Published online Apr 27, 2023. doi: 10.4254/wjh.v15.i4.460
Peer-review started: September 19, 2022
First decision: December 1, 2022
Revised: December 20, 2022
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: April 27, 2023
Processing time: 213 Days and 7.3 Hours
Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer, accounting for 75%-85% of cases. Although treatments are given to cure early-stage HCC, up to 50%-70% of individuals may experience a relapse of the illness in the liver after 5 years. Research on the fundamental treatment modalities for recurrent HCC is moving significantly further. The precise selection of in
Core Tip: Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer, and up to 50%-70% of individuals may experience a relapse of the illness in the liver after 5 years. This review will provide novel approaches to improve the prognosis of patients with recurring HCC with the apparent lack of data to guide the clinical treatment. Neoadjuvant and/or adjuvant therapy methods potentially elevate the opportunity of cure in refractory patients with recurrent HCC and contribute to a better long-term prognosis.
- Citation: Gao YX, Ning QQ, Yang PX, Guan YY, Liu PX, Liu ML, Qiao LX, Guo XH, Yang TW, Chen DX. Recent advances in recurrent hepatocellular carcinoma therapy. World J Hepatol 2023; 15(4): 460-476
- URL: https://www.wjgnet.com/1948-5182/full/v15/i4/460.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i4.460
With an expected 906000 new cases and over 800000 fatalities, primary liver cancer is ranked as the sixth most commonly diagnosed malignancy and the third most prevalent cause of cancer-related deaths worldwide in 2020[1]. Hepatocellular carcinoma (HCC) accounts for 75%-85% of cases of primary liver cancer[2]. As medical care has improved, liver transplantation (LT) has emerged as the best option for individuals with HCC that is either incurable or who have progressive liver damage as a result of their HCC[3]. Although patients receive treatments for early stage HCC intending to cure the disease, up to 50%-70% of patients may experience disease relapse in the liver after 5 years[4,5]. This is not only related to the inadequacy of the surgery (i.e., positive surgical margin) but is also frequently associated with the development of de novo tumors as the disease progresses. Additionally, 70% of patients with recurrent HCC experience an early relapse within 2 years of surgery, which is nearly incurable and has been linked to apoor prognosis[6]. The molecular mechanisms underlying the prompt relapse of HCC are still unclear.
In a small percentage of HCC patients with multifocal intra or extrahepatic relapse, liver function impairment, and tumors that cannot be removed, rehepatectomy is necessary[7]. According to reports, HCC patients with tumors that meet the Milan criteria had excellent 5-year survival rates and minimal risks of relapse after LT[8]. Until the disease worsened or tolerance was established, monotherapy was thought to be the only course of therapy. At least two treatments administered simultaneously or within 4 wk of each other were considered multimodal therapy[9]. Preclinical findings suggest that neoadjuvant and adjuvant dosages should be used in combination instead of using either of them individually[10].
Therefore, it is crucial to devise the best treatment plans and fully comprehend the mechanism of HCC relapse. As a result of these problems, numerous researchers have looked into the benefits of neoadjuvant and adjuvant therapeutic approaches to lower relapse rates and enhance prognosis. Adjuvant therapy is not typically advised following curative treatment since its benefits are unclear[11]. It is clear that some additional treatment modalities are necessary, and in this regard, either neoadjuvant or adjuvant approaches are mostly taken into consideration. These include adjuvant antiviral therapy, repeated excision, transarterial chemoembolization (TACE), transarterial radioembolization, radiofrequency ablation (RFA), LT, tyrosine kinase inhibitors, and immunotherapy. Here, we will discuss the current state of knowledge on and recent advances in the therapy of recurrent HCC in this narrative review.
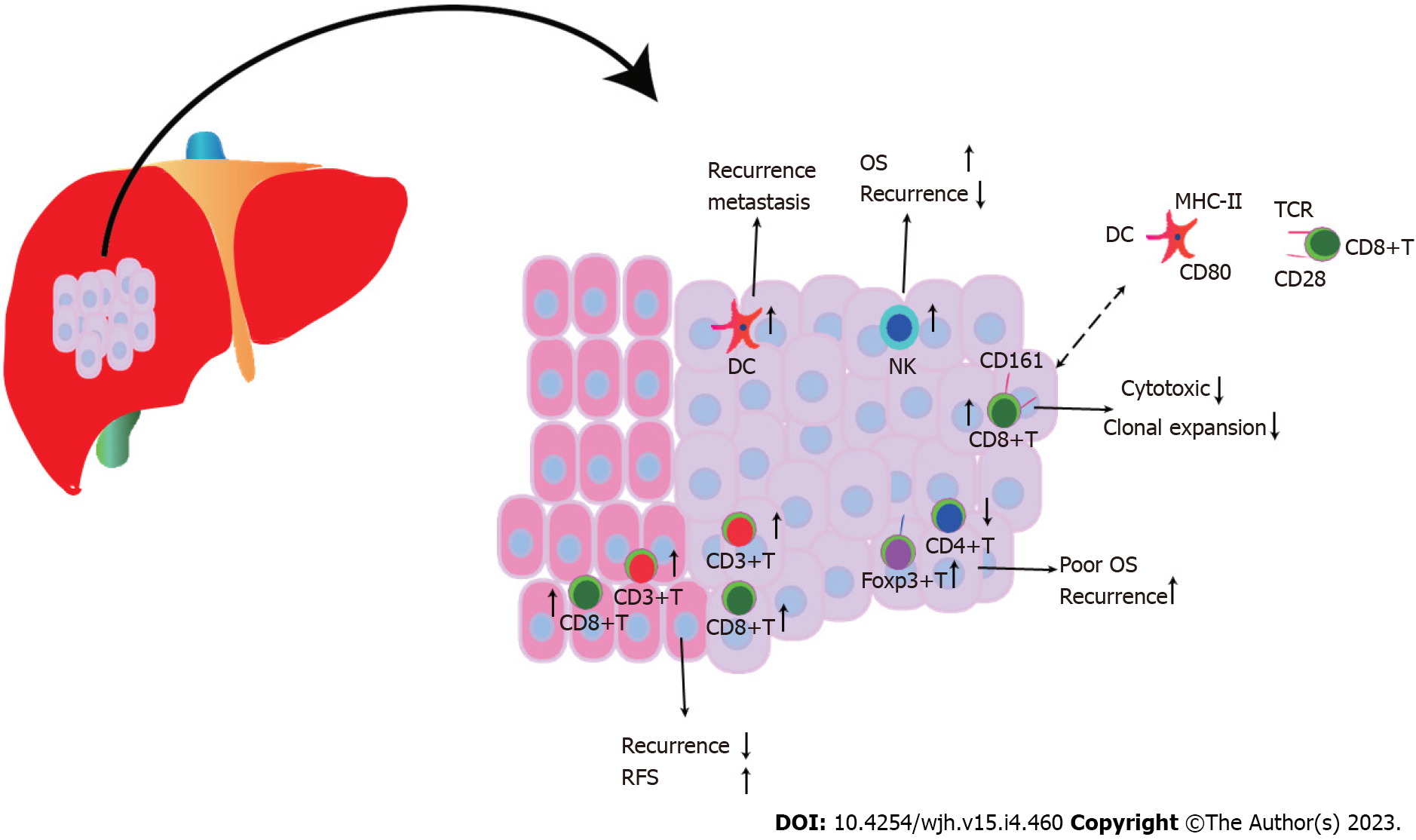
Up to 70% of early HCC recurrence cases were thought to manifest within the first two years following curative therapy; relapses that occur after this point are referred to as late HCC recurrences[12]. Malignant, immunological, and stromal cells are made up of heterogeneous cell types that interact spatiotemporally in complex tumor ecosystems[13]. Recent research has found similarities between the genetic variants of primary and early recurrent HCC[14]. Nevertheless, explanations for differences between the cellular ecosystems of primary and recurrent HCC are still being sought after. Early-relapse HCC displayed decreased regulatory T cells (Tregs) and higher dendritic cells (DCs) and CD8+ T cells compared to primary HCC, which were associated with a poor prognosis[15], as shown in Figure 1. Treg recruitment is a characteristic of the immunosuppressive milieu of primary HCC[16]. In contrast to the traditional depletion state in primary HCC, CD8+ T cells in relapsed HCC displayed higher CD161 expression, a low cytotoxic state that was innate, and reduced clonal expansion. This is significant because the immune escape mechanism underlying HCC relapse was connected to the inhibition of DC antigen presentation and infiltration of innate-like CD8+ T cells[15], which caused intrahepatic dissemination of HCC. Tregs and intragraft Toll-like receptor 4/C-X-C motif chemokine 10/CXCR3 C-X-C motif chemokine receptor 3 levels were higher in patients with HCC recurrence following LT, which was further substantiated in a rat transplantation model[17].
According to a meta-analysis, HCC patients with high Foxp3+ T cell infiltration had worse 1-, 3-, and 5-year survival rates and a greater rate of recurrence than patients with low Foxp3+ T cell infiltration[18]. The frequency of CD8+ tumor-infiltrating lymphocytes in the intratumor and margin area was positively correlated with overall survival (OS) and disease-free survival (DFS) in two HCC cohorts (a combined total of 449), and a larger proportion of CD8+ tumor-infiltrating lymphocytes was associated with a lower recurrence rate[19]. As a result of their inflammatory condition, increased densities of CD8+T lymphocytes that infiltrate the liver in HCC patients contribute to tumor recurrence and carcinogenesis[20]. High CD3+ and CD8+ T cell densities were associated with minimal relapse and extended relapse-free survival in both the tumor center and margin[21]. In HCC patients, increased FoxP3(+) regulatory T cells encouraged a gradual decline in CD4+ cytotoxic T cells, which contributed to poorer survival and high recurrence rates[22]. A high Foxp3/CD8 ratio indicated a higher Edmondson-Steiner nuclear grade, relapse, and shorter OS and DFS, along with worse differentiation[23]. In HCC patients who received LT but not Foxp3+ T-lymphocytes, a correlation between CD4/CD8 ratio and tumor recurrence was established[24]. After surgical excision, high DC infiltration in HCC nodules can be used as a predictor of the recurrence and metastasis of the disease[25]. The response to sorafenib improved relapse-free survival, and OS in patients was significantly influenced by the increased density of natural killer (NK) cells[26].
In HCC patients after surgery, higher interleukin (IL-11) levels enhanced tumor expansion, and in genetic mouse model, suppression of IL-11-STAT3 signaling greatly reduced cell proliferation and post-surgical recurrences of HCC tumors[27]. Local recurrence is caused by the invasion of local tumor blood flow and peritumoral diffusion, whereas systemic dissemination is caused by the “rehoming” of circulating tumor cells that have spread from the initial nodules[28]. HCC recurrence can occur as a result of tumor cells that are circulating or at rest, evading the host’s immune responses. The total somatic mutations and copy number depletion of WNK2 (WNK lysine deficient protein kinase 2) were associated with low levels of WNK2 protein expression, premature tumor relapse, and poor cumulative survival in patients with HCC following curative excision, indicating a tumor-suppressor role of WNK2[29]. WNK2 inactivation results in the recruitment of tumor-associated macrophages, ERK1/2 signaling activation, tumor growth, and metastasis.
Following therapy, HCC shows pathological modifications; therapy induced pathological variation, particularly sarcomatous transformation, results in random and frequent recurrences after RFA[30]. It is generally accepted that recurrent HCC following curative therapy was caused by both initial incomplete treatment as well as current technological and biomarker limitations that make it difficult to detect preexisting microscopic tumors[31]. Occasionally, local therapies like TACE result in the direct diffusion of tumor cells from the RFA needle, which eventually causes a relapse of HCC[32]. However, multicentric origin HCC developed from de novo carcinogenic effect following curative excision, and the latter has a better OS than the former[33], consistent with the results of Kuo et al[34]. The incidence of intrahepatic metastasis and multicentric recurrent HCC was 59.4% and 27.5%, respectively, which were accompanied by loss of heterozygosity (63.8%) and microsatellite instability (30.0%) between primary and recurrent tumors[34]. Concerning previously unidentified circulating tumor cells or preexisting metastasis caused by the current technology that contribute to extrahepatic relapse, metastatic tumor lesions in the graft are originally formed from circulating cells or extrahepatic locales, providing a greater potential for biological advancement[35].
Adjuvant antiviral therapy has been shown to decrease multicentric HCC recurrence, which in turn reduces post-treatment recurrence[33]. However, the ideal time for starting therapy with direct-acting antivirals (DAAs) for hepatitis C virus (HCV)-related HCC patients following surgical resection, and the impact of DAA on HCC recurrence remain unclear. Low risk of HCC recurrence after DAA treatment was suggested in some studies, while others have reported contrasting outcomes. Furthermore, there is conflicting evidence concerning HCV-related HCC recurrence in previously cured patients following virus elimination with DAAs. With a 5.7-mo follow-up in 20 DAA-treated HCV patients, a high rate of early HCV-related HCC recurrence was observed[36]. Even though DAA treatment was not associated with HCC or early HCC recurrence, a higher proportion of DAA-treated patients accepted potential curative therapy for recurrent HCV-related HCC compared to untreated patients (32.0% vs 24.6%) and developed a non-significant complete or partial response (45.3% vs 41.0%)[37]. A systematic review has highlighted an association of HCC recurrence with the status of previous HCC recurrences and the shorter interval between HCV-related HCC complete response and initiation of DAA treatment, and similar recurrences in patients treated with DAAs, those treated with interferon, or untreated patients[38]. HCV-related HCC patients, who had a shorter interval between HCC treatment and DAA therapy (less than 4 mo), appeared to be at greater risk, with a relapse rate of 41.2%[39]. DAA treatment following curative HCC therapy was not associated with early or advanced cancer recurrence[40]. DAA treatment is not associated with a high risk of recurrence in LT patients with HCV and HCC who achieved an original complete response to local-regional therapy, but rather involves a low risk of waitlist dropout due to cancer aggression or death[41]. In three separate prospective cohorts, no increased risk of HCC recurrence following DAA therapy was found, particularly in patients who received curative treatment, such as LT[42].
Even though the impact of DAAs on HCV-related HCC recurrence remains debatable, the results of anti-hepatitis B virus (HBV) treatment following HCC therapy showed that NAs might potentially inhibit HCC recurrence after curative hepatectomy in patients with HBV-related HCC[43]. Managing viral conditions and reactivation of viral replication plays a major role in suppressing HCC recurrence, maintaining liver function, and improving survival for HBV-related HCC post-therapy[44]. After curative therapy, NAs significantly improved recurrence-free survival and OS in HBV-related HCC patients, and entecavir was on par with other NAs, including lamivudine and adefovir, in this regard[45]. Another study discovered that after curative therapy, antiviral therapy with NA could increase survival and reduce early recurrence in patients with HBV-related HCC[46]. NA with or without anti-HBs immunoglobulins was significantly effective in inhibiting post-LT HBV recurrence[47]. In a limited sample cohort, NA did not lower the short-term recurrence rate but increased the elimination of postoperative serum HBV and remnant liver volume, which resulted in significantly improved tolerance to follow-up treatment for HCC recurrence[48]. In a large cohort of 4569 patients with HBV-related HCC who underwent curative resection, the anti-HBV therapy cohort had a significantly lower 6-year HCC recurrence rate than the control cohort [anti-HBV therapy, 45.6%; 95% confidence interval (CI): 36.5%-54.6% vs control, 54.6%; 95%CI: 52.5%-56.6%][49]. According to a previous study, recipients who accepted LT by removing HBV-infected initial liver at undetected serum HBV DNA levels continue to have an elevated risk for posttransplant recurrent HBV due to the absence of any particular treatment[50]. In comparison to lamivudine for HCC after curative therapy, entecavir is associated with a four-fold higher one-year OS rate and lower HCC recurrence, suggesting that entecavir may be more suitable for HBV-related HCC patients[51].
Only a small proportion of patients with recurrent HCC are candidates for repeat hepatectomy due to recurrent multifocal tumors and compromised liver function[52]. Twenty-two patients with recurrent HCC following LT (2 intrahepatic HCC patients and 20 extrahepatic HCC patients) received complete hepatectomy and had a longer median survival of 35 mo than unresected patients with a median survival of 15 mo[53], suggesting a less aggressive tumor biology. According to a retrospective cohort study, 15 patients with HCC recurrence who underwent LT had a better 5-year OS rate and 5-year DFS rate than the patients with RFA treatment (35% vs 28%, and 16% vs 0%, respectively)[54]. A recent study reported that repeat laparoscopic liver resection (LR) for recurrent HCC is both feasible and suitable with promising short-term results[55]. Laparoscopic repeat LR was associated with shorter hospitalization and prolonged operation time compared to open repeat LR for recurrent HCC but they had similar perioperative results for primary HCC except for a longer operation time[56]. Patients who underwent wedge resection during laparoscopic repeat LR showed a significantly lower postoperative complication rate than open repeat LR (7.2% vs 21.8%)[57]. Even though patients with open LR have a higher morbidity rate than those who underwent LR for primary HCC, there are no striking differences in the clinical characteristics of repeat laparoscopic LR based on prior resection method (open or laparoscopic) or tumor location (segments 7 and 8 or other)[55].
A meta-analysis of 767 patients, 334 of who had repeat laparoscopic hepatectomy and 433 of whom had repeat open hepatectomy, discovered that repeat laparoscopic hepatectomy resulted in less intraoperative blood loss, fewer major complications, shortened hospitalization, and a higher rate of R0 resection[58]. The repeat-surgery group had better liver function, long recurrence-free survival (16.5 mo vs 11.4 mo), and better 5-year survival after recurrence (repeat surgery group vs non-surgery group: overall, 53.0% vs 25.7%; intrahepatic recurrence, 73.8% vs 37.2%; extrahepatic recurrence, 30.0% vs 0%; intrahepatic and extrahepatic recurrence, 34.1% vs 10.6%) compared to the non-surgery group[59] for recurrent HCC. Patients with recurrences within 6 mo of resection had poor survival outcomes than those who experienced recurrences later, and patients with intrahepatic-only recurrences had a better prognosis than those with either extrahepatic-only or intra and extrahepatic recurrences[60]. Additionally, repeated resection of recurrences with a remediable objective produced better outcomes than other therapy options[60]. After 18 mo of initial hepatectomy, repeat hepatectomy may be suggested as a treatment for recurrent HCC. When compared to patients with intrahepatic metastasis, repeat hepatectomy improves survival rates in HCC patients with multicentric occurrence[61]. Although RFA is associated with lower grade 3 morbidity and shorter hospital stay, repeated hepatic resection resulted in a longer median recurrence-free survival vs RFA (23.6 mo vs 15.2 mo) in patients with recurrent HCC[62]. Resection can be advised as a treatment option for patients with extrahepatic recurrent HCC in conjunction with local treatment for intrahepatic recurrent HCC due to the superior outcomes[63]. At the third (71.3% vs 65.7%), fifth (59.9% vs 45.4%), and tenth (35.4% vs 32.2%) year follow-up, repeat hepatectomy improved long-term OS more than RFA and showed a late survival advantage for patients with recurrent HCC despite a higher morbidity rate[64].
According to reports, the results of salvage LT (SLT) for recurrent HCC following hepatectomy are comparable to the outcomes of initial transplantation, even when examined on an intention-to-treat basis[65]. LT seems to be the most effective treatment for HCC patients to remove both tumors and underlying liver diseases, but the scarcity of organ donors available globally and stringent criteria for patients who are not eligible for transplantation are the major challenges. However, recurrent HCC patients following LT have a poorer prognosis, with a median OS of 10-13 mo as opposed to 2-3 years for patients who had hepatectomy[66-68]. In 2000, Majno et al[69] made the first suggestion for SLT, which was used in patients with recurrent HCC or liver dysfunction following primary hepatectomy as initial treatment. Fortunately, liver transplants were an option for 80% of patients with recurrent HCC following curative hepatectomy[70]. A case of salvage living donor LT in a patient with tumor recurrence following surgical resection of combined HCC and cholangiocarcinoma has multiple tumor recurrences after 21 mo due to the more aggressive tumor biology of this type of cancer[71]. The patients receiving SLT therapy demonstrated better DFS than those receiving re-resection or RFA, which is a beneficial strategy for intrahepatic recurrent HCC, particularly for patients with multicentric occurrence that is related to better long-term outcomes than the intrahepatic metastasis pattern[72].
SLT (n = 16) revealed poorer short-term perioperative results than repeat LR (n = 16), with a higher incidence of morbidity (57.8% vs 5.4%), reoperations (39.1% vs 0%), renal dysfunction (30.1% vs 3%), bleeding (19.8% vs 2.2%), prolonged intensive care unit stay (4 d vs 0 d), and hospitalization (19.8 d vs 7.1 d) but significantly decreased recurrence (15.4% vs 70.3%) and 5-year cumulative incidence of recurrences (19.4% vs 68.4%) to improve long-term survival outcomes for recurrent HCC[73]. SLT was found in a meta-analysis to have higher blood loss, longer hospital stays and surgeries, increased DFS, and elevated risk of postoperative morbidity than repeat LR, while there was no clear difference in postoperative mortality or OS[74] for recurrent HCC. In terms of disease-specific and recurrence-free survival of patients with intrahepatic HCC recurrence, SLT with transplantable patients is superior to repeat resection, even in patients with Child-Pugh class A liver cirrhosis[75]. Only 56% of cases can be cured using the SLT strategy. A successful SLT strategy is predicted by higher end-stage liver disease scores at the start of the strategy and the absence of pre-resection TACE[76]. Even though SLT is associated with a higher rate of surgical complications, SLT for recurrent HCC following primary hepatic resection is still an efficient and safe treatment that increases survival and reduces tumor recurrence compared to patients with HCC exceeding the Milan criteria who accepted primary orthotopic LT[77]. After hepatectomy, HCC patients with larger tumor sizes were more likely to experience relapse, even with SLT. As a result, LT should be recommended as soon as possible, ideally within a year, for patients with recurrent HCC after LR, followed by meeting the requirements for transplantation[78]. For patients with recurrent HCC after hepatectomy, SLT has a poorer OS and RFS, as well as a higher risk of recurrence and death compared to primary LT, particularly for those who meet the Milan criteria[79]. Another study discovered no difference between patients receiving primary LT and SLT for HCC recurrence following primary treatment with LR or RFA in terms of the 5-year risk of recurrence and 5-year actuarial survival[80]. SLT for relapsed HCC patients after initial LR followed by SLT showed overall and recurrence-free survival rates on par with primary LT. Despite this, there are higher rates of Child-Pugh class A, more than three transplant treatments, and reoperation for postsurgical bleeding[81]. Patient background possibly has various effects on therapy, as Hong Kong patients with recurrent HCC following LR who received SLT, but not Roman patients, showed an increased recurrence rate[82].
Clinical therapy for HCC frequently involves ablation. Following ablation, the tumor experiences residual and local recurrence due to asymmetrical heat diffusion and heat absorption via circulating blood or air around the tumor[83]. For HCC patients who experience recurrence but cannot undergo a suitable operation, ablation is used as a safe and efficient therapy[84]. With ablation alone, the 5-year recurrence rate of HCC patients was 70%[85]. Although a small set of 11 patients with relapsing HCC following LT embraced microwave ablation without serious side effects, this safe technique still needs to be validated in larger studies or compared with other treatment options[86]. RFA and repeat resection are better choices for late-relapsing HCC patients post-curative hepatectomy who meet the Milan criteria[87]. Although the 1-, 3-, and 5-year OS (90.7%, 69.04%, and 55.6% vs 87.7%, 62.9%, and 38.1%, respectively) and progression-free survival (PFS) (56.5%, 27.9%, and 14.6% vs 50.2%, 21.9%, and 19.2%, respectively) were comparable between the RFA and repeat resection groups for locally recurrent HCC following primary resection, the former was superior to the latter in term of complications and hospitalization[88]. In a different study, repeat resection was found to increase survival for recurrent HCC, particularly for patients who had relapsed within two years and whose primary tumor burden exceeded the Milan criteria[89]. Primary HCC (94.8%, 75.7%, 61.6%, and 47.3%, respectively) and recurrent HCC (91.9%, 71.2%, 58.7%, and 45.2%, respectively) did not differ in the 1-, 3-, 5-, or 10-year OS rate[90]. RFA offers comparable long-term survival whether treatment is for the first-time or recurrent HCC that is 5 cm or less. Although LR with long-term survival results is superior to RFA for recurrent HCC patients, RFA is a good alternative to LR in patients with small-sized recurrence or patients with a limited number of recurrent nodules, even though LR has better long-term survival outcomes for patients with recurrent HCC[91]. Multiprobe stereotactic RFA as first-line therapy for recurrent HCC following LR has such low morbidity that the OS and DFS rates at 1, 3, and 5 years were 94.0%, 70.2%, and 53.3%, and 52.6%, 19.7%, and 15.8%, respectively[92]. RFA is beneficial and effective for intrahepatic recurrent HCC with 1-, 3-, and 5-year OS rates of 68.5%, 40.3%, and 40.3%, respectively, particularly for recurrent HCC following LT in the absence of finite extrahepatic metastases[93]. Due to its advantages of being less invasive, extreme selectivity, and reproducibility, RFA is suggested as a better therapy for intrahepatic HCC recurrence given that it is associated with a lower recurrence-free survival than LT[94].
In patients with recurrent HCC (tumor size < 3 cm, tumor number ≤ 2), a phase III non-inferiority trial found that the 2-, 3-, and 4-year local PFS of proton beam radiotherapy was comparable to that for RFA. However, the most common adverse outcomes were radiation pneumonitis (32.5%) and decreased leukocyte counts (23.8%) for proton beam radiotherapy, and increased alanine aminotransferase levels (96.4%) and abdominal pain (30.4%) for RFA[95], which suggested that proton beam radiotherapy was tolerable and safe with long PFS values comparable to those of RFA.
TACE is generally considered a standard therapeutic method for patients with unresectable HCC[11]. The most widely used treatment for postoperative recurrence is TACE, especially when there is a large mass or multifocal relapsed HCC[96]. The outcomes of TACE in the neoadjuvant setting are debatable. OS showed no difference between 71 patients treated with TACE before surgery and 21 patients who underwent surgery without TACE[97]. In a retrospective study, 1457 patients were evaluated, of whom 120 were treated with preoperative TACE, and it was found that 5-year DFS was improved in patients treated with TACE[98]. Patients with primary HCC who undergo embolization have a strikingly higher chance of survival than those with recurrent HCC. A study revealed that primary HCC patients who received TACE had a median survival of 30 mo and a 29% 3-year survival rate[99]. The results of treating patients with recurrent HCC, however, showed a low median survival time of 19 mo and an 11% survival rate. Patients with primary HCC and microvascular invasion (MVI) experience recurrence after resection, and TACE treatment is more effective than resection and RFA for recurrent HCC[100]. There was no significant difference in prognostic factors or OS between the initial and recurrent TACE groups[101]. TACE was administered to 28 patients with recurrent HCC following LT; 19 of these patients (67.9%) experienced tumor-shrinking by over 25%. However, the 1-, 3-, and 5-year survival rates were lower (47.9%, 6.0%, and 0%, respectively) due to extrahepatic metastases or intrahepatic recurrences[102]. According to a different study, patients who underwent chemoembolization without experiencing any serious side effects had a significantly longer OS time following the diagnosis of HCC recurrence post-LT than those who did not receive the treatment[103]. The 1-, 3-, and 5-year OS rates did not significantly differ between the repeat resection or RFA and the TACE groups, suggesting that TACE likely was as effective as repeat resection or RFA for preventing early intrahepatic relapse following curative resection of HCC[87]. Although there is no obvious difference between RFA and TACE treatment for isolated intrahepatic recurrent HCC following LT in terms of 2-year DFS rate (20% vs 14%) and 4-year OS rate (33% vs 25%), TACE treatment seems to be more beneficial in isolated intrahepatic recurrent HCC patients following LT when RFA therapy is not suitable[104]. In contrast to TACE-alone treatment for intrahepatic recurrent HCC after hepatectomy, apatinib, a vascular endothelial growth factor receptor 2 inhibitor, in conjunction with TACE significantly improved the median PFS, short-term objective responses, and disease control rate, and had a tendency of increasing the 1- and 2-year OS rates[105]. The patients who received TACE-RFA for recurrent HCC that was less than 5 cm following LT had a higher DFS than those who received TACE alone[106]. After a follow-up of 24 mo, the median OS for patients with the first recurrence of HCC treated with multimodality therapy was 40 mo (range 8-85 mo), far exceeding that of patients with LR/ablation (27 mo, range 4-75 mo), TACE/XRT (13 mo, range 4-68 mo), and systemic treatments (26 mo, range 3-59 mo)[9].
Sorafenib, a multitarget tyrosine kinase inhibitor (TKI) and the first approved drug for HCC patients, is most frequently used as an adjuvant therapy in resected HCC patients[107] and as a frontline systemic treatment in patients with HCC recurrence after LT. However, the current data are mainly based on observational research due to the exclusion of Randomized Protocol studies and Asia-Pacific trials of sorafenib from the registered studies for HCC[107,108]. Sorafenib has a few drawbacks, including poor oral bioavailability and drug toxicities, and its OS is only marginally improved by 2.8 mo[107,109]. The impacts of sorafenib in patients with recurrent HCC who underwent an incurable liver transplant have been estimated in several retrospective studies. Based on a retrospective cohort study of 50 patients with recurrent HCC following liver transplants who initially accepted sorafenib, an objective response rate of 16% and stable disease in 50% of this population were observed, and the median OS was 18 mo[110]. Patients with HCC recurrence following LT treated with sorafenib had a better median survival of 42 mo compared to 16.2 mo for patients not receiving sorafenib, supporting the notion that sorafenib increases survival[111].
According to this study, patients with relapsed HCC have a better chance of longer OS and a better prognosis by receiving the sorafenib treatment. For patients with recurrent HCC, sorafenib-lenvatinib continuous treatment and radical resection together with nonoperative therapy were both independent favorable factors for post-recurrence survival[112]. According to Lee et al[113], sorafenib for recurrent HCC is associated with a better prognosis because it involves smaller intrahepatic HCC combined with favorable liver function in LT recipients, which may explain why the median OS (16.8 mo vs 7.1 mo) and time to development were higher in 42 HCC patients in the LT group than in 790 non-LT patients. Sequential sorafenib treatments are similarly common in recurrent HCC patients following LT. These treatments improve OS compared to non-LT treatments and do not suppress systemic treatments with concurrent antirejection strategy[35]. Treatment with sorafenib and TACE was associated with a higher 5-year OS and PFS compared to those treated with TACE alone in patients with recurrent intermediate-stage HCC and lesions positive for MVI, but patients with MVI-negative lesions did not show a survival benefit from combined therapy[114]. Treatment with sorafenib plus TACE improves hepatic reserve, leads to a better OS, and results in longer intervals between TACE rounds in TACE-refractory patients with recurrent advanced HCC than repeated TACE treatments[115]. The RFA plus sorafenib treatment resulted in a significantly improved OS than RFA alone (1-, 3-, and 5-year OS rates of RFA-sorafenib vs RFA: 97.7%, 83.7%, and 54.7% vs 93.1%, 61.3%, and 30.9%, respectively), suggesting that adjuvant sorafenib combined with RFA was superior to RFA alone in improving survival results in patients with recurrent HCC who meet the Milan criteria after initial LT[116]. Prussian blue (PB) nanomaterial is safe and has multiple roles as an antidote to thallium poisoning[83]. With minimal injury to surrounding healthy tissues, photothermal therapy is a highly effective and noninvasive therapeutic option[117]. By using human and mouse HCC cell lines, Zhou et al[118] developed HCC-targeted SP94 peptide and cyanine (Cy) 5.5-conjugated PB nanoparticles loaded with sorafenib for HCC-targeted multimodality imaging and combined photothermal therapy/sorafenib treatment. These nanoparticles accumulated in HCC tumor sites and then controlled the release of sorafenib to eradicate the tumor without any local recurrence and with a minimal amount of toxic side effects.
The United States and Europe approved lenvatinib in the first line, cabozantinib, and ramucirumab in the second line as a potential systemic therapeutic approach for liver transplant recipients with relapsed HCC. A retrospective multicenter study discovered that regorafenib, a multitarget TKI, was safe and effective for patients with recurrent HCC following LT who were tolerable to sorafenib, with a median OS of 12.9 mo[119]. Lenvatinib, as a TKI, has been used as an optional frontline treatment strategy. Patients with recurrent HCC treated with lenvatinib who are tolerable to sorafenib have a longer median OS (19.5 mo), far exceeding those who are receiving intermittent sorafenib or regorafenib following sorafenib failure (12 mo)[112]. Cabozantinib, a TKI of vascular endothelial growth factor receptor 2 (VEGFR2), is used as an effective and safe monotherapy to proceed with third-line systemic therapies in advanced HCC[120]. Patients with recurrent HCC who received lenvatinib treatment had decreased expression of programmed death ligand 1 (PD-L1) and Treg infiltration in the tumor compared to the matched primary tumor, suggesting that lenvatinib targets fibroblast growth factor receptor 4 to increase the antitumor immune response of anti-programmed cell death-1 (PD-1) treatment, which is accompanied by decreased expression of tumor PD-L1 and Treg infiltration[121]. As a multi-kinase inhibitor, cabozantinib is expected to be an effective treatment for advanced HCC patients with sorafenib tolerance[122]. A case study identified a patient with recurrent HCC who had more than 10 years of survival after receiving an intensive multimodal therapeutic strategy that included surgery, RFA, and systemic therapy with cabozantinib as the second-line therapy in living-donor LT[123]. In patients with HCC recurrence following LT with sorafenib tolerance, regorafenib treatment resulted in a longer median OS (28.8 mo) than best supportive care (15.3 mo). This makes regorafenib a safe and effective second-line treatment option[124].
Although the importance of immune evasion in the progression of HCC recurrence was widely acknowledged, the lack of effective medications to reverse cancer-related immune suppression remained an untreatable condition until recently. Programmed cell death receptors on T cells and their ligands PDL-1 and PDL-2 on tumor cells are the targets of immune checkpoint inhibitors (ICPIs). Only 15–20% of patients benefit from anti-PD-L1 monoclonal antibodies (mAbs), which block interactions with PD-1 and PD-L1 and restore the roles of T cells in the tumor microenvironment[125,126]. Stimulation-induced immune surveillance has notable antitumoral outcomes in advanced and recurrent HCC, with significant response rates and even complete responses. Despite their promising prospects, ICPIs must be used with caution in transplant patients due to the complexity of HCC. In particular, HCC patients with multifocal tumors, higher AFP levels, larger tumor volume, and poorer differentiation presented a high risk of post-LT relapse when given neoadjuvant ICPIs[127]. The perioperative nivolumab vs ipilimumab/nivolumab combination had fine effects, according to a phase II study, with a 29% complete response rate[128]. The immune checkpoint blockade remedy resulted in only 16%-20% response rates among patients with advanced HCC[129]. Combination therapy with anti-PD-1 plus RFA for recurrent HCC achieved a superior recurrence-free survival compared to RFA monotherapy[130]. By combining anti-PD-L1 mAb with SP94-PB-sorafenib-Cy5.5 nanoparticles plus near-infrared therapy, Zhou et al[118] also observed the production of extraordinary results, such as suppression of distant metastases and obstruction of cancer relapse. Note that, different from primary HCC, the therapeutic strategy for recurrent HCC following LT has to be discrete due to the higher risk of allograft rejection or graft loss[131,132]. For early HCC recurrence after radical resection, TKIs combined with PD-1 therapy demonstrated a better survival benefit than TKIs alone[133]. In a patient with recurrent, refractory, metastatic HCC following LT, PD-1 inhibitor eliminated lung metastases and resulted in a partial radiological response of metastatic retroperitoneal lymph nodes after 13 cycles[134].
Chimeric or classical T-cell receptors (TCRs)-redirected T cells target HBV antigens/epitopes expressed on HBV-infected hepatocytes or in HCC cells as an immunotherapeutic approach. According to a case study, the HBV antigen was expressed in the metastases of a patient with HBV-related HCC after LT[135]. To treat extrahepatic metastases of chemotherapy resistance, HCC autologous T cells were genetically redirected to express an HBsAg-specific T cell receptor. This resulted in decreased HBsAg levels without worsening liver inflammation or other toxicity[135]. In two patients with metastatic recurrence of HBV-related HCC after LT, immunotherapy of HBV-specific TCRs was safe and did not cause any damage to liver function over a year[136]. Notably, a patient appeared to have a reduced volume in 5 of 6 pulmonary metastases during the first year of T-cell management[136]. HBV-specific TCR T-cells transiently escape the immunosuppressive effects of tacrolimus and mycophenolate mofetil owing to the activation of CD39+ Ki67+ peripheral blood mononuclear cells, which are positively correlated to clinical outcomes in patients with HBV-related HCC relapses following LT[137].
Cytokine-induced killing (CIK) cell-based immunotherapy has gained popularity as a promising new adjuvant therapy approach. CIK cells are a mixture of T lymphocytes, which are ex vivo amplified with cytokines and comprised of CD3+/CD56+ and CD3+/CD56- T cells, as well as CD3-/CD56+ NK cells, which have potent antitumor activity with the combined ability of both T cells and NK cells and minimal cytotoxicity to normal cells, but tremendous specificity to cancer cells[138]. Multiple clinical trials revealed that CIK cell-based immunotherapy increased RFS in HCC patients who underwent surgical resection[139,140]. The production of an individual autologous CIK cell-based immunotherapeutic agent involves activating peripheral blood mononuclear cells from the relevant patients with IL-2 and anti-CD3 antibodies[141]. According to research by Lee et al[141], the average RFS for HCC patients who accepted the CIK cell-based agent after curative therapy was 44.0 mo, as opposed to 30.0 mo for those who did not receive adjuvant immunotherapy. The results of a meta-analysis reported that the results of DC-based immunotherapy increased antitumor immunity, enhanced survival rate, and improved survival times in HCC patients[142]. Another meta-analysis listed 22 distinct studies with 3756 HCC patients that received DC-based vaccine and/or CIK-based adoptive therapy after receiving different HCC interventional therapies. These studies showed a prolonged OS (6 mo, 1, 3, and 5 years) and reduced mortality and recurrence at 1, 2, and 3 years but not 5 years[143]. For HCC patients, a personalized neoantigen vaccine served as a safe, practical, and effective anti-recurrence treatment[144]. After a radical operation on seven postoperative HCC patients who had received all of the planned neoantigen vaccinations, five of them showed neoantigen-activated cell responses and longer RFS than the other five patients, who had only received primary vaccination and had propensity scores that matched with those of control patients[144]. After curative resection or RFA in the first stage, the personalized neoantigen-loaded DC vaccine and neoantigen-activated T-cell therapy were successfully used on ten patients with HCC without unexpected delay or grade 3 therapy-related side effects[145]. New circulating multiclonal neoantigen-specific T-cell responses, activated neoantigen-specific immunity, an upregulated immune stimulatory signature, increased immune-cell infiltration, and elevated T-cell inflammatory gene expression, were produced in 70% of patients who had improved DFS compared to non-responders, and 71.4% of patients were without relapse for 2 years after curative treatment. Neoantigen depletion (immunoediting) also increased in recurrent tumors compared to primary tumors, suggesting that immune evasion developed as a result of immunological therapy[145].
With its unique characteristics, recurrent HCC is still a difficult disease to treat. Every stage of the disease calls for a multidisciplinary approach, which is still predominantly evolving. LT and hepatectomy remain successful therapeutic strategies for patients with recurrent HCC. Additionally, neoadjuvant and/or adjuvant therapy techniques may improve the long-term prognosis and increase the chance of cure in refractory patients with recurrent HCC. Relying on the tumor biology and possible hepatic reserve, multimodality therapy should be used in patients with recurrent HCC. By simultaneously optimizing oncologic outcomes and minimal side effects, this therapy helps these patients have better OS and tolerability.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hori T, Japan; Pop TL, Romania S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64615] [Article Influence: 16153.8] [Reference Citation Analysis (176)] |
| 2. | Gao YX, Yang TW, Yin JM, Yang PX, Kou BX, Chai MY, Liu XN, Chen DX. Progress and prospects of biomarkers in primary liver cancer (Review). Int J Oncol. 2020;57:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3026] [Article Influence: 432.3] [Reference Citation Analysis (3)] |
| 4. | Yi Y, Sun BY, Weng JL, Zhou C, Zhou CH, Cai MH, Zhang JY, Gao H, Sun J, Zhou J, Fan J, Ren N, Qiu SJ. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: A retrospective study. Front Oncol. 2022;12:1046584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6056] [Article Influence: 865.1] [Reference Citation Analysis (3)] |
| 6. | Zheng J, Kuk D, Gönen M, Balachandran VP, Kingham TP, Allen PJ, D'Angelica MI, Jarnagin WR, DeMatteo RP. Actual 10-Year Survivors After Resection of Hepatocellular Carcinoma. Ann Surg Oncol. 2017;24:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Song H, Ding H, Zhu C. CT-Guided Percutaneous Microwave Ablation of Sclerosing Hepatic Carcinoma. Can J Gastroenterol Hepatol. 2020;2020:8881978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, Busuttil RW, Roberts J, Emond JC, Samstein B, Brown RS Jr, Najjar M, Chapman WC, Doyle MM, Florman SS, Schwartz ME, Llovet JM. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022;157:779-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Sanmamed MF, Berraondo P, Rodriguez-Ruiz ME, Melero I. Charting roadmaps towards novel and safe synergistic immunotherapy combinations. Nat Cancer. 2022;3:665-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Fronda M, Mistretta F, Calandri M, Ciferri F, Nardelli F, Bergamasco L, Fonio P, Doriguzzi Breatta A. The Role of Immediate Post-Procedural Cone-Beam Computed Tomography (CBCT) in Predicting the Early Radiologic Response of Hepatocellular Carcinoma (HCC) Nodules to Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE). J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Li Z, Tan C, Liu X, Feng Z, Li K. Early and late recurrence after hepatectomy in patients with low-level HBV-DNA hepatocellular carcinoma under antiviral therapy. Infect Agent Cancer. 2022;17:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 13. | Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, Rees M, Ramaswamy A, Muenst S, Soysal SD, Jacobs A, Windhager J, Silina K, van den Broek M, Dedes KJ, Rodríguez Martínez M, Weber WP, Bodenmiller B. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell. 2019;177:1330-1345.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 551] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 14. | Ding X, He M, Chan AWH, Song QX, Sze SC, Chen H, Man MKH, Man K, Chan SL, Lai PBS, Wang X, Wong N. Genomic and Epigenomic Features of Primary and Recurrent Hepatocellular Carcinomas. Gastroenterology. 2019;157:1630-1645.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 15. | Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404-421.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 530] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 16. | Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342-1356.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1503] [Article Influence: 187.9] [Reference Citation Analysis (0)] |
| 17. | Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Zheng X, Jin W, Wang S, Ding H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front Immunol. 2021;12:729705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 19. | Ambrozkiewicz F, Trailin A, Červenková L, Vaclavikova R, Hanicinec V, Allah MAO, Palek R, Třeška V, Daum O, Tonar Z, Liška V, Hemminki K. CTNNB1 mutations, TERT polymorphism and CD8+ cell densities in resected hepatocellular carcinoma are associated with longer time to recurrence. BMC Cancer. 2022;22:884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Dash S, Aydin Y, Widmer KE, Nayak L. Hepatocellular Carcinoma Mechanisms Associated with Chronic HCV Infection and the Impact of Direct-Acting Antiviral Treatment. J Hepatocell Carcinoma. 2020;7:45-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Qu WF, Tian MX, Qiu JT, Guo YC, Tao CY, Liu WR, Tang Z, Qian K, Wang ZX, Li XY, Hu WA, Zhou J, Fan J, Zou H, Hou YY, Shi YH. Exploring pathological signatures for predicting the recurrence of early-stage hepatocellular carcinoma based on deep learning. Front Oncol. 2022;12:968202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 22. | Zaki MYW, Fathi AM, Samir S, Eldafashi N, William KY, Nazmy MH, Fathy M, Gill US, Shetty S. Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Zeng Z, Jiang X, Pan Z, Zhou R, Lin Z, Tang Y, Cui Y, Zhang E, Cao Z. Highly expressed centromere protein L indicates adverse survival and associates with immune infiltration in hepatocellular carcinoma. Aging (Albany NY). 2021;13:22802-22829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Zhang L, Yang Z, Zhang S, Zhou K, Zhang W, Ling S, Sun R, Tang H, Wen X, Feng X, Song P, Xu X, Xie H, Zheng S. Polyploidy Spectrum Correlates with Immunophenotype and Shapes Hepatocellular Carcinoma Recurrence Following Liver Transplantation. J Inflamm Res. 2022;15:217-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Li FP, Liu GH, Zhang XQ, Kong WJ, Mei J, Wang M, Dai YH. Overexpressed SNRPB/D1/D3/E/F/G correlate with poor survival and immune infiltration in hepatocellular carcinoma. Am J Transl Res. 2022;14:4207-4228. [PubMed] |
| 26. | Wu M, Mei F, Liu W, Jiang J. Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed Pharmacother. 2020;121:109637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Wang D, Zheng X, Fu B, Nian Z, Qian Y, Sun R, Tian Z, Wei H. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine. 2019;46:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Carissimi F, Barbaglia MN, Salmi L, Ciulli C, Roccamatisi L, Cordaro G, Mallela VR, Minisini R, Leone BE, Donadon M, Torzilli G, Pirisi M, Romano F, Famularo S. Finding the seed of recurrence: Hepatocellular carcinoma circulating tumor cells and their potential to drive the surgical treatment. World J Gastrointest Surg. 2021;13:967-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 29. | Zhou SL, Zhou ZJ, Hu ZQ, Song CL, Luo YJ, Luo CB, Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, Cao Y, Fan J, Zhou J. Genomic sequencing identifies WNK2 as a driver in hepatocellular carcinoma and a risk factor for early recurrence. J Hepatol. 2019;71:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Cao S, Lyu T, Fan Z, Guan H, Song L, Tong X, Wang J, Zou Y. Long-term outcome of percutaneous radiofrequency ablation for periportal hepatocellular carcinoma: tumor recurrence or progression, survival and clinical significance. Cancer Imaging. 2022;22:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Minami Y, Minami T, Ueshima K, Yagyu Y, Tsurusaki M, Okada T, Hori M, Kudo M, Murakami T. Three-Dimensional Radiological Assessment of Ablative Margins in Hepatocellular Carcinoma: Pilot Study of Overlay Fused CT/MRI Imaging with Automatic Registration. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Guo Y, Ren Y, Dong X, Kan X, Zheng C. An Overview of Hepatocellular Carcinoma After Insufficient Radiofrequency Ablation. J Hepatocell Carcinoma. 2022;9:343-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 33. | Xie DY, Fan HK, Ren ZG, Fan J, Gao Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin Transl Gastroenterol. 2019;10:e00006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Kuo MJ, Mo LR, Chen CL. Factors predicting long-term outcomes of early-stage hepatocellular carcinoma after primary curative treatment: the role of surgical or nonsurgical methods. BMC Cancer. 2021;21:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Tovoli F, Pallotta DP, Sansone V, Iavarone M, De Giorgio M, Ielasi L, Di Costanzo GG, Giuffrida P, Sacco R, Pressiani T, Di Donato MF, Trevisani F, Fagiuoli S, Piscaglia F, Granito A. Outcomes of Sorafenib for Recurrent Hepatocellular Carcinoma After Liver Transplantation in the Era of Combined and Sequential Treatments. Transplantation. 2023;107:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 37. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 38. | Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Kolly P, Waidmann O, Vermehren J, Moreno C, Vögeli I, Berg T, Semela D, Zeuzem S, Dufour JF. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: A European multicentre study. J Hepatol. 2017;67:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Baumert TF, Berg T, Lim JK, Nelson DR. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology. 2019;156:431-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 41. | Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology. 2018;68:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Mori Y, Matsuda S, Sato M, Muraoka M, Suzuki Y, Tatsumi A, Nakayama Y, Inoue T, Maekawa S, Enomoto N. The Impact of Antiviral Therapy for Hepatitis C Virus on the Survival of Patients after Hepatocellular Carcinoma Treatment. Intern Med. 2022;61:2721-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Pazgan-Simon M, Simon KA, Jarowicz E, Rotter K, Szymanek-Pasternak A, Zuwała-Jagiełło J. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin Exp Hepatol. 2018;4:210-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Jeng LB, Li TC, Hsu SC, Chan WL, Teng CF. Association of Low Serum Albumin Level with Higher Hepatocellular Carcinoma Recurrence in Patients with Hepatitis B Virus Pre-S2 Mutant after Curative Surgical Resection. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Liu GM, Huang XY, Shen SL, Hu WJ, Peng BG. Adjuvant antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after curative treatment: A systematic review and meta-analysis. Hepatol Res. 2016;46:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Xia BW, Zhang YC, Wang J, Ding FH, He XD. Efficacy of antiviral therapy with nucleotide/nucleoside analogs after curative treatment for patients with hepatitis B virus-related hepatocellular carcinoma: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Durand F, Francoz C. The future of liver transplantation for viral hepatitis. Liver Int. 2017;37 Suppl 1:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Xia Z, He L, Xiong L, Wen T. The comparison of different antiviral therapies on the prognosis of hepatitis B virus-related hepatocellular carcinoma after curative treatments: A network meta-analysis. Medicine (Baltimore). 2020;99:e20877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Prognostic Impact of MAFLD Following Surgical Resection of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Nationwide Cohort Study. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 50. | Roche B, Bauhofer A, Gomez Bravo MÃ, Pageaux GP, Zoulim F, Otero A, Prieto M, Baliellas C, Samuel D. Long-Term Effectiveness, Safety, and Patient-Reported Outcomes of Self-Administered Subcutaneous Hepatitis B Immunoglobulin in Liver Post-Transplant Hepatitis B Prophylaxis: A Prospective Non-Interventional Study. Ann Transplant. 2022;27:e936162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Jin H, Wang H, Li G, Hou Q, Wu W, Liu F. Risk factors for early postoperative recurrence in single and small hepatitis B virus-associated primary hepatocellular carcinoma. J Int Med Res. 2020;48:300060520961260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 52. | Morise Z. Developments and perspectives of laparoscopic liver resection in the treatment of hepatocellular carcinoma. Surg Today. 2019;49:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Onoe T, Yamaguchi M, Irei T, Ishiyama K, Sudo T, Hadano N, Kojima M, Kubota H, Ide R, Tazawa H, Shimizu W, Suzuki T, Shimizu Y, Hinoi T, Tashiro H. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg Endosc. 2020;34:4574-4581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Goh BKP, Syn N, Teo JY, Guo YX, Lee SY, Cheow PC, Chow PKH, Ooi LLPJ, Chung AYF, Chan CY. Perioperative Outcomes of Laparoscopic Repeat Liver Resection for Recurrent HCC: Comparison with Open Repeat Liver Resection for Recurrent HCC and Laparoscopic Resection for Primary HCC. World J Surg. 2019;43:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Nomi T, Kaibori M, Tanaka S, Hirokawa F, Hokuto D, Noda T, Ueno M, Nakai T, Ikoma H, Iida H, Matsui K, Komeda K, Hayami S, Eguchi H, Matsumoto M, Morimura R, Maehira H, Yoshikawa T, Kubo S. Short- and long-term outcomes of laparoscopic vs open repeat liver resection for hepatocellular carcinoma: A multicenter study. J Hepatobiliary Pancreat Sci. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Liang Y, Lin C, Zhang B, Cao J, Chen M, Shen J, Feng X, Xiao G, Pan L, Chen K, Maher H, Cai X. Perioperative outcomes comparing laparoscopic with open repeat liver resection for post-hepatectomy recurrent liver cancer: A systematic review and meta-analysis. Int J Surg. 2020;79:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg. 2021;273:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 60. | Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, Zhong CQ, Wang K, Chai ZT, Wei XB, Shi J, Guo WX, Wu MC, Lau WY, Cheng SQ. Patterns, treatments, and prognosis of tumor recurrence after resection for hepatocellular carcinoma with microvascular invasion: a multicenter study from China. HPB (Oxford). 2022;24:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Bednarsch J, Czigany Z, Heij LR, Amygdalos I, Heise D, Bruners P, Ulmer TF, Neumann UP, Lang SA. The role of re-resection in recurrent hepatocellular carcinoma. Langenbecks Arch Surg. 2022;407:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 62. | Zhong JH, Xing BC, Zhang WG, Chan AW, Chong CCN, Serenari M, Peng N, Huang T, Lu SD, Liang ZY, Huo RR, Wang YY, Cescon M, Liu TQ, Li L, Wu FX, Ma L, Ravaioli M, Neri J, Cucchetti A, Johnson PJ, Li LQ, Xiang BD. Repeat hepatic resection vs radiofrequency ablation for recurrent hepatocellular carcinoma: retrospective multicentre study. Br J Surg. 2021;109:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Midorikawa Y, Takayama T, Nakayama H, Moriguchi M, Aramaki O, Yamazaki S, Teramoto K, Yoshida N, Kobayashi N, Tsuji S, Higaki T. Favorable outcomes of surgical resection for extrahepatic recurrent hepatocellular carcinoma. Hepatol Res. 2020;50:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Chua DW, Koh YX, Syn NL, Chuan TY, Yao TJ, Lee SY, Goh BKP, Cheow PC, Chung AY, Chan CY. Repeat hepatectomy vs radiofrequency ablation in management of recurrent hepatocellular carcinoma: an average treatment effect analysis. Ann Surg Oncol. 2021;28:7731-7740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 658] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 66. | Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 67. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 68. | Matsumoto M, Yanaga K, Shiba H, Wakiyama S, Sakamoto T, Futagawa Y, Gocho T, Ishida Y, Ikegami T. Treatment of intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Ann Gastroenterol Surg. 2021;5:538-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Zheng S, Xie Q, Cheng J. Salvage liver transplant for hepatocellular carcinoma: rescues and benefits. Transl Gastroenterol Hepatol. 2018;3:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Kim M, Hwang S, Song GW, Ahn CS, Moon DB, Jung DH, Park GC, Lee SG. Salvage living donor liver transplantation for post-resection recurrence of combined hepatocellular carcinoma-cholangiocarcinoma. Korean J Transplant. 2021;35:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 72. | Zhang X, Li C, Wen T, Peng W, Yan L, Yang J. Outcomes of Salvage Liver Transplantation and Re-resection/Radiofrequency Ablation for Intrahepatic Recurrent Hepatocellular Carcinoma: A New Surgical Strategy Based on Recurrence Pattern. Dig Dis Sci. 2018;63:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Guo Y, Tan EK, Syn NL, Krishnamoorthy TL, Tan CK, Lim R, Lee SY, Chan CY, Cheow PC, Chung AYF, Jeyaraj PR, Goh BKP. Repeat liver resection vs salvage liver transplant for recurrent hepatocellular carcinoma: A propensity score-adjusted and -matched comparison analysis. Ann Hepatobiliary Pancreat Surg. 2019;23:305-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Kostakis ID, Machairas N, Prodromidou A, Stamopoulos P, Garoufalia Z, Fouzas I, Sotiropoulos GC. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Transplant Proc. 2019;51:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Yoon YI, Song GW, Lee S, Moon D, Hwang S, Kang WH, Cho HD, Ha SM, Kim MJ, Kim SH, Na BG, Yang G, Min Kim S, Hyun Shim J, Park JI. Salvage living donor liver transplantation vs repeat liver resection for patients with recurrent hepatocellular carcinoma and Child-Pugh class A liver cirrhosis: A propensity score-matched comparison. Am J Transplant. 2022;22:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | de Haas RJ, Lim C, Bhangui P, Salloum C, Compagnon P, Feray C, Calderaro J, Luciani A, Azoulay D. Curative salvage liver transplantation in patients with cirrhosis and hepatocellular carcinoma: An intention-to-treat analysis. Hepatology. 2018;67:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Chan KM, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Lee WC. Salvage living donor liver transplantation for posthepatectomy recurrence: a higher incidence of recurrence but promising strategy for long-term survival. Cancer Manag Res. 2019;11:7295-7305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Chan KM, Wu TH, Cheng CH, Lee CF, Wu TJ, Chou HS, Lee WC. Advantage of early liver transplantation whenever indicated for hepatocellular carcinoma recurrence after primary liver resection. Biomed J. 2019;42:335-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Shan Y, Huang L, Xia Q. Salvage Liver Transplantation Leads to Poorer Outcome in Hepatocellular Carcinoma Compared with Primary Liver Transplantation. Sci Rep. 2017;7:44652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Muaddi H, Al-Adra DP, Beecroft R, Ghanekar A, Moulton CA, Doyle A, Selzner M, Wei A, McGilvray ID, Gallinger S, Grant DR, Cattral MS, Greig PD, Kachura J, Cleary SP, Sapisochin G. Liver Transplantation is Equally Effective as a Salvage Therapy for Patients with Hepatocellular Carcinoma Recurrence Following Radiofrequency Ablation or Liver Resection with Curative Intent. Ann Surg Oncol. 2018;25:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Abe T, Tashiro H, Teraoka Y, Hattori M, Tanimine N, Kuroda S, Tahara H, Ohira M, Tanaka Y, Kobayashi T, Ide K, Ishiyama K, Ohdan H. Efficacy and Feasibility of Salvage Living Donor Liver Transplantation after Initial Liver Resection in Patients with Hepatocellular Carcinoma. Dig Surg. 2016;33:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Roddy H, Meyer T, Roddie C. Novel Cellular Therapies for Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 83. | Li J, Wang S, Fontana F, Tapeinos C, Shahbazi MA, Han H, Santos HA. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact Mater. 2023;23:471-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 84. | Yang Y, Yu H, Tan X, You Y, Liu F, Zhao T, Qi J, Li J, Feng Y, Zhu Q. Liver resection vs radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2021;38:875-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4102] [Article Influence: 586.0] [Reference Citation Analysis (6)] |
| 86. | Pelizzaro F, Gambato M, Gringeri E, Vitale A, Cillo U, Farinati F, Burra P, Russo FP. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Zhang X, Li C, Wen T, Yan L, Li B, Yang J, Wang W, Xu M, Lu W, Jiang L. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, Zhao X, Wang L, Xiao F, Lv H, Liu T, Qi J, Li J, Zhong N, Wang C, Feng H, Liang B, Ren W, Qin C, Nguyen MH, Zhu Q. Radiofrequency ablation vs repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur Radiol. 2020;30:6357-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, Chen MS, Zhang YF, Guo RP. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection vs radiofrequency ablation. Cancer Med. 2020;9:2997-3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 90. | Bai XM, Cui M, Yang W, Wang H, Wang S, Zhang ZY, Wu W, Chen MH, Yan K, Goldberg SN. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary vs Recurrent HCC. Radiology. 2021;300:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 91. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 92. | Schullian P, Laimer G, Putzer D, Levy E, Braunwarth E, Stättner S, Bale R. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol. 2020;46:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Liu B, Huang G, Xie X, Zhao Q, Su L, Liu M, Li X, Long J, Kuang M. Feasibility and outcomes of percutaneous radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma after liver transplantation: a single-center experience. Int J Hyperthermia. 2020;37:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Chen X, Chen Y, Li Q, Ma D, Shen B, Peng C. Radiofrequency ablation vs surgical resection for intrahepatic hepatocellular carcinoma recurrence: a meta-analysis. J Surg Res. 2015;195:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, Park JW. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol. 2021;74:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 96. | Jeon MY, Kim HS, Lim TS, Han DH, Kim BK, Park JY, Kim DY, Ahn SH, Choi GH, Choi JS, Han KH, Kim SU. Refractoriness to transarterial chemoembolization in patients with recurrent hepatocellular carcinoma after curative resection. PLoS One. 2019;14:e0214613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 98. | Manjunatha N, Ganduri V, Rajasekaran K, Duraiyarasan S, Adefuye M. Transarterial Chemoembolization and Unresectable Hepatocellular Carcinoma: A Narrative Review. Cureus. 2022;14:e28439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 100. | Qi YP, Zhong JH, Liang ZY, Zhang J, Chen B, Chen CZ, Li LQ, Xiang BD. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Cerban R, Ester C, Iacob S, Grasu M, Pâslaru L, Dumitru R, Lupescu I, Constantin G, Croitoru A, Gheorghe L. Predictive Factors of Tumor Recurrence and Survival in Patients with Hepatocellular Carcinoma treated with Transarterial Chemoembolization. J Gastrointestin Liver Dis. 2018;27:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Sposito C, Citterio D, Virdis M, Battiston C, Droz Dit Busset M, Flores M, Mazzaferro V. Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma. World J Gastroenterol. 2022;28:4929-4942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Kim SS, Kang TW, Song KD, Cho SK, Lee MW, Rhim H, Sinn DH, Jung SH. Radiofrequency ablation and transarterial chemoembolisation as first-line treatment for recurrent hepatocellular carcinoma or isolated intrahepatic recurrent hepatocellular carcinoma in transplanted livers. Clin Radiol. 2017;72:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Gu H, Li J, You N, Wu K, Wang Z, Wang L, Zhu Y, Liu Q, Peng X, Zheng L. Efficacy and safety of apatinib combined with transarterial chemoembolization (TACE) in treating patients with recurrent hepatocellular carcinoma. Ann Transl Med. 2020;8:1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Song Q, Ren W, Fan L, Zhao M, Mao L, Jiang S, Zhao C, Cui Y. Long-Term Outcomes of Transarterial Chemoembolization Combined with Radiofrequency Ablation Versus Transarterial Chemoembolization Alone for Recurrent Hepatocellular Carcinoma After Surgical Resection. Dig Dis Sci. 2020;65:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Sun KX, Cao SS, Shi FH, Guan Y, Tang M, Zhao MN, Jian YF, Cui B, Li ZY, Wang JW, Yu F, Ding Y. First-line treatments for advanced hepatocellular carcinoma: a network meta-analysis and cost-effectiveness analysis in China and the United States. Therap Adv Gastroenterol. 2022;15:17562848221140662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 108. | Hur MH, Kim YJ. Evolution of systemic therapy for advanced-stage hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Farinha D, Migawa M, Sarmento-Ribeiro A, Faneca H. A Combined Antitumor Strategy Mediated by a New Targeted Nanosystem to Hepatocellular Carcinoma. Int J Nanomedicine. 2021;16:3385-3405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, Antonelli B, Airoldi A, Manini MA, Sangiovanni A, Rossi G, Donato MF, Saverio Belli L, Lampertico P. Experience With Early Sorafenib Treatment With mTOR Inhibitors in Hepatocellular Carcinoma Recurring After Liver Transplantation. Transplantation. 2020;104:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 111. | Alsina AE, Makris A, Nenos V, Sucre E, Arrobas J, Franco E, Kemmer N. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? Am Surg. 2014;80:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Yang Z, Wang S, Tian XY, Xie QF, Zhuang L, Li QY, Chen CZ, Zheng SS. Impact of treatment modalities on patients with recurrent hepatocellular carcinoma after liver transplantation: Preliminary experience. Hepatobiliary Pancreat Dis Int. 2020;19:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Lee SK, Jang JW, Nam H, Sung PS, Kim HY, Kwon JH, Lee SW, Song DS, Kim CW, Song MJ, Choi HJ, You YK, Bae SH, Choi JY, Yoon SK. Sorafenib for advanced hepatocellular carcinoma provides better prognosis after liver transplantation than without liver transplantation. Hepatol Int. 2021;15:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Peng Z, Chen S, Xiao H, Wang Y, Li J, Mei J, Chen Z, Zhou Q, Feng S, Chen M, Qian G, Peng S, Kuang M. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology. 2019;292:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 115. | Kaibori M, Matsushima H, Ishizaki M, Kosaka H, Matsui K, Kariya S, Yoshii K, Sekimoto M. The Impact of Sorafenib in Combination with Transarterial Chemoembolization on the Outcomes of Intermediate-Stage Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2021;22:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Zhou Q, Wang X, Li R, Wang C, Wang J, Xie X, Li Y, Li S, Mao X, Liang P. Sorafenib as adjuvant therapy following radiofrequency ablation for recurrent hepatocellular carcinoma within Milan criteria: a multicenter analysis. J Gastroenterol. 2022;57:684-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 118. | Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, Du Y, Fang C, Tian J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano. 2020;14:12679-12696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 119. | Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, Manini MA, López MF, Anders M, Pinter M, Rodríguez MJB, Cristóbal MR, Soteras GA, Piñero F, Villadsen GE, Weinmann A, Crespo G, Mazzaferro V, Regnault H, Giorgio M, González-Diéguez ML, Donato MF, Varela M, Wörns MA, Bruix J, Lampertico P, Reig M. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 120. | Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, Charmsaz S, Lin D, Quong D, Wilt B, Kamel IR, Weiss M, Philosophe B, Burkhart R, Burns WR, Shubert C, Ejaz A, He J, Deshpande A, Danilova L, Stein-O'Brien G, Sugar EA, Laheru DA, Anders RA, Fertig EJ, Jaffee EM, Yarchoan M. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat Cancer. 2021;2:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 121. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 122. | Azhie A, Grant RC, Herman M, Wang L, Knox JJ, Bhat M. Phase II clinical trial of cabozantinib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Future Oncol. 2022;18:2173-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 123. | Mahn R, Sadeghlar F, Bartels A, Zhou T, Weismüller T, Kupczyk P, Meyer C, Gaertner FC, Toma M, Vilz T, Knipper P, Glowka T, Manekeller S, Kalff J, Strassburg CP, Gonzalez-Carmona MA. Multimodal and systemic therapy with cabozantinib for treatment of recurrent hepatocellular carcinoma after liver transplantation: A case report with long term follow-up outcomes. Medicine (Baltimore). 2021;100:e27082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, Fraile-López M, Czauderna C, Di Costanzo G, Bhoori S, Pinter M, Manini MA, Amaddeo G, Yunquera AF, Piñero F, Blanco Rodríguez MJ, Anders M, Aballay Soteras G, Villadsen GE, Yoon PD, Cesarini L, Díaz-González Á, González-Diéguez ML, Tortora R, Weinmann A, Mazzaferro V, Romero Cristóbal M, Crespo G, Regnault H, De Giorgio M, Varela M, Prince R, Scudeller L, Donato MF, Wörns MA, Bruix J, Sapisochin G, Lampertico P, Reig M. Regorafenib Efficacy After Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Retrospective Study. Liver Transpl. 2021;27:1767-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 125. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10346] [Article Influence: 795.8] [Reference Citation Analysis (34)] |
| 126. | Gao Y, Xu Q, Li X, Guo Y, Zhang B, Jin Y, Zhu C, Shen Y, Yang P, Shi Y, Jin R, Liu D, Ouyang Y, Liu X, Wang W, Chen D, Yang T. Heterogeneity induced GZMA-F2R communication inefficient impairs antitumor immunotherapy of PD-1 mAb through JAK2/STAT1 signal suppression in hepatocellular carcinoma. Cell Death Dis. 2022;13:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 127. | Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, Kneteman NM. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 128. | Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, Yavuz BG, Mohamed YI, Qayyum A, Jindal S, Duan F, Basu S, Yadav SS, Nicholas C, Sun JJ, Singh Raghav KP, Rashid A, Carter K, Chun YS, Tzeng CD, Sakamuri D, Xu L, Sun R, Cristini V, Beretta L, Yao JC, Wolff RA, Allison JP, Sharma P. Perioperative nivolumab monotherapy vs nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 129. | Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (1)] |
| 130. | Wang X, Liu G, Chen S, Bi H, Xia F, Feng K, Ma K, Ni B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia. 2021;38:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 131. | Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, Farmer DG, Busuttil RW, Federman N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 132. | Hoffman D, Mehta N. Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev Gastroenterol Hepatol. 2021;15:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 133. | Li Z, Han N, Ren X, Zhang Y, Chu X. Effectiveness of TKI Inhibitors Combined With PD-1 in Patients With Postoperative Early Recurrence of HCC: A Real-World Study. Front Oncol. 2022;12:833884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 134. | Qiu J, Tang W, Du C. Immune Checkpoint Inhibitors in Patients with Recurrent Hepatocellular Carcinoma after Liver Transplantation: A Case Report and Literature Review. Curr Cancer Drug Targets. 2020;20:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 135. | Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, Moriconi F, Farzhenah F, Mazzoni A, Chan L, Morris E, Thrasher A, Maini MK, Bonino F, Stauss H, Bertoletti A. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 136. | Tan AT, Yang N, Lee Krishnamoorthy T, Oei V, Chua A, Zhao X, Tan HS, Chia A, Le Bert N, Low D, Tan HK, Kumar R, Irani FG, Ho ZZ, Zhang Q, Guccione E, Wai LE, Koh S, Hwang W, Chow WC, Bertoletti A. Use of Expression Profiles of HBV-DNA Integrated Into Genomes of Hepatocellular Carcinoma Cells to Select T Cells for Immunotherapy. Gastroenterology. 2019;156:1862-1876.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 137. | Hafezi M, Lin M, Chia A, Chua A, Ho ZZ, Fam R, Tan D, Aw J, Pavesi A, Krishnamoorthy TL, Chow WC, Chen W, Zhang Q, Wai LE, Koh S, Tan AT, Bertoletti A. Immunosuppressive Drug-Resistant Armored T-Cell Receptor T Cells for Immune Therapy of HCC in Liver Transplant Patients. Hepatology. 2021;74:200-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 138. | Tao L, Wang S, Kang G, Jiang S, Yin W, Zong L, Li J, Wang X. PD-1 blockade improves the anti-tumor potency of exhausted CD3(+)CD56(+) NKT-like cells in patients with primary hepatocellular carcinoma. Oncoimmunology. 2021;10:2002068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 139. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 140. | Cassese G, Han HS, Lee B, Lee HW, Cho JY, Panaro F, Troisi RI. Immunotherapy for hepatocellular carcinoma: A promising therapeutic option for advanced disease. World J Hepatol. 2022;14:1862-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 141. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 142. | Chen C, Ma YH, Zhang YT, Zhang F, Zhou N, Wang X, Liu T, Li YM. Effect of dendritic cell-based immunotherapy on hepatocellular carcinoma: A systematic review and meta-analysis. Cytotherapy. 2018;20:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 143. | Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2019;25:3649-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 144. | Cai Z, Su X, Qiu L, Li Z, Li X, Dong X, Wei F, Zhou Y, Luo L, Chen G, Chen H, Wang Y, Zeng Y, Liu X. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol Cancer. 2021;20:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 145. | Peng S, Chen S, Hu W, Mei J, Zeng X, Su T, Wang W, Chen Z, Xiao H, Zhou Q, Li B, Xie Y, Hu H, He M, Han Y, Tang L, Ma Y, Li X, Zhou X, Dai Z, Liu Z, Tan J, Xu L, Li S, Shen S, Li D, Lai J, Peng B, Peng Z, Kuang M. Combination Neoantigen-Based Dendritic Cell Vaccination and Adoptive T-Cell Transfer Induces Antitumor Responses Against Recurrence of Hepatocellular Carcinoma. Cancer Immunol Res. 2022;10:728-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |