Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1258
Peer-review started: January 22, 2022
First decision: March 16, 2022
Revised: April 6, 2022
Accepted: May 12, 2022
Article in press: May 12, 2022
Published online: June 27, 2022
Processing time: 152 Days and 14.7 Hours
Spontaneous bacterial empyema (SBE) occurs when a hepatic hydrothorax becomes infected and runs a course similar to spontaneous bacterial peritonitis (SBP). It remains underdiagnosed as patients with cirrhosis do not routinely undergo diagnostic thoracentesis. Current understanding is limited by small cohorts, while studies reporting its association with ascites/SBP are conflicting.
To explore the incidence of SBE, to determine its association with ascites, and to summarize what is known regarding treatment and outcomes for patients with SBE.
Major databases were searched until June 2021. Outcomes include the incidence of SBE in pleural effusions, SBP in peritoneal fluid, and SBE in patients without ascites within our cohort of patients with cirrhosis. We performed a meta-analysis using a random-effects model with pooled proportions and 95% confidence intervals (CI). We assessed heterogeneity using I2 and classic fail-safe to determine bias.
Eight studies with 8899 cirrhosis patients were included. The median age ranged between 41.2 to 69.7 years. The majority of the patients were Child-Pugh B and C. Mean MELD score was 18.6 ± 8.09. A total of 1334 patients had pleural effusions and the pooled incidence of SBE was 15.6% (CI 12.6-19; I2 50). Amongst patients diagnosed with SBE, the most common locations included right (202), left (64), and bilateral (8). Amongst our cohort, a total of 2636 patients had ascites with a pooled incidence of SBP of 22.2% (CI 9.9-42.7; I2 97.8). The pooled incidence of SBE in patients with cirrhosis but without concomitant ascites was 9.5% (CI 3.6-22.8; I2 82.5).
SBE frequently occurs with concurrent ascites/SBP; our results suggest high incidence rates of SBE even in the absence of ascites. The pleura can be an unrecognized nidus and our findings support the use of diagnostic thoracentesis in patients with decompensated cirrhosis after exclusion of other causes of pleural effusion. Thoracentesis should be considered particularly in patients without ascites and when there is a high suspicion of infection. The need for diagnostic thorac
Core Tip: Identification of risk factors for developing spontaneous bacterial empyema and characterization of spontaneous bacterial empyema are lacking. This is a systematic review and meta-analysis describing spontaneous bacterial empyema and the relationship to ascites in patients with cirrhosis. We investigated the incidence of spontaneous bacterial empyema, the incidence of spontaneous bacterial peritonitis, and the incidence of spontaneous bacterial empyema without ascites in a meta-analysis including eight studies.
- Citation: Reiche W, Deliwala S, Chandan S, Mohan BP, Dhindsa B, Ramai D, Perisetti A, Rangray R, Mukherjee S. Spontaneous bacterial empyema in cirrhosis: A systematic review and meta-analysis. World J Hepatol 2022; 14(6): 1258-1268
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1258.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1258
Hepatic hydrothorax (HH) is one of the pulmonary complications observed in cirrhotic patients and attributed to portal hypertension which leads to a transudative effusion. The true prevalence of HH in cirrhotic patients is unclear but estimated to be at 10%. Infection of the HH (pleura and pleural fluid) is termed spontaneous bacterial empyema (SBE) and represents a distinct and underdiagnosed infectious etiology in patients with decompensated cirrhosis. This entity’s existence has frequently been debated; the true nature is uncertain[1-4]. Knowledge regarding this complication has been limited due to a lack of clinical studies. At the same time, several studies suggest SBE is not spontaneous but occurs due to ruptured pleuroperitoneal blebs, which cause diaphragm defects. The negative pressure created in the pleural cavity creates unidirectional flow into the pleural space where low protein ascitic fluid can accumulate and propagate infection[5]. Conversely, other studies have found that SBE can develop without spontaneous bacterial peritonitis (SBP) and can even be diagnosed in patients without ascitic fluid, representing an overlooked infection nidus[5-8]. More robust data is needed within SBE to guide efficient clinical decision-making due to the significant burden on patients and healthcare resources[9,10]. Patients inflicted by pleural pathology can have prolonged stay with increased mortality[7,11,12].
The exudative nature of SBE is well established; however, risk factors for developing this condition are less clearly elucidated. Several studies have found that patients who develop HH or SBE are more likely to have lower levels of pleural fluid protein and a higher Child-Pugh or MELD score to support the diagnosis[13,14]. Additionally, the concurrence of hydrothorax and ascites remains unknown. We aim to fill the current understanding by performing a systematic review and incidence meta-analysis exploring the incidence of SBE and its association with ascites.
This review has been in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) and Meta-analyses of Observational Studies in Epidemiology reporting standards (Supplementary Tables 1 and 2)[15].
An expert librarian conducted a systematic literature search using a priori protocol to identify studies reporting the incidence, associations, and outcomes of SBE in patients with cirrhosis. The search strategies included “spontaneous bacterial empyema,” “SBE,” and “SBEM.” The search was run in June 2021 across multiple databases, including Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+ including epub ahead of print, in-process, and other non-indexed citations), Scopus (1970+), Web of Science (1975+), and PubMed. The search was restricted to articles in English and identified searches were exported to a reference manager (EndNote). We cross-checked reference lists of identified sources for additional relevant studies. Any discrepancy was resolved by a third reviewer (SC). Detailed search strategy presented as Supplementary material.
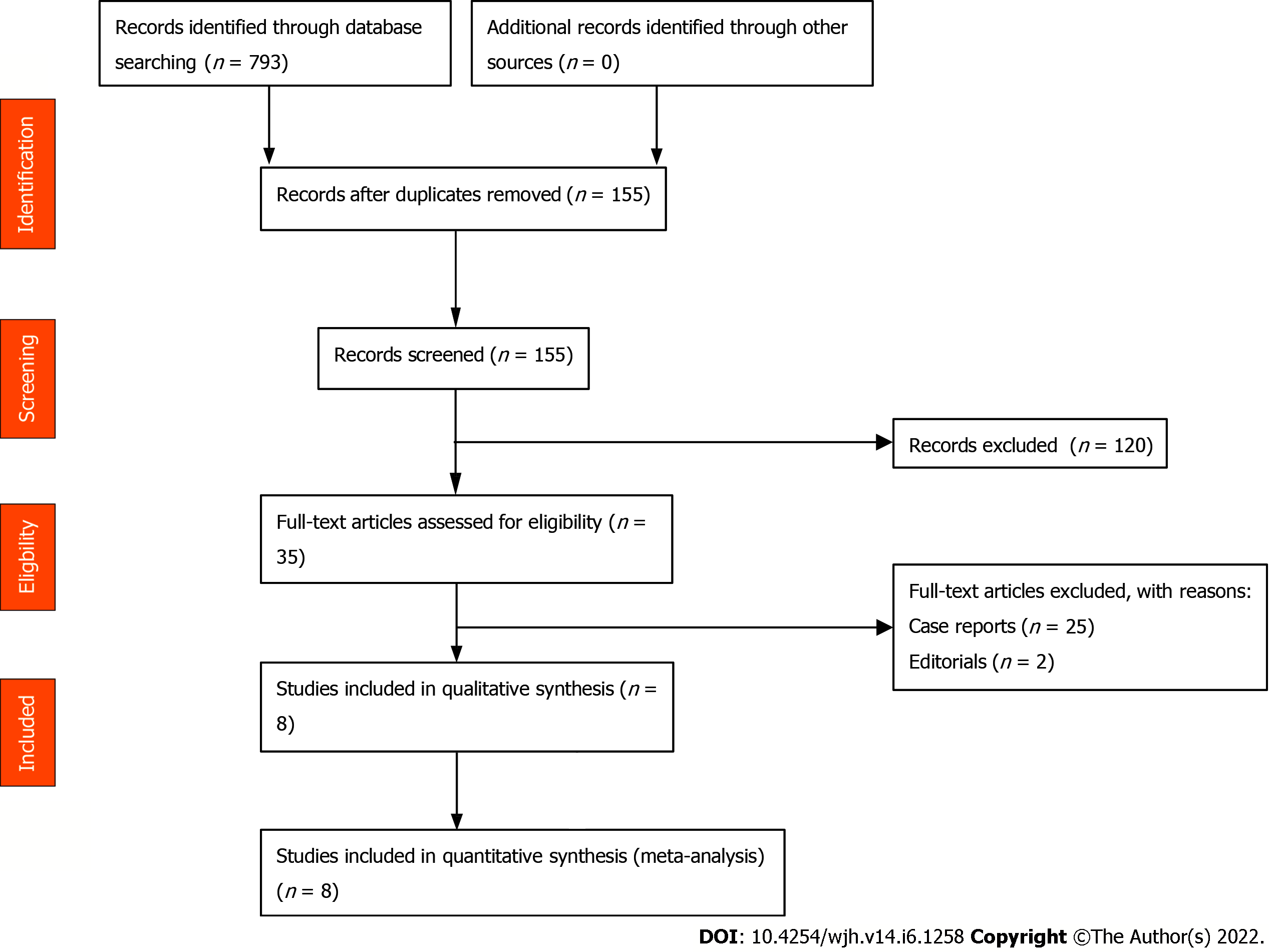
This meta-analysis included studies that evaluated patients with SBE. SBE was defined as positive pleural fluid culture and polymorphonuclear leukocytes (PMN) count > 250 cells/mm3 or negative pleural fluid culture and PMN count > 500 cells/mm3, without evidence of pneumonia/parapneumonic effusion on imaging[5-7]. Studies reporting performance in pediatric age groups (< 18 years), conference abstracts, case reports, and non-English studies were excluded. Studies were restricted to full-text. Two authors decided on the final selection (WR, SC). Details presented in PRISMA flow diagram, Figure 1.
Two reviewers (WR, SD) independently extracted eligible information into an a priori designed Google excel spreadsheet. The Qumseya scale for quality assessment of cohort studies for systematic reviews and meta-analyses consisted of nine questions (Supplementary Figure 1). We assessed each study for its design, measurements, outcomes, and patient characteristics. Each risk of bias had a maximum score of 10. Studies with less than six were considered low, 6-7 were moderate, and > 8 were deemed to be high quality[16].
(1) Incidence of SBE in patients with cirrhosis; (2) Incidence of SBP in patients with cirrhosis; (3) Incidence of SBE in patients without concomitant ascites.
Statistical analysis was performed using Comprehensive Meta-Analysis (CMA 3.0) software (Biostat, Englewood, NJ). Pooled estimates and corresponding 95% confidence intervals (CI) for dichotomous variables were calculated using the random-effects inverse variance method[17]. Heterogeneity was measured by Cochrane Q and I2 statistics, with values of < 30%, 31%-60%, 61%-75%, and > 75% suggesting low, moderate, substantial, and considerable heterogeneity, respectively[18,19]. A funnel plot combined with Egger’s tests was performed to assess publication bias. A p-value of 0.05 or less combined with asymmetry in the funnel plots was used to measure significant publication bias. If < 0.05, the trim-and-fill computation was used to evaluate the effect of publication bias on the interpretation of the results. Three levels of impact were reported based on the concordance between the reported results and the actual estimate if there was no bias. The impact was reported as minimal if both versions were estimated to be the same, modest if the effect size changed substantially. Still, the final finding would remain the same and severe if the bias threatens the conclusion of the analysis[20]. To evaluate an individual study's effect on the collective outcome, sensitivity analysis was completed.
An initial search identified 155 publications after removing duplicates. After screening full-text articles, eight studies were eligible for qualitative and quantitative synthesis, as shown in Supplemen-tary Figure 1. Study locations included Spain, Taiwan, Egypt, and Pakistan between 1988-2017. Among eight studies, 8899 patients (270 males and 110 females; not all studies reported sex); were included, with the median age between 41.2 to 69.7 years. Most of the patients were Child-Turcott Pugh B and C, while the average MELD score was 18.6 ± 8.09. 202 cases were seen in the right pleural space, while 64 cases were seen in the left pleural space, eight cases of bilateral pleural effusions were reported. Study and baseline clinical characteristics have been summarized in Tables 1 and 2.
| Ref. | Year, country, study type | Total patients | Patients w/ PE | Patients w/ SBE | Patients w/ ascites | Patients with SBP | SBE w/o ascites | Age(mean) | Sex(m/f) | R PE | L PE | B/l PE | Treated patients | MELD score | CP score | Mortality |
| Xiol et al[8], 1996 | 1996, Spain, Prospective | 120 | 120/120 | 16/120 | 95/120 | 14/18 | 6/24/ | 19/24 | 10.67 (1.20) | |||||||
| Chen et al[21], 2003 | 2003, Taiwan, Prospective | 862 | 132/862 | 17/132 | 451/862 | 104/451 | 2/411 | 53.7 (13.2) [17n] | 13/4[17n] | 17/17 | 11.5 (1.6) [17n] | |||||
| Chen et al[7], 2011 | 2011, Taiwan, Retrospective | 3390 | 508/3390 | 81/508 | 1729/3390 | 44/1729 | 14/81 | 60.0 (12.8) [81n] | 55/26 [81n] | 60/81 | 21/81 | 58/81 | 20.5 (8.0) | 9.7 (2.1) | 31/81 | |
| Makhlouf et al[5], 2013 | 2012, Egypt, Prospective | 901 | 61/901 | 16/61 | 45/901 | 9/45' | 4/16' | 51.1 (11.00) [16n] | 15/1 [16n] | 53/61 | 5/61 | 3/61 | 0 [CP A], 1 [CP B], 15 [CP C]//11.8 (1.3) | 4/16 | ||
| Mansour et al[22], 2013 | 2013, Egypt, Prospective | 98 | 98/98 | 14/98 | 94/98 | 16/94 | 1/14' | 69.7 (16.5) [14n] | 8/6 [14n] | 12/14 | 1/14 | 1/14 | 27.2 (5.7) | |||
| Emam et al[24], 2015 | 2015, Egypt, Prospective | 322 | 322/322 | 46/322 | 108/322 | 0/46 | 56.76 (6.23) [46n] | 30/16 [46n] | 42/46 | 2/46 | 2/46 | 0 [CP A], 4 [CP B], 42 {CP C] | ||||
| Abbasi et al[6], 2016 | 2016, Pakistan, Prospective | 206 | 23/206 | 7/23' | 152/206 | 5/23' | 41.25 (13.593) [206n] | 149/57 [206n] | 18/23 | 3/23 | 2/23 | 62 [CP A], 61 [CP B], 83 [CP C] | ||||
| Mohamed et al[23], 2017 | 2017, Egypt, Prospective | 3000 | 70/3000 | 5/70' | 70/3000 | 17/70 |
| Ref. | Xiol et al[8], 1996 | Chen et al[21], 2003 | Chen et al[7], 2011 | Makhlouf et al[5], 2013 | Mansour et al[22], 2013 | Emam et al[24], 2015 | Abbasi et al[6], 2016 | Mohamed et al[23], 2017 |
| Year, country, study type | Spain, Prospective | Taiwan, Prospective | Taiwan, Retrospective | Egypt, Prospective | Egypt, Prospective | Egypt, Prospective | Pakistan, Prospective | Egypt, Prospective |
| SBE-diagnostic criteria | Positive PF culture and a PMN cell count > 250 cells/mm3 or negative PF culture, compatible clinical course, and a | Positive PF culture and a PMN cell count > 250 cells/mm3 or PMN cell count > 500 cells/mm3; no pneumonia on CXR or CT; PF transudate characteristics during infection or evidence of pleural effusion before the infected episode | Positive PF culture and a PMN cell count > 250 cells/mm3 or, negative PF culture, PMN cell | Positive PF culture and a PMN count of 250 cells/mm3 or, if a negative culture, a PF PMN count of > 500 cells/mm3 and the absence of pneumonia or a contiguous infection process on CXR | Positive PF culture or, if negative, a PF PMN count > 500 cells/µL without radiographic evidence of pneumonia | Positive PF culture or, if negative, a PF PMN count > 500 cells/mm3 without radiographic evidence of pneumonia or a contiguous infection process on CXR | PF with PMN cell count > 500 cells/mm or positive culture with PMN cell count > 250 cells/mm3 with exclusion of a parapneumonic effusion | Positive PF culture and PMN count > 250 cells/mm3 or negative PF culture and PMN |
Scores for methodological quality assessment are shown in Supplementary Figure 2. Amongst eight studies, one was prospective,[8] five retrospective,[6,7,21-23] and two were cross-sectional[5,24]. All studies were performed in single-centers.
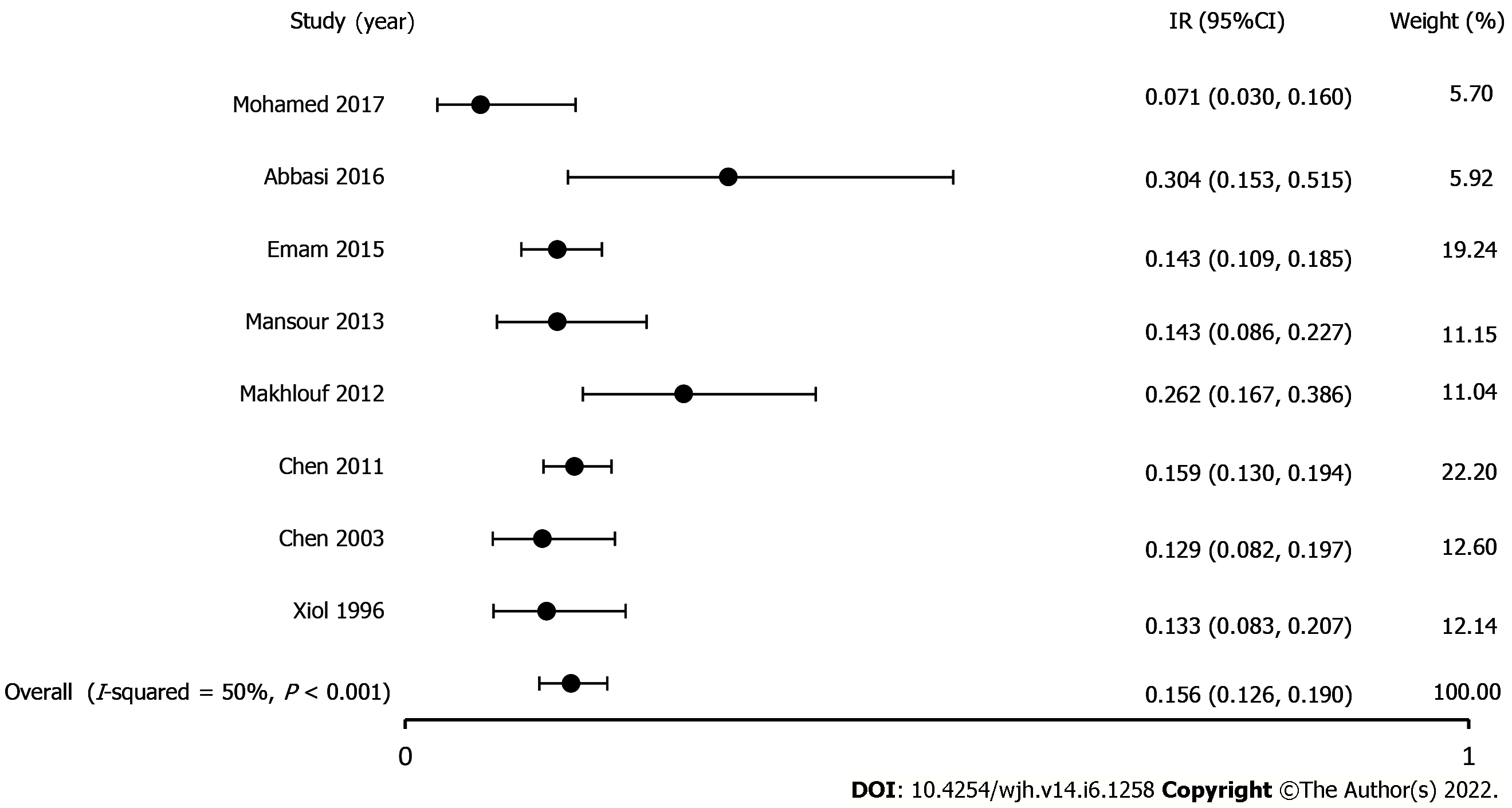
Incidence of SBE in patients with cirrhosis: All eight studies reported the incidence of SBE in patients with cirrhosis[5-8,21-24]. A total of 1334 patients had pleural effusions, and the pooled incidence of SBE was 15.6% (CI, 12.6-19; P < 0.001, I2 50%). The true effect size in 95% of all comparable populations falls in the interval 0.12-0.21 (Figure 2).
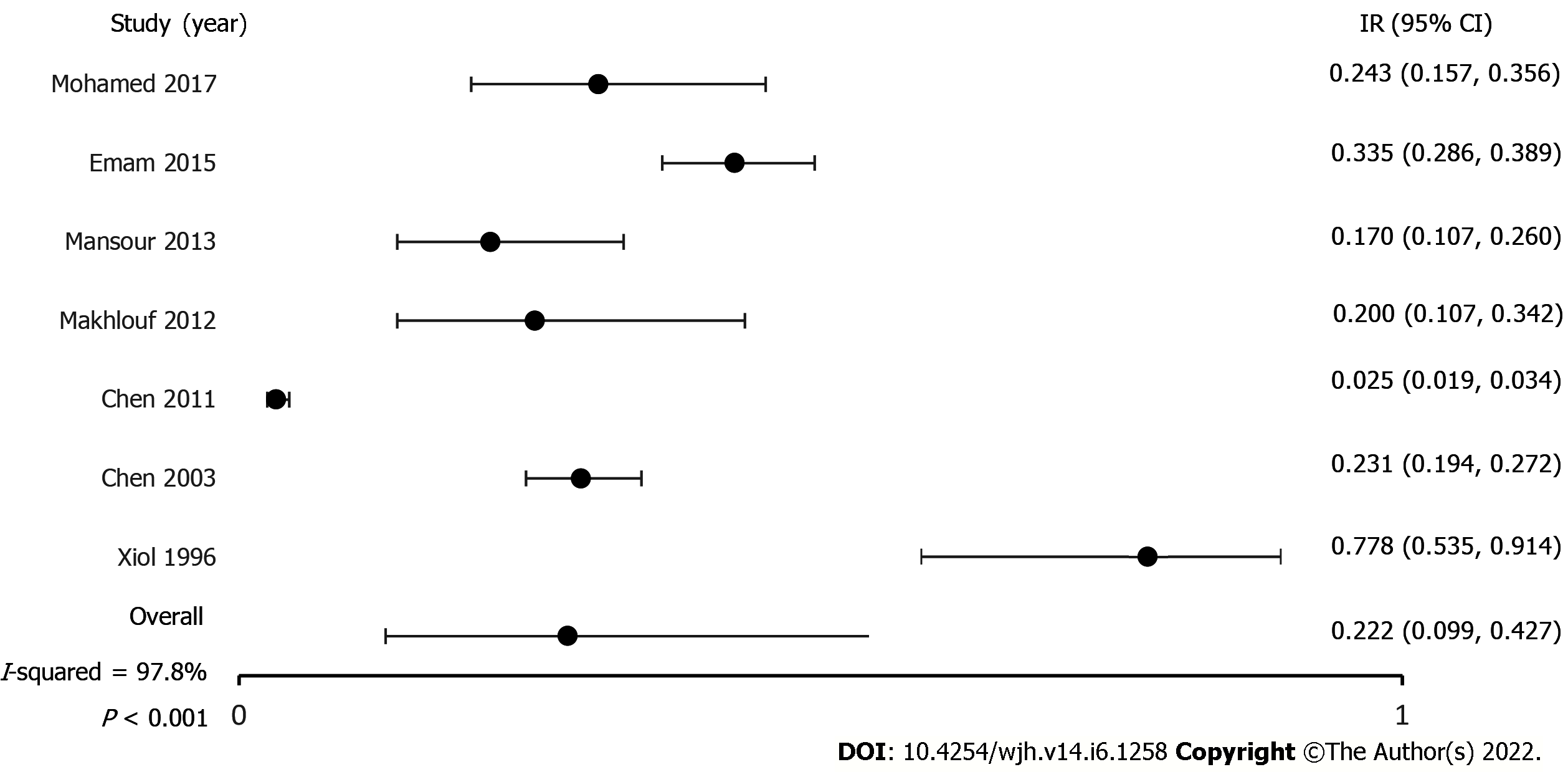
Incidence of SBP in patients with cirrhosis: Seven studies reported ascites and incidence of SBP[5-7,21-24]. After pooling the results of 2636 patients, the incidence of SBP was 22.2% (CI, 9.9-42.7; P < 0.001, I2 97.8%). The true effect size in 95% of all comparable populations falls in the interval 0.01-0.90 (Figure 3).
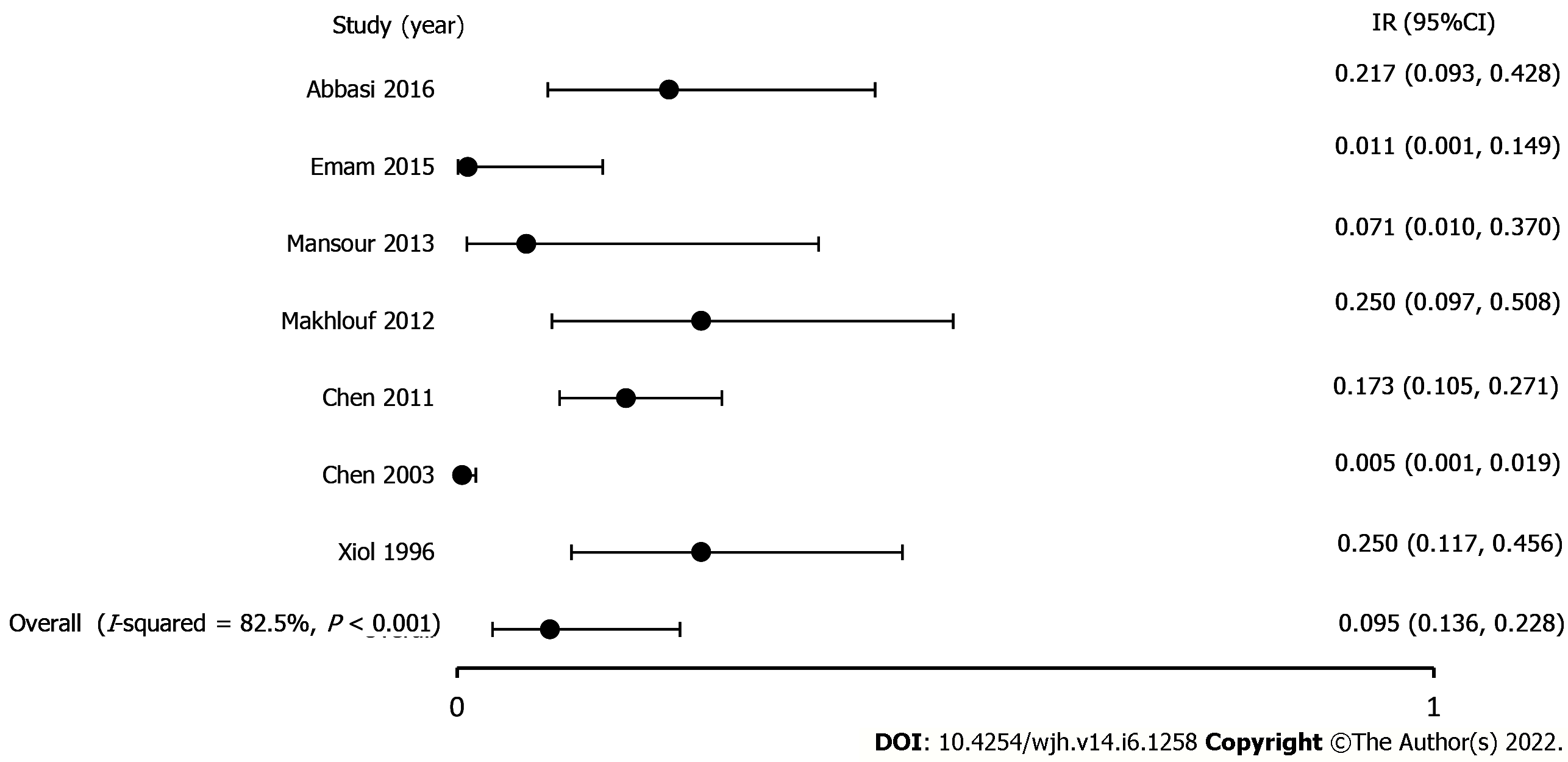
Incidence of SBE in patients without concomitant ascites: Six studies reported SBE without concomitant ascites[5-8,21,22]. The pooled incidence of SBE in patients without concomitant ascites was 9.5% (CI, 3.6-22.8; P < 0.001, I2 82.5 %). The true effect size in 95% of all comparable populations falls in the interval 0-0.76 (Figure 4).
Sensitivity analysis: We completed a one-study removal sensitivity analysis to assess if one study had a dominant effect on the meta-analysis. Statistical significance and direction of findings for all outcomes remained unchanged.
Heterogeneity: The I2 was consistently between 50%-75% across most outcomes suggesting considerable heterogeneity of our sample.
Publication bias: A publication bias analysis and estimated symmetry could not be completed because fewer than ten studies were included.
To our knowledge, this is the first systematic review and meta-analysis exploring the incidence of SBE in patients with cirrhosis. The pleural space is a potential pocket for infection and often can be overlooked in cases of septic decompensation. SBE is recommended to be managed without a chest tube and requires the delivery of appropriate antibiotics and exclusion of pneumonia, placing importance on timely diagnostic thoracentesis. Our study includes one prospective, five retrospective, and two cross-sectional studies amongst four countries with 8899 patients and 1334 cases of pleural effusions. The criteria for diagnosis of SBE were consistent throughout most of the studies and the parameters for cell count and culture results were identical to widely accepted definitions of SBE[25]. Our results support the current understanding that SBE most commonly occurs in patients with ascites or concomitant SBP. Studies have been conflicting on its association with ascites/SBP. Our results uncovered SBE at 9.5%, which was previously unknown and demonstrates the high incidence. In our cohort, roughly 22% had ascites and SBP, suggesting that the high SBE rates near SBP incidence-indicating that the pleural space is a potential space for infection and should be considered to complete a thorough evaluation.
Just as the peritoneal fluid is susceptible to translocation and infection leading to SBP, the development of HH in the pleura is a risk factor for SBE. SBE without HH occurs at less than 3%, but it increases up to 30% with underlying HH. Although HH prevalence is 10%, this is likely underestimated, as patients with HH do not routinely undergo thoracentesis[8,26]. Indications for thoracentesis include patients with HH who develop fever, pleuritic pain, encephalopathy, or a sharp drop in renal function[5]. Pleural fluid characteristics to diagnose HH include a total cell count of PMN < 250/uL, total protein < 2.5 g/dL, albumin gradient > 1.1g/dL, protein quotient < 0.5, or LDH gradient < 0.6. A PMN count > 250/uL with a positive pathogen detected or > 500/uL and a negative pathogen confirm SBE. Computed tomography (CT) can often be helpful in the setting of SBE to detect pleural abscesses that may require more immediate drainage. SBE development often occurs spontaneously or due to the flow of infected ascites from the peritoneal to pleural space. Infected ascites develops from a variety of mechanisms predominantly related to portal hypertension including (but not limited to) bacterial translocation from increased gastrointestinal permeability and bacterial overgrowth from intestinal dysmotility. SBE must be suspected in every patient with HH, as its symptomatology varies greatly. In our study, cohorts from Egypt and Spain mainly exhibited fever and dyspnea, while the remaining cohorts had cough, dyspnea, pleuritic pain, or tachypnea[5,8,22-24].
Amongst the included studies, sterile effusions were most common, while positive cultures commonly reported enteric organisms-Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and enterococcus on pleural studies. The distinction between SBE and empyema secondary to pneumonia is important as treatment differs greatly. 3rd generation cephalosporins such as cefotaxime and ceftriaxone were most used, followed by cefazolin, ampicillin/sulbactam, fluoroquinolones, and meropenem. Carbapenems should be used for possible extended-spectrum beta-lactamase-producing strains in high-risk patients. Aspiration and pigtail catheters were used in a minority of studies, often in cases of frank pus and were not associated[5,24]. Repeat thoracentesis is not routinely performed and is only undertaken in non-responding cases. Albumin infusion at 1.5g/kg on day 1 and 1g/kg on day 3 has shown benefit in SBP and has been used in SBE; none in our included studies. Antibiotic duration based on SBP experience has been recommended; however, the evidence was based on a few cohorts and case-control studies[27]. In our meta-analysis, two studies reported a duration of seven to ten days followed by a control thoracentesis[7,8]. Antibiotic response varied; one included study found SBE resolved in 72% of patients; however, the need for aspiration and second-line antibiotic therapy is frequent. This same study found 43% of patients died before second line therapy could be initiated[7]. A chest tube was only used in one of the patients and this patient had biochemical analysis suggestive of empyema[5,7].
Just as hepatic hydrothorax is known to decrease survival, SBE is known to impact mortality negatively[28]. In our meta-analysis we found the mortality rate of SBE in patients receiving treatment ranged from 20%-38%[5,21]. Compared to patients without SBE, patient with SBE have been shown to have a higher likelihood of death or liver transplantation at one year[26]. First-line treatment failure, odds ratio (OR) 7.56 followed by ICU admission (OR 5.53), and concomitant bacteremia (OR 4.32), concomitant SBP (OR 2.51), CPS (OR 1.59), and MELD-Na (OR 1.21) correlated to increased mortality[7]. MELD-Na has been shown to most accurately predict SBE associated hospital mortality with an area under the curve of 0.793, followed by serum sodium-0.778 and CPS-0.744. INR, pleural total protein, sex, creatinine, followed by diabetes mellitus, MELD-Na, MELD, bilirubin have been identified as predictors of dual SBP and SBE infection[23]. Five patients underwent an orthotopic liver transplant (OLT) a few months after SBE and all were alive at follow-up five years after OLT[8]. HH management is based on therapeutic principles of treating ascites-diuretics, sodium restriction, and fluid removal in symptomatic cases. Transjugular intrahepatic portosystemic shunt (TIPS) has been beneficial in cases of recurrent HH by reducing portal hypertension pressures. Indwelling pleural catheters (IPCs) may be an option for patients who are not TIPS candidates. IPCs have been associated with fewer complications compared to chest tubes.[29] Chest tubes have been associated with increased mortality unless pus has been demonstrated in the pleural space[26,27]. The development of SBE is significant for patients in the peri-transplantation period, as a few studies have suggested that independent SBE be considered an indication for liver transplantation evaluation and MELD exception points due to its impact on outcomes[8,26,27].
A limitation for determining the incidence of SBE was the lack of studies and a small number of included patients[8,22]. Despite this, we used a newer quality assessment scale to elicit the performance characteristics of the included studies. Follow-up data, including mortality, antibiotic duration, and the number of successfully treated patients, were only reported in two studies[7,8]. Majority of included patients were Child-Pugh class B or C, while a majority lacked a MELD score. MELD and Child-Pugh scores were reported in two studies[22,23]. There was considerable heterogeneity in the included studies attributed to study location, patient selection, and characteristics. To illustrate the range of true effects, we additionally provided prediction intervals to our outcomes[30]. The lack of long-term results in our studies translates to our current limited understanding of this disease process and its impact on respiratory mechanisms and overall mortality. A publication bias was not provided due to fewer than ten studies.
This study highlights the importance of considering SBE and HH in the differential for patients with cirrhosis who have pleural effusion. HH in the setting of cirrhosis is not routinely evaluated. The pleura can be an unrecognized nidus and our findings support the use of diagnostic thoracentesis in patients with decompensated cirrhosis after exclusion of other causes of pleural effusion. Thoracentesis should be considered particularly in patients without ascites and when there is a high suspicion of infection. It helps rule out empyema due to pneumonia and allows for targeted antibiotic therapy against enteric organisms. Additionally, as rates of multi-drug resistant (MDR) organisms increase globally, the need for organism identification for targeted treatment will become even more crucial, making timely thoracentesis of key importance[31]. Future observational and long-term studies will help elucidate further the mortality rates, optimal treatment route and duration, and risk factors for SBE.
Spontaneous bacterial empyema (SBE) is analogous to spontaneous bacterial peritonitis (SBP); however, much less is understood regarding its incidence rate, treatment strategies, and management.
The current understanding of SBE is limited by small sample size and results regarding its association with ascites are conflicting. Previous studies have noted patients who have cirrhosis and SBE may have poorer outcomes therefore more information regarding its association with ascites/SBP, incidence, treatment, and effect on outcomes are needed.
To identify the incidence of SBE in patients with cirrhosis, the incidence of SBP in patients with cirrhosis, and the incidence of SBE in patients without concomitant ascites. Additionally, we performed a systematic review of the treatment and outcomes of SBE.
We performed a meta-analysis using a random-effects model with pooled proportions and 95% confidence intervals (CI). We assessed heterogeneity using I2 and classic fail-safe to determine bias.
A total of 1334 patients had pleural effusions and the pooled incidence of SBE was 15.6% (CI 12.6-19; I2 50). Amongst patients diagnosed with SBE, the most common locations included right (202), left (64), and bilateral (8). Amongst our cohort, a total of 2636 patients had ascites with a pooled incidence of SBP of 22.2% (CI 9.9-42.7; I2 97.8). The pooled incidence of SBE in patients with cirrhosis but without concomitant ascites was 9.5% (CI 3.6-22.8; I2 82.5).
SBE frequently occurs with concurrent ascites/SBP; our results suggest high incidence rates of SBE even in the absence of ascites. The pleura can be an unrecognized nidus and our findings support the use of diagnostic thoracentesis in patients with decompensated cirrhosis after exclusion of other causes of pleural effusion. Thoracentesis should be considered particularly in patients without ascites and when there is a high suspicion of infection. The need for diagnostic thoracentesis will continue to be important as rates of multi-drug resistant bacterial infections increase and antibiotic susceptibility information is required for adequate treatment.
This study suggests the baseline incidence of SBE is high in patients with cirrhosis and diagnostic thoracentesis should be considered after underlying pulmonary and cardiac causes have been ruled out, especially when there is high concern for infection. High index of suspicion for SBE must be maintained especially in cirrhosis patients with pleural effusions and without underlying ascites. Timely treatment is warranted given high associated mortality of SBE. Future prospective studies are needed, as it remains unclear if long term prophylaxis against SBE is warranted in patients with decompensated cirrhosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, 58606; American Association for the Study of Liver Diseases, 259789.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Garbuzenko DV, Russia; Manrai M, India S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Lai YK, Eiger G, Fischer RA. Point: does spontaneous bacterial empyema occur? Chest. 2015;147:1207-1208. [PubMed] |
| 2. | Lai YK, Eiger G, Fischer RA. Rebuttal from Dr Lai et al. Chest. 2015;147:1210-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Nguyen TA, Liendo C, Owens MW. Counterpoint: does spontaneous bacterial empyema occur? Chest. 2015;147:1208-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Nguyen TA, Liendo C, Owens MW. Rebuttal from Dr Nguyen et al. Chest. 2015;147:1211-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Makhlouf HA, Morsy KH, Makhlouf NA, Eldin EN, Khairy M. Spontaneous bacterial empyema in patients with liver cirrhosis in Upper Egypt: prevalence and causative organisms. Hepatol Int. 2013;7:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Abbasi A, Bhutto AR, Alam MT, Aurangzaib M, Masroor M. Frequency of Hepatic Hydrothorax and its Association with Child Pugh Class in Liver Cirrhosis Patients. J Coll Physicians Surg Pak. 2016;26:566-569. [PubMed] |
| 7. | Chen CH, Shih CM, Chou JW, Liu YH, Hang LW, Hsia TC, Hsu WH, Tu CY. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Xiol X, Castellví JM, Guardiola J, Sesé E, Castellote J, Perelló A, Cervantes X, Iborra MJ. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 127] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 10. | Bajaj JS, Kamath PS, Reddy KR. The Evolving Challenge of Infections in Cirrhosis. N Engl J Med. 2021;384:2317-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 836] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 12. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 13. | Sese E, Xiol X, Castellote J, Rodríguez-Fariñas E, Tremosa G. Low complement levels and opsonic activity in hepatic hydrothorax: its relationship with spontaneous bacterial empyema. J Clin Gastroenterol. 2003;36:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Tu CY, Chen CH. Spontaneous bacterial empyema. Curr Opin Pulm Med. 2012;18:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17526] [Article Influence: 1095.4] [Reference Citation Analysis (1)] |
| 16. | Qumseya BJ. Quality assessment for systematic reviews and meta-analyses of cohort studies. Gastrointest Endosc. 2021;93:486-494.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25779] [Article Influence: 1120.8] [Reference Citation Analysis (0)] |
| 18. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 19. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9082] [Article Influence: 363.3] [Reference Citation Analysis (0)] |
| 20. | Viechtbauer, W. , Publication bias in meta-analysis: Prevention, assessment and adjustments. Psychometrika. 2007;72:269-271. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Chen TA, Lo GH, Lai KH. Risk factors for spontaneous bacterial empyema in cirrhotic patients with hydrothorax. J Chin Med Assoc. 2003;66:579-586. [PubMed] |
| 22. | E Mansour A, AA El-Rahman, T Besheer. Prevalence and risk factors for spontaneous bacterial pleuritis in cirrhotic patients with hydrothorax. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62:435-438. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Mohamed A, Atef M, Alsebaey A, Musa Elhabshy M, Salama M. Combined spontaneous bacterial empyema and peritonitis in cirrhotic patients with ascites and hepatic hydrothorax. Arab J Gastroenterol. 2017;18:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Emam M. Study of Frequency of Spontaneous Bacterial Empyema in Cirrhotic Patients With Hepatic Hydrothorax. Journal of Gastroenterology and Hepatology Research. 2015;4:1569-1572. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1815] [Article Influence: 259.3] [Reference Citation Analysis (2)] |
| 26. | Jiménez-Gutiérrez JM, García-Juárez I, Olivas-Martinez A, Ruiz I. One-year outcome of patients with cirrhosis who developed spontaneous bacterial empyema: A cohort study. J Dig Dis. 2021;22:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Al-Zoubi RK, Abu Ghanimeh M, Gohar A, Salzman GA, Yousef O. Hepatic hydrothorax: clinical review and update on consensus guidelines. Hosp Pract (1995). 2016;44:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Matei D, Craciun R, Crisan D, Procopet B, Mocan T, Pasca S, Zaharie R, Popovici B, Sparchez Z. Hepatic Hydrothorax-An Independent Decompensating Event Associated with Long-Term Mortality in Patients with Cirrhosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Avula A, Acharya S, Anwar S, Narula N, Chalhoub M, Maroun R, Thapa S, Friedman Y. Indwelling Pleural Catheter (IPC) for the Management of Hepatic Hydrothorax: The Known and the Unknown. J Bronchology Interv Pulmonol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 688] [Cited by in RCA: 1227] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 31. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |