Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1459
Peer-review started: February 23, 2022
First decision: April 17, 2022
Revised: April 20, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: July 27, 2022
Processing time: 153 Days and 21.1 Hours
Challenging lesions, difficult to diagnose through non-invasive methods, constitute an important emotional burden for each patient regarding a still uncertain diagnosis (malignant x benign). In addition, from a therapeutic and prognostic point of view, delay in a definitive diagnosis can lead to worse outcomes. One of the main innovative trends currently is the use of molecular and functional methods to diagnosis. Numerous liver-specific contrast agents have been developed and studied in recent years to improve the performance of liver magnetic resonance imaging (MRI). More recently, one of the contrast agents introduced in clinical practice is gadoxetic acid (gadoxetate disodium).
To demonstrate the value of the hepatobiliary phases using gadoxetic acid in MRI for the characterization of focal liver lesions (FLL) in clinical practice.
Overall, 302 Lesions were studied in 136 patients who underwent MRI exams using gadoxetic acid for the assessment of FLL. Two radiologists independently reviewed the MRI exams using four stages, and categorized them on a 6-point scale, from 0 (lesion not detected) to 5 (definitely malignant). The stages were: stage 1- images without contrast, stage 2- addition of dynamic phases after contrast (analogous to usual extracellular contrasts), stage 3- addition of hepatobiliary phase after 10 min (HBP 10’), stage 4- hepatobiliary phase after 20 min (HBP 20’) in addition to stage 2.
The interobserver agreement was high (weighted Kappa coefficient: 0.81- 1) at all stages in the characterization of benign and malignant FLL. The diagnostic weighted accuracy (Az) was 0.80 in stage 1 and was increased to 0.90 in stage 2. Addition of the hepatobiliary phase increased Az to 0.98 in stage 3, which was also 0.98 in stage 4.
The hepatobiliary sequences improve diagnostic accuracy. With growing potential in the era of precision medicine, the improvement and dissemination of the method among medical specialties can bring benefits in the management of patients with FLL that are difficult to diagnose.
Core Tip: The translational objective was to determine the value of hepatobiliary phases using gadoxetic acid as a liver-specific agent in magnetic resonance imaging (MRI) in the characterization of benign and malignant focal liver lesions (FLL) in clinical practice. Morphofunctional MRI with gadoxetic acid in addition to the usual dynamic phases after contrast medium (arterial, portal and transitional/ equilibrium) increased the proportion of hits for differentiation between benign and malignant FLL in relation to the definitive diagnosis. The results suggest a relevant impact on the definition of strategies for the approach of focal hepatic lesions, as well as in the assessment of the treatment employed.
- Citation: Fernandes DA, Dal Lago EA, Oliver FA, Loureiro BMC, Martins DL, Penachim TJ, Barros RHO, Araújo Filho JAB, Eloy da Costa LB, da Silva ÁMO, de Ataíde EC, Boin IFSF, Caserta NMG. Hepatobiliary phases in magnetic resonance imaging using liver-specific contrast for focal lesions in clinical practice. World J Hepatol 2022; 14(7): 1459-1469
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1459.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1459
The accurate characterization of focal liver lesions (FLL) has great clinical relevance. Although ultrasonography (US) and computed tomography (CT) are the most important diagnostic tools for screening FLL, magnetic resonance imaging (MRI) is a well-established diagnostic imaging method in clinical practice and produces images without ionizing radiation, with good spatial resolution and excellent tissue resolution, thus allowing a very reliable assessment. Challenging lesions, difficult to diagnose through non-invasive methods, constitute an important emotional burden for each patient regarding a still uncertain diagnosis (malignant x benign). In addition, from a therapeutic and prognostic point of view, delay in a definitive diagnosis can lead to worse outcomes. One of the main innovative trends currently is the use of molecular and functional methods. Combined with diffusion and dynamic studies of the liver after administration of a contrast medium, MRI stands out as the most accurate non-invasive imaging method for the detection and characterization of FLL[1].
Numerous liver-specific contrast agents have been developed and studied in recent years to improve the performance of liver MRI, specifically those that are captured by liver cells by hepatocytes (gadolinium-based compounds), such as gadobenate dimeglumine (Gd-BOPTA), mangafodipir trisodium (Mn-DPDP), or by Kupffer cells which are particles of super magnetic iron oxide. Recently, one of the contrast agents introduced in clinical practice is gadoxetic acid (gadoxetate), formed by gadolinium and the ligand ethoxybenzyl-diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)[2]. The gadoxetic acid has hepatocellular uptake and biliary excretion (about 50% in healthy patients), which allows to carry out routine three-phase dynamic studies at first (arterial, portal and transitional/ equilibrium), with the characteristics of the liver parenchyma and FLL similar to the extracellular gadolinium, such as gadopentetate dimeglumine (Gd-DTPA), followed by hepatobiliary assessment in the same exam[3-6]. Given the particular importance in each patient's outcome of the correct diagnosis of a challenging focal liver lesion, the recent introduction of this contrast medium in MRI and its potential uses, the objective was to determine the value of hepatobiliary phases (HBP) using gadoxetic acid as a liver-specific agent in MRI in addition to the non-contrast and dynamic phases after contrast in the characterization of benign and malignant FLL in clinical practice, including hepatocellular carcinoma (HCC) and metastases.
Controlled diagnostic clinical trial. Identification of the study under the Universal Trial Number (UTN): U1111-1247-9655.
Abdominal MRI exams with the use of a liver-specific contrast agent for the assessment of FLL characterized as challenging- assessments that had already been identified in previous exams (US and CT with contrast and/or MRI with conventional gadolinium), but that remained undetermined, requiring diagnostic complementation for clarification.
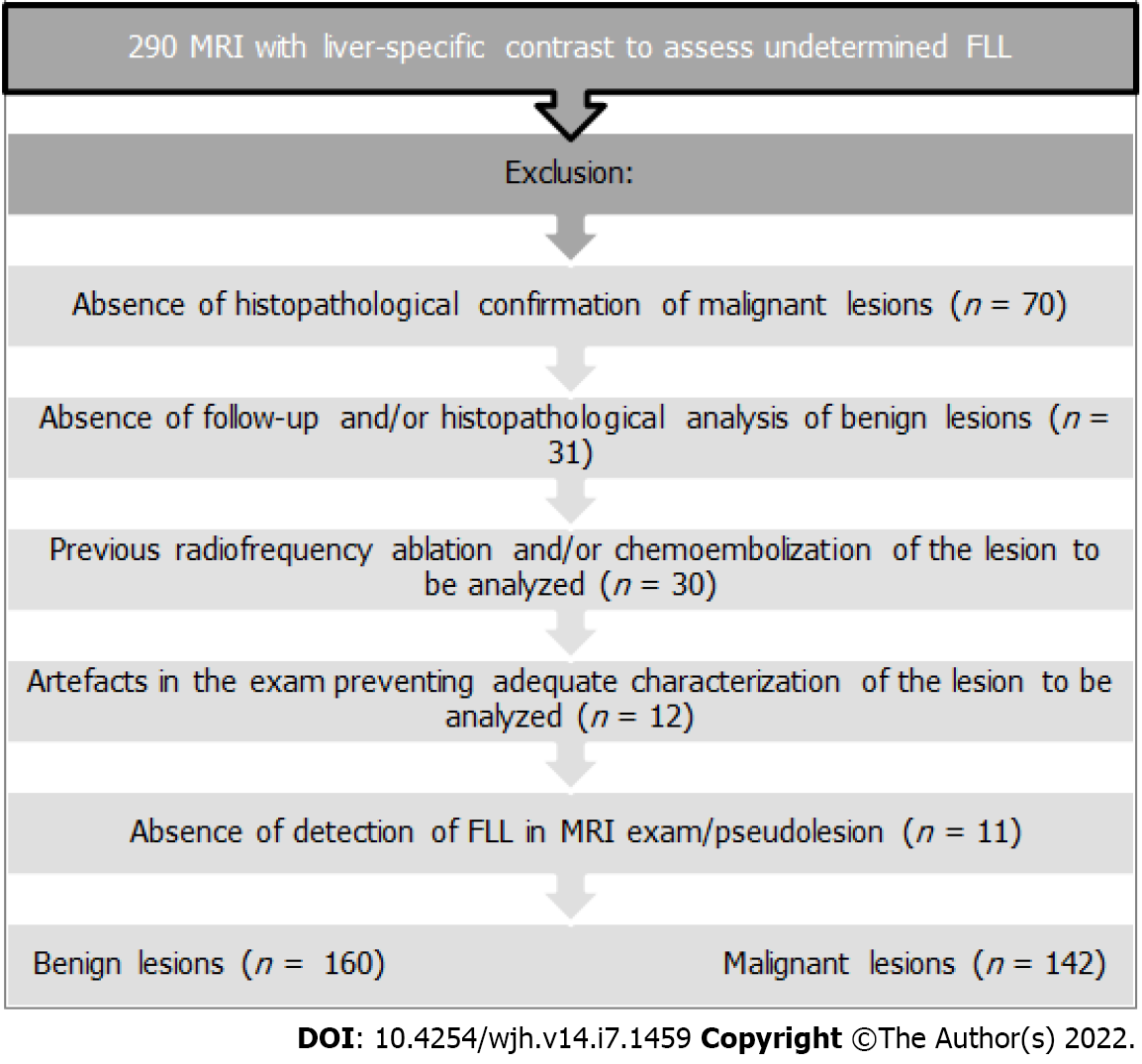
(1) Absence of definitive diagnostic criteria for FLL; (2) Previous radiofrequency ablation and/or chemoembolization of the lesion to be analyzed; (3) Artifacts in the exam preventing adequate characterization of the lesion to be analyzed; and (4) Absence of detection of FLL in the MRI exam.
The definitive diagnostic criterion for malignant lesions [liver metastases and HCC) and adenomas was based on anatomopathological confirmation. The histopathological slides were blindly reviewed by an experienced pathologist at the liver transplant unit of the hospital. The criteria used for the definitive diagnosis of other benign lesions [focal nodular hyperplasia (FNH), cysts, and hemangiomas] was the histopathological assessment or the absence of changes in the imaging follow-up (CT or MRI) of two years without treatment.
The exams were performed in a 1.5 T (Tesla) MRI scanner, with a 4-channel body sense coil. The patients were required to fast for 6 h, prior to scanning. Non-contrast T1-weighted sequences, in-phase and out-of-phase, and T2-weighted coronal sequences were performed. A dynamic study was conducted following injection of the contrast medium with T1-weighted sequences with fat saturation before and after intravenous injection of the contrast medium, with a dose of 0.1 mL/kg of weight (equivalent to 0.025 mmol/kg) in bolus, using an automatic injector, at a rate of 1.5 mL/s, followed by a flush of 20 mL of saline solution at the same rate of infusion. After the injection of gadoxetic acid, axial images and T1-weighted gradient echo sequences with fat saturation were obtained in these dynamic phases: arterial within 15 to 20 s after the start of the intravenous injection, portal after 60 s, transition after 120 s, and in the hepatobiliary phase within 10 and 20 min after the start of the intravenous injection. Between the transition phase and the hepatobiliary phase, T2-weighted images with and without fat saturation and diffusion-weighted sequences (DWI, b-value 1000) were acquired. The technical parameters used in each sequence are shown in Table 1.
| Parameter | T2 | T2 with fat saturation | T1 “in-phase” and “out-of-phase” | Diffusion | T1-weighted images without contrast and after contrast |
| Sequence | Fast spin-echo | Fast spin-echo | Gradient- echo FFE | EPI | Gradient- echo 3D/ TFE |
| Free breathing | Yes | Yes | No | No | No |
| Matrix | 268 × 184 | 300 × 261 | 236 × 161 | 152 × 150 | 168 × 228 |
| Thickness (mm) | 6.5 | 7 | 7 | 7 | 2.5 |
| Spacing (Gap) | 1.5 | 1 | 1 | 1 | - |
| Turning angle | 90 | 90 | 80 | 90 | 10 |
| Field of view (AP, LL, CC) | 297 × 335 × 222 | 363 × 400 × 223 | 353 × 400 × 223 | 380 × 380 × 239 | 295 × 400 × 225 |
| Repeat time (ms) | 5299 | 1299 | 104 | 2160 | 4.1 |
| Echo time (ms) | 160 | 80 | 4.6/2.3 | 80 | 2.0 |
| Acquisition time | 02:48 | 02:24 | 00:21 | 02:57 | 00:15 |
| Number of excitations | 2 | 2 | 1 | 4 | 1 |
Two radiologists (radiologist A with 5 years of experience in abdominal radiology, while radiologist B has more than 10 years) independently assessed the four stages of images in the following order: Stage 1: Non-contrast images (T1-pre-contrast; T2-weighted images with and without fat saturation; DWI, b-value 1000); Stage 2: Non-contrast images and dynamic phases following injection of gadoxetic acid (arterial, portal, and transition phase); Stage 3: Addition of hepatobiliary phase ten minutes (HBP10’) following the injection of gadoxetic acid in stage 2; Stage 4: Addition of hepatobiliary phase twenty minutes (HBP 20’) following the injection of gadoxetic acid in stage 2. A 6-point scale was created by the author for the assessment of each focal liver lesion in each stage as follows: Score 0: Lesion not detected in this stage; Score 1: Definitely benign; Score 2: Probably benign; Score 3: Undetermined; Score 4: Probably malignant; Score 5: Definitely malignant. The total time of analysis for each observer was three months, respecting the time interval of fifteen days between stages to avoid the influence of previous findings, to thus obtain an independent assessment of each stage. The two radiologists blindly assessed clinical-laboratory data and definitive diagnoses, and each issued its own report according to the parameters proposed by the researcher. The objective was to carry out an independent double assessment and subsequent comparison. Each observer reported the number of lesions diagnosed for each stage, the location (Couinaud segmentation[7]), and the proposed scores for each stage. The findings of each observer were analyzed with an assessment of the interobserver agreement. The cases of disagreement were discussed, and a consensus was reached.
Only lesions that appeared in the same location at the different stages of MRI and in the criteria for definitive diagnosis were considered correctly detected and characterized by the observers. The method of generalized estimating equations (GEE)[8] was used to compare the stages. The estimates were calculated by maximum likelihood to weight the difference in the number of repetitions for each patient. The statistical review of the study was performed by a medical statistician. The receiver operating characteristic (ROC) curve for repeated measurements was used to assess the accuracy of each stage in relation to the definitive diagnosis[9]. The observations in each patient are not independent, and intra-patient correlation and variation were introduced in the analyses using a generalized linear mixed model. The accuracy of each stage was compared estimating a logistic regression model for repeated measurements using the method of GEE[10]. A level of significance was adopted to be 5%.
After approval of the project by the Institutional Research Ethics Committee, it was found that 290 MRI exams had been performed consecutively during the study period in patients over 18 years of age who had used gadoxetic acid in the characterization of FLL that had already been identified in previous exams (US and CT and/or MRI with conventional gadolinium), that had undetermined characterization, requiring diagnostic complementation. The exclusion criteria are shown in Figure 1. Therefore, the final sample according to the criteria used for the definitive diagnosis was composed of 302 Lesions from 136 patients who performed MRI exams using gadoxetic acid for the assessment of FLL, with 160 benign lesions (53.0%) and 142 malignant lesions (47.0%). Benign lesions included: FNH (n = 90; 56.2%); cysts (n = 36; 22.5%); hemangiomas (n = 22; 13.7%); adenomas (n = 12; 7.5%). Malignant lesions included: metastases (n = 87; 61.3%) and hepatocellular carcinomas- HCCs (n = 55; 38.7%). The number of lesions according to the criteria for the definitive diagnosis in each patient ranged from 1-5 Lesions (mean 2.4; SD 1.8). The diameter of the 160 benign lesions ranged from 0.4 cm to 8.8 cm (mean 2.7 cm; SD 1.9 cm). The diameter of the 142 malignant lesions ranged between 0.4 cm and 7.8 cm (mean 2.1 cm; SD 1.7 cm).
The final sample, based on the criteria used for the definitive diagnosis, was composed of 302 Lesions from 136 patients who performed MRI exams using gadoxetic acid for the assessment of FLL. Of these 136 patients, 80 (58.8%) were female, with a mean age of 43 years (SD 19). Personal history of cancer was present in 52.9% of patients (colorectal 95.5%; gastric 11.8%; breast 8.8%; prostate 8.1%; melanoma 7.3%; pheochromocytoma 4.4%).
The weighted Kappa coefficient is used to describe the agreement between two or more observers when performing a nominal or ordinal assessment of the same sample and demonstrated high agreement (between 0.81 and 1) for all stages in the characterization of benign and malignant FLL. Of the total 302 Lesions, there was disagreement between observers in ten lesions in stage 1; eight lesions in stage 2; seven lesions in stages 3 and 4. For lesions where in there was no agreement between observers, the consensus of the radiologists was used for the final definition.
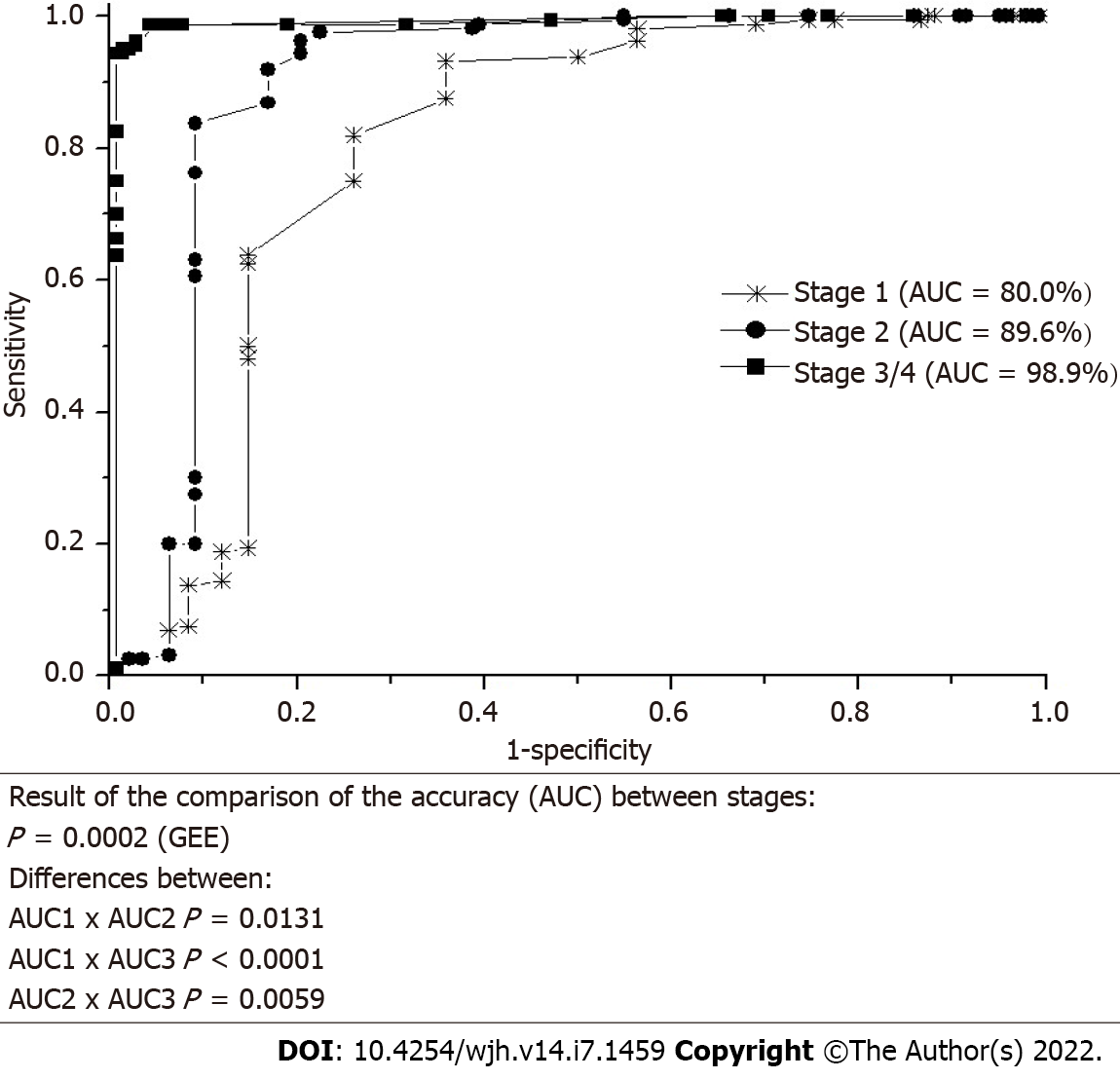
The accuracy weighted by the number of repetitions of lesions in each patient showed a good proportion of correct answers for differentiating between benign and malignant lesions (Figure 2). There were significant differences between the accuracy of the four stages (P = 0.0002, GEE, Figure 2).
The comparison of the weighted accuracy [area under the curve (AUC)] showed that the accuracy of stage 1 was lower than the accuracy of stages 2 and 3/4. The accuracy of stage 2 was lower than the accuracy of stages 3 and 4. There were no significant differences between stages 3 and 4 (Figure 2).
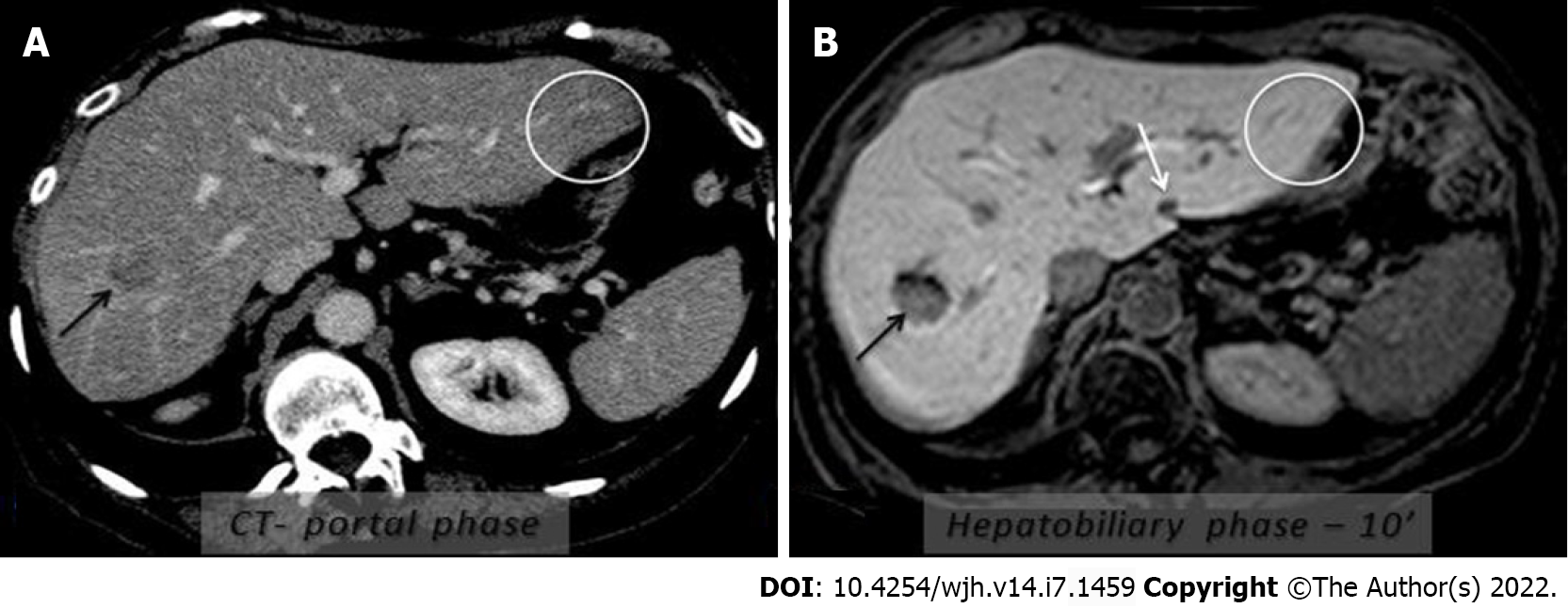
The characterizations in the stages of only the malignant lesions were associated with the numerical size (in cm) of the FLL. Each unit of increase in the size of the malignant lesion increases the chance of characterization with higher scores by 1.26 at each of all stages. Characterizations in the stages of only malignant lesions were associated with the size of the FLL categorized as < 1 cm and ≥ 1.0 cm. Malignant lesions ≥ 1 cm are 2.4 times more likely to be characterized with higher scores at all stages than lesions < 1 cm (Table 2). Figure 3 shows subcentimetric metastasis in a cancer patient detected only in the hepatobiliary phases and a pseudo lesion.
| Size effect | General P value | Benign P value | Malignant P value |
| Numerical (cm) | 0.3785 | 0.1766 | 0.0025 OR = 1.2561 95%CI (1.0824; 1.4577) |
| (≥ 1 cm) x (< 1 cm) | 0.2361 | 0.1476 | 0.0058 OR = 2.3691 95%CI (1.3001; 4.3171) |
The present study found a significant increase in the diagnostic reliability of malignant lesions (HCC and metastases) with the inclusion of stage 3 compared to stage 2. An ideal diagnostic tool by liver imaging should have a high diagnostic accuracy to provide an adequate therapeutic approach in malignant and benign cases. The MRI with gadoxetic acid has revealed excellent diagnostic performance for detecting metastases in recent meta-analyses[11-13]. The combined use of diffusion weighted sequences (DWI) and hepatobiliary phases in clinical practice is recommended in patients with potentially resectable liver metastases[14,15].
Still on the significant increase in the diagnostic reliability in the characterization of malignant lesions found in our study, the HCC is one of the few malignancies that can be diagnosed by imaging alone, without the need for confirmation by biopsy when the image is typical. Different guidelines established by medical groups and entities are used in patients at risk for HCC and reflect clinical and epidemiological differences, underlying etiologies of liver disease, socioeconomic background, and specificities of each region, such as surveillance and available therapeutic options[16-20]. The additional benefit of diffusion and a liver-specific contrast is recognized by the American College of Radiology (ACR) and is incorporated into the Liver Imaging Reporting and Data System (LIRADS)[21-25].
There was an increase in diagnostic reliability in the characterization of benign lesions with the addition of the hepatobiliary phases (stage 3) compared to stage 2. For benign lesions, a recent systematic review concludes that the low signal intensity in the hepatobiliary phases can help distinguish between adenomas and FNH[26].
Our research also showed the value of morphofunctional MRI with gadoxetic acid as a liver-specific contrast in the diagnosis of pseudo lesions, since 11 exams were excluded (3.8%) from the initial sample of 290 due to the absence of detectable lesions in the MRI exam. The lesions had been observed in other previous imaging methods, remaining undetermined. It is also noteworthy that 12 exams (4.1%) were excluded from the 290 of the initial sample due to artifacts preventing adequate characterization of the lesion to be analyzed, such as the phenomenon of “transient dyspnea”. Studies relate this artifact to the use of gadoxetic acid, although the data is not consistent and the pathophysiology is not yet fully elucidated[27-29].
In this study, stages 3 and 4 showed identical results in the characterization of FLL. Although recommendations point to the acquisition of HBP20’, some evidences suggest the possibility of earlier acquisition (HBP10’) in the assessment of part of the cases of FLL[15,31-33]. Other cases individualized according to the diagnostic suspicion in clinical practice may require phases after 20 or possibly up to 30 minutes after contrast medium injection, for example the differentiation between biliary lesions and extra biliary cysts that do not communicate with bile ducts, such as duodenal duplication cysts, duodenal diverticula and pseudo cysts. The liver-specific contrast delineates the biliary tract demon
Some considerations should be made about this study. The assessed MRI exams are from patients who are part of a cohort at the institutional FLL outpatient clinic; thus, the results of this research with an institutional-based sample may differ from results with population-based samples. Moreover, all images were acquired with the same parameter and the observers are familiar with the specific technical protocols, as in the usual clinical routine conditions.
Given the reality of the higher cost of liver-specific contrast in most countries, we highlight the value of morphofunctional MRI with the hepatobiliary phase, notably in specific situations after, for example, the diagnosis of a FLL has remained undetermined in previous exams (US and CT with contrast and/or MRI with extracellular contrast routinely used), as in the screening of patients in our study. The use and additional analysis in clinical practice of hepatobiliary stages (steps 3 and 4 in this study) as a criterion for information aggregation in relation to other sequences routinely performed in CT and MRI scans (stage 1: Non-contrast images and stage 2: Dynamic phases after contrast, analogous to the phases with extracellular contrast- arterial, portal and equilibrium/transition) may benefit a specific group of patients. Good cost-effective practices for the use of this methodology in morphofunctional MRI with liver-specific contrast may include, therefore, (1) The elucidation of possible pseudo-lesions (perfusion alterations x HCC, for example; most HCCs, except the well-differentiated ones, present hypo signal in the hepatobiliary phases) and and/or problem solving in patients with lesions with atypical characteristics by imaging; (2) The diagnosis of small metastatic lesions in potential patients for surgical treatment; (3) The search to complement information to increase diagnostic assertiveness in benign lesions still undetermined (hepatocellular x non-hepatocellular origin; or biliary lesions x extra biliary cysts); and (4) The definitive diagnosis in the non-invasive era of malignant lesions hitherto uncharacteristic in previous exams with routine extracellular contrast agents (either through the potential increase in the LIRADS category in hepatocellular carcinomas or through a more assertive diagnosis of secondary liver involvement), as demonstrated herein. These applications mentioned above refer to the context more focused on FLL, without including the other important potential indications like those mentioned in the discussion of this study.
Other potential benefits in living laboratories integrating translational research and technological innovations have brought to light new uses of this methodology in morphofunctional MRI with liver-specific contrast, such as imaging biomarkers, outcome predictions and co-creation intelligences for the resolution and/or amelioration of specific diseases to patients, emerging as promising prospects. Further potential liver-specific contrast applications include assesment of liver fibrosis, the evaluation of the functional hepatic reserve before partial hepatectomy; evaluation of live donor's hepatic function as well as evaluation of early liver failure after transplantation. In another active area of investigation, morphofunctional MRI with liver-specific contrast may provide a system for stratifying patients according to risk of recurrence with a likely influence on the outcomes of locoregional HCC treatments[36]. The congruence of different knowledge is evident in medical practice and in the necessary advances.
The value of morphofunctional MRI with gadoxetic acid as a liver-specific contrast in addition to the usual dynamic phases after contrast medium (arterial, portal and transitional/equilibrium) was to increase the proportion of hits for differentiation between benign and malignant FLL in relation to the definitive diagnosis. The interobserver agreement was high (0.81-1). With growing potential in the era of precision medicine, the improvement and dissemination of the method among medical specialties can bring benefits in the management of patients with focal liver lesions that are difficult to diagnose.
The accurate characterization of focal liver lesions (FLL) has great clinical relevance. Although ultrasonography (US) and computed tomography (CT) are the most important diagnostic tools for screening FLL, magnetic resonance imaging (MRI) is a well-established diagnostic imaging method in clinical practice and produces images without ionizing radiation, with good spatial resolution and excellent tissue resolution, thus allowing a very reliable assessment. One of the main innovative trends currently, is the use of molecular and functional methods.
Challenging lesions, difficult to diagnose through non-invasive methods, constitute an important emotional burden for each patient regarding a still uncertain diagnosis (malignant x benign). In addition, from a therapeutic and prognostic point of view, delay in a definitive diagnosis can lead to worse outcomes. Numerous liver-specific contrast agents have been developed and studied in recent years to improve the performance of liver MRI. More recently, one of the contrast agents introduced in clinical practice is gadoxetic acid (gadoxetate disodium).
To determine the value of hepatobiliary phases (HBP) using gadoxetic acid as a liver-specific agent in MRI in addition to the non-contrast and dynamic phases after contrast in the characterization of benign and malignant FLL in clinical practice, including hepatocellular carcinoma and metastases.
Controlled diagnostic clinical trial. Two radiologists independently assessed the four stages of images in the following order: Stage 1: Non-contrast images (T1-pre-contrast; T2-weighted images with and without fat saturation; DWI, b-value 1000); Stage 2: Non-contrast images and dynamic phases following injection of gadoxetic acid (arterial, portal, and transitional phase); Stage 3: Addition of hepatobiliary phase ten minutes (HBP10’) following the injection of gadoxetic acid in stage 2; Stage 4: Addition of hepatobiliary phase twenty minutes (HBP 20’) following the injection of gadoxetic acid in stage 2. A 6-point scale was created by the author for the assessment of each focal liver lesion in each stage. The method of Generalized Estimating Equations (GEE) was used to compare the stages. The estimates were calculated by maximum likelihood to weight the difference in the number of repetitions for each patient. The receiver operating characteristic (ROC) curve for repeated measurements was used to assess the accuracy of each stage in relation to the definitive diagnosis.
The interobserver agreement was high (weighted Kappa coefficient: 0.81-1) at all stages in the characterization of benign and malignant FLL. The diagnostic weighted accuracy (Az) was 0.80 in stage 1 and was increased to 0.90 in stage 2. Addition of the hepatobiliary phase increased Az to 0.98 in stage 3, which was also 0.98 in stage 4.
The value of morphofunctional MRI with gadoxetic acid as a liver-specific contrast in addition to the usual dynamic phases after contrast medium (arterial, portal and transitional/equilibrium) was to increase the proportion of hits for differentiation between benign and malignant FLL in relation to the definitive diagnosis.
With growing potential in the era of precision medicine, the improvement and dissemination of the method among medical field can bring benefits in the management of patients with focal liver lesions that are difficult to diagnose. With the accumulation of experience, the use demonstrated herein and other potentials of morphofunctional MRI with liver-specific contrast as a new potential imaging tumor biomarker may be established, benefiting patients with challenging focal liver lesions. Other potential benefits in living laboratories have brought to light new uses of this methodology, such as outcome predictions and co-creation intelligences for the resolution and/or amelioration of specific diseases to patients, emerging as promising prospects. Further potential liver-specific contrast applications include assesment of liver fibrosis, the evaluation of the functional hepatic reserve before partial hepatectomy; evaluation of live donor's hepatic function as well as evaluation of early liver failure after transplantation. In another active area of investigation, morphofunctional MRI with liver-specific contrast may provide a system for stratifying patients according to risk of recurrence with a likely influence on the outcomes of locoregional HCC treatments. Also new translational studies similar to this one in other parts of the world added to the socioeconomic background and specificities of each region may bring benefits to this group of patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Colégio Brasileiro de Radiologia; Sociedade Brasileira de Patologia; Colégio Brasileiro de Cirurgiões.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iwao Y, Japan; Liu ET, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Purysko AS, Remer EM, Veniero JC. Focal liver lesion detection and characterization with GD-EOB-DTPA. Clin Radiol. 2011;66:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Ronot M, Clift AK, Vilgrain V, Frilling A. Functional imaging in liver tumours. J Hepatol. 2016;65:1017-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 4. | Palmucci S. Focal liver lesions detection and characterization: The advantages of gadoxetic acid-enhanced liver MRI. World J Hepatol. 2014;6:477-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Jeong WK, Kim YK, Song KD, Choi D, Lim HK. The MR imaging diagnosis of liver diseases using gadoxetic acid: emphasis on hepatobiliary phase. Clin Mol Hepatol. 2013;19:360-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 6. | Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, Santillan CS, Wolfson T, Gamst A, Sirlin CB. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY). 2016;41:71-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Couinaud C. Le foie: études anatomiques et chirurgicales. Paris: Masson; 1957. |
| 8. | Brown H, Prescott R. Applied Mixed Models in Medicine, 2nd Edition. Publisher: John Wiley & Sons Ltda. Inglaterra. 2006. |
| 9. | Liu H, Wu T. Estimating the Area under a Receiver Operating Characteristic Curve For Repeated Measures Design. J Statistical Software. 2003;8:1-18. |
| 10. | Kuss O. How to use SAS® for logistic regression with correlated data. Proceedings of the 27th Annual SAS® Users Group International Conference (SUGI 27). Orlando, Florida. SAS Institute Inc 2002: 261–327. |
| 11. | Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Chen L, Zhang J, Zhang L, Bao J, Liu C, Xia Y, Huang X, Wang J. Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One. 2012;7:e48681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Mao Y, Chen B, Wang H, Zhang Y, Yi X, Liao W, Zhao L. Diagnostic performance of magnetic resonance imaging for colorectal liver metastasis: A systematic review and meta-analysis. Sci Rep. 2020;10:1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26:4595-4615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Jeong HT, Kim MJ, Park MS, Choi JY, Choi JS, Kim KS, Choi GH, Shin SJ. Detection of liver metastases using gadoxetic-enhanced dynamic and 10- and 20-minute delayed phase MR imaging. J Magn Reson Imaging. 2012;35:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3241] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 17. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 18. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 19. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 20. | Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'Kontchou G, Rode A, Radenne S, Cassinotto C, Vergniol J, Bricault I, Leroy V, Ronot M, Castera L, Michalak S, Esvan M, Vilgrain V; CHIC Group. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37:1515-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: A meta-analysis. Liver Transpl. 2017;23:1505-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Lan H, Lin G, Zhong W. A meta-analysis of the added value of diffusion weighted imaging in combination with contrast-enhanced magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma lesser or equal to 2 cm. Oncol Lett. 2020;20:2739-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Fernandes DA, Martins DL, Penachim TJ, Barros RHO, Costa LBED, Ataíde EC, Boin IFSF, Caserta NMG. The value of morphofunctional magnetic resonance imaging with hepatospecific contrast agent in the characterization of hepatocellular carcinoma in a non-cirrhotic patient with hepatitis C. Rev Assoc Med Bras (1992). 2020;66:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Santillan C, Fowler K, Kono Y, Chernyak V. LI-RADS major features: CT, MRI with extracellular agents, and MRI with hepatobiliary agents. Abdom Radiol (NY). 2018;43:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Chernyak V, Tang A, Flusberg M, Papadatos D, Bijan B, Kono Y, Santillan C. LI-RADS® ancillary features on CT and MRI. Abdom Radiol (NY). 2018;43:82-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Guo Y, Li W, Cai W, Zhang Y, Fang Y, Hong G. Diagnostic Value of Gadoxetic Acid-Enhanced MR Imaging to Distinguish HCA and Its Subtype from FNH: A Systematic Review. Int J Med Sci. 2017;14:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014;272:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Well L, Weinrich JM, Adam G, Bannas P. Transient Severe Respiratory Motion Artifacts After Application of Gadoxetate Disodium: What We Currently Know. Rofo. 2018;190:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol. 2009;50:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Zech CJ, Ba-Ssalamah A, Berg T, Chandarana H, Chau GY, Grazioli L, Kim MJ, Lee JM, Merkle EM, Murakami T, Ricke J, B Sirlin C, Song B, Taouli B, Yoshimitsu K, Koh DM. Consensus report from the 8th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol. 2020;30:370-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | van Kessel CS, Veldhuis WB, van den Bosch MA, van Leeuwen MS. MR liver imaging with Gd-EOB-DTPA: a delay time of 10 minutes is sufficient for lesion characterisation. Eur Radiol. 2012;22:2153-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Motosugi U, Ichikawa T, Tominaga L, Sou H, Sano K, Ichikawa S, Araki T. Delay before the hepatocyte phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur Radiol. 2009;19:2623-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 35. | Schramm C, Eaton J, Ringe KI, Venkatesh S, Yamamura J; MRI working group of the IPSCSG. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology. 2017;66:1675-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Erstad DJ, Tanabe KK. Hepatocellular carcinoma: early-stage management challenges. J Hepatocell Carcinoma. 2017;4:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |