Published online May 27, 2022. doi: 10.4254/wjh.v14.i5.1016
Peer-review started: March 26, 2021
First decision: August 18, 2021
Revised: September 17, 2021
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: May 27, 2022
Processing time: 423 Days and 23.6 Hours
Portal vein thrombosis (PVT) after liver resection is rare but can lead to life-threatening liver failure. This prospective study evaluated patients using contrast-enhanced computed tomography (E-CT) on the first day after liver resection for early PVT detection and management.
To evaluate patients by E-CT on the first day after liver resection for early PVT detection and immediate management.
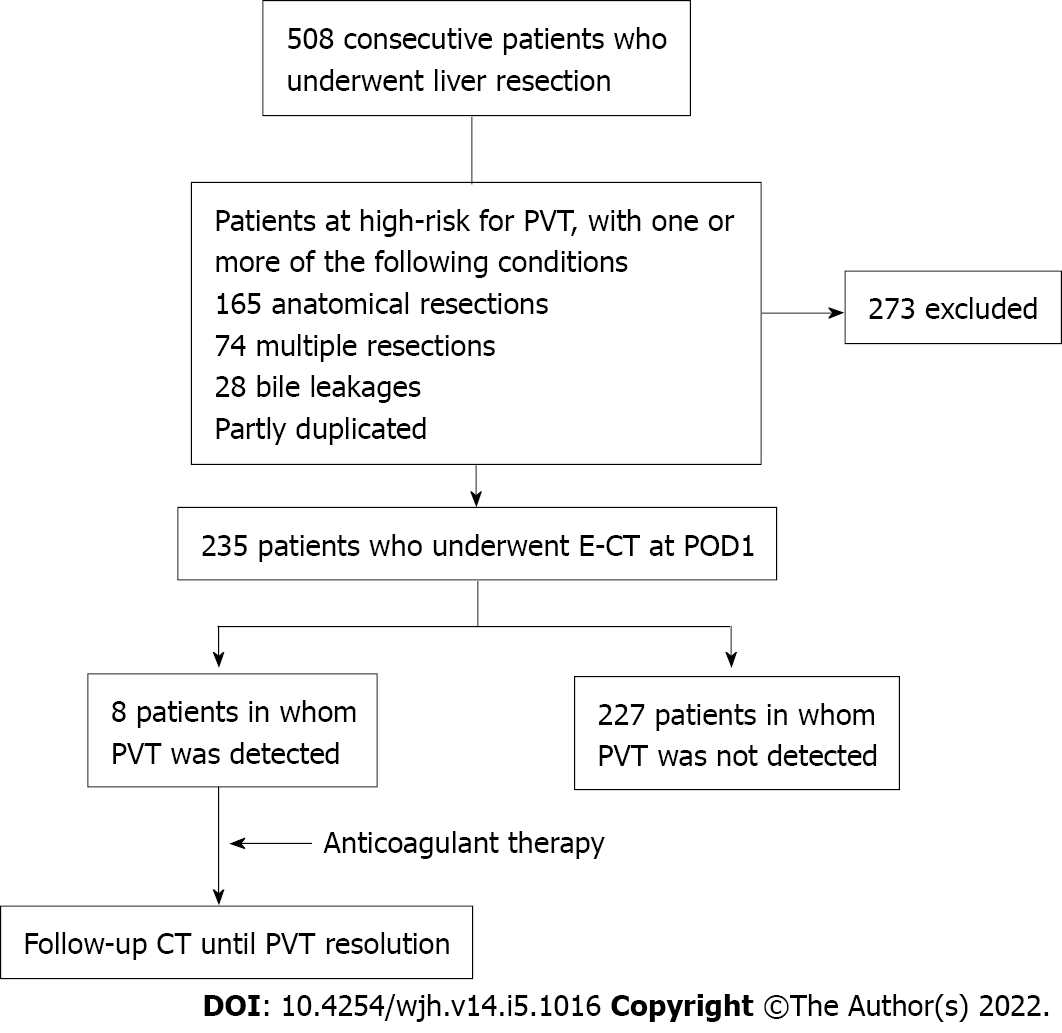
Patients who underwent liver resection for primary liver cancer from January 2015 were enrolled. E-CT was performed on the first day after surgery in patients undergoing anatomical resection, multiple resections, or with postoperative bile leakage in the high-risk group for PVT. When PVT was detected, anticoagulant therapy including heparin, warfarin, and edoxaban was administered. E-CT was performed monthly until PVT resolved.
The overall incidence of PVT was 1.57% (8/508). E-CT was performed on the first day after surgery in 235 consecutive high-risk patients (165 anatomical resections, 74 multiple resections, and 28 bile leakages), with a PVT incidence of 3.4% (8/235). Symptomatic PVT was not observed in the excluded cohort. Multivariate analyses revealed that sectionectomy was the only independent predictor of PVT [odds ratio (OR) = 12.20; 95% confidence interval (CI): 2.22-115.97; P = 0.003]. PVT was found in the umbilical portion of 75.0% (6/8) of patients, and sectionectomy on the left side showed the highest risk of PVT (OR = 14.10; 95%CI: 3.17-62.71; P < 0.0001).
Sectionectomy on the left side should be chosen with caution as it showed the highest risk of PVT. E-CT followed by anticoagulant therapy was effective in managing early-phase PVT for 2 mo without adverse events.
Core Tip: This prospective study evaluated patients by contrast-enhanced computed tomography (E-CT) on the first day after liver resection for early portal vein thrombosis (PVT) detection and immediate management. Sectionectomy on the left side should be treated with caution as it showed the highest risk of PVT. E-CT on the first day and immediate anticoagulant therapy were effective in managing early-phase PVT for 2 mo without adverse events.
- Citation: Yoshida N, Yamazaki S, Masamichi M, Okamura Y, Takayama T. Prospective validation to prevent symptomatic portal vein thrombosis after liver resection. World J Hepatol 2022; 14(5): 1016-1024
- URL: https://www.wjgnet.com/1948-5182/full/v14/i5/1016.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i5.1016
The rate of portal vein thrombosis (PVT) after liver resection occurs in 2.1%-11.1% of cases[1-5] and was unchanged in recent series[6-8] .While no symptoms are detected in the early phase, severe PVT can lead to life-threatening liver failure[9,10]. Immediate anticoagulant therapy after surgery is recommended in some cases[11]. However, patients who undergo liver resection may have coagulation disorders such as hepatocellular carcinoma, which makes it difficult to administer routine anticoagulant therapy. Therefore, early detection and immediate intervention for asymptomatic PVT are essential. The risk factors for PVT after liver resection include hepatic clumping and total operation time[1,2,4,7,8], resection scale and location[1,3], splenectomy[3], and portal vein reconstruction[4,5].
Studies have reported the occurrence of postoperative PVT in high-risk[1-3,7,8] or symptomatic cases in the first 5-7 d after liver resection[4,6]. However, there is a lack of information regarding the timing of PVT occurrence, approaches for its early detection, and the efficacy of early interventions. No study has focused on early detection and intervention before symptomatic PVT. Therefore, we conducted a prospective study and assessed patients considered to be at high risk for PVT after liver resection using screening with contrast-enhanced computed tomography (E-CT). E-CT was performed on the first day after liver resection, and immediate anticoagulant therapy was started when PVT was detected. We assessed the frequency, predictive factors, and efficacy of early intervention for PVT.
This study was conducted in patients who underwent liver resection for primary liver cancer from January 2015 onward. E-CT was performed on the first day after surgery for patients at high risk of PVT, including those undergoing: Anatomical resection, multiple resections, or postoperative bile leakage. In previous studies, the frequency of PVT was hypothesized to be 5%. Consequently, a total of 400 resections was expected to detect 20 PVT events[1]. Our institution performs an average of 150 liver resections for primary liver cancer annually; thus, the study was estimated to take 3 years to complete. This study excluded patients with metastatic liver cancer, bile duct or portal vein reconstruction, laparoscopic surgery, contrast medium allergy, renal dysfunction, or thrombus requiring anticoagulant therapy. The liver, lungs, and legs of patients with abnormal preoperative serum D-dimer levels were screened by ultrasound and E-CT.
Liver resection was performed under general anesthesia with hypoventilation during liver transection. Total hepatic inflow occlusion was performed using Pringle’s maneuver, in which the protocol called for a 15-min blood flow clamp and a 5-min release[12,13]. The liver parenchyma was transected using the crush-clamping method, and the vessels were ligated using an energy device or silk thread[14-16]. At the end of the operation, routine intraoperative ultrasonography was performed to detect any thrombus in the liver. A drain was placed on the resected stump of the liver and removed 3 d after the operation[17]. Elastic bandages and intermittent air compression on the legs were routinely used to prevent thrombosis.
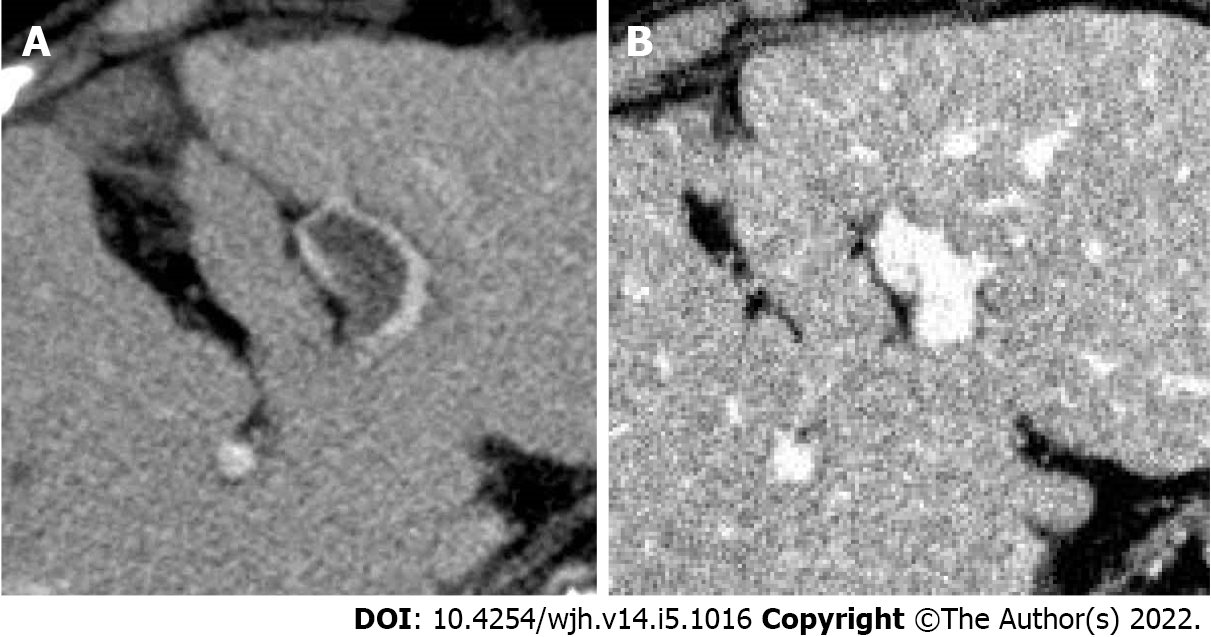
PVT was defined as a defect image that was confirmed in the portal phase of dynamic CT. The images were checked by a surgeon (Yoshida N) and 2 independent radiologists. When PVT was detected, anticoagulant therapy was performed according to the following protocol: Unfractionated heparin (10000-12000 units/d), warfarin (2 mg/d), and direct oral anticoagulant edoxaban tosylate hydrate (LIXIANA®, 30 mg/d). The target range of anticoagulant therapy was an activated partial thromboplastin time of 50-60 s and a prothrombin time-international normalized ratio of 1.5-2.0. In small cases of PVT, short half-life heparin was used just after the detection of PVT by E-CT, whereas in cases of large PVT, intravenous heparin combined with oral anticoagulation (warfarin or direct oral anticoagulant) was used as an initial treatment. Heparin was then changed to an oral anticoagulant 10 d after the operation. E-CT was performed in all treated cases for 1 wk after starting anticoagulant therapy to confirm the severity or new appearance of PVT. Anticoagulant therapy was continued when the PVT had disappeared by monthly E-CT after discharge. Patients who had no PVT on E-CT the first day after surgery did not undergo E-CT until discharge. All patients received E-CT at 2 or 3 mo after discharge as a routine follow-up post liver resection.
Statistical analyses were performed using JMP 10.0.2 (SAS Institute Inc., Cary, NC, United States). Data are expressed as the mean ± standard error of the mean. Categorical variables were compared using χ2 tests, whereas continuous variables were compared using nonparametric Wilcoxon or parametric t-tests. Statistical significance was set at P ≤ 0.05. Risk factors evaluated by univariate logistic regression (P < 0.05) were included in the multivariate analysis to determine the independent risk factors. This study protocol was approved by the ethics committee of Nihon University School of Medicine (Protocol No. RK-170214-3; Tokyo, Japan) and was registered in the UMIN Clinical Trials Registry under entry number UMIN000047362.
This study was started in January 2015 and discontinued in December 2018 after 4 years because the occurrence of PVT was lower than our hypothesis. The incidence of PVT was 8 (1.57%) among the 508 participants over the study period. E-CT was performed on the first day after liver resection in 235 patients (165 for anatomical resections, 74 for multiple resections, and 28 for bile leakage). The incidence of PVT in the high-risk group was 3.4% (8/235) (Figure 1). No cases of symptomatic PVT occurred in patients who met the exclusion criteria.
In univariate analyses, the occurrence of PVT was significantly higher in women (50.0% vs 79.7%; P = 0.04) and in patients who had undergone sectionectomy (75.0% vs 24.2%; P = 0.001). In multivariate analyses, sectionectomy [odds ratio (OR) = 12.2, 95% confidence interval (CI): 2.22-115.97; P = 0.003] was the only independent predictor of PVT occurrence (Table 1). There were 2 postoperative deaths, neither of which was related to PVT. One patient with pneumonia underwent a pathological autopsy and did not show thrombus anywhere. One patient with liver failure and no PVT was observed on E-CT during the study.
| Variables | Univariate analyses | Multivariate analyses | ||||
| PVT (+), n = 8 | PVT (-), n = 227 | P value | OR | 95%CI | P value | |
| Back ground characteristics | ||||||
| Age | 69.9 ± 3.4 | 67.8 ± 0.6 | 0.54 | |||
| Male sex | 4 (50.0%) | 181 (79.7%) | 0.04 | 0.25 | 0.05-1.29 | 0.10 |
| Hepatocellular carcinoma (+) | 7 (87.5%) | 190 (73.7%) | 0.77 | |||
| Multiple tumor | 2 (25.0%) | 72 (31.7%) | 0.69 | |||
| Heavy drinker | 2 (25.0%) | 55 (24.2%) | 0.96 | |||
| Portal vein embolization | 0 | 12 (5.3%) | 0.50 | |||
| Body mass index | 24.8 ± 1.2 | 23.7 ± 0.2 | 0.39 | |||
| Preoperative anticoagulant therapy | 1 (12.5%) | 10 (4.4%) | 0.29 | 7.95 | 0.32-106.81 | 0.17 |
| Diabetes mellitus | 2 (25.0%) | 92 (40.5%) | 0.38 | |||
| HBsAg (+) | 1 (12.5%) | 39 (17.2%) | 0.73 | |||
| HCVAb (+) | 2 (25.0%) | 52 (22.9%) | 0.89 | |||
| Platelet count as × 104/μL | 18.6 ± 7.8 | 21.4 ± 1.5 | 0.72 | |||
| Albumin in g/dL | 4.2 ± 0.2 | 4.2 ± 0.03 | 0.79 | |||
| Total bilirubin in mg/dL | 0.7 ± 0.2 | 0.7 ± 0.03 | 0.92 | |||
| Prothrombin time, % | 93.8 ± 3.8 | 93.4 ± 0.7 | 0.94 | |||
| D-dimer in ng/mL | 1.7 ± 0.8 | 1.7 ± 0.2 | 0.97 | |||
| ICGR15, % | 15.0 ± 2.2 | 11.7 ± 0.4 | 0.14 | 0.91 | 0.82-1.02 | 0.09 |
| Liver damage B | 0 | 10 (6.3%) | 0.53 | |||
| Procedure | ||||||
| Anatomical resection | 6 (75.0%) | 159 (70.0%) | 0.76 | |||
| Segmentectomy | 0 | 48 (21.2%) | 0.14 | |||
| Sectiontectomy | 6 (75.0%) | 55 (24.2%) | 0.001 | 12.20 | 2.22-115.97 | 0.003 |
| Hemihepatectomy | 0 | 56 (24.7%) | 0.11 | |||
| Surgical outcomes | ||||||
| Operatin time in min | 324 ± 40 | 374 ± 8 | 0.21 | |||
| Hepatic clamping time in min | 76 ± 15 | 89 ± 3 | 0.38 | 1.01 | 0.99-1.03 | 0.56 |
| Blood loss in g | 517 ± 186 | 455 ± 35 | 0.74 | |||
| RBC transfusion | 1 (12.5%) | 13 (5.7%) | 0.43 | |||
| Surgical results | ||||||
| Complication CD ≥ 3a | 3 (37.5%) | 50 (22.0%) | 0.30 | |||
| Bile leakage | 2 (25.0%) | 26 (11.5%) | 0.25 | 3.12 | 0.35-21.58 | 0.28 |
| Mortality | 0 | 2 (0.9%) | 0.79 | |||
| Histological cirrhosis | 1 (12.5%) | 32 (14.1%) | 0.90 | |||
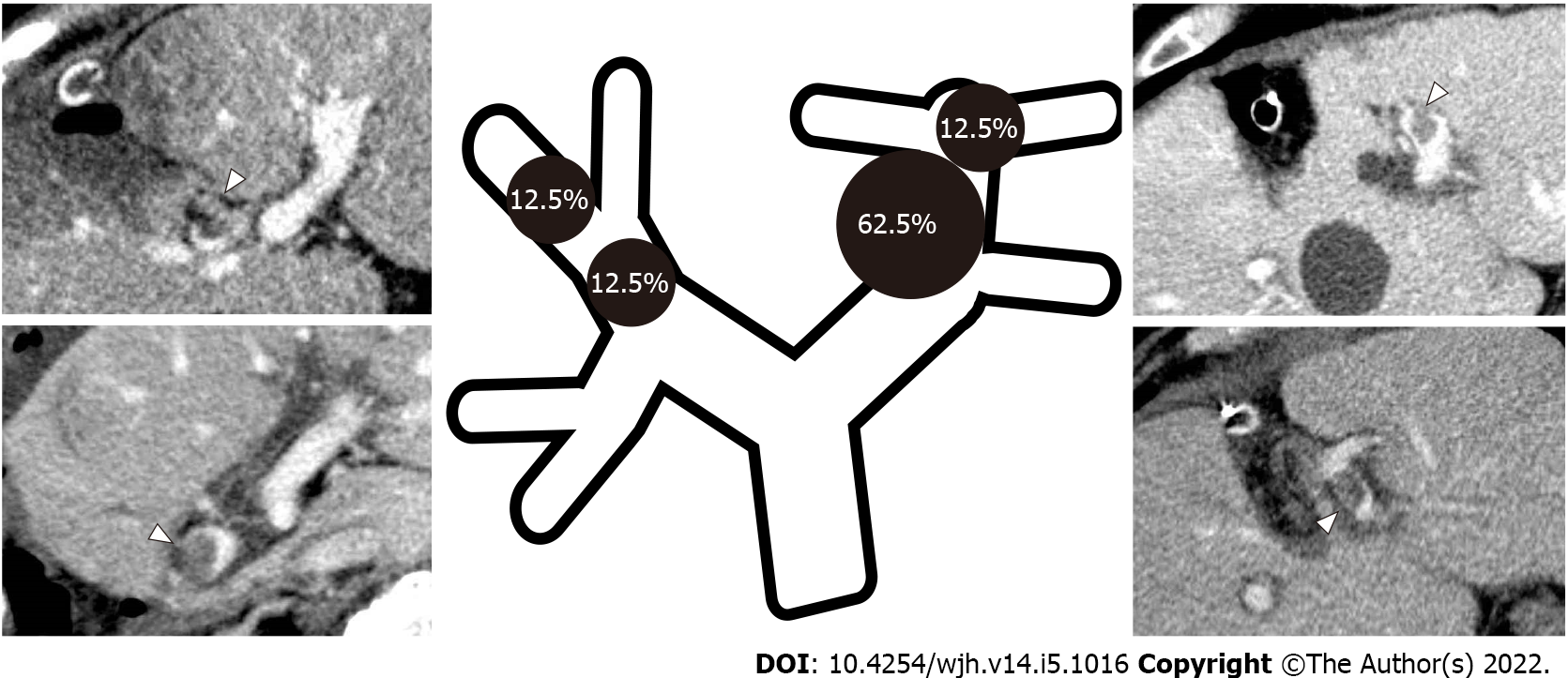
Six of the eight patients with PVT underwent anatomical resection. Most instances of PVT were found on the left side of the liver, with 62.5% (5/8) around the umbilical portion (UP) and one in the apex of the UP (12.5%). Only one instance was found at the resection stump of segment 8 and one in the anterior branch (Figure 2). All PVTs at the UP were found in the second-to third-order branches, with preserved peripheral blood flow from the thrombus at the time of detection. Among the procedures, 3 patients among 26 who underwent left lateral sectionectomy developed PVT (11.5%). PVT also developed in 2 of 3 patients undergoing left medial sectionectomy (66.7%) and in 1 of the 25 patients who underwent right posterior sectionectomy (4%). Among the procedures, sectionectomy on the left side was associated with the highest risk of PVT (OR = 14.10; 95%CI: 3.17-62.71; P < 0.0001) (Table 2).
| PVT (+), n = 8 | PVT (-), n = 227 | P value | OR | 95%CI | ||
| Partial resection | 2 (25.0%) | 68 (30.0%) | 0.76 | 0.78 | 0.15-3.96 | |
| Segmentectomy | Left | - | 8 (3.5%) | 0.59 | ||
| Right | - | 40 (17.6%) | 0.19 | |||
| Sectiontectomy | Left | 5 (62.5%) | 24 (10.6%) | < 0.0001 | 14.10 | 3.17-62.71 |
| Right | 1 (12.5%) | 31 (13.7%) | 0.93 | 0.90 | 0.11-7.60 | |
| Hemihepatectomy | Left | - | 37 (16.3%) | 0.21 | ||
| Right | - | 19 (8.4%) | 0.39 |
No adverse events were observed with anticoagulant therapy, and all patients were successfully treated (Table 3 and Figure 3). One patient (case 5) stopped treatment because the thrombus did not extend to the central side. The median period of PVT treatment was 55 d (range: 37-140 d).
| Case | No. of patients | Age | Sex | Diagnosis | Extent of resection | PVT location | Anticoagulation | Outcome | PVT treatment period in d |
| 1 | 65 | 71 | Male | HCC | Left medial sectionectomy | UP | Heparin, warfarin | Resolved | 53 |
| 2 | 135 | 83 | Male | ICC | Left lateral sectionectomy | Lt branch, UP | Heparin, warfarin | Resolved | 37 |
| 3 | 151 | 50 | Female | HCC | Left lateral sectionectomy, partial resection (S1) | UP | Heparin, warfarin | Resolved | 63 |
| 4 | 214 | 75 | Female | HCC | Left lateral sectionectomy | UP | Warfarin | Resolved | 59 |
| 5 | 385 | 78 | Female | HCC | Partial resection (S8) | P8 stump | Edoxaban | Stable | 46 |
| 6 | 391 | 70 | Male | HCC | Right posterior sectionectomy | Anterior branch | Heparin, warfarin | Resolved | 44 |
| 7 | 417 | 75 | Female | HCC | Partial resection (S4, S7) | UP | Heparin, edoxaban | Resolved | 140 |
| 8 | 475 | 57 | Male | HCC | Left medial sectionectomy | UP | Heparin, edoxaban | Resolved | 58 |
The incidence of PVT was lower (1.56%) in this study than in previous reports[3,4,6]. In the high-risk group, E-CT on the first day after surgery had an incidence of the initial phase of asymptomatic PVT of 3.4% (8/235), which was successfully treated with anticoagulant therapy. The results of this study demonstrated that PVT formed as early as the first day after liver resection. To date, most studies have found PVT at 5-7 d after liver resection[1-3,7,8]. In this phase, peripheral blood flow is obstructed and impaired by a large thrombus. Anticoagulant therapy takes a long time to resolve the thrombus, and some patients experience severe liver failure[3,6]. Early anticoagulant therapy is an ideal option for patients at high-risk for PVT; however, no study has reported the initiation of PVT. Consequently, in this study, we aimed to detect early-stage PVT in a high-risk group of patients using E-CT. This enabled the prompt initiation of anticoagulant therapy and thus preserved peripheral blood flow from PVT.
The risk factors for PVT after liver resection include operating procedure or scale[1,3], operation or hepatic clamping time[1,2,4,7,8], portal vein reconstruction, and complications[3,5]. Sectionectomy is the only independent predictor of PVT occurrence. Sectionectomy requires ligation of the second branch root of the liver, which is more complex than hemihepatectomy. With regard to the sites of PVT, six of the eight PVTs identified in this study were found in the UP and 5 patients had undergone sectionectomy on the left side. A previous report suggested a higher PVT incidence in the UP than in other sites, and that left lateral sectionectomy was a risk factor for PVT[6]. Thrombi occur due to three factors (i.e., stasis of blood flow, hypercoagulable state, and endothelial injury), known as Virchow’s triad[18]. Blood stasis occurs due to the shape of the UP, which flows dorsal to ventral and caudal to cranial, especially following liver transection. The initial site of PVT is unknown, but this study detected small PVTs in the UP. Turbulent flow caused by reduced liver parenchyma after liver resection and a change in the shape of the UP in cases of sectionectomy on the left side may increase the risk of PVT.
Although this was a prospective study, it had several limitations. First, the incidence of PVT was lower than expected. The statistical power to analyze the risk factors for PVT was not optimal therefore, this protocol is ongoing to analyze larger population in the future. Second, E-CT and anticoagulant therapy were contraindicated. Third, cost-effectiveness is low for E-CT; therefore, routine ultrasonography may be more practical for the high-risk group in our study. Fourth, E-CT alone was insufficient to detect a small PVT on day 1. Therefore, it is necessary to combine coagulation makers and images.
In conclusion, sectionectomy on the left side should be cautiously managed, as this patient group showed the highest risk of PVT. Immediate anticoagulant therapy from the first day after surgery is recommended for patients at high risk for PVT.
Portal vein thrombus (PVT) is one of the potentially lethal complication after liver resection; however, its etiology and the way for immediate treatment is unsettled.
Based on our experience, we tried to resolve hepatic failure due to huge PVT.
The study was conducted in patients who underwent open liver resection for cancer in our institution.
Retrospective but retrospectively collected cohort.
In a total of 235 patients, 8 had major PVT. We successfully treated the patients with anticoagulant therapy without adverse events. No hepatic failure observed through this study.
Performing enhanced computed tomography (CT) on post-operative day 1 is an effective option to find a thrombi at the portal vein close to the surgical site.
The early detection of PVT by enhanced CT is a promising way to avoid hepatic failure after liver resection.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Li HL, China; Zhuge YZ, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Yoshiya S, Shirabe K, Nakagawara H, Soejima Y, Yoshizumi T, Ikegami T, Yamashita Y, Harimoto N, Nishie A, Yamanaka T, Maehara Y. Portal vein thrombosis after hepatectomy. World J Surg. 2014;38:1491-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Yamashita Y, Bekki Y, Imai D, Ikegami T, Yoshizumi T, Ikeda T, Kawanaka H, Nishie A, Shirabe K, Maehara Y. Efficacy of postoperative anticoagulation therapy with enoxaparin for portal vein thrombosis after hepatic resection in patients with liver cancer. Thromb Res. 2014;134:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Kuboki S, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Takayashiki T, Takano S, Okamura D, Suzuki D, Sakai N, Kagawa S, Miyazaki M. Incidence, risk factors, and management options for portal vein thrombosis after hepatectomy: a 14-year, single-center experience. Am J Surg. 2015;210:878-85.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Han JH, Kim DS, Yu YD, Jung SW, Yoon YI, Jo HS. Analysis of risk factors for portal vein thrombosis after liver resection. Ann Surg Treat Res. 2019;96:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Uchida T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, Aramaki T, Uesaka K. Prediction of Portal Vein Thrombosis Following Hepatectomy for Perihilar Cholangiocarcinoma: Efficacy of Postoperative Portal Vein Diameter Ratio and Angle. Anticancer Res. 2019;39:5019-5026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Mori A, Arimoto A, Hamaguchi Y, Kajiwara M, Nakajima A, Kanaya S. Risk Factors and Outcome of Portal Vein Thrombosis After Laparoscopic and Open Hepatectomy for Primary Liver Cancer: A Single-Center Experience. World J Surg. 2020;44:3093-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Onda S, Furukawa K, Shirai Y, Hamura R, Horiuchi T, Yasuda J, Shiozaki H, Gocho T, Shiba H, Ikegami T. New classification-oriented treatment strategy for portal vein thrombosis after hepatectomy. Ann Gastroenterol Surg. 2020;4:701-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Takata H, Hirakata A, Ueda J, Yokoyama T, Maruyama H, Taniai N, Takano R, Haruna T, Makino H, Yoshida H. Prediction of portal vein thrombosis after hepatectomy for hepatocellular carcinoma. Langenbecks Arch Surg. 2021;406:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, Grandone E, Balzano A. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 391] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Lertpipopmetha K, Auewarakul CU. High incidence of hepatitis B infection-associated cirrhosis and hepatocellular carcinoma in the Southeast Asian patients with portal vein thrombosis. BMC Gastroenterol. 2011;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J. 2011;75:1258-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 841] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 13. | Takayama T, Makuuchi M, Inoue K, Sakamoto Y, Kubota K, Harihara Y. Selective and unselective clamping in cirrhotic liver. Hepatogastroenterology. 1998;45:376-380. [PubMed] |
| 14. | Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, Kokudo N, Makuuchi M. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Gotohda N, Yamanaka T, Saiura A, Uesaka K, Hashimoto M, Konishi M, Shimada K. Impact of energy devices during liver parenchymal transection: a multicenter randomized controlled trial. World J Surg. 2015;39:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Ichida A, Hasegawa K, Takayama T, Kudo H, Sakamoto Y, Yamazaki S, Midorikawa Y, Higaki T, Matsuyama Y, Kokudo N. Randomized clinical trial comparing two vessel-sealing devices with crush clamping during liver transection. Br J Surg. 2016;103:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Mitsuka Y, Yamazaki S, Yoshida N, Masamichi M, Higaki T, Takayama T. Prospective Validation of Optimal Drain Management "The 3 × 3 Rule" after Liver Resection. World J Surg. 2016;40:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow's contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010;8:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |