Published online Jul 27, 2023. doi: 10.4254/wjh.v15.i7.925
Peer-review started: March 26, 2023
First decision: May 15, 2023
Revised: June 12, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: July 27, 2023
Processing time: 116 Days and 22.3 Hours
Irritable bowel syndrome (IBS) is associated with obesity and metabolic syndro
To systematically review their association according to the Preferred Reporting Items for Systemic Review and Meta-analyses guidelines.
PubMed, EMBASE and Cochrane Database of Systematic Reviews were searched for relevant papers. Manual searches were also performed.
Six studies were included. Both IBS and NAFLD subjects had significantly more metabolic risk factors like hypertension, obesity, dyslipidaemia and diabetes. Our review showed that 23.2% to 29.4% of NAFLD patients had IBS. IBS was signifi
Further prospective studies are warranted to evaluate the relationship and shared pathways between IBS and NAFLD, potentially leading to the development of future therapeutics.
Core Tip: The relationship between irritable bowel syndrome (IBS) and non-alcoholic fatty liver disease (NAFLD) is increasingly recognised but their shared mechanisms remain poorly elucidated. We evaluate the association between IBS and NAFLD and discuss the risk factors and possible common mechanistic pathways including the brain-gut-liver axis, gut dysbiosis and translocation, altered hypothalamic-pituitary-adrenal axis and sleep quality.
- Citation: Ng JJJ, Loo WM, Siah KTH. Associations between irritable bowel syndrome and non-alcoholic fatty liver disease: A systematic review. World J Hepatol 2023; 15(7): 925-938
- URL: https://www.wjgnet.com/1948-5182/full/v15/i7/925.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i7.925
The global surge in obesity has heralded the increasing prevalence of non-alcoholic fatty liver disease (NAFLD), which is the hepatic manifestation of the metabolic syndrome. NAFLD has emerged as the most common chronic liver disease and is increasingly recognised as a leading cause of morbidity and mortality[1]. It affects up to 25%-30% of the general population but is highly prevalent (up to 50%-90%) in patients with obesity and features of the metabolic syndrome[1,2]. Irritable bowel syndrome (IBS) is a disorder of gut-brain interaction, characterised by chronic abdominal pain and altered bowel movements without an organic cause[3]. Given the degree of heterogeneity in criteria used for the diagnosis of IBS, the true global prevalence of IBS remains elusive but is estimated to affect 1 in 10 people globally[4,5]. IBS is a complex condition that is caused by a myriad of factors with interplay of genetics, epigenetics, immune activation, gut dysbiosis, abnormal gut-brain interactions, visceral hypersensitivity, altered gut motility, eating behaviours, psychological stressors, and environmental factors[6,7]. To date, studies have found an interesting relationship between obesity and IBS. As NAFLD is closely linked with obesity, insulin resistance and the metabolic risk factors, emerging evidence has shown a possible correlation between NAFLD and IBS due to purported shared underlying pathophysiological links[8].
IBS is significantly associated with a higher prevalence of the metabolic syndrome and a multitude of studies have determined a link and common pathogenic mechanisms between these two conditions. Obesity and metabolic syndrome are found more frequently in IBS patients compared with controls[9]. A cross-sectional study from Japan showed a positive association between IBS and increased prevalence of metabolic syndrome and triglyceride levels. The odds ratio (OR) (95%CI) in IBS subjects were 2.01 (1.13-3.55) and 1.50 (1.03-2.18) respectively as compared with non-IBS subjects[10]. There is a higher occurrence of pre-diabetes and higher low-density lipoprotein (LDL) levels in patients with IBS[11,12]. In a large population-based cohort study, increased bowel movement frequency was associated with elevated risks of cardiovascular disease, diabetes, heart failure and chronic kidney disease[13]. In IBS subjects, an elevated body mass index (BMI) is associated with significantly faster colonic and rectosigmoid transit and higher bowel frequency[14].
Obesity and the metabolic syndrome are linked with insulin resistance, oxidative stress, chronic low-grade inflammation, abnormal lipid metabolism and gut microbiota alterations, all of which play key roles in the pathogenesis of NAFLD and the more progressive non-alcoholic steatohepatitis (NASH)[15,16]. The gut-liver axis is implicated in the development of NAFLD and similar mechanisms have also been shown to be pivotal to the pathogenesis of IBS[17,18]. Several studies have highlighted the correlation between NAFLD and IBS. Lee et al[19] demonstrated an increased prevalence of elevated alanine aminotransferase and gamma-glutamyl transferase levels and metabolic syndrome in IBS patients[19].
In this systematic review, we aim to evaluate the association between IBS and NAFLD including the common mechanistic pathways and overlapping risk factors.
The Preferred Reporting Items for Systemic Review and Meta-analyses (PRISMA) guidelines were used for the purposes of this systematic review. A two-step approach was adopted to identify studies: (1) A systematic search of electronic databases; and (2) A manual search of direct citations from potentially relevant papers and other peer-reviewed journals.
The following electronic libraries-PubMed, Embase and the Cochrane Database of Systemic Reviews were searched from inception to March 2023 to identify papers studying the associations between IBS and NAFLD. The uses of search strategies were described in Table 1.
| Database | |
| PubMed | (Irritable bowel syndrome[Mesh] OR "Irritable bowel syndrome"[tiab] OR "irritable colon*"[tiab] OR IBS[tiab]) AND (Fatty Liver[Mesh] OR (fatty[tiab] AND (liver*[tiab] OR hepat*[tiab]) OR steatohepat*[tiab] OR NAFL*[tiab] OR NASH*[tiab])) |
| EMBASE | ('irritable colon'/exp OR 'irritable bowel syndrome':ab,ti OR ‘irritable colon’:ab,ti OR ‘IBS’:ab,ti) AND (‘Fatty Liver’/exp OR (fatty:ab,ti AND (liver*:ab,ti OR hepat*:ab,ti) OR steatohepat*:ab,ti OR NAFL*:ab,ti OR NASH*:ab,ti)) |
| CENTRAL | #1 MeSH descriptor: [Irritable Bowel Syndrome] explode all trees |
| #2 ('irritable bowel syndrome' OR ‘irritable colon’ OR ‘IBS’):ti,ab,kw | |
| #3 MeSH descriptor: [Fatty Liver] explode all trees | |
| #4 fatty:ab,ti AND (liver*:ab,ti OR hepat*:ab,ti) OR steatohepat*:ab,ti OR NAFL*:ab,ti OR NASH*:ab,ti | |
| #5 (#1 or #2) and (#3 or #4) |
Manual searches of direct citations from potentially relevant papers and other peer-reviewed journals were also performed to identify additional studies not included in the systemic search. No filters were used to refine search results. No linguistic or geographical restrictions were imposed.
The titles of all papers retrieved by the literature search were screened for relevance and any studies of obvious irrelevance were excluded. All full manuscripts of relevant papers were then retrieved and subsequently screened in its entirety. The entire screening process was done by two independent researchers to identify studies that met the study selection criteria. Consensus was reached regarding any discrepancies that arose without the need for a third independent reviewer.
Studies that met the following inclusion criteria were included: (1) Observational studies that investigated the relevance between IBS and NAFLD that included cross-sectional, case-control or cohort studies; (2) Population studies that showed the relevance between IBS and NAFLD; and (3) Human studies. Due to predictions of limited number of studies regarding the topic of interest, a planned option of expansion of criteria was put in place to include studies published in the Chinese language, of which all of the authors are also proficient in.
The data extraction process was done independently as per the PRISMA checklist. Publication details were first extracted, which included: (1) Author’s name; (2) Year and Country of study; and (3) Methodology (sample characteristics, study design, and modalities used to diagnose IBS and NAFLD). Further data extraction included any risk factors that predisposed patients to the incidence of IBS and NAFLD if present that included risk estimate, including OR with corresponding 95%CIs about the association between IBS and NAFLD.
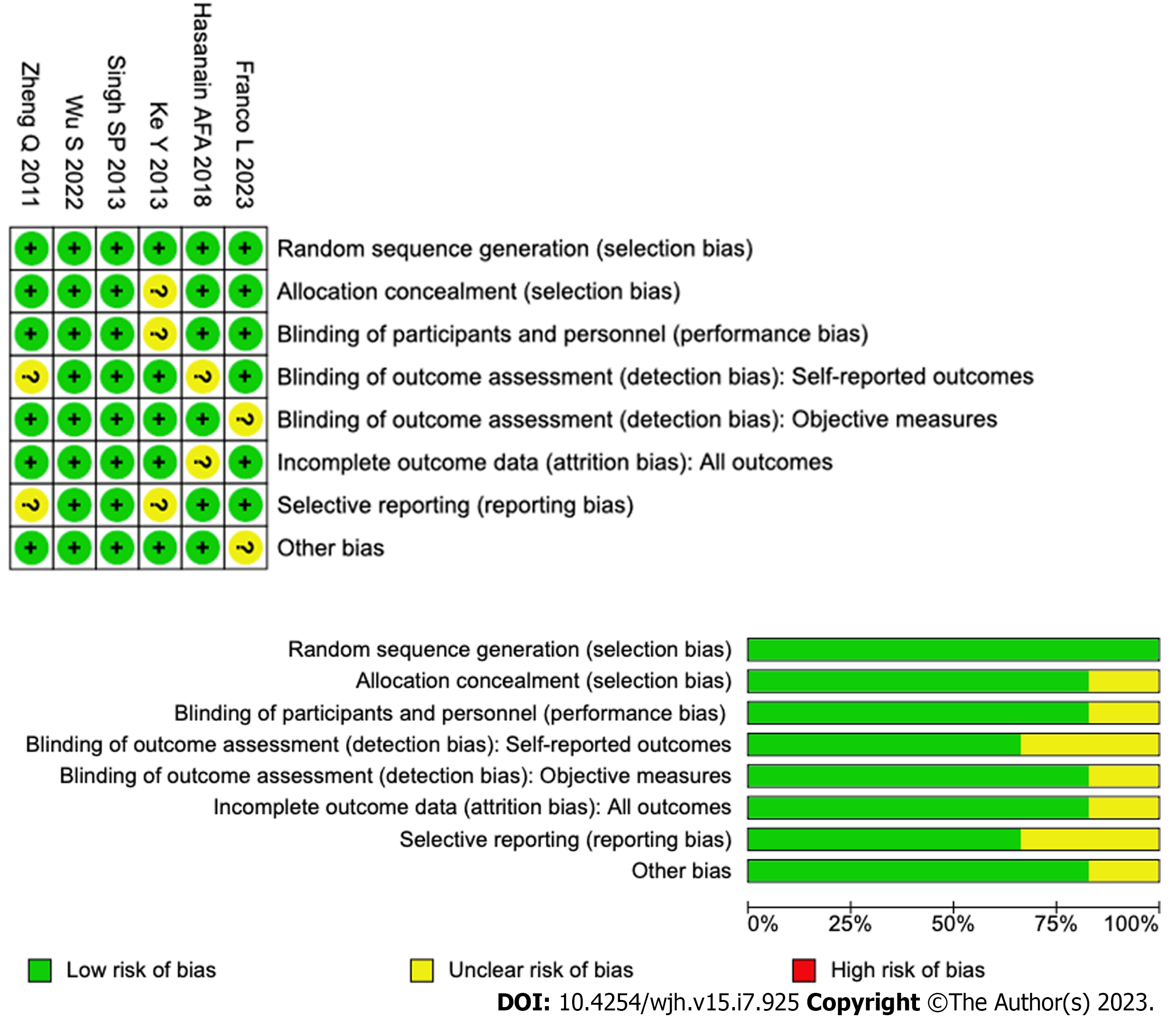
All eligible studies were assessed for risk of bias using Cochrane Risk of Bias Assessment (Figure 1).
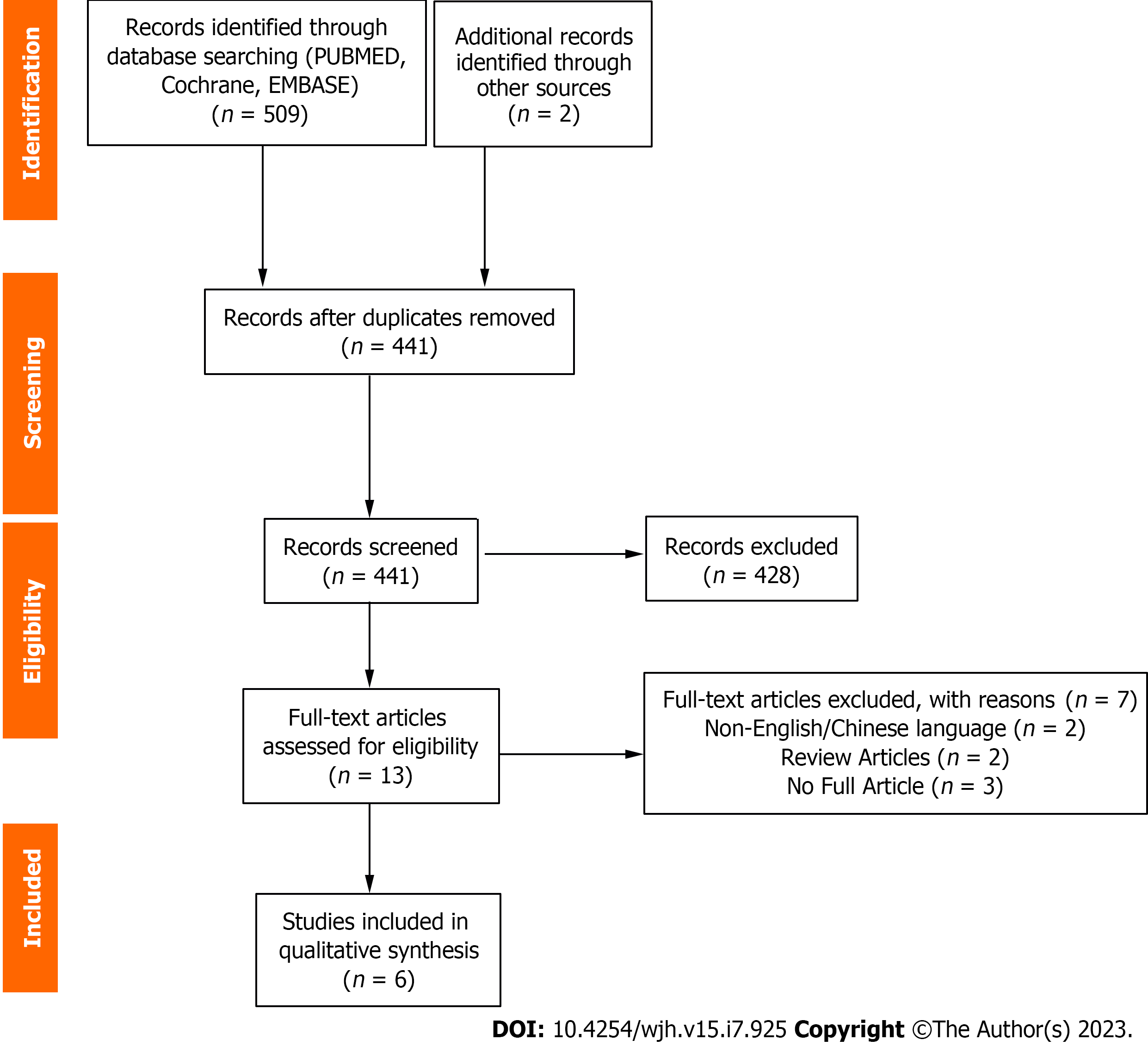
The search from PubMed, Embase and Cochrane identified 509 studies along with 2 studies via manual search. 441 studies were screened for after the removal of 70 duplicates. 428 studies were excluded and 13 studies were retrieved for and screened in full. Among these 13 studies, 7 studies were excluded for reasons that included (1) Review articles (2) No full papers available; and (3) Non-English or non-Chinese language (Figure 2). The final 6 studies were included in this systematic review (Table 2).
| Ref. | Location | Sample characteristics | Study design | IBS diagnosis | NAFLD diagnosis | IBS and NAFLD overlap | Associated risk factors |
| Ke et al[20], 2013 | China | No. of patients: 945. NAFLD population: 470 (226 males, 222 females). Total without NAFLD: 475 (198 males, 234 females). Note: DM was in exclusion criteria | Cross-sectional study | ROME III | Ultrasound; Chinese Society of Hepatology, Chinese Medical Association diagnosis criteria for NAFLD | IBS incidence: 104 (23.2%) of NAFLD patients vs 54 (12.5%) of patients without NAFLD. NAFLD incidence in patients with IBS symptoms: 65.8% of patients with IBS-like symptoms had NAFLD. Higher detection rate of IBS-like symptoms with more severe NAFLD (P < 0.05). Mild NAFLD (Group A): 24 out of 212 (11.3%). Moderate NAFLD (Group B): 52 out of 188 (27.7%). Severe NAFLD (Group C): 28 out of 48 (58.3%) | Risk factors for IBS-like symptoms: BMI (P = 0.049). Triglycerides (P = 0.034). Fatty liver (P = 0.047). Ethnicity-Han, Uygur (P = 0.008) |
| Hasanian et al[21], 2018 | Egypt | 100 consecutive patients diagnosed with IBS (49 males, 51 females): 45% IBS-C 23% IBS-D 32% IBS-M. Further divided into: Mild IBS (n = 42); Moderate IBS (n = 43); Severe IBS (n = 15) | Cross-sectional study | ROME III | Ultrasound | 74% of IBS patients had NAFLD. Moderate/severe NAFLD significantly associated with moderate/severe IBS. 22.4% moderate/severe IBS patients (95%CI: 15.8%-31.5%) vs 4.8% mild IBS patients: (95%CI: 2.7%-7.9%) (P = 0.001) | Predictors of moderate/severe IBS: NAFLD (P = 0.026); Metabolic Syndrome (P = 0.011) |
| Zheng et al[22], 2011 | China | No. of patients: 200 (89 males, 111 females) (1) Both NAFLD and IBS: 25; (2) Either NAFLD or IBS: 36; and (3) Neither IBS nor NAFLD: 139 | Cross-sectional study | ROME III | Ultrasound; NAFLD diagnosis based on Chinese Society of Hepatology, Chinese Medical Association | 25 subjects (12.5%) had both NAFLD and IBS | NAFLD and IBS overlap group compared to group without NAFLD and IBS Overlap had higher: BMI (P = 0.045); TG (P = 0.035); TC (P = 0.038); HDL-C (P = 0.045); LDL-C (P = 0.031); FBG (P = 0.023). NAFLD and IBS overlap group compared to the either NAFLD or IBS group had higher: Hypertension (P = 0.041); Obesity (P = 0.034); Dyslipidemia (P = 0.020); Diabetes (P = 0.037); Digestive system diseases (P = 0.037). GIT sensory threshold: NAFLD and IBS overlap group had lower threshold of the following compared to either NAFLD or IBS group: FSV (P = 0.034); DSV (P = 0.032); MTV (P = 0.035); PSV (P = 0.027) |
| Franco et al[23], 2022 | United States | No. of patients: 130 patients with NAFLD (49 males, 81 females) | Cross-sectional study | ROME IV | ICD-10 Code | 38 (29.2%) patients with NAFLD fulfilled Rome IV IBS criteria | Increased prevalence of depression (18.4% vs 5.4%, P = 0.01) and anxiety (31.6% vs 9.8%, P = 0.002) in NAFLD patients with IBS compared to those without IBS. Independent predictors of IBS in NAFLD: Female gender (OR: 5.69, P = 0.001); Depression (OR: 1.23, P < 0.001); BMI (OR: 0.90, P = 0.02) |
| Wu et al[24], 2022 | United Kingdom | No. of patients: 396838 (189759 males, 207079 females). NAFLD population: 153203 | Prospective cohort study; United Kingdom Biobank | ICD-10 Code | Fatty Liver Index | 7129 cases of incident IBS in NAFLD patients (cumulative incidence rate of 1.49 (95%CI: 1.46–1.53) per 1000 person-years). NAFLD patients showed a 13% higher risk of developing IBS (HR = 1.13, 1.05-1.17). Increased risk of IBS in higher FLI quartile | Subgroup analysis showed increased IBS risk for: Age (P < 0.003); Gender (Female) (P < 0.001) |
| Singh et al[25], 2013 | India | No. of patients: 632 patients with incidentally detected NAFLD (484 males, 148 females) | Retrospective analysis | Not documented | Ultrasound diagnosis and grading of liver steatosis by two radiologists in a blinded study | 186 (29.4%) out of 632 NAFLD patients had IBS | - |
The studies that were included demonstrated associations between NAFLD and IBS. The criteria used to identify IBS patients included ROME III criteria (Ke et al[20], Hasanian et al[21], Zheng et al[22]) and ROME IV criteria (Franco
All the included studies demonstrate overlap between IBS and NAFLD, either via a substantial proportion of sample population having both IBS and NAFLD, or via meta-analysis that showed increased risk of also having IBS or NAFLD should either condition be present. Two studies reported that patients with IBS had increased risk of metabolic syndrome, including higher BMI, dyslipidemia and diabetes mellitus[20,21]. One study demonstrated the same for the IBS and NAFLD overlap group compared to the group that had either IBS or NAFLD[22].
The 100 IBS patients were included in the study by Hasanian et al[21] according to the ROME III diagnostic criteria[21]. BMI and waist circumference were obtained. Laboratory investigations included fasting serum glucose levels, lipid profile, liver chemistry profile, international normalized ratio and a complete blood count. NAFLD was diagnosed in 74% of the study population using abdominal ultrasonography. Furthermore, it was noted that a higher prevalence of moderate/severe NAFLD were found among patients with moderate/severe IBS compared to mild IBS (22.4% vs 4.8%). This could signify a potential association between higher grades of NAFLD with more severe IBS. Multi-variate analysis affirmed the association of moderate/severe NAFLD with moderate/severe IBS which was independent of other risk factors of IBS (OR: 2.4, 95%CI: 1.3-62.7, P = 0.026). Metabolic syndrome was also found to be independently associated with moderate/severe IBS (OR: 3.1, 95%CI: 1.8-54.6, P = 0.011). The study observed that the most frequent metabolic parameter in IBS patients was high BMI (89%)[21].
Ke et al[20] aimed to detect the prevalence of IBS in NAFLD patients as well as normal patients. Patients who underwent health examination in Urumqi, China were randomised into 2 groups: NAFLD and normal controls. IBS was diagnosed using the ROME III criteria. NAFLD was diagnosed using ultrasound based on the 2006 Revised Diagnostic criteria by Fatty Liver and Alcoholic Liver Disease Group of Chinese Medical association Hepatology Branch. 65.8% of adults with IBS-like symptoms had NAFLD while the detection rate of IBS was higher in NAFLD patients compared to normal (23.2% vs 12.5%, P < 0.01), suggesting an association between NAFLD and IBS-like symptoms. NAFLD patients were subsequently subdivided based on severity into: (1) Mild: 212; (2) moderate: 188; and (3) severe: 48. The prevalence of IBS in NAFLD patients increased with the severity of NAFLD as noted by the IBS detection rate for the groups being 11.3%, 27.7% and 58.3% respectively. Further analysis showed that more IBS symptoms were experienced with increasing severity of NAFLD. Multivariate logistic regression analysis demonstrated that IBS-like symptoms were closely related to ethnicity (OR: 0.316, 95%CI: 0.134-0.745, P = 0.008), fatty liver (OR: 0.525; 95%CI: 0.278-0.991, P = 0.047), BMI (OR: 0.918; 95%CI: 0.844-1.000, P = 0.049) and triglyceride levels (OR: 0.855; 95%CI: 0.739-0.988; P = 0.034)[20].
A cross-sectional study by Franco et al[23] included 130 NAFLD patients of which up to 29.2% of patients had co-existing IBS according to ROME IV criteria[23]. A higher prevalence of depression (18.4%) and anxiety (31.6%) was detected using the Hospital Anxiety Depression Scale in NAFLD patients with IBS compared to those without IBS (5.4% and 9.8%) respectively. Female gender (OR: 5.69, 95%CI: 2.01-16.12, P = 0.001) and depression (OR: 1.23, 95%CI: 1.10-1.38, P < 0.001) were independent risk factors for IBS[23].
A recent prospective cohort study followed 153203 patients diagnosed with NAFLD using FLI over 12.4 years[24]. IBS patients were determined via ICD-10 codes and diagnosis was based on self-report, primary care and hospital admission data. 7129 cases of incident IBS were detected with a cumulative incidence rate of 1.49 (95%CI: 1.46-1.53) per 1000 person-years. NAFLD patients showed a 13% higher risk of developing IBS (HR = 1.13, 1.05-1.17) compared with non-NAFLD patients. The highest FLI quartile was associated with a significant increase in risk of IBS compared to the lowest FLI quartile (HR q4 vs q1 = 1.21, 1.13-1.30, P < 0.001). This positive association between NAFLD and IBS was also observed by per SD change of FLI (adjusted HR = 1.08, 1.05-1.10) and predominantly in females[24].
Singh et al[25] showed that a proportion of NAFLD patients initially presented with IBS, though this was not elaborated upon. Out of 16225 patients with various gastrointestinal complaints, 632 patients with NAFLD were included. These patients attended the clinic not because of NAFLD but rather for a variety of gastrointestinal symptoms and the initial reason for evaluation for 29.4% (186) of these individuals with NAFLD was IBS[25].
Zheng et al[22] grouped patients into those with IBS and NAFLD overlap and those without[22]. 200 subjects were recruited and split into 3 groups: (1) Both IBS and NAFLD; (2) either IBS or NAFLD; and (3) neither NAFLD nor IBS. IBS was diagnosed according to the ROME III criteria while NAFLD was diagnosed based on the Chinese Society of Liver Diseases on NAFLD. Out of 200 subjects, 25 (12.5%) had both IBS and NAFLD while 36 (18%) had either NAFLD or IBS. The rest of the 139 (69.5%) subjects had neither IBS nor NAFLD. Results suggested that the combined effect of IBS and NAFLD can implicate the metabolic parameters of patients. The IBS and NAFLD overlap group had a significant higherly incidence of hypertension (8% vs 5.56% P = 0.041), obesity (12% vs 5.56%, P = 0.034), dyslipidemia (12% vs 2.78%, P = 0.020), diabetes (4.0% vs 2.78%, P = 0.037) and digestive illnesses (24.0% vs 11.11%, P = 0.037) compared to the group with either IBS or NAFLD. The extent of dyslipidemia was greater in the IBS and NAFLD overlap group compared to the group with either IBS or NAFLD as higher triglycerides (2.34 ± 1.22 vs 1.71 ± 0.98, P = 0.035), total cholesterol (5.78 ± 1.57 vs 4.99 ± 1.06, P = 0.038), LDL cholesterol (LDL-C) (3.34 ± 1.12 vs 2.90 ± 0.99, P = 0.023) were noted. The IBS and NAFLD overlap group had a higher BMI (26.30 ± 3.03 vs 25.12 ± 2.59, P = 0.045) and higher fasting blood glucose levels (6.02 ± 1.01 vs 5.11 ± 0.97, P = 0.023)[22].
Anorectal manometry assessment was also performed which suggested that when both IBS and NAFLD were present the multiple risk factors from both conditions had a synergistic effect in irritation of the gastrointestinal tract. There was decreased gastrointestinal volume sensory threshold for patients in the IBS and NAFLD overlap group in comparison with the group that had either IBS or NAFLD as indicated by the decreased first sensation volume (22.56 ± 6.04 vs 30.27 ± 5.38, P = 0.034), defaecating sensation volume (63.22 ± 5.29 vs 78.34 ± 6.41, P = 0.032), maximum tolerable volume (82.39 ± 7.45 vs 131.78 ± 23.22, P = 0.035) and painful sensation volume (132.56 ± 19.29 vs 228.32 ± 17.36, P = 0.027) in the IBS and NAFLD overlap group[22].
The results overall support the possible association between IBS and NAFLD. NAFLD was more prevalent in patients with IBS compared to those without IBS. Individuals with both IBS and NAFLD overlap had more metabolic risk factors including high BMI, hypertension, dyslipidemia, and diabetes. The proportion of NAFLD patients with IBS increased along with the severity. IBS patients were three times more likely to have NAFLD compared with non-IBS patients (P < 0.001) with a significant correlation between the severity of IBS and NAFLD.
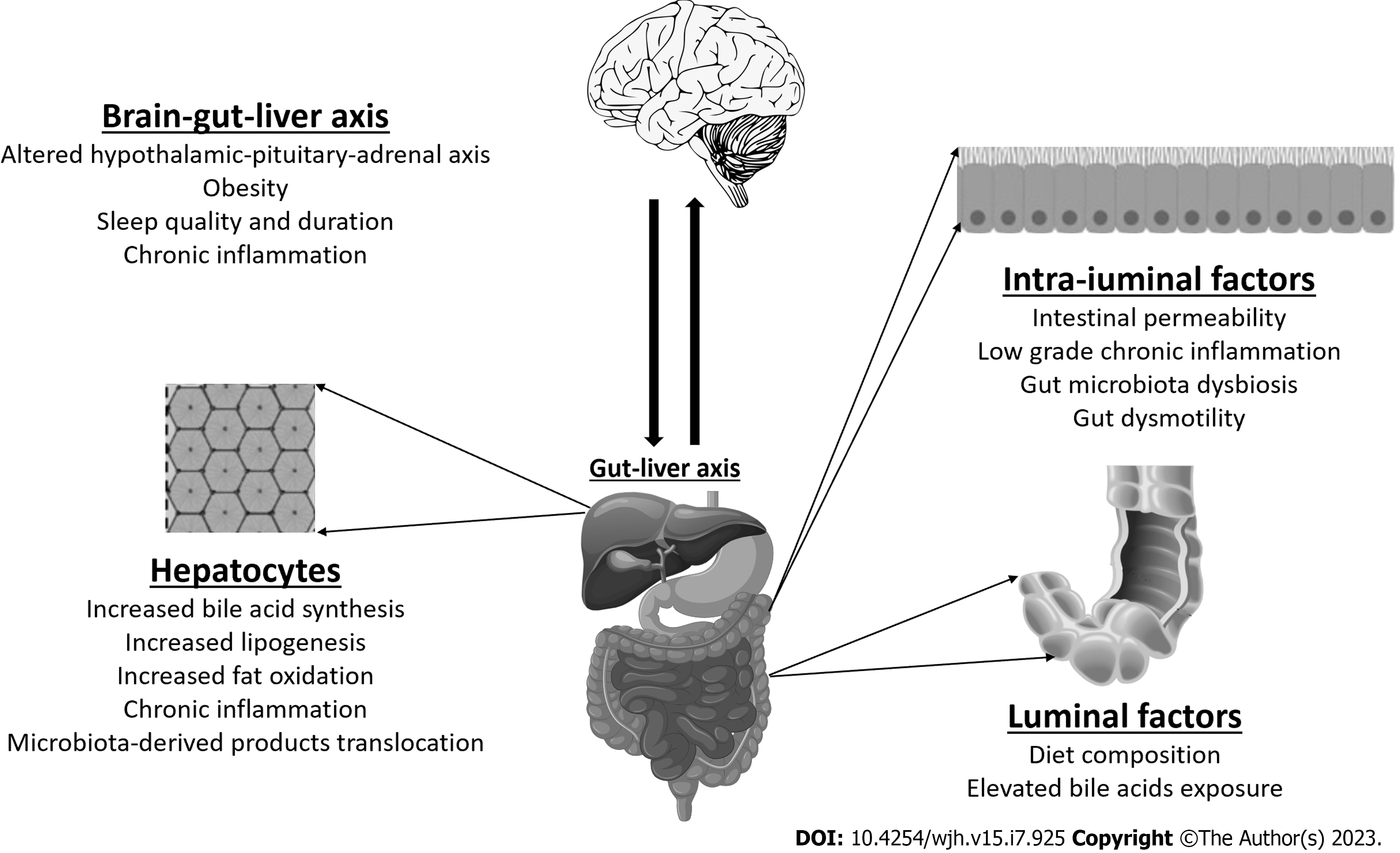
IBS and NAFLD have been postulated to share similar characteristics which are further discussed (Figure 3).
The bidirectional relationship between the gut and the liver is well-established. The gut-liver axis involving gut dysbiosis, intestinal barrier dysfunction, intestinal dysmotility plays a vital role in the pathogenesis of NAFLD[18,26]. Changes in the intestinal microbiota is associated with severity of hepatic fat deposition through several mechanisms: Increasing low-grade mucosal inflammation, immune system activation, altering intestinal permeability, bile acid metabolism, dietary choline metabolism, and generating endogenous ethanol[27,28]. The brain-gut axis likewise shares a bidirectional relationship and similar changes in the brain-gut axis are implicated in the pathogenesis of IBS. The hypothalamic-pituitary-adrenal (HPA) axis and serotonin (5-HT) signalling are some of the pathways affected by dysregulation in the brain-gut axis[28,29]. These alterations lead to abnormal gut motility and visceral hypersensitivity[30]. Emerging evidence suggests a link between NAFLD and IBS symptoms such as diarrhoea. Population-based data from the National Health and Nutrition Examination Surveys revealed that NAFLD and diabetes were independently associated with diarrhoea as opposed to constipation or normal bowel patterns even after adjusting for BMI[31]. This is consistent with our results showing a correlation between IBS and NAFLD with the common mechanism of gut dysbiosis.
The gut microbiota has a significant role in the regulation of the various mechanisms and dysregulation of the gut microbiome contributes to the development of both NAFLD and IBS[26,32]. NAFLD patients have been shown to have increased Firmicutes, Proteobacteria, Actinobacteria, Escherichia, Clostridium, and Bacteroides, and decreased Bifdobacterium, Prevotella and Faecalibacterium[27,33-35]. Somewhat similarly, IBS patients have shown increased abundance of Ruminococcus, Streptococci, Firmicutes, and decreased Bifidobacterium, Faecalibacerium, and Lactobacillus[18,36-38]. However, no specific microbial signature exists for IBS and NAFLD patients to distinguish them from healthy controls, in part due to the variability of sequencing techniques and population groups, as well as various confounding factors such as dietary habits[35,39].
Activation of the innate and adaptive immune pathways has been implicated in the pathogenesis of IBS, both in the intestinal mucosa and neuroinflammation via the brain-gut axis. This involves an overall state of inflammatory overdrive, a dysregulated HPA axis and serotonergic signaling[40]. IBS patients display persistent signs of low-grade mucosal inflammation with increased counts of CD3+, CD4+ and CD8+ T-lymphocytes, mast cells[41]. Enhanced expression of pro-inflammatory cytokines including interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6, IL-8 are observed in IBS patients[18,41,42]. Higher baseline TNF-α, lipopolysaccharide-induced TNF-α, IL-6 and IL-8 levels were significantly correlated with increased bowel frequency and severity of IBS symptoms[42,43].
NAFLD shares similar points of contact with IBS with a low-grade chronic inflammation as a main driver of disease progression in NAFLD[18,44]. Obesity triggers activation of innate and adaptive immune pathways and adipose tissue inflammation exacerbates NASH[18]. Liver metabolism is affected directly via circulating free fatty acids (FFA) from food, adipose tissue, intestinal bacteria, and indirectly via pro-inflammatory cytokines[18,45]. Higher levels of IL-6, C-reactive protein, TNF-α are detected in NAFLD subjects[18,46]. FFA binds toll-like receptors (TLR) on immune cells and in the liver, contributing to activity of the immune system. FFA are also involved in production of reactive oxygen species, mitochondrial dysfunction and endoplasmic reticulum stress in the liver[18,46]. Increased natural killer (NK) cell and NKT cell activity is linked with hepatic expression of inflammatory cytokines and activation of Kupffer cells[47]. All these mediators drive the inflammatory cascade and consequent fibrogenesis in NAFLD.
Bile acid-mediated mechanisms are involved in the pathophysiology of both NAFLD and IBS. There is bile acid signaling dysregulation resulting in increased bile acid production and bile acid malabsorption[48]. Altered bile acid metabolism with defective farnesoid X receptor (FXR) and fibroblast growth factor 19 (FGF19) contributes to abnormal hepatic lipid metabolism in NAFLD[48,49]. Patients with IBS have higher colonic bile acid exposure compared with healthy controls which affects bowel habits in IBS patients, predominantly in the IBS-D subgroup[50,51]. This stimulates colonic motility with acceleration of colonic transit, activation of visceral fluid sensation and fluid secretion[52].
In NAFLD, patients were found to have higher total faecal bile acid levels, increased rates of bile acid synthesis in the liver and a predominance of primary bile acids in the stool[53]. Primary unconjugated faecal bile acids correlated with the degree of hepatic steatosis, the presence of ballooning and severity of fibrosis in NASH subjects[53]. A retrospective study described an increased prevalence of NAFLD in individuals with bile acid diarrhoea[54]. These findings were further confirmed in a prospective study of 127 NAFLD patients that showed a correlation between increased hepatic bile acid production and diarrhoea with increased NAFLD fibrosis scores[49].
A higher prevalence of small intestinal bacterial overgrowth (SIBO) has been reported in patients with IBS[55]. Studies have demonstrated that IBS patients were more likely to develop SIBO compared with healthy controls, predominantly of the diarrhoea subtype[56,57]. SIBO is also found to be more prevalent in NAFLD patients attributed to proposed mechanisms including endotoxaemia and induction of TLR and pro-inflammatory cytokines[58,59]. A study by Sabaté et al[60] showed an association between SIBO and severity of hepatic steatosis in obese individuals[60].
Impaired intestinal permeability is a key factor in the development of IBS[61]. Alterations in gut barrier function were observed in IBS patients which correlated with severity of symptoms[62,63]. A subgroup of IBS-D patients with increased intestinal permeability experienced more severe IBS symptoms and visceral hypersensitivity[62]. This is in part due to bacterial translocation and inflammatory agents through disruption of the epithelial tight junctions[7,18].
NAFLD is similarly associated with increased gut permeability which has an important role in the pathogenesis of NASH[64]. Several studies have described increased intestinal permeability in correlation with the degree of hepatic steatosis[65,66]. Impaired intestinal permeability allows for translocation of bacterial-derived products into the portal circulation and increasing hepatic exposure to harmful substances resulting in inflammation and fibrosis[66]. Conversely, a study by Luther et al[67] suggests that initial hepatic injury may contribute to impaired intestinal permeability although the mechanism is undetermined[67].
A multitude of studies denotes the association between IBS and obesity including the metabolic syndrome. Talley et al[68] demonstrated that older age, less early satiety, increased stool frequency and heartburn were all independently associated with increasing BMI[68]. Visceral abdominal obesity is correlated with an increased risk of developing IBS, primarily diarrhoea-predominant IBS. This is attributed to alteration in visceral fat metabolism which triggers production of adipokines and immunologic factors[69]. IBS patients have an augmented visceral perception of luminal stimuli, dysmotility and abdominal pain related to increased visceral adiposity[70]. Conversely, recent studies have shown that IBS subjects with morbid obesity achieved significant improvement in bowel symptoms after undergoing weight loss intervention[71].
The interplay between NAFLD, obesity and the metabolic syndrome with insulin resistance as a key pathogenic driver has been well-established. A meta-analysis by Li et al[72] showed that obese individuals had a 3.5-fold increased risk of developing NAFLD which has a dose-dependent relationship with BMI[72]. A high pooled prevalence of NAFLD was found in type 2 diabetes patients, with 60% of diabetes patients being diagnosed with NAFLD[73].
Poor sleep quality and circadian misalignment have been implicated in the pathogenesis of IBS[74]. Exacerbation of symptoms have been observed after a night of poor sleep in IBS patients[74]. Likewise, impaired sleep quality, short sleep duration and daytime sleepiness are associated with NAFLD risk with correlation with insulin resistance[75,76]. The hypothalamus-pituitary-adrenal axis can also be affected with impaired cortisol metabolism leading to hepatic steatosis[75]. In addition, obstructive sleep apnoea could have deleterious effects on liver metabolism in the disease progression of NAFLD[76].
In view of the postulated shared mechanisms underlying IBS and NAFLD, therapeutic strategies for IBS may also be beneficial for patients with NAFLD and vice versa. Lifestyle modification with diet and exercise leading to weight loss remains the cornerstone of NAFLD management[77]. Weight loss in IBS subjects showed marked improvement in bowel symptoms along with subjective well-being[71].
Rifaximin has been established as an effective drug in improving global IBS symptoms and bloating[78]. The administration of Rifaximin in NAFLD demonstrated effects including the reduction of serum endotoxin, pro-inflammatory cytokines, NAFLD-liver fat score and improvement in insulin resistance[79,80]. The postulated mechanism could be due to Rifaximin’s effect on gram-negative bacteria leading to the inhibition of endotoxin proinflammatory cytokine production in NAFLD patients[79]. Several studies have shown that probiotics can lead to decrease in serum cytokine levels, oxidative stress markers, liver fat and biochemistry in NAFLD[81-83]. Similarly, studies have identified that probiotics can aid in alleviating IBS symptoms[84,85].
Obeticholic acid (OCA), a semi-synthetic FXR agonist, has been shown to stimulate FGF19 and decrease bile acid synthesis, improve stool form and diarrhoea in patients with IBS-D symptoms[86]. The promising impact of OCA on NASH and its associated metabolic features has been described with significant improvement in fibrosis and NASH histology[87]. Lubiprostone has been demonstrated to be effective in treating global IBS-C symptoms[88]. It has also been shown to decrease hepatic enzyme levels in NAFLD patients with constipation. Greater reduction in hepatic steatosis levels and levels of endotoxin were seen in those with improved intestinal permeability[89]. This may provide a basis for the role of Lubiprostone in a subset of NAFLD patients.
There are several limitations. The number of studies included was small given the lack of data on this topic to date. Different ultrasonographic diagnostic criteria was used for NAFLD. One study used codes from the International Disease Classification of diseases for identification of IBS while the other did not disclose how IBS patients were diagnosed, resulting in heterogeneity among the studies. Statistical analysis was unable to be carried out as not all studies assessed OR and 95%CI.
In conclusion, the evidence supports the association between IBS and NAFLD. IBS and NAFLD may co-exist and patients with IBS should be assessed for NAFLD and vice versa. Given the common postulated pathophysiology of both conditions, this may form the basis for further studies to assess suitability and benefits of utilising known therapeutics for IBS to treat NAFLD. This may guide future therapeutic strategies, especially in patients who suffer from both conditions. However, further prospective studies are required to confirm this association.
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease with the rise of obesity and metabolic syndrome. Functional gastrointestinal disorders like irritable bowel syndrome (IBS) are increasing in prevalence.
At present, there is limited understanding regarding the links between the two conditions despite there being suggestions of possible overlap between IBS and NAFLD. We hope to explore literature to assess this overlap and also possible common pathophysiological links.
To review the current literature regarding the overlap of NAFLD and IBS and potentially identify common pathophysiological links which may show potential for utilizing common therapeutics to treat both conditions.
A systematic search was done to assess current literature showing overlap between NAFLD and IBS in human subjects from PubMed, EMBASE and Cochrane.
We identified studies showing overlap between NAFLD and IBS. Both IBS and NAFLD patients demonstrated more metabolic risk factors like obesity, hypertension, dyslipidaemia and diabetes. IBS was seen to be more common in NAFLD patients and vice versa. Common pathophysiological links included the brain-gut-liver axis, intestinal permeability, gut microbiota dysbiosis, bile acid signalling dysregulation, obesity and metabolic syndrome.
Our systematic review summarizes the current literature regarding IBS and NAFLD and demonstrates overlap between the two conditions. Common pathophysiological links were identified between both conditions.
The evidence supports the association between IBS and NAFLD. With common postulated pathophysiology of both conditions discussed, further studies would be useful to further strengthen the association between both conditions and also look into possible common therapeutics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lv L, China; Sitkin S, Russia; Sun X, China S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7477] [Article Influence: 830.8] [Reference Citation Analysis (0)] |
| 2. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4911] [Article Influence: 701.6] [Reference Citation Analysis (8)] |
| 3. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1016] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 4. | Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 353] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 5. | Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 6. | Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 368] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 7. | Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 441] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 8. | Purssell H, Whorwell PJ, Athwal VS, Vasant DH. Non-alcoholic fatty liver disease in irritable bowel syndrome: More than a coincidence? World J Hepatol. 2021;13:1816-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 9. | Bayrak M. Metabolic syndrome, depression, and fibromyalgia syndrome prevalence in patients with irritable bowel syndrome: A case-control study. Medicine (Baltimore). 2020;99:e20577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Guo Y, Niu K, Momma H, Kobayashi Y, Chujo M, Otomo A, Fukudo S, Nagatomi R. Irritable bowel syndrome is positively related to metabolic syndrome: a population-based cross-sectional study. PLoS One. 2014;9:e112289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Gulcan E, Taser F, Toker A, Korkmaz U, Alcelik A. Increased frequency of prediabetes in patients with irritable bowel syndrome. Am J Med Sci. 2009;338:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Aasbrenn M, Høgestøl I, Eribe I, Kristinsson J, Lydersen S, Mala T, Farup PG. Prevalence and predictors of irritable bowel syndrome in patients with morbid obesity: a cross-sectional study. BMC Obes. 2017;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Yang S, Yu C, Guo Y, Bian Z, Fan M, Yang L, Du H, Chen Y, Yan S, Zang Y, Chen J, Chen Z, Lv J, Li L; China Kadoorie Biobank Collaborative Group. Bowel movement frequency and risks of major vascular and non-vascular diseases: a population-based cohort study among Chinese adults. BMJ Open. 2020;10:e031028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Sadik R, Björnsson E, Simrén M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Lim S, Oh TJ, Koh KK. Mechanistic link between nonalcoholic fatty liver disease and cardiometabolic disorders. Int J Cardiol. 2015;201:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Machado MV, Cortez-Pinto H. Gut microbiota and nonalcoholic fatty liver disease. Ann Hepatol. 2012;11:440-449. [PubMed] |
| 17. | Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 18. | Scalera A, Di Minno MN, Tarantino G. What does irritable bowel syndrome share with non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:5402-5420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lee SH, Kim KN, Kim KM, Joo NS. Irritable Bowel Syndrome May Be Associated with Elevated Alanine Aminotransferase and Metabolic Syndrome. Yonsei Med J. 2016;57:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ke Y, Yang T, Yao P. Association between nonalcoholic fatty liver disease and irritable bowel syndrome in populations undergoing health examination in Urumqi. Shijie Huaren Xiaohua Zazhi. 2013;21: 4164-4169. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (3)] |
| 21. | Hasanian AF, Abdel-Rahman ME, Ali AM, Abdel-Aal SM. Nonalcoholic fatty liver disease among patients with irritable bowel syndrome: prevalence and contribution to disease severity. Gastroenterol Hepatol Endoscopy. 2018;3:1-4. |
| 22. | Zheng Q, Huang Y, Peng L, Hong M. The discussion of correlation between irritable bowel syndrome and non-alcoholic fatty liver disease. Zhongguo Yiyao Daobao. 2011;8:29-31. [DOI] [Full Text] |
| 23. | Franco L, Jones-Pauley M, Tamimi O, Neshatian L, Nguyen D, Graviss E, Quigley EM, Victor D 3rd. Irritable Bowel Syndrome Symptoms in Nonalcoholic Fatty Liver Disease Patients Are an Indicator of Depression and Anxiety. J Clin Gastroenterol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 24. | Wu S, Yuan C, Yang Z, Liu S, Zhang Q, Zhang S, Zhu S. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: a prospective cohort study. BMC Med. 2022;20:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 25. | Singh SP, Kar SK, Panigrahi MK, Misra B, Pattnaik K, Bhuyan P, Meher C, Agrawal O, Rout N, Swain M. Profile of patients with incidentally detected nonalcoholic fatty liver disease (IDNAFLD) in coastal eastern India. Trop Gastroenterol. 2013;34:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 26. | Ding JH, Jin Z, Yang XX, Lou J, Shan WX, Hu YX, Du Q, Liao QS, Xie R, Xu JY. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26:6141-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (6)] |
| 27. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1042] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 28. | Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20:16452-16463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2403] [Cited by in RCA: 2935] [Article Influence: 225.8] [Reference Citation Analysis (1)] |
| 30. | Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Shin A, Xu H, Imperiale TF. Associations of chronic diarrhoea with non-alcoholic fatty liver disease and obesity-related disorders among US adults. BMJ Open Gastroenterol. 2019;6:e000322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 746] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 33. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 34. | Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, Sung JJ, Chan HL. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8:e62885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 35. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 676] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 36. | Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol. 2014;20:2470-2481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 38. | Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887-2892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 206] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 39. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8015] [Article Influence: 616.5] [Reference Citation Analysis (2)] |
| 40. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (2)] |
| 41. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Zhen Y, Chu C, Zhou S, Qi M, Shu R. Imbalance of tumor necrosis factor-α, interleukin-8 and interleukin-10 production evokes barrier dysfunction, severe abdominal symptoms and psychological disorders in patients with irritable bowel syndrome-associated diarrhea. Mol Med Rep. 2015;12:5239-5245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 44. | Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773-4783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 45. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 46. | Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, Zhang Q, Du Z, Gai Z, Wu J, Li Y. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Biomed Res Int. 2020;2020:4020249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 47. | Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 48. | Weaver MJ, McHenry SA, Sayuk GS, Gyawali CP, Davidson NO. Bile Acid Diarrhea and NAFLD: Shared Pathways for Distinct Phenotypes. Hepatol Commun. 2020;4:493-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Appleby RN, Moghul I, Khan S, Yee M, Manousou P, Neal TD, Walters JRF. Non-alcoholic fatty liver disease is associated with dysregulated bile acid synthesis and diarrhea: A prospective observational study. PLoS One. 2019;14:e0211348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 51. | Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0151829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 54. | Appleby RN, Nolan JD, Johnston IM, Pattni SS, Fox J, Walters JR. Novel associations of bile acid diarrhoea with fatty liver disease and gallstones: a cohort retrospective analysis. BMJ Open Gastroenterol. 2017;4:e000178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 56. | Ghoshal UC, Nehra A, Mathur A, Rai S. A meta-analysis on small intestinal bacterial overgrowth in patients with different subtypes of irritable bowel syndrome. J Gastroenterol Hepatol. 2020;35:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018;53:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Shanab AA, Scully P, Crosbie O, Buckley M, O'Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 59. | Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, Ray P, Dhiman RK, Chawla Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M, Coffin B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. 2014;49:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, De Winter BY, Grover M. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 64. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1096] [Article Influence: 68.5] [Reference Citation Analysis (1)] |
| 65. | De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, Koek GH. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40:2906-2916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 66. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 67. | Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 68. | Talley NJ, Quan C, Jones MP, Horowitz M. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Lee CG, Lee JK, Kang YS, Shin S, Kim JH, Lim YJ, Koh MS, Lee JH, Kang HW. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol. 2015;110:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Emerenziani S, Guarino MPL, Trillo Asensio LM, Altomare A, Ribolsi M, Balestrieri P, Cicala M. Role of Overweight and Obesity in Gastrointestinal Disease. Nutrients. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Aasbrenn M, Lydersen S, Farup PG. A Conservative Weight Loss Intervention Relieves Bowel Symptoms in Morbidly Obese Subjects with Irritable Bowel Syndrome: A Prospective Cohort Study. J Obes. 2018;2018:3732753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 73. | Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, Lai Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017;96:e8179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 74. | Orr WC, Fass R, Sundaram SS, Scheimann AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 75. | Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H, Ryu S. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 76. | Bernsmeier C, Weisskopf DM, Pflueger MO, Mosimann J, Campana B, Terracciano L, Beglinger C, Heim MH, Cajochen C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS One. 2015;10:e0143293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3167] [Article Influence: 351.9] [Reference Citation Analysis (4)] |
| 78. | Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28-35; quiz 36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 79. | Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 80. | Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elzehery R, Elhelaly R, Zalata K, Abdelsalam M, Eldeeb AA, Awad M, Elgamal A, Attia A, El-Wakeel N, Eldars W. Rifaximin in nonalcoholic fatty liver disease: hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. 2018;30:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 81. | Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090-1095. [PubMed] |
| 82. | Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256-262. [PubMed] |
| 83. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 84. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 85. | Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. 2018;35:289-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 86. | Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 87. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 913] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 88. | Drossman DA, Chey WD, Johanson JF, Fass R, Scott C, Panas R, Ueno R. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 89. | Kessoku T, Imajo K, Kobayashi T, Ozaki A, Iwaki M, Honda Y, Kato T, Ogawa Y, Tomeno W, Kato S, Higurashi T, Yoneda M, Kirikoshi H, Kubota K, Taguri M, Yamanaka T, Usuda H, Wada K, Kobayashi N, Saito S, Nakajima A. Lubiprostone in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Gastroenterol Hepatol. 2020;5:996-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |