Published online Apr 27, 2023. doi: 10.4254/wjh.v15.i4.538
Peer-review started: December 28, 2022
First decision: January 22, 2023
Revised: February 3, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 27, 2023
Processing time: 112 Days and 23.9 Hours
The biliary system consists of intrahepatic and extrahepatic bile ducts lined by biliary epithelial cells (cholangiocytes). Bile ducts and cholangiocytes are affected by a variety of disorders called cholangiopathies, which differ in aetiology, path
Core Tip: A wide range of non-neoplastic and neoplastic conditions affects the small intrahepatic bile ducts. Cholangiopathies account for significant morbidity and mortality and represent an important indication for liver transplantation in adult and paediatric populations. Pathogenesis of most cholangiopathies is complex, and likely involves both environmental and genetic factors. Understanding the underlying pathogenetic mechanisms, knowledge of basic morphological patterns, and an ability to correlate microscopic findings with results obtained by imaging and laboratory methods are important steps in forming an overall clinical picture and selecting the optimal therapeutic approach in patients with biliary diseases. Our minireview addresses some important morphological aspects of small-duct cholangiopathies relevant to the diagnostic process, and also provides a brief overview of the most clinically significant conditions in light of recent progress.
- Citation: Sticova E, Fabian O. Morphological aspects of small-duct cholangiopathies: A minireview. World J Hepatol 2023; 15(4): 538-553
- URL: https://www.wjgnet.com/1948-5182/full/v15/i4/538.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i4.538
The biliary system is a three-dimensional complex of developmentally, anatomically, and functionally different ductal structures. The extrahepatic bile ducts are composed of the right and left hepatic ducts, their confluence, the common hepatic duct, and the common bile duct. The intrahepatic biliary tree is divided according to size into the large intrahepatic bile ducts (area and segmental) and the small intrahepatic bile ducts (interlobular and septal).
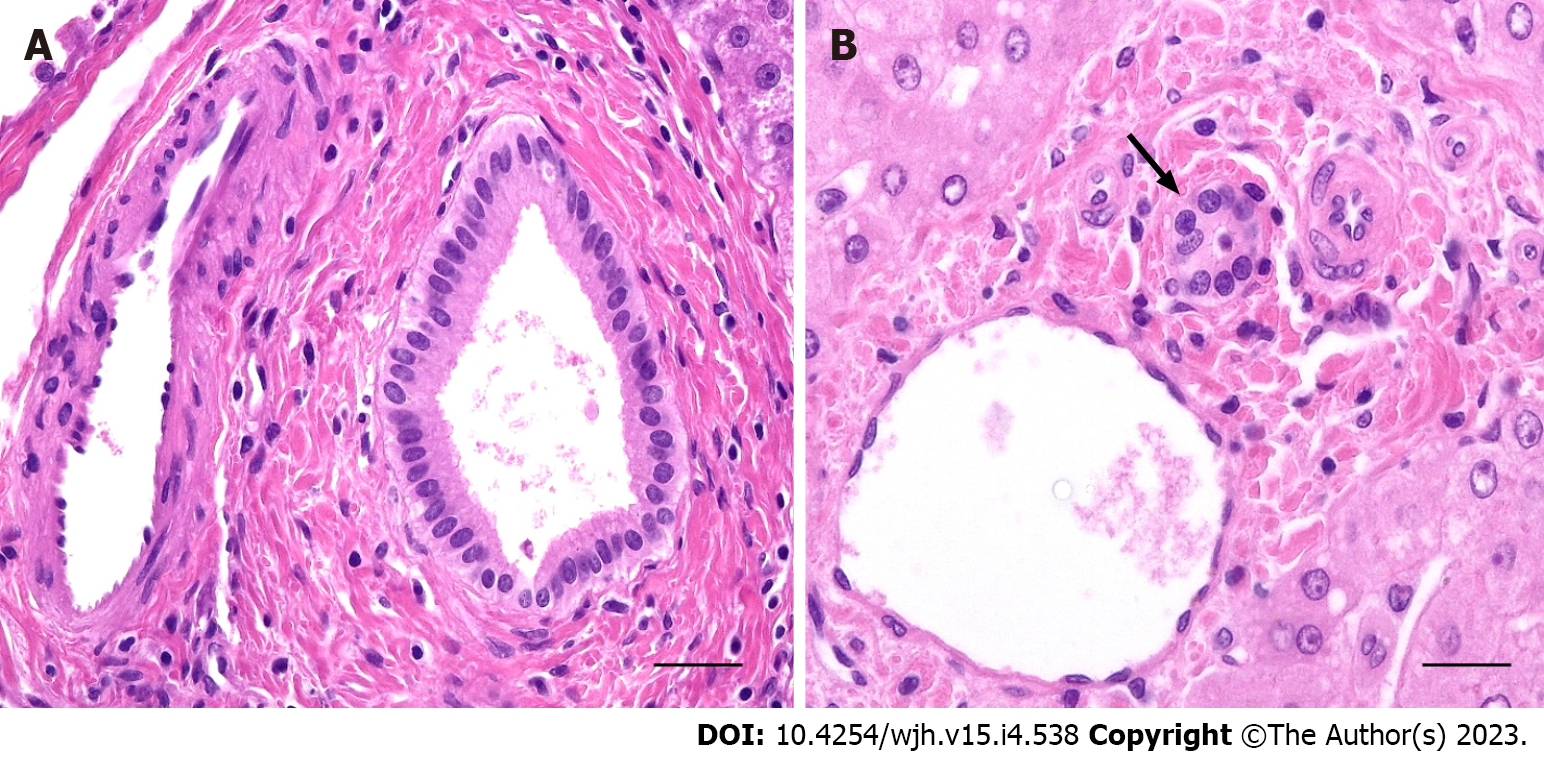
Septal bile ducts (> 100 µm in diameter) are lined by tall columnar cells with basal nuclei supported by a fibrous duct wall (Figure 1A). Interlobular bile ducts (15-100 µm) are thin-walled structures lined by cuboidal epithelial cells resting on a basal membrane (Figure 1B). Interlobular bile ducts are connected to a complex network by ductules (< 15 µm diameter), lined by low cuboidal cells, and the canals of Hering, which are partly composed of the biliary epithelium and partly of hepatocytes.
In humans, biliary epithelial cells (cholangiocytes) account for 3%-5% of the total liver cell mass. Cholangiocytes of the intrahepatic biliary tree are derived from the endodermal cells of the hepatic primordium, while epithelial cells lining the extrahepatic bile ducts derive from the caudal portion of the hepatic diverticulum[1,2].
Biliary epithelial cells along with a variety of receptors and transporters play important roles in the secretion and reabsorption of water, electrolytes, bile acids, and many other bile compounds. As well as modifying bile composition, cholangiocytes secreting mucin and HCO3- are important elements in the biliary self-defence system. In addition, biliary epithelial cells are involved in a wide range of physiological processes, including intracellular and intercellular signalling, liver regeneration, and immune-mediated reactions[3,4].
Bile ducts and cholangiocytes can be affected by a variety of disorders generally referred to as cholangiopathies. Under pathological circumstances, cholangiocytes can undergo morphological and functional changes, which differ according to the size of the affected segment of the biliary tree. Large bile duct disorders are primarily diagnosed by radiology imaging and liver biopsy is generally not required. However, in the case of small bile duct injuries, imaging methods are not adept at detecting alterations of the biliary tree. Therefore, histomorphological examination should be considered a relevant part of the diagnostic process.
To increase the diagnostic yield of a liver biopsy and arrive at an optimal therapeutic approach, the results of histopathological examination must be interpreted by the presiding clinician. This requires knowledge and understanding of the basic morphological patterns of hepatobiliary injury.
This minireview briefly addresses some important morphological aspects of small-duct cholangiopathies relevant to the diagnostic process, and also provides a brief overview of the most clinically significant conditions in light of recent progress.
Biliary diseases are classified according to aetiological aspects, pathogenic mechanisms, and the predominant morphological pattern of bile duct injury.
Based on the underlying pathogenic mechanism, cholangiopathies can be classified as follows[5,6]: (1) Immune-mediated cholangiopathy; (2) genetic cholangiopathy; (3) drug- and toxin-induced cholangiopathy; (4) ischaemic cholangiopathy; (5) infectious cholangiopathy; and (6) neoplastic cholangiopathy. From a morphological point of view, diseases that target bile ducts and cholangiocytes are categorised as either cholangitis (inflammatory bile duct injury) or cholangiopathy (non-inflammatory bile duct injury). Based on the predominant inflammatory cell type and the presence of associated periductal fibrosis, cholangitis can assume a suppurative (neutrophilic) form, predominantly infective, or a non-suppurative form, e.g. lymphocytic, pleomorphic, granulomatous, or sclerosing (Table 1)[5,6].
| Pattern of biliary injury | Disease | |
| Cholangitis | Neutrophilic | Infection, sterile |
| Lymphocytic | PBC, GVHD, allograft rejection | |
| Pleomorphic | AIH, PBC, DILI | |
| Granulomatous | PBC, sarcoidosis, DILI | |
| Sclerosing | PSC, IgG4-sclerosing cholangitis, DILI | |
| Bile duct loss and ductopenia | See Table 2 | |
| Ductal plate malformation | See Table 3 | |
| Neoplastic | BilIN, IPN, cholangiocarcinoma |
The most clinically important patterns of non-inflammatory bile duct lesions are ductal plate malformation, bile duct loss and ductopenia (vanishing bile duct syndrome), and – in a broader context – neoplastic biliary lesions[5].
Several common features of cholangiopathies mainly affecting the small bile ducts have been observed. Considering that early histomorphological changes are often subtle and focal, they can be easily overlooked in a percutaneous liver biopsy. Early alterations secondary to impaired bile flow include focal mild portal oedema accompanied by mild periductal inflammation. Periportal ductular proliferation is commonly detectable at the periphery of some portal areas. Focal and mild juxtaportal copper and copper-associated protein depositions due to prolonged cholestasis can be highlighted by Schmorl’s reaction and orcein or rhodanine staining. And although these changes are not pathognomonic, they can indicate a diagnosis of early-stage chronic cholangiopathy[7,8].
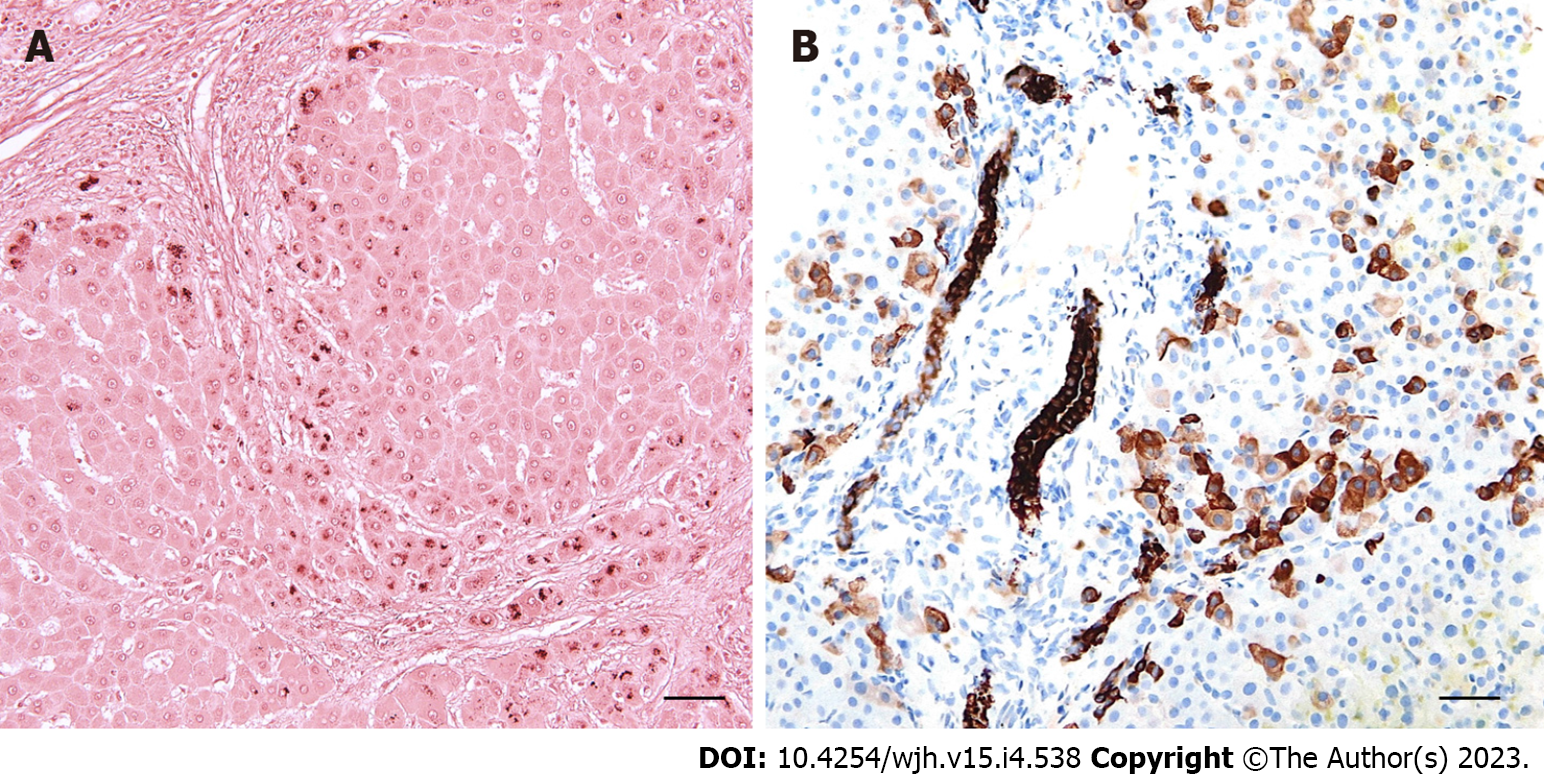
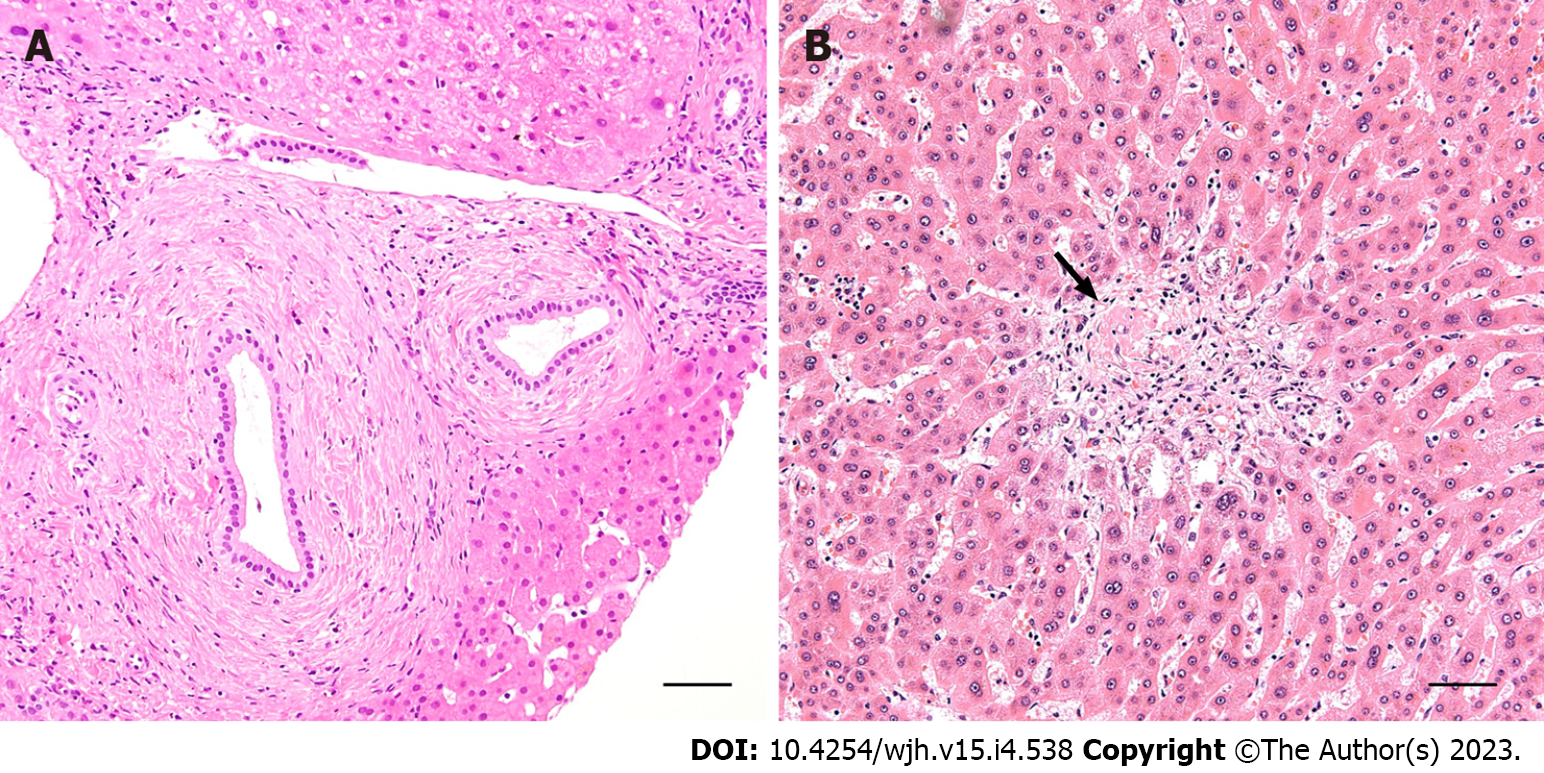
Morphological features of prolonged periportal bile salt accumulation (cholate stasis) can be accompanied by biliary interface activity, a complex reaction composed of periportal ductular proliferation with mild neutrophilic inflammation, aberrant cytokeratin 7 expression in hepatocytes (cholangiolar or ductular metaplasia), and varying degrees of fibrosis (Figure 2). Late stages of the disease are characterised by prominent cholate stasis with oedema and “feathery” degeneration of periportal hepatocytes containing Mallory-Denk hyaline inclusions and depositions of copper and copper-associated protein (Figure 2). This results in the formation of a signature “halo” effect at the peripheries of portal areas and septa (Figure 3A). Biliary-type fibrosis can also progress, with a typically uneven distribution of fibrous changes in the liver tissue. Periportal changes may be accompanied by bilirubinostasis in liver lobules, predominantly in the centrilobular zone (zone 3)[7,8,9].
Some cholangiopathies are associated with progressive small bile duct destruction and loss. Hepatic ductopenia, an uncommon but potentially serious cholestatic liver disease, is defined as a loss of the interlobular bile ducts in at least 50% of portal areas[7,10,11]. Ten portal tracts in a liver biopsy specimen are usually considered sufficient for an evaluation of bile duct loss. Vanishing bile duct syndrome is a rare condition in which progressive bile duct loss results in chronic cholestasis and ductopenia[10,11]. A heterogeneous group of conditions, comprising immune-mediated processes, drug- and toxin-induced hepatobiliary injury, ischaemia, infections, and some hereditary diseases, has been implicated in bile duct destruction (Table 2)[11,12].
| Infantile and childhood diseases | Bile duct paucity (syndromic/non-syndromic) |
| Extrahepatic biliary atresia | |
| Progressive familial intrahepatic cholestasis | |
| Fibropolycystic disease | |
| Alpha-1 antitrypsin deficiency | |
| Immune-mediated diseases | Primary biliary cholangitis |
| Primary sclerosing cholangitis | |
| Chronic graft-versus-host disease | |
| Chronic hepatic allograft rejection | |
| Hepatic sarcoidosis | |
| Vascular diseases | Ischaemic cholangiopathy |
| Portal biliopathy | |
| Infectious diseases | Ascending cholangitis |
| Protozoal and parasitic infestations | |
| Drug- or toxin-induced biliary injury | |
| Neoplastic diseases | Hodgkin’s lymphoma |
| Langerhans cell histiocytosis | |
| Systemic mastocytosis | |
| Idiopathic | Idiopathic adulthood ductopenia |
The major histomorphological features of various small-duct cholangiopathies are briefly discussed below.
Primary biliary cholangitis: Primary biliary cholangitis (PBC) is an autoimmune disease that selectively affects the small intrahepatic bile ducts. Typical clinical features of PBC include marked female preponderance, middle-to-elderly age range, frequent association with other autoimmune disorders, pruritus, and skin hyperpigmentation. Increased activity of serum alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT), elevated serum IgM, and the presence of M2-type antimitochondrial antibodies (AMA) are also commonly observed[13-15].
According to the classic histological systems proposed by Scheuer and Ludwig, microscopical changes are categorised into the following four stages[16,17]: Stage 1: Focal, destructive, lymphocytic and/or granulomatous cholangitis of the interlobular bile ducts (florid ductal lesion) with inflammation generally confined within portal tract boundaries (Figure 3B); Stage 2: Ongoing bile duct injury with portal tract expansion due to periportal ductular proliferation (biliary interface activity), inflammatory interface activity, and periportal fibrosis; Stage 3: Bridging portoportal fibrosis with persistent biliary interface activity and ongoing small bile duct loss; Stage 4: Cirrhosis with ductopenia and chronic cholestasis (cholate stasis) discernible by a periportal “halo” (Figure 3A).
The above classic staging systems are simple and easily reproducible. However, they do not reflect all of the morphological features of disease activity and progression that should be considered when determining the optimal therapeutic approach. In addition, the heterogeneous distribution of diagnostic changes in the liver parenchyma and the potential for sampling errors during percutaneous needle biopsy reduce their applicability and practical use. These shortcomings have been overcome by a novel complex system applicable to needle liver biopsy specimens proposed by Nakanuma et al[18,19]. Three components – fibrosis, bile duct loss, and chronic cholestasis – are used in this PBC staging system, while necroinflammatory activity of PBC is graded based on chronic cholangitis and hepatitis activity[18,19].
Apart from portal and portal-parenchymal interface changes, small cell changes in periportal hepatocytes and nodular regenerative hyperplasia (NRH) can occur during non-cirrhotic stages of PBC. NRH may explain the hepatomegaly in PBC and the development of clinically significant portal hypertension long before cirrhosis develops[20,21].
The following PBC variants have been recognised: AMA-negative PBC (autoimmune cholangitis) represents 5% of PBC cases. Subtle differences in the clinical, laboratory, and immunological features of AMA-negative PBC have been reported in a number of studies. However, the basic histopathological findings and responses to ursodeoxycholic acid are similar to those observed in AMA-positive patients[13,14].
PBC-AIH overlap syndrome is characterised by the features of both PBC and autoimmune hepatitis coexisting in a single patient. Diagnostic criteria for PBC-AIH overlap syndrome have been proposed by Chazouillères et al[22], while other guidelines have been issued by the International Autoimmune Hepatitis Group. These include the presence of at least two of three diagnostic features of PBC (1-3) and at least two of three diagnostic features of AIH (4-6)[23]: (1) GGT ≥ 5× upper limit of normal (ULN) or ALP ≥ 2× ULN; (2) positive AMA; (3) florid bile duct lesion on histology; (4) increased alanine transaminase (ALT) levels ≥ 5× ULN; (5) serum IgG levels ≥ 2× ULN or positive anti-smooth muscle antibody (SMA); and (6) moderate or severe lymphocytic interface activity on histology.
Nevertheless, as opposed to genuine overlap syndrome, most cases are best regarded as either PBC with unusually prominent inflammatory activity (PBC with hepatitis features) or as classical AIH with PBC-like bile duct injury.
Rare cases of overlapping features involving PBC and primary sclerosing cholangitis (PSC), IgG4-related sclerosing cholangiopathy, and sarcoidosis have been reported in the medical literature[14,22].
PSC is a chronic cholestatic condition characterised by non-specific inflammatory fibrosis in bile duct walls of all sizes, with irregular areas of stricturing and beading on cholangiography. Unlike PBC, PSC can occur in infancy and childhood, has a male preponderance, and is closely associated with inflammatory bowel disease (IBD). An increased risk of hepatobiliary malignancy in PSC patients has also been observed[13,14,24,25].
PSC typically involves extrahepatic and large intrahepatic bile ducts with variable involvement of small ducts. Nonetheless, in 5%–10% of cases, the disease is confined to the small intrahepatic bile ducts (small-duct PSC or sdPSC)[24,25,26]. The aetiopathogenesis of sdPSC is poorly understood. A study by Naess et al[27] evaluated sdPSC components in a subset of patients with and without concomitant IBD. They found that patients with sdPSC and IBD may represent precursor lesions indicative of classic PSC, while sdPSC patients without IBD could point to a different biliary disease process.
Since a cholangiography is negative in sdPSC, histopathological evaluation of liver tissue specimens is usually required to establish a diagnosis. Although most studies have reported that sdPSC has a similar histopathological picture to large-duct PSC, its pathognomonic features may be subtle and randomly distributed within the liver tissue[7,24,26]. The distinctive features of PSC are fibro-obliterative lesions characterised by ‘onion-skin’ periductal fibrosis and replacement of the bile duct by a dense acellular fibrous scar (Figure 4). Biliary changes are frequently associated with mild portal tract oedema, low-grade periductal inflammatory infiltrate, and periportal ductular proliferation accompanied by juxtaportal copper and copper-binding protein deposition. As the disease advances, fibrous expansion of portal areas may progress to bridging septal fibrosis and eventual biliary-type cirrhosis[7,24,26].
Histopathological changes in PSC are divided into four stages (1-4) based on the same classification systems as proposed for PBC (see above).
Autoimmune sclerosing cholangitis (PSC-AIH overlap syndrome) is a variant syndrome characterised by features of sclerosing cholangitis and AIH serology[15,23,28]. Importantly, biliary features detected by histopathological examination may be the first indication for biliary tree imaging in patients originally diagnosed with uncomplicated AIH. The syndrome is more common in children and adolescents, with these groups also displaying higher prevalence of sdPSC[7,15,28].
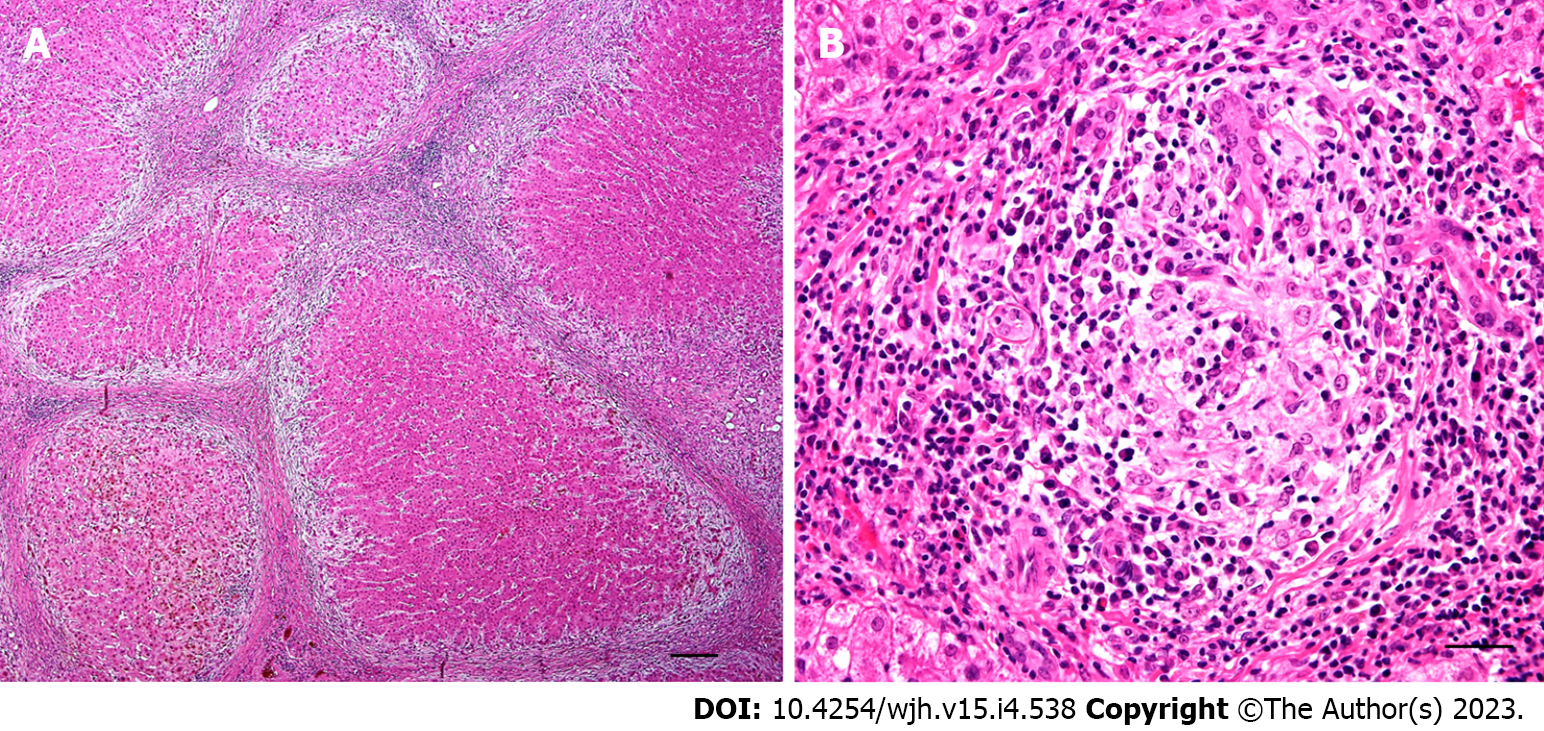
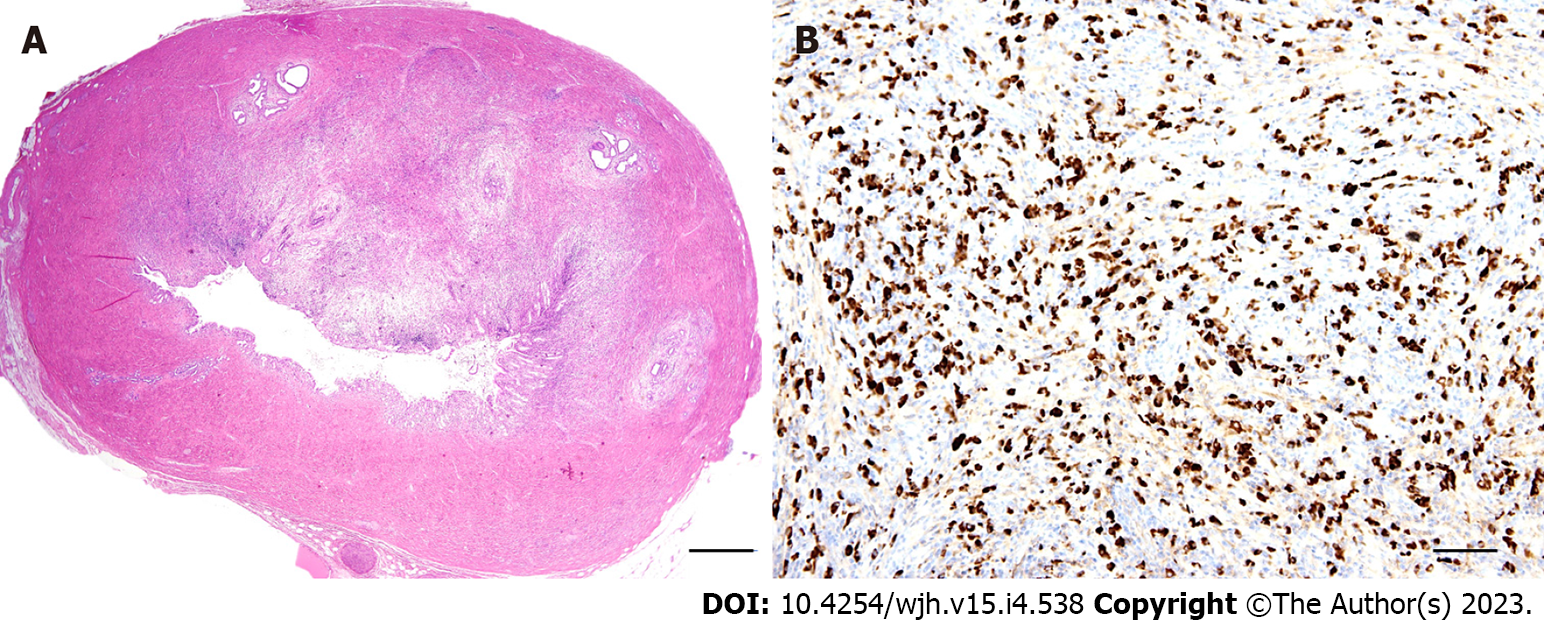
IgG4-related sclerosing cholangitis is now considered a biliary manifestation of systemic IgG4-related disease. This condition is commonly associated with IgG4-related lesions in other organs, most often with type 1 autoimmune pancreatitis[29,30]. The affected extrahepatic, hilar and perihilar ducts show diffuse and uniform tube-like thickening on gross examination. Histological characteristics of large duct lesions are transmural inflammation, obliterative phlebitis, and heavy lymphoplasmacytic infiltration with a predominance of IgG4-positive cells and abundant eosinophilic leukocytes (Figure 5)[29,30]. In up to 30% of patients, intrahepatic disease involving the small bile ducts is detectable by liver biopsy. Morphological findings include plasma cell-rich portal inflammation and portal fibrosis, interlobular bile duct damage, lobular inflammation, and features of cholestasis with periportal copper-associated protein deposition. A distinctive portal-based fibroinflammatory nodule composed of inflammatory cells and fibroblasts was observed in 50% of liver biopsies obtained from patients with IgG4-related sclerosing cholangitis[30,31,32]. While differentiating the condition from PSC by needle biopsy is challenging, the presence of more than ten IgG4+ plasma cells on liver biopsy can serve as a diagnostic aid[30,31].
Cholangiocytes and small interlobular bile ducts are targets of an immune reaction associated with liver allograft rejection and graft-versus-host disease (GVHD)[5,33].
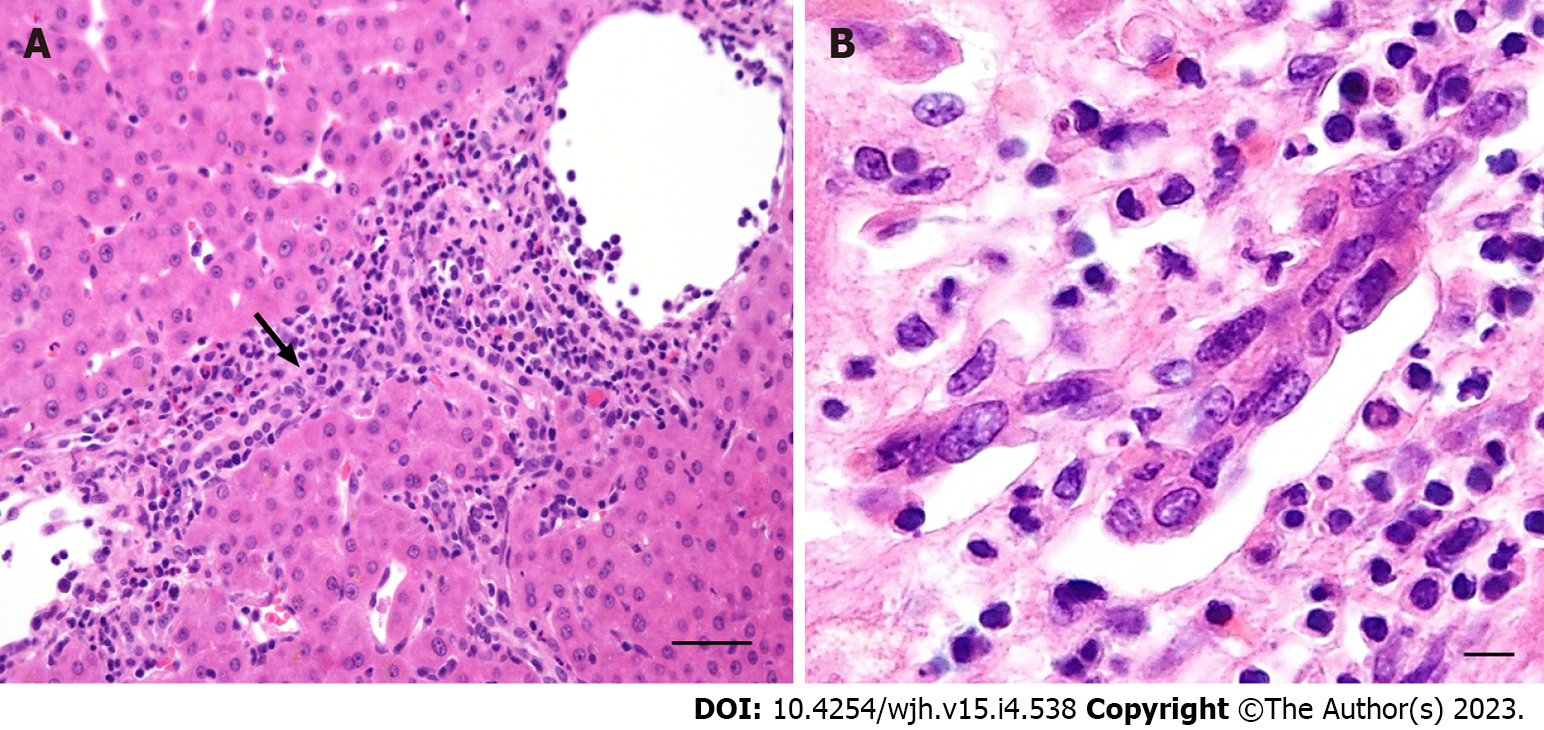
Biliary lesions in acute T cell-mediated rejection are characterised by periductal and intraepithelial lymphocytic infiltration, degenerative changes to biliary epithelial cells with cytoplasmic vacuolisation, increased N:C ratios, disordered nuclear polarity, and eventual luminal disruption (Figure 6A). Senescence-related changes to biliary epithelial cells (eosinophilic cytoplasmic transformation, uneven nuclear spacing, nuclear hyperchromasia) and progressive interlobular bile duct loss indicate chronic (ductopenic) rejection (Figure 6B)[33-35].
Immune-mediated small bile duct injury, degenerated dysplastic-like cholangiocytes, and progressive bile duct destruction are the most characteristic histological features of GVHD, a frequent complication of haematopoietic cell transplantation. Lymphocytic infiltration of the biliary epithelium in GVHD is usually minimal to mild[36].
Genetic factors are variably implicated in most cholangiopathies. In addition to ductal plate malformation (hereditary fibropolycystic disease), neonatal sclerosing cholangitis, and various conditions within the spectrum of familial intrahepatic cholestasis, cholangiopathy can also occur in cystic fibrosis, a multisystemic disease caused by a homozygous or compound heterozygous mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene[37]. Alpha-1 antitrypsin deficiency (AATD) is an autosomal recessive storage disease caused by pathogenic mutations in the SERPINA1 gene. Besides pulmonary emphysema, AATD can manifest as a progressive cholestatic liver disease involving hypoplasia of the small bile ducts, mostly occurring in paediatric patients[38].
Ductal plate malformation is a common feature in ciliopathy-associated liver diseases, characterised by a spectrum of biliary hamartomatous lesions, segmental bile duct dilatations and cysts. All of the above conditions stem from aberrant remodelling of the embryonal ductal plate (Figure 7A)[39,40]. Liver disease is often associated with the involvement of other organs, mainly the kidneys. Over the past decade, a number of genes (and their corresponding protein products) involved in several of these disorders have been identified (Table 3)[40,41].
| Disorder | Gene | Product |
| ARPKD | PKHD | Fibrocystin/polyductin |
| ADPKD | PKD1 | Polycystin 1 |
| PKD2 | Polycystin 2 | |
| GANAB/PKD3 | Glucosidase II alpha subunit | |
| PCLD | PRKCSH | Glucosidase II beta subunit |
| ALG8 | Alpha-1,3-glucosyltransferase | |
| SEC61B | SEC61 translocon subunit beta | |
| SEC63 | Translocon component in the endoplasmic reticulum | |
| LRP5 | LDL receptor-related protein 5 | |
| CHF-SC | ZFYVE19 | Zinc finger FYVE-type containing 19 |
Alagille syndrome (ALGS) is an autosomal dominant multisystem disorder accompanied by severe cholestasis due to congenital maldifferentiation of the intrahepatic bile ducts. The syndrome is caused by mutations in the JAG1 gene, which encodes the intercellular signalling protein JAGGED1 (type 1 ALGS), and, rarely, the NOTCH2 gene (type 2 ALGS)[42,43]. Common manifestations include paucity of the intrahepatic bile ducts with obstructive-type cholestasis, skeletal abnormalities (butterfly-like vertebrae), congenital heart disease (usually peripheral pulmonary stenosis), ocular anomalies, and facial dysmorphism[42,44].
ATP-binding cassette (ABC) subfamily B member 4 (ABCB4), also known as multidrug resistance protein 3 (MDR3), is a membrane-associated transport protein. Almost exclusively expressed in the liver, ABCB4 is principally involved in biliary phospholipid secretion[45]. The ABCB4 alteration has deleterious effects on the hepatobiliary system, mostly caused by toxic bile with a potent detergent and lithogenic profile. The morphological spectrum of biliary lesions connected with ABCB4/MDR3 deficiency varies widely. The most commonly associated conditions are gallbladder disease-1 (GBD1), also known as low phospholipid-associated cholelithiasis (LPAC) syndrome, secondary sclerosing cholangitis (characterised by fibro-obliterative lesions and progressive small bile duct loss), and adult biliary-type liver fibrosis/cirrhosis[46,47].
GBD1 (LPAC syndrome) should be suspected in patients with symptomatic cholelithiasis and at least one of the following criteria: under 40 years of age at onset of symptoms; recurrence after chole
Neonatal sclerosing cholangitis (NSC) is a rare and severe genetic cholangiopathy, commonly progressing to liver failure. The disease usually manifests in the first weeks of life. Clinical features include jaundice, hepatosplenomegaly, pale stools, coagulopathy, signs of gastrointestinal bleeding, and high serum GGT levels. Percutaneous cholangiography typically reveals an intrahepatic sclerosing cholangiopathy[49,50].
Mutations in the doublecortin domain-containing 2 (DCDC2) gene have been recently associated with NSC induction. DCDC2 encodes doublecortin domain-containing protein 2 (the DCDC2 protein), which binds tubulin and facilitates microtubule polymerisation. Histologically, DCDC2 defects are generally expressed in the form of small-duct cholangiopathies, featuring focal concentric periductal lamellar fibrosis and progressive destruction of the small bile ducts[51,52]. Immunohistochemical examination typically confirms the absence of both the DCDC2 protein and the primary ciliary protein ACALT (acetylated alpha-tubulin), especially in septal and perihilar bile ducts, along with focal irregular expression in small interlobular bile ducts. Simple liver cysts have also been observed in some patients with DCDC2 deficiency[51,52].
Drug-induced liver injury (DILI) encompasses a wide variety of acute and chronic forms of hepatobiliary injury that can mimic virtually any liver disease, including primary biliary conditions such as PBC or PSC. Bile duct loss, ductopenia, and biliary-type fibrosis/cirrhosis are well-documented consequences of exposure to various drugs, remedies, and toxins[53-55].
In general, drug-induced injury can be dose-dependent (intrinsic) or dose-independent (idiosyncratic). Some drugs and remedies selectively damage larger bile ducts, predominantly by dose-dependent mechanisms, and can induce biliary abnormalities with cholangiographic and histomorphological features of sclerosing cholangiopathy[49]. In most cases, however, drug-induced mechanisms are dose-independent, unpredictable, and affect cholangiocytes of small bile ducts[53].
Drug-induced cholangiopathy usually manifests in fatigue, upper abdominal pain, and features of clinical and biochemical cholestasis. Histopathological findings depend on the severity and stage of disease progression. Mixed portal-based inflammation with eosinophils, inflammatory bile-duct injury, and centrilobular hepatocanalicular bilirubinostasis are the most common features reported in the acute phase. The chronic stage is characterised by degenerative changes of cholangiocytes, prominent bile duct injury and loss, as well as acute and chronic cholestasis (cholate stasis). Periportal ductular proliferation, associated with fibrous portal tract expansion and relatively mild portal inflammation, also occurs. Small bile duct injury can eventually progress to drug-induced ductopenia. In addition, drug-induced portal and periductal granulomata are not uncommon[53-55].
The most common drugs implicated in bile duct injury are neuroleptics, tricyclic antidepressants, anticonvulsants, antibiotics, and non-steroidal anti-inflammatory drugs. Recently, significant bile duct pathology was observed in patients treated with immune checkpoint inhibitors (CPI). Non-infective cholangitis with predominant neutrophilic or lymphocytic infiltrates, PBC-like changes, and fibrosing cholangitis mimicking sdPSC are the most common patterns of CPI-related cholangiopathy, which responds poorly to immunosuppression and can eventually lead to bile duct loss[56,57].
Diagnosis of DILI requires clinical suspicion, knowledge of the clinicopathological patterns of injury associated with the suspected agent, and exclusion of other possible causes. Underlying genetic defects and predispositions to adverse drug reactions should also be considered in certain cases.
Supplied by branches of the hepatic artery, the rich peribiliary vascular plexus is vulnerable to any disruption in arterial flow. Ischaemic cholangiopathy, a form of secondary sclerosing cholangiopathy (SSC), is a well-documented complication of transcatheter arterial chemoembolisation (TACE), intra-arterial infusion of chemotherapeutic agents, irradiation, radiofrequency ablation of hepatocellular carcinoma, arterial spasm after cocaine use, sickle cell crisis, HELLP syndrome, and hepatic artery thrombosis after liver transplantation[58,59]. Ischaemic cholangiopathy is characterised by injury to cholangiocytes, cytoplasmic vacuolisation, pyknotic nuclei, and subsequent desquamation of the biliary epithelial lining. Advanced lesions can lead to necrosis of the biliary tree resulting in bile leak, multiple fibrous strictures of the extrahepatic and intrahepatic bile ducts, cholangiectasis, and progressive bile duct loss. Bacterial and fungal infections are common complications[59].
Ischaemia of the intrahepatic bile ducts is likely involved in secondary sclerosing cholangitis in critically ill patients (SSC-CIP), a newly recognised form of SSC observed in patients after long-term treatment in intensive care units[60,61]. In addition to ischaemia, infections and toxic bile compounds are understood to contribute to the development of SSC-CIP. Interestingly, a cholangiopathy associated with some features of SSC-CIP was documented in patients with critical coronavirus disease 2019 (COVID-19) (see below).
The most frequent symptoms of SSC-CIP typically persist after recovery from the primary illness. These include jaundice, pruritus, and abdominal discomfort localised in the right upper quadrant of the abdomen. Recurrent biliary infections are also commonly observed. SSC-CIP is associated with rapid progression to liver cirrhosis[60,61,62].
Endoscopic retrograde cholangiopancreatography and magnetic resonance cholangiopancreatography (MRCP) are considered the gold standards for diagnosing SSC-CIP. Characteristic initial findings are multiple ribbon-like intraductal filling defects (biliary casts). In later stages, multiple irregular strictures, dilatations, thickening of the bile duct walls, and destruction of the intrahepatic bile ducts (except for the common bile duct, which results in a ‘pruned tree’ appearance) are routinely observed. Intrahepatic bile ducts are affected in all SSC-CIP patients, with abnormalities of the extrahepatic biliary tree occurring only in a minority of individuals[60,61].
Histopathological changes in SSC-CIP patients include degenerative epithelial alterations to the interlobular bile ducts, mild-to-moderate periductal inflammatory infiltrate, portal oedema, and periportal ductular reactions. Portal changes are accompanied by parenchymal hepatocanalicular bilirubinostasis, bile infarcts, and features of cholate stasis. Fibrous expansion of portal areas and rapid progression to advanced portoseptal fibrosis/cirrhosis have also been reported[61,62].
A variety of microbial agents can directly impact the hepatobiliary system in both normal and immunocompromised hosts. Bacterial and viral infections are understood to play an important role in the progression of ischaemic and immune-mediated bile duct injury such as GVHD and hepatic allograft rejection. Cytomegalovirus (CMV) and hepatitis C virus (HCV) infections after interferon/ribavirin treatment have been shown to trigger ductopenic rejection after orthotopic liver transplantation[63]. Hepatitis-associated bile duct lesions featuring prominent swelling and vacuolisation of cholangiocytes alongside periductal lymphocytic aggregates have been observed in association with HCV and hepatitis E virus infections. These lesions are mostly reversible and typically occupy individual segments along the circumferences of small bile ducts[64,65]. CMV infection of biliary epithelial cells in the presence of typical viral inclusions is not an uncommon finding in immunocompromised adults and neonates with CMV hepatitis[66].
Human immunodeficiency virus (HIV)-associated cholangiopathy is a biliary obstruction caused by a benign stricture of the biliary system in patients with advanced acquired immune deficiency syndrome (AIDS)[67,68]. Although the aetiology of this disease remains unclear, it is understood to occur in association with various opportunistic infections, such as CMV, Cryptosporidium spp., and Giardia lamblia. In most patients, MRCP usually results in an accurate diagnosis of HIV-associated cholangiopathy based on identification of characteristic ductal abnormalities (multiple intrahepatic and/or extrahepatic biliary strictures, papillary stenosis). Liver biopsy and histomorphological examination are reserved for inconclusive and complicated cases[68].
Recently, a syndrome of progressive bile duct injury characterised by a marked elevation in serum ALP was reported in some patients recovering from severe COVID-19[69,70]. Although the pathogenesis of post-Covid-19 cholangiopathy has not been fully elucidated, it is likely that some of its pathogenic mechanisms resemble those observed in SSC-CIP. A combination of ischaemic cholangiopathy related to microvascular coagulopathy and an imbalance between the deleterious effects of bile components and biliary protective mechanisms likely contribute to bile duct injury. In addition, direct virus-mediated alteration of the biliary epithelium may also be involved in the pathogenesis of cholangiopathy[70,71].
The most common MRCP findings are intrahepatic and extrahepatic bile duct beading with multiple short segmental strictures and intervening dilatations together with bile duct wall thickening and hyper-enhancement, all of which are consistent with SSC. Histology typically reveals fibro-oedematous expansion of portal tracts, prominent ductular reaction, neutrophilic inflammation, and severe parenchymal cholestasis[70,71].
Biliary system is affected by a variety of tumour-forming and neoplastic processes, both benign and malignant.
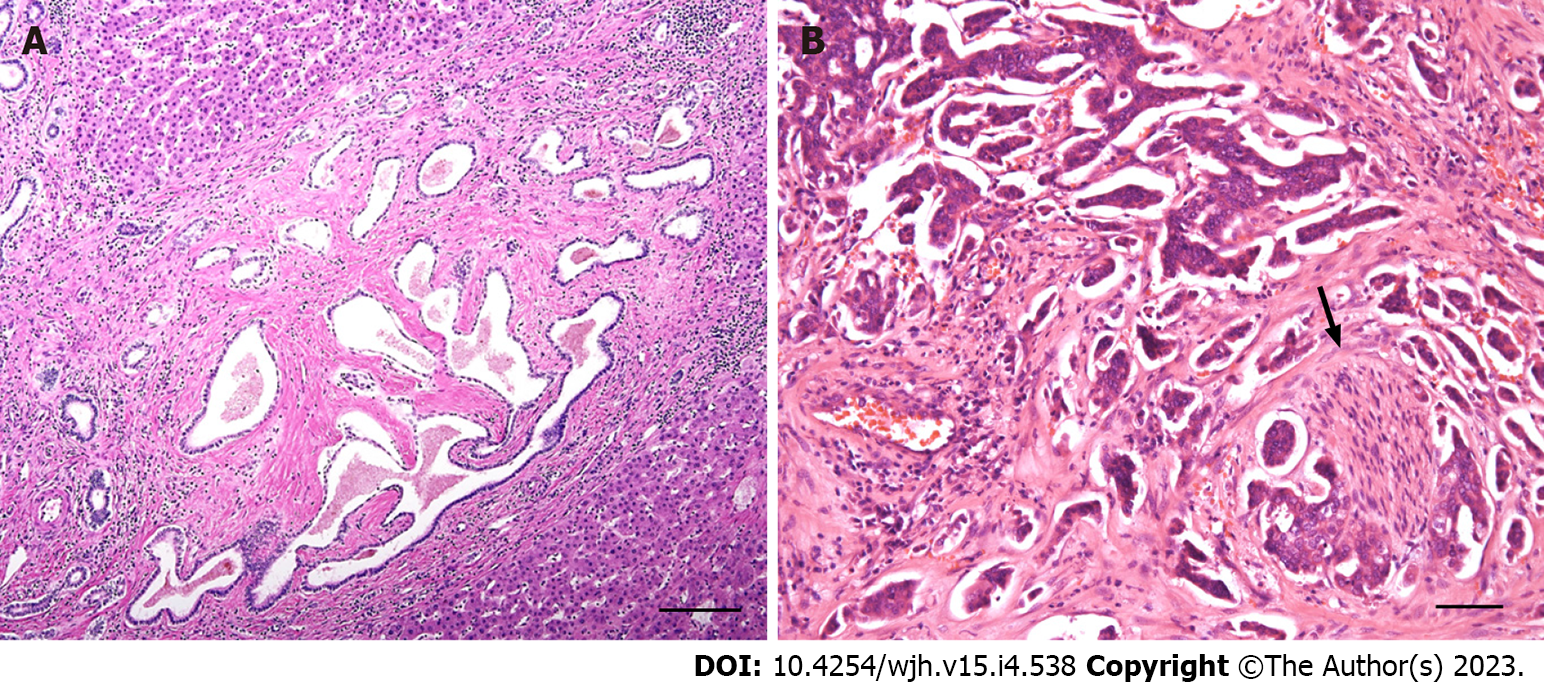
Von Meyenburg complex is a common hamartomatous tumour-like lesion likely representing a form of ductal plate malformation (Figure 7A). Biliary hamartomas are typically found adjacent to a portal area and may be multiple[72,73].
Bile duct adenoma (BDA) is a benign lesion composed of proliferating small bile ducts. The origin of BDA is controversial and a reactive process, a hamartomatous origin and a neoplastic aetiology are considered[73].
Many cases of cholangiocarcinoma have been reported to develop through multistep carcinogenesis. The current World Health Organization classification of tumours proposes several precursor lesions of the biliary tract that may precede invasive malignancy[74,75].
Dysplastic epithelial changes can take the form of microscopic, non-invasive lesions occurring in the extrahepatic and intrahepatic bile ducts and gallbladder. This condition is known as biliary intraepithelial neoplasia (BilIN). Based on the extent of cytoarchitectural atypia, BilIN can be subdivided into low-grade (former BilIN-1 and BilIN-2) and high-grade dysplasia (former BilIN-3)[74,75].
Intraductal papillary neoplasm of the bile duct (IPNB) is a macroscopic premalignant neoplastic process with multifocal intraductal papillary or villous projections covered by a single- to multi-layered biliary-type epithelium. While the epithelial lining is usually columnar, it can also exhibit pancreaticobiliary, gastric, intestinal, or oncocytic differentiation along with varying degrees of dysplasia. Resection is the standard therapy for IPNB. However, the multifocal nature of these tumours makes complete resection difficult; therefore, tumour recurrence is a frequent complication. Tubular and mucinous forms of invasive adenocarcinoma are associated with IPNB[75,76,77].
Mucinous cystic neoplasm (MCN) is a rare lesion occurring almost exclusively in women. These tumours are typically multilocular, with cystic spaces lined by the columnar mucinous epithelium overlying ovarian-like stroma. Based on the degree of dysplasia, MCN can be subdivided into low-grade and high-grade categories. This type of lesion can also progress to invasive adenocarcinoma[75,76,78].
Cholangiocarcinoma (CC) is a malignant epithelial neoplasm with biliary differentiation. CC may originate from intrahepatic bile ducts (intrahepatic or peripheral CC), the confluence of the right and left hepatic ducts (hilar CC or Klatskin tumour), or distal extrahepatic bile ducts (extrahepatic distal CC). Although these subtypes differ in overall clinical outcome, they share the basic histomorphological features (Figure 7B)[75,79].
Intrahepatic CC is the second most common primary hepatic malignancy and accounts for 10%-15% primary liver cancers. Incidence is highest in south-east Asia (> 80 cases/100000 persons per year in Thailand) but lowest in Europe (0.2-1.8 cases/100000 per year)[80].
Intrahepatic CC has two main subtypes: large duct CC, originating from the larger intrahepatic bile ducts near the hepatic hilus, and small duct CC, which preferentially occurs in the periphery of the liver parenchyma[75,79].
Aetiology in most cases of CC is unknown. However, several risk factors with a high geographic prevalence have been established. Large duct CC typically originates against a background of liver fluke infection, PSC, hepaticolithiasis, biliary tract malformations, and other rare conditions, mostly associated with chronic inflammation of the biliary tract. On the other hand, the risk factors for small duct intrahepatic CC are similar to those for primary hepatocellular carcinomas, including chronic viral hepatitis and/or non-biliary fibrosis and cirrhosis[80,81].
Recent research on intrahepatic CC has identified many molecular alterations, including KRAS, TP53, ARID1A, IDH1/2, BAP1, BRAF, and other mutations[75].
Both types of intrahepatic CC are aggressive carcinomas with high mortality and poor survival rates. Resectability of the tumour indicates better prognosis, but most patients present with unresectable tumours. Adjuvant therapy, usually a combination of gemcitabine and cisplatin, is recommended for patients with node-positive disease and positive resection margins. Other therapies such as radiation, TACE and ablation have been successful to varying degrees in unresectable cases. Furthermore, a combination of targeted therapy and immunotherapy has shown promise in the treatment of patients with CC[82,83].
The small intrahepatic bile ducts are affected by a wide range of non-neoplastic and neoplastic conditions and vary considerably in clinical and morphological presentation. Given their progressive nature and limited curative options, biliary diseases account for significant morbidity and mortality in both the adult and paediatric populations. Cholangiopathies often result in end-stage liver disease requiring liver transplantation.
Although the majority of cholangiopathies are long established, recent entities such as SSC-CIP and CPI-induced cholangiopathy have complicated the application of new therapeutic agents and approaches[56,60,61]. Cholangiopathy has also developed in some critically ill patients infected by β-coronavirus severe acute respiratory syndrome coronavirus 2, isolated for the first time in Wuhan in December 2019[69,71].
Although the pathogenesis of most cholangiopathies is complex and poorly understood, environmental and genetic factors are likely to be involved. Moreover, given that each cholangiopathy has a heterogeneous pathogenesis and a variable natural history, individual responses to therapy will be different[61,71].
Early identification of the pathological mechanisms that compromise cholangiocytes and the small bile ducts is crucial in determining appropriate treatment. While large bile duct pathology is usually visualised by imaging methods, liver biopsy is still considered an effective tool in the diagnosis of small bile duct injury. However, discrepancies between histomorphological, clinical, and biochemical presentations of small bile duct disorders can hinder the diagnostic process. Clinically clear cholestatic conditions manifesting in pruritus and elevated serum ALP, GGT, cholesterol and bile salts can progress without significant bilirubinostasis on biopsy. In addition, pathognomonic morphological signs of small duct cholangiopathies are often focal and may be easily overlooked during percutaneous liver biopsy. On the other hand, early-stage cholangiopathy with a fully developed histomorphological pattern may be accompanied by only minimal and non-specific biochemical abnormalities.
Uneven fibrosis progression within the liver parenchyma is another factor that complicates the diagnostic process, notably when staging fibrosis in a chronic biliary disease. The application of standard semiquantitative scoring systems can serve to underestimate or overestimate the fibrosis stage, particularly in a limited tissue specimen. The clinician should consider not only the data obtained from the liver biopsy but also the results of imaging and other methods (FibroScan, elastography) relevant to the assessment of liver fibrosis.
The implementation of new, predominantly non-invasive diagnostic tools and methods that bridge the shortcomings of liver biopsy is needed. Moreover, further studies are required to elucidate the environmental, genetic, and epigenetic background of the processes affecting the biliary tree and to improve the clinical management of both hereditary and acquired small duct cholangiopathies.
Understanding the underlying pathogenetic mechanisms, familiarity with basic morphological patterns, and the ability to correlate microscopical findings with clinical and laboratory results are important elements when forming an overall clinical picture and selecting the optimal therapeutic approach in patients with biliary diseases. To that end, the close cooperation of all medical specialists participating in the diagnostic process is recommended.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nugrahani AD, Indonesia; Parekh M, India; Tantau AI, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | de Jong IEM, van den Heuvel MC, Wells RG, Porte RJ. The heterogeneity of the biliary tree. J Hepatol. 2021;75:1236-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Yoo KS, Lim WT, Choi HS. Biology of Cholangiocytes: From Bench to Bedside. Gut Liver. 2016;10:687-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 5. | Nakanuma Y. Tutorial review for understanding of cholangiopathy. Int J Hepatol. 2012;2012:547840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Nakanuma Y, Demetris AJ, Ueno Y, Quaglia A. Cholangiopathy: genetics, mechanism, and pathology. Int J Hepatol. 2012;2012:950713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Portmann B, Zen Y. Inflammatory disease of the bile ducts-cholangiopathies: liver biopsy challenge and clinicopathological correlation. Histopathology. 2012;60:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Pollheimer MJ, Fickert P, Stieger B. Chronic cholestatic liver diseases: clues from histopathology for pathogenesis. Mol Aspects Med. 2014;37:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc. 2015;90:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (37)] |
| 10. | Desmet VJ. Destructive intrahepatic bile duct diseases. Recenti Prog Med. 1990;81:392-398. [PubMed] |
| 11. | Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008;12:203-217, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 12. | Izzo P, Gallo G, Codacci Pisanelli M, D'Onghia G, Macci L, Gabriele R, Polistena A, Izzo L, Izzo S, Basso L. Vanishing Bile Duct Syndrome in an Adult Patient: Case Report and Review of the Literature. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Park JW, Kim JH, Kim SE, Jung JH, Jang MK, Park SH, Lee MS, Kim HS, Suk KT, Kim DJ. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Sarcognato S, Sacchi D, Grillo F, Cazzagon N, Fabris L, Cadamuro M, Cataldo I, Covelli C, Mangia A, Guido M. Autoimmune biliary diseases: primary biliary cholangitis and primary sclerosing cholangitis. Pathologica. 2021;113:170-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Sherlock S. Primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune cholangitis. Clin Liver Dis. 2000;4:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, To KF. Evaluation of histological staging systems for primary biliary cirrhosis: correlation with clinical and biochemical factors and significance of pathological parameters in prognostication. Histopathology. 2014;65:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Namisaki T, Moriya K, Kitade M, Kawaratani H, Takeda K, Okura Y, Takaya H, Nishimura N, Seki K, Kaji K, Sato S, Sawada Y, Yamao J, Mitoro A, Uejima M, Mashitani T, Shimozato N, Nakanishi K, Furukawa M, Saikawa S, Kubo T, Yoshiji H. Clinical significance of the Scheuer histological staging system for primary biliary cholangitis in Japanese patients. Eur J Gastroenterol Hepatol. 2017;29:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Harada K, Hsu M, Ikeda H, Zeniya M, Nakanuma Y. Application and validation of a new histologic staging and grading system for primary biliary cirrhosis. J Clin Gastroenterol. 2013;47:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Nakanuma Y, Zen Y, Harada K, Sasaki M, Nonomura A, Uehara T, Sano K, Kondo F, Fukusato T, Tsuneyama K, Ito M, Wakasa K, Nomoto M, Minato H, Haga H, Kage M, Yano H, Haratake J, Aishima S, Masuda T, Aoyama H, Miyakawa-Hayashino A, Matsumoto T, Sanefuji H, Ojima H, Chen TC, Yu E, Kim JH, Park YN, Tsui W. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int. 2010;60:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Colina F, Pinedo F, Solís JA, Moreno D, Nevado M. Nodular regenerative hyperplasia of the liver in early histological stages of primary biliary cirrhosis. Gastroenterology. 1992;102:1319-1324. [PubMed] [DOI] [Full Text] |
| 21. | Warnes TW, Roberts SA, Smith A, Cope VM, Vales P, Haboubi NY, McMahon RF. Portal hypertension in primary biliary cholangitis: prevalence, natural history and histological correlates. Eur J Gastroenterol Hepatol. 2021;33:1595-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Chazouillères O. Overlap Syndromes. Dig Dis. 2015;33 Suppl 2:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E; International Autoimmune Hepatitis Group. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 24. | Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology. 2002;35:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Deliwala S, Sundus S, Haykal T, Elbedawi MM, Bachuwa G. Small Duct Primary Sclerosing Cholangitis: An Underdiagnosed Cause of Chronic Liver Disease and Cirrhosis. Cureus. 2020;12:e7298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Nguyen CM, Kline KT, Stevenson HL, Khan K, Parupudi S. Small duct primary sclerosing cholangitis: A discrete variant or a bridge to large duct disease, a practical review. World J Hepatol. 2022;14:495-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 27. | Naess S, Björnsson E, Anmarkrud JA, Al Mamari S, Juran BD, Lazaridis KN, Chapman R, Bergquist A, Melum E, Marsh SG, Schrumpf E, Lie BA, Boberg KM, Karlsen TH, Hov JR. Small duct primary sclerosing cholangitis without inflammatory bowel disease is genetically different from large duct disease. Liver Int. 2014;34:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Olsson R, Glaumann H, Almer S, Broomé U, Lebrun B, Bergquist A, Björnsson E, Prytz H, Danielsson A, Lindgren S. High prevalence of small duct primary sclerosing cholangitis among patients with overlapping autoimmune hepatitis and primary sclerosing cholangitis. Eur J Intern Med. 2009;20:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Tanaka A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver. 2019;13:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Lee HE, Zhang L. Immunoglobulin G4-related hepatobiliary disease. Semin Diagn Pathol. 2019;36:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Nakanuma Y, Ishizu Y, Zen Y, Harada K, Umemura T. Histopathology of IgG4-Related Autoimmune Hepatitis and IgG4-Related Hepatopathy in IgG4-Related Disease. Semin Liver Dis. 2016;36:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Jang SY, Han YS, Lee SY, Han JR, Kweon YO, Tak WY, Park SY, Lee YR, Ryeom HK, Cha JG, Hong J, Kang YN. A Case of Hepatic Immunoglobulin G4-Related Disease Presenting as an Inflammatory Pseudotumor and Sclerosing Cholangitis. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Demetris AJ, Bellamy C, Hübscher SG, O'Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ, Del Bello A, Reinholt FP, Haga H, Adeyi O, Czaja AJ, Schiano T, Fiel MI, Smith ML, Sebagh M, Tanigawa RY, Yilmaz F, Alexander G, Baiocchi L, Balasubramanian M, Batal I, Bhan AK, Bucuvalas J, Cerski CTS, Charlotte F, de Vera ME, ElMonayeri M, Fontes P, Furth EE, Gouw ASH, Hafezi-Bakhtiari S, Hart J, Honsova E, Ismail W, Itoh T, Jhala NC, Khettry U, Klintmalm GB, Knechtle S, Koshiba T, Kozlowski T, Lassman CR, Lerut J, Levitsky J, Licini L, Liotta R, Mazariegos G, Minervini MI, Misdraji J, Mohanakumar T, Mölne J, Nasser I, Neuberger J, O'Neil M, Pappo O, Petrovic L, Ruiz P, Sağol Ö, Sanchez Fueyo A, Sasatomi E, Shaked A, Shiller M, Shimizu T, Sis B, Sonzogni A, Stevenson HL, Thung SN, Tisone G, Tsamandas AC, Wernerson A, Wu T, Zeevi A, Zen Y. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 431] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 34. | Rastogi A, Nigam N, Gayatri R, Bihari C, Pamecha V. Biliary Epithelial Senescence in Cellular Rejection Following Live Donor Liver Transplantation. J Clin Exp Hepatol. 2022;12:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Trussoni CE, O'Hara SP, LaRusso NF. Cellular senescence in the cholangiopathies: a driver of immunopathology and a novel therapeutic target. Semin Immunopathol. 2022;44:527-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Salomao M, Dorritie K, Mapara MY, Sepulveda A. Histopathology of Graft-vs-Host Disease of Gastrointestinal Tract and Liver: An Update. Am J Clin Pathol. 2016;145:591-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Debray D, Corvol H, Housset C. Modifier genes in cystic fibrosis-related liver disease. Curr Opin Gastroenterol. 2019;35:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Odièvre M, Martin JP, Hadchouel M, Alagille D. Alpha1-antitrypsin deficiency and liver disease in children: phenotypes, manifestations, and prognosis. Pediatrics. 1976;57:226-231. [PubMed] |
| 39. | Raynaud P, Tate J, Callens C, Cordi S, Vandersmissen P, Carpentier R, Sempoux C, Devuyst O, Pierreux CE, Courtoy P, Dahan K, Delbecque K, Lepreux S, Pontoglio M, Guay-Woodford LM, Lemaigre FP. A classification of ductal plate malformations based on distinct pathogenic mechanisms of biliary dysmorphogenesis. Hepatology. 2011;53:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Lee-Law PY, van de Laarschot LFM, Banales JM, Drenth JPH. Genetics of polycystic liver diseases. Curr Opin Gastroenterol. 2019;35:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Luan W, Hao CZ, Li JQ, Wei Q, Gong JY, Qiu YL, Lu Y, Shen CH, Xia Q, Xie XB, Zhang MH, Abuduxikuer K, Li ZD, Wang L, Xing QH, Knisely AS, Wang JS. Biallelic loss-of-function ZFYVE19 mutations are associated with congenital hepatic fibrosis, sclerosing cholangiopathy and high-GGT cholestasis. J Med Genet. 2021;58:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Kohut TJ, Gilbert MA, Loomes KM. Alagille Syndrome: A Focused Review on Clinical Features, Genetics, and Treatment. Semin Liver Dis. 2021;41:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 43. | Gilbert MA, Bauer RC, Rajagopalan R, Grochowski CM, Chao G, McEldrew D, Nassur JA, Rand EB, Krock BL, Kamath BM, Krantz ID, Piccoli DA, Loomes KM, Spinner NB. Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification. Hum Mutat. 2019;40:2197-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Ayoub MD, Kamath BM. Alagille Syndrome: Current Understanding of Pathogenesis, and Challenges in Diagnosis and Management. Clin Liver Dis. 2022;26:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 45. | Lincke CR, Smit JJ, van der Velde-Koerts T, Borst P. Structure of the human MDR3 gene and physical mapping of the human MDR locus. J Biol Chem. 1991;266:5303-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Sticova E, Jirsa M. ABCB4 disease: Many faces of one gene deficiency. Ann Hepatol. 2020;19:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Reichert MC, Lammert F. ABCB4 Gene Aberrations in Human Liver Disease: An Evolving Spectrum. Semin Liver Dis. 2018;38:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Wang HH, Portincasa P, Liu M, Wang DQ. Genetic Analysis of ABCB4 Mutations and Variants Related to the Pathogenesis and Pathophysiology of Low Phospholipid-Associated Cholelithiasis. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Mieli-Vergani G, Vergani D. Sclerosing cholangitis in the paediatric patient. Best Pract Res Clin Gastroenterol. 2001;15:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Feldman AG, Sokol RJ. Neonatal Cholestasis. Neoreviews. 2013;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Grammatikopoulos T, Sambrotta M, Strautnieks S, Foskett P, Knisely AS, Wagner B, Deheragoda M, Starling C, Mieli-Vergani G, Smith J; University of Washington Center for Mendelian Genomics, Bull L, Thompson RJ. Mutations in DCDC2 (doublecortin domain containing protein 2) in neonatal sclerosing cholangitis. J Hepatol. 2016;65:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Girard M, Bizet AA, Lachaux A, Gonzales E, Filhol E, Collardeau-Frachon S, Jeanpierre C, Henry C, Fabre M, Viremouneix L, Galmiche L, Debray D, Bole-Feysot C, Nitschke P, Pariente D, Guettier C, Lyonnet S, Heidet L, Bertholet A, Jacquemin E, Henrion-Caude A, Saunier S. DCDC2 Mutations Cause Neonatal Sclerosing Cholangitis. Hum Mutat. 2016;37:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Dobreva I, Karagyozov P. Drug-induced Bile Duct Injury - A Short Review. Curr Drug Metab. 2020;21:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Björnsson ES, Andrade RJ. Long-term sequelae of drug-induced liver injury. J Hepatol. 2022;76:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 56. | Berry P, Kotha S, Zen Y, Papa S, El Menabawey T, Webster G, Joshi D, Heneghan M. Immune checkpoint inhibitor-related cholangiopathy: Novel clinicopathological description of a multi-centre cohort. Liver Int. 2023;43:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 57. | Zhang HC, Wang LS, Miller E. Hepatobiliary and Pancreatic Adverse Events. Adv Exp Med Biol. 2021;1342:339-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008;28:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Goria O, Archambeaud I, Lemaitre C, Dutheil D, Plessier A, Rautou PE, Hernandez-Gea V, Valla D. Ischemic cholangiopathy: An update. Clin Res Hepatol Gastroenterol. 2020;44:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Gudnason HO, Björnsson ES. Secondary sclerosing cholangitis in critically ill patients: current perspectives. Clin Exp Gastroenterol. 2017;10:105-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Martins P, Verdelho Machado M. Secondary Sclerosing Cholangitis in Critically Ill Patients: An Underdiagnosed Entity. GE Port J Gastroenterol. 2020;27:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Eurich D, Faber W, Neuhaus P, Seehofer D. Secondary Sclerosing Cholangitis in Critically Ill Patients: Clinical Presentation, Cholangiographic Features, Natural History, and Outcome: A Series of 16 Cases. Medicine (Baltimore). 2015;94:e2188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Stanca CM, Fiel MI, Kontorinis N, Agarwal K, Emre S, Schiano TD. Chronic ductopenic rejection in patients with recurrent hepatitis C virus treated with pegylated interferon alfa-2a and ribavirin. Transplantation. 2007;84:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Poulsen H, Christoffersen P. Abnormal bile duct epithelium in liver biopsies with histological signs of viral hepatitis. Acta Pathol Microbiol Scand. 1969;76:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Dhingra S, Ward SC, Thung SN. Liver pathology of hepatitis C, beyond grading and staging of the disease. World J Gastroenterol. 2016;22:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 66. | Oku T, Maeda M, Waga E, Wada Y, Nagamachi Y, Fujita M, Suzuki Y, Nagashima K, Niitsu Y. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol. 2005;40:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Devarbhavi H, Sebastian T, Seetharamu SM, Karanth D. HIV/AIDS cholangiopathy: clinical spectrum, cholangiographic features and outcome in 30 patients. J Gastroenterol Hepatol. 2010;25:1656-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Tonolini M, Bianco R. HIV-related/AIDS cholangiopathy: pictorial review with emphasis on MRCP findings and differential diagnosis. Clin Imaging. 2013;37:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 69. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 70. | Caballero-Alvarado J, Zavaleta Corvera C, Merino Bacilio B, Ruiz Caballero C, Lozano-Peralta K. Post-COVID cholangiopathy: A narrative review. Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 71. | Yanny B, Alkhero M, Alani M, Stenberg D, Saharan A, Saab S. Post-Covid- 19 Cholangiopathy:A Systematic Review. J Clin Exp Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 72. | Torbenson MS. Hamartomas and malformations of the liver. Semin Diagn Pathol. 2019;36:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Aishima S, Tanaka Y, Kubo Y, Shirabe K, Maehara Y, Oda Y. Bile duct adenoma and von Meyenburg complex-like duct arising in hepatitis and cirrhosis: pathogenesis and histological characteristics. Pathol Int. 2014;64:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Nakanuma Y, Kakuda Y, Sugino T, Sato Y, Fukumura Y. Pathologies of Precursor Lesions of Biliary Tract Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 75. | Lendvai G, Szekerczés T, Illyés I, Dóra R, Kontsek E, Gógl A, Kiss A, Werling K, Kovalszky I, Schaff Z, Borka K. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol Oncol Res. 2020;26:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 76. | Nakanuma Y, Uesaka K, Miyayama S, Yamaguchi H, Ohtsuka M. Intraductal neoplasms of the bile duct. A new challenge to biliary tract tumor pathology. Histol Histopathol. 2017;32:1001-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 77. | Nakanuma Y, Kakuda Y, Uesaka K. Characterization of Intraductal Papillary Neoplasm of the Bile Duct with Respect to the Histopathologic Similarities to Pancreatic Intraductal Papillary Mucinous Neoplasm. Gut Liver. 2019;13:617-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 78. | Van Treeck BJ, Lotfalla M, Czeczok TW, Mounajjed T, Moreira RK, Allende DS, Reid MD, Naini BV, Westerhoff M, Adsay NV, Kerr SE, Rizvi SH, Smoot RL, Liu Y, Davila J, Graham RP. Molecular and Immunohistochemical Analysis of Mucinous Cystic Neoplasm of the Liver. Am J Clin Pathol. 2020;154:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Guedj N. Pathology of Cholangiocarcinomas. Curr Oncol. 2022;30:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (35)] |
| 80. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 501] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 81. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 82. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 83. | Liao P, Cao L, Chen H, Pang SZ. Analysis of metastasis and survival between extrahepatic and intrahepatic cholangiocarcinoma: A large population-based study. Medicine (Baltimore). 2021;100:e25635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |