.62 | 5.44 ± 1.81 | 5.93 ± 1.65 | |
| HbA1c (%) | 5.91 ± 0.96 | 5.52 ± 0.63 | 5.90 ± 0.95 | 5.59 ± 0.72 | 5.92 ± 0.95 | |
| BUN, mmol/L | 4.97 ± 1.23 | 4.71 ± 1.31 | 4.95 ± 1.23 | 4.75 ± 1.31 | 4.97 ± 1.23 | |
| SUA, μmol/L | 385.23 ± 85.79 | 312.61 ± 79.14 | 372.25 ± 85.03 | 328.43 ± 87.85 | 375.02 ± 85.10 | |
| Platelets (×109/L) | 227.84 ± 54.33 | 225.02 ± 55.02 | 229.55 ± 54.92 | 224.64 ± 54.61 | 229.55 ± 55.11 | |
| Total bile acid, μmol/L | 4.36 ± 5.89 | 3.96 ± 5.25 | 4.24 ± 5.13 | 4.07 ± 5.71 | 4.29 ± 5.25 | |
| Creatinine, μmol/L | 77.32 ± 16.64 | 78.80 ± 43.80 | 75.62 ± 17.34 | 72.46 ± 41.16 | 75.84 ± 17.52 | |
| ≥ 2 metabolic abnormalities | ||||||
| Yes | 38399 | 27536 (79.85) | 10863 (21.40) | 19018 (72.03) | 19381 (32.94) | 19018 (79.56) |
| No | 46843 | 6949 (20.15) | 39894 (78.60) | 7385 (27.97) | 39458 (67.06) | 4887 (20.44) |
| Lifestyle management | ||||||
| Do you often eat late night snacks | ||||||
| Never | 56798 | 23097 (66.99) | 33701 (66.40) | 19221 (72.81) | 37577 (63.87) | 17377(72.70) |
| Occasionally | 25624 | 10204 (29.59) | 15420 (30.38) | 6579 (24.92) | 19045 (32.37) | 5971 (24.98) |
| Often | 2811 | 1179 (3.42) | 1632 (3.22) | 600 (2.27) | 2211 (3.76) | 554 (2.32) |
| Crapulent | ||||||
| Yes | 5750 | 3175 (9.21) | 2575 (5.07) | 1799 (6.81) | 3951 (6.72) | 1706 (7.14) |
| No | 79484 | 31305 (90.79) | 48179 (94.93) | 24600 (93.19) | 54884 (93.28) | 22195 (92.86) |
| Food preference | ||||||
| Light | 35389 | 12278 (35.61) | 23111 (45.54) | 10875 (41.20) | 24514 (41.67) | 9665 (40.44) |
| Briny | 26194 | 12755 (36.99) | 13439 (26.48) | 8694 (32.93) | 17500 (29.74) | 8014 (33.53) |
| Unclear | 23649 | 9446 (27.40) | 14203 (27.98) | 6829 (25.87) | 16820 (28.59) | 6221 (26.03) |
| Drink beverage | ||||||
| Never | 46065 | 18399 (82.24) | 27666 (82.51) | 13920 (81.90) | 32145 (82.27) | 12602 (81.58) |
| Occasionally | 9198 | 3653 (16.33) | 5545 (16.54) | 2830 (16.65) | 6368 (16.30) | 2624 (16.99) |
| Often | 806 | 320(1.43) | 320 (0.95) | 246 (1.45) | 560 (1.43) | 222 (1.44) |
| Exercise frequency | ||||||
| 1-2 times/wk | 21380 | 8441 (39.52) | 12939 (41.37) | 6477 (39.69) | 14903 (41.04) | 5820 (39.48) |
| 3-5 times/wk | 21162 | 8672 (40.60) | 12490 (39.94) | 6503 (39.85) | 14659 (40.36) | 5887 (39.93) |
| > 5 times/wk | 10093 | 4247 (19.88) | 5846 (18.69) | 3338 (20.46) | 6755 (18.60) | 3036 (20.59) |
| Exercise training | ||||||
| Yes | 52829 | 21444 (62.20) | 31385 (61.84) | 16404 (62.15) | 36425 (61.91) | 14825 (62.03) |
| No | 32400 | 13033 (37.80) | 19367 (38.16) | 9991 (37.85) | 22409 (38.09) | 9073 (37.97) |
| Exercise duration | ||||||
| < 30 min | 12701 | 4950 (23.17) | 7751 (24.78) | 4085 (25.03) | 8616 (23.72) | 3662 (24.84) |
| 30-60 min | 30669 | 12575 (58.87) | 18094 (57.85) | 9475 (58.06) | 21194 (58.36) | 8568 (58.12) |
| > 60 min | 9266 | 3836 (17.96) | 5430 (17.36) | 2758 (16.90) | 6508 (17.92) | 2513 (17.05) |
| Labour intensity | ||||||
| Light physical labor | 77907 | 31651 (91.78) | 46256 (91.13) | 24186 (91.60) | 53721 (91.30) | 21844 (91.38) |
| Moderate physical labor | 6282 | 2463 (7.14) | 3819 (7.52) | 1940 (7.35) | 4342 (7.38) | 1808 (7.56) |
| Heavy physical labor | 1053 | 371 (1.08) | 682 (1.34) | 277 (1.05) | 776 (1.32) | 253 (1.06) |
| Psychological states | ||||||
| Irritability | ||||||
| Never | 43964 | 18836 (54.63) | 25128 (49.51) | 14745 (55.85) | 29219 (49.67) | 13428 (56.18) |
| Occasionally | 35294 | 13631 (39.53) | 21663 (42.68) | 10152 (38.46) | 25142 (42.74) | 9103 (38.09) |
| Often | 5973 | 2012 (5.84) | 3961 (7.80) | 1502 (5.69) | 4471 (7.60) | 1370 (5.73) |
| Tense and unrelaxed | ||||||
| Never | 54907 | 22753 (65.99) | 31154 (61.38) | 17813 (67.47) | 36094 (61.35) | 16156 (67.59) |
| Occasionally | 26438 | 10081 (29.24) | 16357 (32.23) | 7379 (27.95%) | 19059 (32.39) | 6638 (27.77%) |
| Often | 4890 | 1647 (4.78) | 3243 (6.39) | 1208 (4.58) | 3682 (6.26) | 1108 (4.64) |
| Anxious | ||||||
| Never | 55837 | 23594 (68.43) | 32243 (63.53) | 18363 (69.56) | 37474 (63.69) | 16670 (69.75) |
| Occasionally | 25399 | 9578 (27.78) | 15821 (31.17) | 7059 (26.74) | 18340 (31.17) | 6337 (26.51) |
| Often | 3999 | 1307 (3.79) | 2692 (5.30) | 977 (3.70) | 3022 (5.14) | 894 (3.74) |
| Depress | ||||||
| Never | 59871 | 25192 (73.06) | 34679 (68.32) | 19610 (74.28) | 40261 (68.43) | 17811 (74.52) |
| Occasionally | 22155 | 8306 (24.09) | 13849 (27.29) | 6040 (22.88) | 16115 (27.39) | 5410 (22.63) |
| Often | 3210 | 982 (2.85) | 2228 (4.39) | 750 (2.84) | 2460 (4.18) | 681 (2.85) |
| Sleep | ||||||
| Well | 33017 | 14188 (41.15) | 18829 (37.10) | 11027 (41.77) | 21990 (37.38) | 10043 (42.02) |
| Moderate | 43242 | 16974 (49.23) | 26268 (51.76) | 12894 (48.84) | 30348 (51.58) | 11627 (48.65) |
| Bad | 8974 | 3318 (9.62) | 5656 (11.14) | 2478 (9.39) | 6496 (11.04) | 2231 (9.33) |
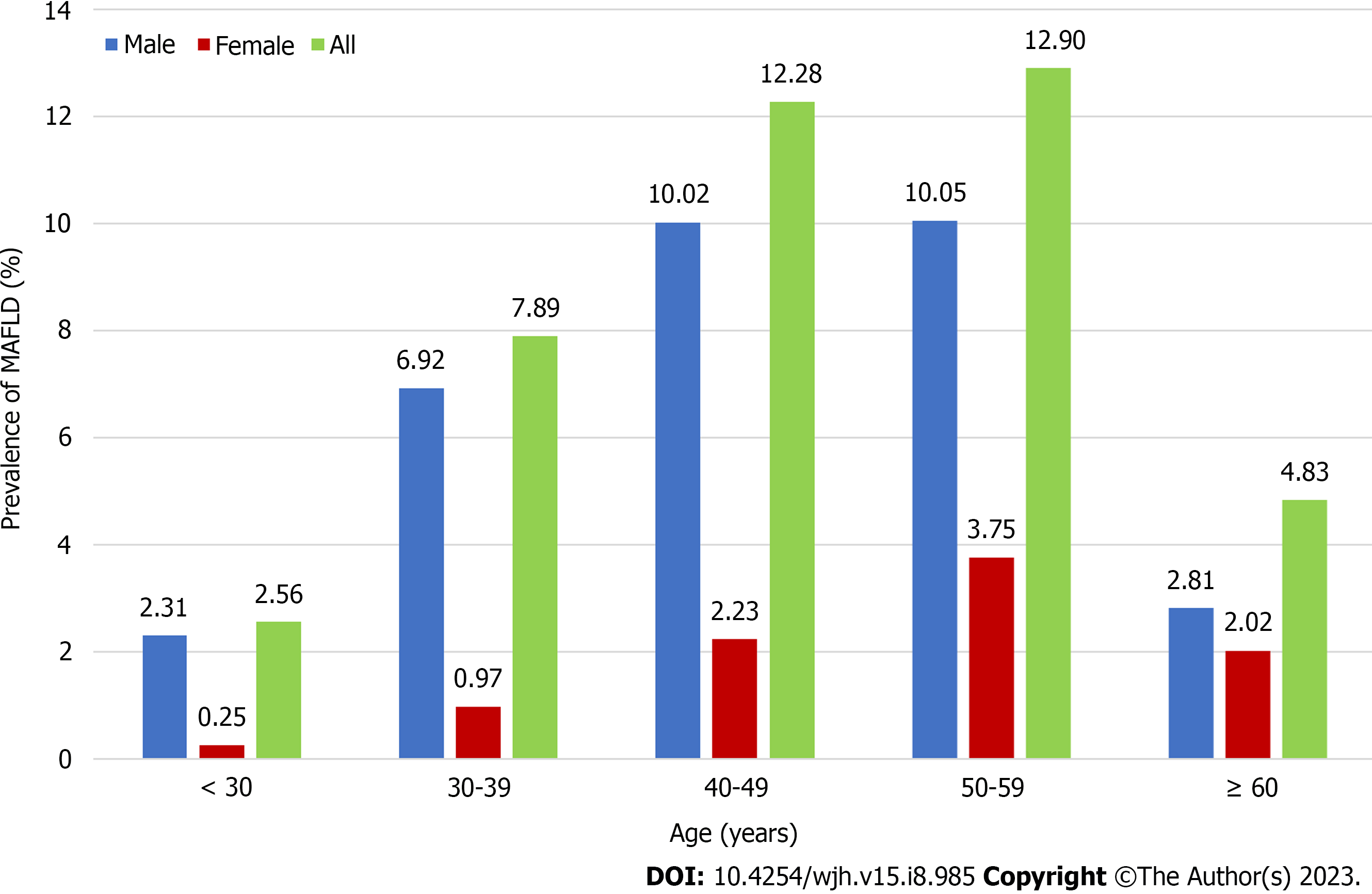
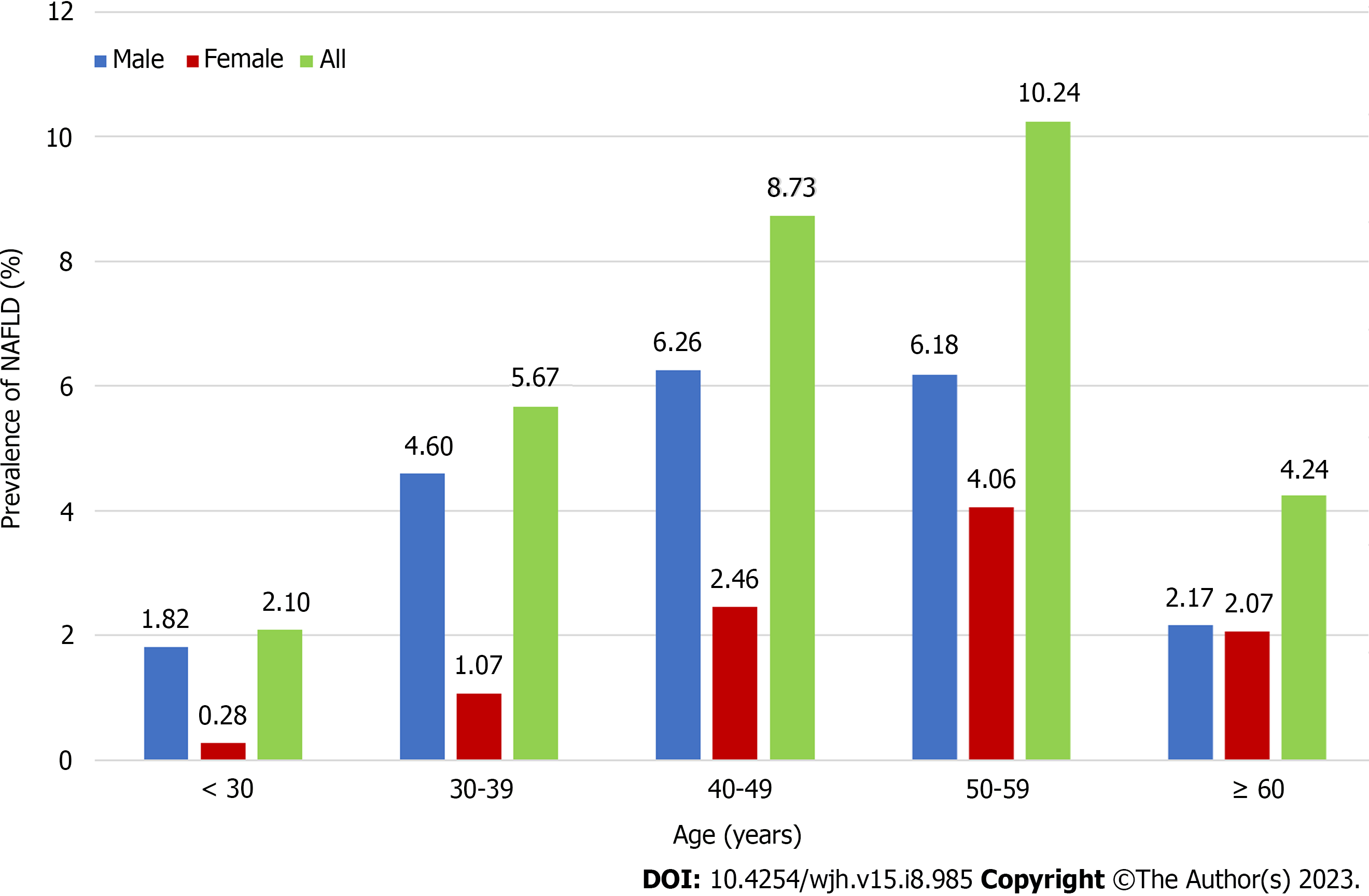
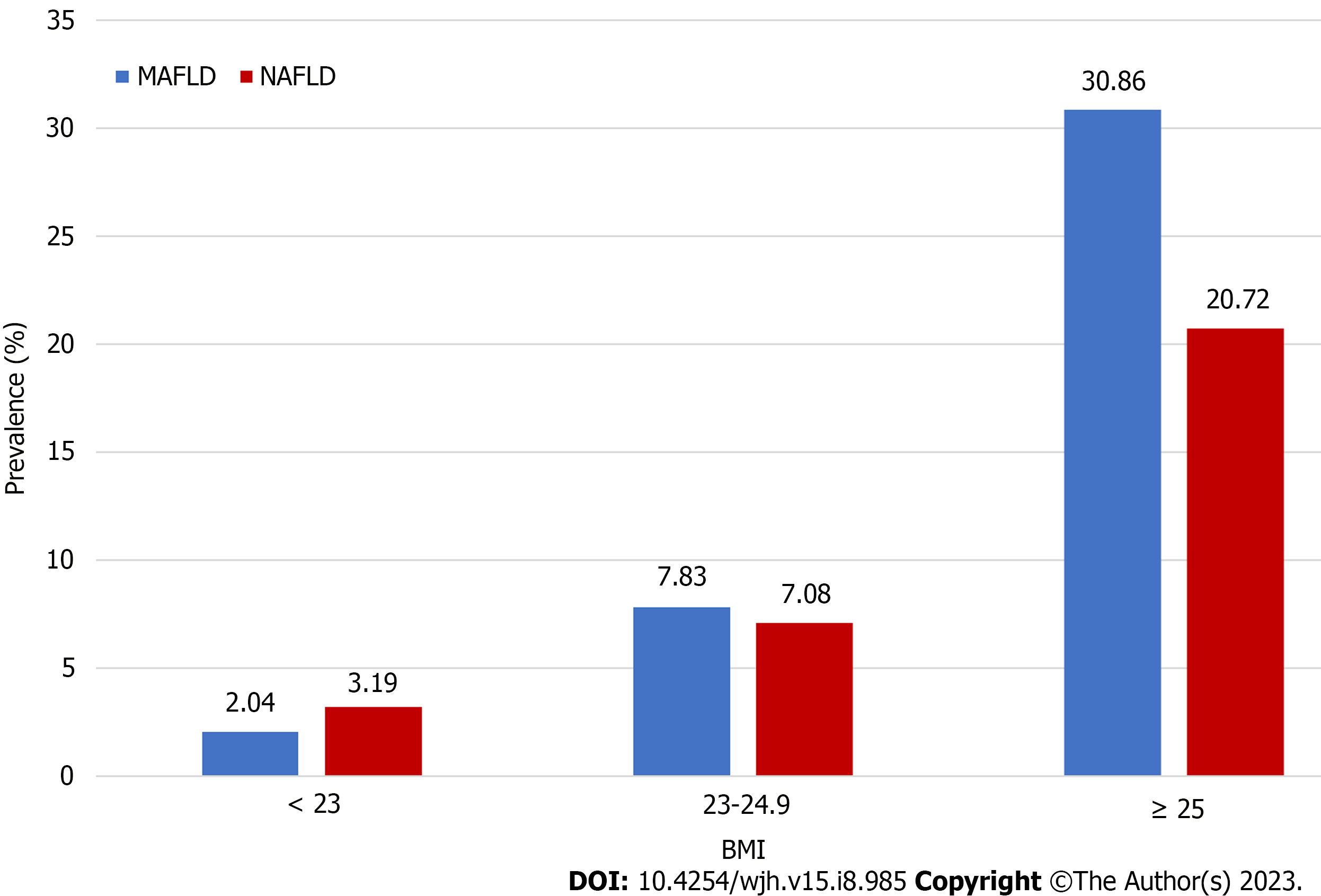
The prevalence of MAFLD was lower among individuals < 30 years of age (approximately 2.56%) and highest among those 50–59 years of age (Figure 4). Disease prevalence was significantly higher among men than women. Changes in age-related prevalence were similar for patients with NAFLD and MAFLD, however, there was a lower overall prevalence of NAFLD than MAFLD (Figure 5). The prevalence of both MAFLD and NAFLD increased with BMI and for patients with a BMI ≥ 25, the risk of NAFLD and MAFLD increased dramatically (Figure 6).
In univariate analysis, male sex, older age, higher BMI, higher diastolic blood pressure, higher waist circumference, lower hip circumference, and alcohol consumption, ≥ 2 metabolic abnormalities, medically knowledgeable, TG, HDL-C, TBIL, AST, ALT, A/G, glycated hemoglobin (HbA1c), SUA, platelet, creatinine, drink beverage, exercise frequency, exercise duration and physical labor intensity were associated with MAFLD. In contrast, systolic blood pressure, smoking, diabetes, TC, LDL-C, blood urea nitrogen (BUN), FPG, total bile acid, inappetence, night snacks, crapulent, food preferences, and psychological characteristics were not significantly associated with this disease (P > 0.05). In multivariate analysis, female sex (OR = 0.67, 95%CI: 0.57–0.80, P = 0.001), older age (OR = 1.01, 95%CI: 1.00–1.02, P = 0.001), higher BMI (OR = 1.45, 95%CI: 1.40–1.51, P < 0.001), diastolic blood pressure (OR = 1.01, 95%CI: 1.00–1.01, P = 0.002), waist circumference (OR = 1.12, 95%CI: 1.11–1.14, P < 0.001), hip circumference (OR = 0.95, 95%CI: 0.93–0.96, P < 0.001), metabolic abnormalities(OR = 3.38, 95%CI: 2.99–3.81, P < 0.001), actively acquire medical knowledge (OR = 1.14, 95%CI: 1.03–1.27, P = 0.014), TG (OR = 1.33, 95%CI: 1.27–1.40, P < 0.001), HDL-C (OR = 0.58, 95%CI: 0.47–0.71, P < 0.001), TBIL(OR = 0.98, 95%CI: 0.98–0.99, P < 0.001), AST OR = 1.01, 95%CI: 1.01–1.01, P < 0.001), ALT (OR = 1.02, 95%CI: 1.02–1.02, P < 0.001), HbA1c (OR = 1.52, 95%CI: 1.47–1.57, P < 0.001), higher SUA level (OR = 1.01, 95%CI: 1.01–1.01, P < 0.001), platelets (OR = 1.00, 95%CI: 1.00–1.00, P < 0.001), creatinine (OR = 0.99, 95%CI: 0.99–0.99, P < 0.001), drink beverages (OR = 0.32, 95%CI: 0.17–0.63, P = 0.001), exercise frequency (OR = 0.82, 95%CI: 0.71–0.95, P = 0.009), exercise duration (OR = 1.24, 95%CI: 1.04-1.47, P = 0.015), and labour intensity (OR = 0.78, 95%CI: 0.65–0.95, P = 0.013) remained as independent variables associated with MAFLD (Table 2).
| Variable | Category | Univariate analysis | Multivariable analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | ||
| General information | |||||
| Sex | Male(ref) | ||||
| Female | 0.69 (0.58-0.84) | < 0.001 | 0.67 (0.57-0.80) | 0.001 | |
| Age | 1.01 (1.01-1.02) | 0.001 | 1.01 (1.00–1.02) | 0.001 | |
| BMI, kg/m2 | 1.46 (1.41-1.52) | < 0.001 | 1.45 (1.40-1.51) | < 0.001 | |
| Systolic blood pressure, mmHg | 1.00 (0.99-1.00) | 0.360 | None | ||
| Diastolic blood pressure, mmHg | 1.01 (1.00-1.02) | 0.007 | 1.01 (1.00–1.01) | 0.002 | |
| Waist circumference, cm | 1.12 (1.11-1.14) | < 0.001 | 1.12 (1.11-1.14) | < 0.001 | |
| Hip circumference, cm | 0.95 (0.93-0.96) | < 0.001 | 0.95 (0.93-0.96) | < 0.001 | |
| Smoke | Never (ref) | 0.409 | None | ||
| Always | 1.18 (0.90-1.56) | 0.236 | None | ||
| Smoke in the past | 1.07 (0.80-1.43) | 0.667 | None | ||
| Passive exposure to secondhand smoke | 1.15 (0.80-1.65) | 0.464 | None | ||
| Alcohol consumption | No(ref) | ||||
| Yes | 0.89 (0.78-1.01) | 0.061 | 1.11 (0.99-1.26) | 0.082 | |
| Diabetes | No(ref) | ||||
| Yes | 1.16 (0.91-1.49) | 0.231 | None | ||
| ≥ 2 metabolic abnormalities | No(ref) | ||||
| Yes | 3.38 (3.00-3.82) | < 0.001 | 3.38 (2.99-3.81) | < 0.001 | |
| Take the initiative to acquire medical knowledge | No(ref) | ||||
| Yes | 1.14 (1.02-1.26) | 0.017 | 1.14 (1.03-1.27) | 0.014 | |
| Laboratory inspection | |||||
| TG, mmol/L | 1.31 (1.15-1.49) | < 0.001 | 1.33 (1.27-1.40) | < 0.001 | |
| TC, mmol/L | 1.09 (0.81-1.45) | 0.578 | None | ||
| LDL- C, mmol/L | 1.00 (0.74-1.35) | 0.997 | None | ||
| HDL-C, mmol/L | 0.50 (0.33-0.73) | < 0.001 | 0.58 (0.47-0.71) | < 0.001 | |
| TBIL, μmol/L | 1.01 (1.00-1.02) | 0.044 | 0.98 (0.98-0.99) | < 0.001 | |
| AST, U/L | 0.98 (0.97-0.98) | < 0.001 | 1.01 (1.01-1.01) | < 0.001 | |
| ALT, U/L | 1.02 (1.02-1.03) | < 0.001 | 1.02 (1.02-1.02) | < 0.001 | |
| A/G | 1.85 (1.53-2.25) | < 0.001 | 0.99 (0.92-1.08) | 0.884 | |
| BUN, mmol/L | 0.98 (0.94-1.02) | 0.388 | None | ||
| FPG, mmol/L | 0.97 (0.91-1.04) | 0.426 | None | ||
| HbA1c (%) | 1.35 (1.20-1.52) | < 0.001 | 1.52 (1.47-1.57) | < 0.001 | |
| Total bile acid, μmol/L | 1.00 (1.00-1.01) | 0.395 | None | ||
| SUA, μmol/L | 1.00 (1.00-1.01) | < 0.001 | 1.01 (1.01-1.01) | < 0.001 | |
| Platelets (×109/L) | 1.00 (1.00-1.00) | < 0.001 | 1.00 (1.00-1.00) | < 0.001 | |
| Creatinine, μmol/L | 0.99 (0.99-1.00) | < 0.001 | 0.99 (0.99-0.99) | < 0.001 | |
| Lifestyle management | |||||
| Inappetence | Never(ref) | 0.597 | None | ||
| Occasionally | 1.13 (0.84-1.52) | 0.409 | None | ||
| Often | 1.16 (0.86-1.57) | 0.322 | None | ||
| Do you often eat late night snacks | Never(ref) | 0.529 | None | ||
| Occasionally | 1.33 (0.78-2.28) | 0.288 | |||
| Often | 1.37 (0.79-2.35) | 0.260 | |||
| Crapulent | No(ref) | ||||
| Yes | 0.93 (0.73-1.20) | 0.586 | None | ||
| Food preference | Light (ref) | 0.868 | None | ||
| Briny | 0.97 (0.85-1.10) | 0.617 | |||
| Unclear | 0.99 (0.85-1.14) | 0.874 | |||
| Drink beverage | Never (ref) | 0.015 | 0.004 | ||
| Occasionally | 2.66 (1.37-5.18) | 0.004 | 1.01 (0.85-1.20) | 0.917 | |
| Often | 2.69 (1.36-5.32) | 0.004 | 0.32 (0.17-0.63) | 0.001 | |
| Exercise frequency | 1-2 times/wk (ref) | 0.048 | 0.025 | ||
| 3-5 times/wk | 1.20 (1.03-1.40) | 0.017 | 0.95 (0.84-1.08) | 0.429 | |
| > 5 times/wk | 1.14 (1.00-1.30) | 0.049 | 0.82 (0.71-0.95) | 0.009 | |
| Exercise training | 0.39 (0.04-3.58) | 0.404 | None | ||
| Exercise duration | < 30 min (ref) | 0.049 | 0.045 | ||
| 30-60 min | 0.81 (0.68-0.96) | 0.017 | 1.07 (0.94-1.22) | 0.283 | |
| > 60 min | 0.87 (0.76-1.00) | 0.045 | 1.24 (1.04-1.47) | 0.015 | |
| Labor intensity | Light physical labor (ref) | 0.049 | 0.026 | ||
| Moderate physical labor | 0.80 (0.66-0.97) | 0.024 | 0.78 (0.65-0.95) | 0.013 | |
| Heavy physical labor | 0.79 (0.51-1.21) | 0.273 | 0.77 (0.50-1.18) | 0.226 | |
| Psychological states | |||||
| Irritability | Never (ref) | 0.637 | None | ||
| Occasionally | 1.01 (0.89-1.15) | 0.851 | |||
| Often | 1.14 (0.87-1.50) | 0.346 | |||
| Tense and unrelaxed | Never (ref) | 0.806 | None | ||
| Occasionally | 0.95 (0.820-1.11) | 0.351 | |||
| Often | 0.96 (0.69-1.35) | 0.828 | |||
| Anxious | Never (ref) | 0.076 | 0.091 | ||
| Occasionally | 0.92 (0.78-1.08) | 0.290 | 0.96 (0.85-1.07) | 0.447 | |
| Often | 1.38 (0.91-2.08) | 0.127 | 1.31 (1.00-1.73) | 0.052 | |
| Depress | Never (ref) | 0.211 | None | ||
| Occasionally | 1.09 (0.92-1.29) | 0.320 | |||
| Often | 0.77 (0.49-1.21) | 0.264 | |||
| Sleep | Well (ref) | 0.221 | None | ||
| Moderate | 0.98 (0.82-1.16) | 0.791 | |||
| Bad | 0.89 (0.74-1.07) | 0.225 | |||
This study found that the prevalence of fatty liver disease was higher when the MAFLD definition was used for diagnosis rather than the NAFLD definition (40.5% vs 31.0%, respectively). In addition, a higher number of factors were associated with MAFLD, including general information (e.g., 8 items such as metabolic abnormalities, diastolic blood pressure), laboratory (e.g., 9 items such as TBIL, SUA), and lifestyle (e.g., 4 items such as drink beverage) characteristics. In contrast, psychological factors were not significantly correlated with MAFLD. Among these significant indicators, we have an interesting finding that three indicators are associated with CKD. Participants with CKD may have elevated SUA levels[15], low TBIL levels[16] and abnormal creatinine values, which may suggest that there is an association between CKD and MAFLD, the exact mechanism need to further analysis.
The prevalence of MAFLD in this study was 40.5%. Several studies have assessed the epidemiology of MAFLD, however, the reported prevalence of this condition varies. While the study[17] demonstrated a lower prevalence of MAFLD (25%-37.3%), a meta-analysis of 2667052 individuals estimated that the global prevalence[18] of this disease was 50.7%. A study using 2017-2018 NHANES data[19] indicated that MAFLD prevalence was 39.1%, a finding similar to that reported here. Consistent with the our study, prior reports have also found that MAFLD[20] is more prevalent in males. Reported[21] variations in the prevalence of MAFLD may be the result of ethnic disparities and environment factors. Differences in the methods used to estimate steatosis (liver ultrasound, elastography, diagnostic scores) may account for some of the heterogeneity.
The prevalence of NAFLD (31.0%) was lower than the prevalence of MAFLD in this study. A total of 23905 participants had overlapping diagnostic criteria for NAFLD and MAFLD. While 2498 patients had NAFLD without metabolic dysregulation, 10580 patients had fatty liver with metabolic abnormalities and alcohol use. A recent study by Lee et al[22] identified a similar number of cases using the MAFLD and NAFLD criteria on population-based data (n = 8962813) from National Health and Nutrition Examination surveys (37.3% vs 28.0%, respectively), a result similar to our findings. It is probable that the high MAFLD prevalence in the current study was primarily caused by the high prevalence of overweight and metabolic dysfunction.
Regardless of age, the prevalence of MAFLD and NAFLD was much higher in males than females, a finding consistent with a study by Ito et al[23]. This may be because males are more prone to poor lifestyle habits, such as smoking and alcohol consumption. The current study also found that the peak prevalence of MAFLD occurred earlier among men (40–49 years) than women (50–59 years), a finding reported previously[24]. Women enter menopause and begin to lose estrogen after they are ≥ 50 years of age. Estrogen is thought to suppress visceral fat accumulation and increase subcutaneous fat accumulation. A higher BMI is linked to a higher prevalence of MAFLD and NAFLD. Thus, individuals with high BMI should be appropriately educated about these conditions.
General information: This study found that as hip circumference increase (OR = 0.95, 95CI%: 0.93–0.96), the risk of MAFLD decreases, a finding consistent with Lin et al[25]. Indeed, fat accumulation on the hips may be beneficial to metabolic health and reduce the risk of metabolic-related diseases[26]. The risk of MAFLD was also 1.14 times higher among those who actively acquired medical knowledge than those who did not. This may be because individuals who are willing to actively acquire knowledge are more likely to attend medical check-ups for early detection and diagnosis. Meanwhile, people who aren’t willing to acquire medical knowledge lack an understanding of self-health management and may be less likely to attend medical check-ups. This could cause an illusion of low MAFLD prevalence.
Laboratory indicators: After correcting for sex, age and BMI confounders, multivariate logistic regression analysis found that TG, HDL-C, TBIL, AST, ALT, glycated hemoglobin, SUA, platelets, and creatinine were associated factors for MAFLD. The risk of MAFLD increases by 1.33 times for each unit increase in TG value, which is consistent with the findings of previous studies[27]. Therefore, regular screening of TG levels and attention to dynamic changes in TG should be performed during routine medical examinations to facilitate screening of people at risk of MAFLD. A high HDL-C level indicates that the body is using cholesterol well and is a sign of good health. The OR value of 0.58 in our study, which suggests that elevated HDL-C may be an important protective factor for MAFLD. ALT and AST are indicators of hepatocellular damage, with ALT being the most sensitive. A number of studies have shown that ALT is an independent risk factor for the development of MAFLD in both obese and non-obese people[28]. In this study, ALT and AST were significantly increased in patients with MAFLD, and they were independent risk factors for the development of MAFLD. The increase in free fatty acids in the liver cells of MAFLD patients led to an increased susceptibility of the liver to inflammatory reactions and the production of oxygen free radicals, which led to hepatocyte degeneration and necrosis, resulting in an increase in serum ALT and AST.
Platelets[29] are elevated during inflammation, and previous studies have found a linear correlation between platelet count and the severity of liver fibrosis[30] in individuals with MAFLD. In our study, we also found that platelet count was mild correlated with MAFLD, consistent with the results of Zeng et al[31], indicating that platelet count may be used as a reference indicator of MAFLD development and the resulting liver fibrosis. And our study found that high glycated hemoglobin values were also strongly associated with a high risk of MAFLD (OR = 1.52, 95%CI: 1.47-1.57), suggesting that glycated hemoglobin is an important reference indicator for screening for MAFLD, and there is previous evidence that patients with MAFLD have significantly higher glycated haemoglobin values compared to the healthy population[32].
In addition to above indicators, we have an interesting finding, SUA, TBIL and creatinine have significance in the multifactorial regression analysis of this study. Participants with CKD may have elevated SUA levels, low TBIL levels and abnormal creatinine values, which may suggest that there is an association between CKD and MAFLD, the exact mechanism need to further analysis. Longitudinal studies[33] have also shown an increased incidence of CKD among NAFLD patients. Despite these findings, however, there is little awareness about CKD in NAFLD, and evidence on the relationship between MAFLD and CKD is even rarer. The current study found that SUA was significantly correlated with MAFLD. While the mechanisms remain unclear, there are a few hypotheses. First, SUA may act as an oxidant and elevated levels may increase oxidative stress, thereby promoting the development of MAFLD. Second, SUA[34] induces adipogenesis through the production of endoplasmic reticulum, activating fatty acid synthase and acetyl coenzyme A carboxylase and leading to the accumulation of fat in hepatocytes. Indeed, low TBIL[35] and creatinine levels may be associated with MAFLD risk. The descend creatinine levels in MAFLD are consistent with the findings of Liu et al[36]. The reduction in creatinine associated with MAFLD may be the result of sarcopenia, which is linked to low skeletal muscle mass and reduced function. MAFLD patients maybe follow lower skeletal muscle mass, especially in lean MAFLD patients. There are differing views on the relationship between TBIL and the risk of MAFLD. Our study showed a mild negative correlation. This may be because TBIL activates toll-like receptor 4 signaling and promotes inflammation.
Lifestyle indicators: Previous studies[37] have shown that consuming sugary beverages may increase the risk of MAFLD, while drinking coffee and tea may reduce the risk. The current study found that individuals who regularly consumed beverages were 0.32 times more likely to develop MAFLD than those who never drank beverages. This may be because coffee and tea, which contain biologically active compounds with anti-oxidant and anti-fibrotic potential, were the most consumed beverages in this population[38]. Our study found that the risk of developing MAFLD when exercising > 5/wk was only 0.82 times that of exercising 1-2/wk. Meanwhile, prior studies have indicated that < 2/wk maybe no effect[39]. However, these findings do not necessarily mean that more frequent exercise is beneficial. It is also important to consider frequency in relation to exercise intensity and length. The risk of MAFLD was found to be 1.24 times higher following exercise lasting > 60 min than exercise lasting < 30 min, suggesting that the benefit of exercise doesn’t increase after a certain length, perhaps due to fatigue that reduces long-term adherence. Finally, labor intensity was a protective factor, with moderate labor intensity is 0.78 times risk incidence of MAFLD than light labor intensity. This finding is consistent with a study by Chen et al[40] and suggests that moderate physical labor is beneficial to health.
To our knowledge, this is the first largest sample study to assess the new nomenclature of MAFLD in the Mid-South region of China. The study has some limitations, however. First, lifestyle information was self-reported by the participants, which may cause recall bias. Second, all the participants were recruited from one medical facility so the findings may not be generalizable to the Chinese population. Additional studies are needed to assess the prevalence and features of MAFLD in other regions of the country. Third, this study lacked histological information on steatosis and fibrosis diagnoses. While ultrasound imaging is highly sensitive and specific for liver fibrosis and steatosis, this technique is not the gold standard for diagnosis. In addition, vibration controlled transient elastography (VCTE) also been recommended for a wide range of studies related to NAFLD[41-45], and VCTE has good diagnostic performance in assessing steatosis. However, there are certain shortcomings that limit its use and make it less widespread than ultrasound, such as high dependence on operator experience, limited sampling range, large overlap in liver fibrosis staging data, and inconsistent delineation of Cut off values.
This study found that MAFLD was significantly more prevalent than NAFLD in our study population. In addition to the usual risk factors, our results suggest that CKD may be related with MAFLD. More research is needed to determine the potential mechanisms underlying the occurrence of MAFLD and to develop interventions to prevent and treat this disease.
Metabolic associated fatty liver disease (MAFLD) is renamed from non-alcoholic fatty liver disease (NAFLD), but there are differences in diagnostic criteria. Since the research on MAFLD is just beginning, however, evidence on its incidence and prevalence in the general population and in specific subpopulations remains limited.
MAFLD proposal is not only a change in nomenclature. On one hand, MAFLD includes patients with concomitant liver diseases and secondary causes of fatty liver. On the other hand, patients with hepatic steatosis but not fulfilling the metabolic criteria are not classified as MAFLD. How these criteria affect our understanding of the epidemiology of MAFLD is unclear. The clinical characteristics and risk factors between MAFLD and NAFLD has not been adequately explored. We aimed to further clarify a possible link and difference between the two diagnostic criteria.
We sought to assess the impact of the new definition on the epidemiology of fatty liver disease and compare MAFLD with NAFLD in a general population. Potential risk factors of MAFLD-diagnosed individuals were also explored.
A total of 85242 adults were selected from the Chinese health management database in 2017–2022. Specifically, the participants were divided into MAFLD group, NAFLD group and MAFLD & NAFLD group for analysis and comparison. Several elements were included such as prevalence, disease characteristics, and risk factors.
We found a higher prevalence of MAFLD than NAFLD. There are differences in clinical features between MAFLD, NAFLD and MAFLD & NAFLD. In addition to the common risk factors, we identified CKD may be related with MAFLD.
MAFLD was more prevalent than NAFLD in the study population, with two-fifths of individuals meeting the diagnosis criteria. Compared to NAFLD, MAFLD has its own disease characteristics and risk factors. Intervention program should address the risk factors for MAFLD and regular screening for the disease is recommended.
Some of the risk factors for MAFLD have been initially identified, but cross-sectional studies of causality are weak. In the future, multi-centre, multi-regional longitudinal studies could be conducted to elucidate disease characteristics, disease trajectory and risk factors in depth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu M, China; Pham TTT, Viet Nam S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken). 2018;11:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 2. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 133.7] [Reference Citation Analysis (2)] |
| 3. | Wang Z, Zhao X, Chen S, Wang Y, Cao L, Liao W, Sun Y, Wang X, Zheng Y, Wu S, Wang L. Associations Between Nonalcoholic Fatty Liver Disease and Cancers in a Large Cohort in China. Clin Gastroenterol Hepatol. 2021;19:788-796.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 4. | Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;15:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Eslam M, George J. Reply to: correspondence regarding "A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement": Bringing evidence to the NAFLD-MAFLD debate. J Hepatol. 2020;73:1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020;17:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 7. | Lee HW, Wong GL, Kwok R, Choi KC, Chan CK, Shu SS, Leung JK, Chim AM, Luk AO, Ma RC, Chan HL, Chan JC, Kong AP, Wong VW. Serial Transient Elastography Examinations to Monitor Patients With Type 2 Diabetes: A Prospective Cohort Study. Hepatology. 2020;72:1230-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1502] [Article Influence: 250.3] [Reference Citation Analysis (0)] |
| 9. | Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, Kim SU, Lee JI. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig Dis Sci. 2022;67:4919-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, Leung JK, Chim AM, Kong AP, Lui GC, Chan HL, Chu WC. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin Gastroenterol Hepatol. 2021;19:2161-2171.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 11. | Survey. NHaNE. Third National Health and Nutrition Examination Survey: Hepatic/Gallbladder Ultrasound and Hepatic Steatosis (HGUHS). 2021; Accessed Aug 23, 2021. Available from: https://wwwn.cdc.gov/nchs/Data/Nhanes3/34A/HGUHS.htm. |
| 12. | National Health and Nutrition Examination Survey (NHANES) III, Hepatic Steatosis Ultrasound Images Assessment Procedures Manual 2010. Accessed Aug 23, 2021. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf. |
| 13. | Boccatonda A, Andreetto L, D'Ardes D, Cocco G, Rossi I, Vicari S, Schiavone C, Cipollone F, Guagnano MT. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 14. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 506] [Article Influence: 56.2] [Reference Citation Analysis (2)] |
| 15. | Bartáková V, Kuricová K, Pácal L, Nová Z, Dvořáková V, Švrčková M, Malúšková D, Svobodová I, Řehořová J, Svojanovský J, Olšovský J, Bělobrádková J, Kaňková K. Hyperuricemia contributes to the faster progression of diabetic kidney disease in type 2 diabetes mellitus. J Diabetes Complications. 2016;30:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Wang J, Wang B, Liang M, Wang G, Li J, Zhang Y, Huo Y, Cui Y, Xu X, Qin X. Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin Epidemiol. 2018;10:121-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Li H, Guo M, An Z, Meng J, Jiang J, Song J, Wu W. Prevalence and Risk Factors of Metabolic Associated Fatty Liver Disease in Xinxiang, China. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, Bruno MJ, de Knegt RJ, Cao W, Peppelenbosch MP, Ghanbari M, Li Z, Pan Q. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin Gastroenterol Hepatol. 2022;20:e573-e582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 19. | Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 20. | Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 21. | Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, Tan DJH, Lim WH, Lin SY, Huang D, Chan M, Khoo CM, Chew NWS, Kaewdech A, Chamroonkul N, Dan YY, Noureddin M, Muthiah M, Eslam M, Ng CH. Global Prevalence and Clinical Characteristics of Metabolic-associated Fatty Liver Disease: A Meta-Analysis and Systematic Review of 10 739 607 Individuals. J Clin Endocrinol Metab. 2022;107:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 22. | Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138-2147.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 23. | Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, Maeda M, Thin KN, Tanaka K, Takahashi Y, Itoh Y, Oniki K, Seko Y, Saruwatari J, Kawanaka M, Atsukawa M, Hyogo H, Ono M, Ogawa E, Barnett SD, Stave CD, Cheung RC, Fujishiro M, Eguchi Y, Toyoda H, Nguyen MH. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. 2021;15:366-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 24. | Huang YP, Zhang S, Zhang M, Wang Y, Wang WH, Li J, Li C, Lin JN. Gender-specific prevalence of metabolic-associated fatty liver disease among government employees in Tianjin, China: a cross-sectional study. BMJ Open. 2021;11:e056260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Lin S, Xian Y, Liu Y, Cai W, Song J, Zhang X. Risk factors and community intervention for nonalcoholic fatty liver disease in community residents of Urumqi, China. Medicine (Baltimore). 2018;97:e0021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 27. | Tong C, Li Q, Kong L, Ni X, Halengbieke A, Zhang S, Wu Z, Tao L, Han Y, Zheng D, Guo X, Yang X. Sex-specific metabolic risk factors and their trajectories towards the non-alcoholic fatty liver disease incidence. J Endocrinol Invest. 2022;45:2233-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1070] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 29. | Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, Onder E. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Pitisuttithum P, Chan WK, Piyachaturawat P, Imajo K, Nakajima A, Seki Y, Kasama K, Kakizaki S, Fan JG, Song MJ, Yoon SK, Dan YY, Lesmana L, Ho KY, Goh KL, Wong VWS, Treeprasertsuk S. Predictors of advanced fibrosis in elderly patients with biopsy-confirmed nonalcoholic fatty liver disease: the GOASIA study. BMC Gastroenterol. 2020;20:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Zeng J, Yang RX, Sun C, Pan Q, Zhang RN, Chen GY, Hu Y, Fan JG. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol. 2020;26:1792-1804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Huang H, Wang Q, Shi X, Chen Y, Shen C, Zhang J, Xu C. Association between Monocyte to High-Density Lipoprotein Cholesterol Ratio and Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. Mediators Inflamm. 2021;2021:6642246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, Ryu S, Chang Y, Lazo M, Guallar E, Cho J, Gwak GY. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J Hepatol. 2017;67:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Chao G, Chen L. Study on the independent effect of thyroid hormone based on uric acid level on NAFLD. J Health Popul Nutr. 2021;40:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Liang C, Yu Z, Bai L, Hou W, Tang S, Zhang W, Chen X, Hu Z, Duan Z, Zheng S. Association of Serum Bilirubin With Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2022;13:869579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Liu L, Shi X, Gao J, Xu C, Liu X. Predictive Risk Factors of Nonalcoholic Fatty Liver Disease in a Lean Chinese Population. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Chhimwal J, Patial V, Padwad Y. Beverages and Non-alcoholic fatty liver disease (NAFLD): Think before you drink. Clin Nutr. 2021;40:2508-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Kennedy OJ, Roderick P, Poole R, Parkes J. Coffee, caffeine and non-alcoholic fatty liver disease? Therap Adv Gastroenterol. 2016;9:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Zheng YC, Wen J. Research progress of exercise prescription for non-alcoholic fatty liver disease. Occupation and Health 2020 36: 569-572, 576 Available from: https://docs.qq.com/pdf/DQmZPS3Z3cE1NZmVU. |
| 40. | Chen SJ, Zhang H, Chang XL, Fu JP, Li HY, Yang HY, Guo YH, Wei M, Wang HL, Song JD. Analysis of new risk factors for metabolic syndrome in Han Chinese population in urban health check-ups in northern China. Zhongguo Quanke Yixue Zazhi. 2010;13:980-983,985. [DOI] [Full Text] |
| 41. | Xie R, Liu M. Relationship Between Non-Alcoholic Fatty Liver Disease and Degree of Hepatic Steatosis and Bone Mineral Density. Front Endocrinol (Lausanne). 2022;13:857110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 42. | Blank V, Petroff D, Boehlig A, Heinze A, Karlas T, Berg T, Wiegand J. Clinical implications of hepatic structure and function evaluation based on vibration-controlled transient elastography and liver maximum function capacity test in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2022;34:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Xie R, Zhang Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: Evidence from NHANES and FNDDS. Nutr Metab Cardiovasc Dis. 2023;33:1179-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 44. | Tang M, Liu M, Zhang Y, Xie R. Association of family income to poverty ratio and vibration-controlled transient elastography quantified degree of hepatic steatosis in U.S. adolescents. Front Endocrinol (Lausanne). 2023;14:1160625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 45. | Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, Liu Q, Zhang Y. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. 2022;13:925690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |