Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.766
Peer-review started: November 8, 2021
First decision: December 12, 2021
Revised: January 4, 2022
Accepted: March 7, 2022
Article in press: March 7, 2022
Published online: April 27, 2022
Processing time: 164 Days and 12.8 Hours
Critical care is rapidly evolving with significant innovations to decrease hospital stays and costs. To our knowledge, there is limited data on factors that affect the length of stay and hospital charges in cirrhotic patients who present with ST-elevation myocardial infarction-related cardiogenic shock (SRCS).
To identify the factors that increase inpatient mortality, length of stay, and total hospital charges in patients with liver cirrhosis (LC) compared to those without LC.
This study includes all adults over 18 from the National Inpatient Sample 2017 database. The study consists of two groups of patients, including SRCS with LC and without LC. Inpatient mortality, length of stay, and total hospital charges are the primary outcomes between the two groups. We used STATA 16 to perform statistical analysis. The Pearson's chi-square test compares the categorical variables. Propensity-matched scoring with univariate and multivariate logistic regression generated the odds ratios for inpatient mortality, length of stay, and resource utilization.
This study includes a total of 35798453 weighted hospitalized patients from the 2017 National Inpatient Sample. The two groups are SRCS without LC (n = 758809) and SRCS with LC (n = 11920). The majority of patients were Caucasian in both groups (67% vs 72%). The mean number of patients insured with Medicare was lower in the LC group (60% vs 56%) compared to the other group, and those who had at least three or more comorbidities (53% vs 90%) were significantly higher in the LC group compared to the non-LC group. Inpatient mortality was also considerably higher in the LC group (28.7% vs 10.63%). Length of Stay (LOS) is longer in the LC group compared to the non-LC group (9 vs 5.6). Similarly, total hospital charges are higher in patients with LC ($147407.80 vs $113069.10, P ≤ 0.05). Inpatient mortality is lower in the early percutaneous coronary intervention (PCI) group (OR: 0.79 < 0.11), however, it is not statistically significant. Both early Impella (OR: 1.73 < 0.05) and early extracorporeal membrane oxygenation (ECMO) (OR: 3.10 P < 0.05) in the LC group were associated with increased mortality. Early PCI (-2.57 P < 0.05) and Impella (-3.25 P < 0.05) were also both associated with shorter LOS compared to those who did not. Early ECMO does not impact the LOS; however, it does increase total hospital charge (addition of $24717.85, P < 0.05).
LC is associated with a significantly increased inpatient mortality, length of stay, and total hospital charges in patients who develop SRCS. Rural and Non-teaching hospitals have significantly increased odds of extended hospital stays and higher adjusted total hospital charges. The Association of LC with worse outcomes outlines the essential need to monitor these patients closely and treat them early on with higher acuity care. Patients with early PCI had both shorter LOS and reduced inpatient mortality, while early Impella was associated with increased mortality and shorter LOS. Early ECMO is associated with increased mortality and higher total hospital charges. This finding should affect the decision to follow through with interventional management in this cohort of patients as it is associated with poor outcomes and immense resource utilization.
Core Tip: This paper was written to identify the predictors of mortality and the effect of liver cirrhosis on patients who develop ST-elevation myocardial infarction-related cardiogenic shock requiring interventional management. We reviewed the effect of liver cirrhosis on mortality, length of stay, and total hospital charges. We hope that this article will help build the foundation for future studies that will benefit this population of patients.
- Citation: Dar SH, Rahim M, Hosseini DK, Sarfraz K. Impact of liver cirrhosis on ST-elevation myocardial infarction related shock and interventional management, a nationwide analysis. World J Hepatol 2022; 14(4): 766-777
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/766.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.766
The understanding of Liver cirrhosis (LC) and its relationship with cardiovascular disease (CVD) has evolved over the last half-century. Initial theories suggested an inverse relationship between LC and CVD development[1,2]. These theories are based on the pathophysiology of LC and its effects on known CVD risk factors such as reduced cholesterol and systemic blood pressure. Autopsy studies that showed that plaque burden and myocardial infarction were less prevalent amongst those with LC support this hypothesis[2]. More recent epidemiologic studies refute this notion and portray a positive correlation between LC and CVD risk[3]. This risk elucidates the increasing incidence of obesity and the emergence of nonalcoholic fatty liver disease as the leading cause of cirrhosis. This disease process shares many risk factors (diabetes, obesity, etc.) with coronary artery disease[4,5].
Patients with liver cirrhosis develop thrombocytopenia, do not synthesize coagulation factors properly, and have delayed clearance of pro and anti-thrombotic factors, placing them at an increased risk of bleeding and thrombosis[6]. The cornerstone of managing ST-elevation myocardial infarction (STEMI) is revascularization and initiation of antiplatelet agents, and clinical trial data to guide the management of patients with LC and STEMI are lacking as most studies exclude this patient population[7]. To complicate matters further, a potential sequela of STEMI is cardiogenic shock, which requires consideration of mechanical circulatory support such as extracorporeal membrane oxygenation (ECMO) or ventricular offloading devices. Abnormal coagulation makes treating patients with LC who present with STEMI challenging. The bleeding risk cirrhosis poses might dissuade providers from pursuing percutaneous coronary intervention (PCI), an intervention shown to decrease mortality in patients with STEMI[8]. The optimal strategy, timing, and whether the benefit of such interventions is the same for those patients with LC as the general population is not well studied. Our study aims to identify the predictors of mortality and observe hospitalization outcomes (length of stay (LOS), mortality, and total hospital charges) in patients with LC who develop STEMI-related cardiogenic shock and receive standard revascularization and circulatory support interventions, including PCI, Impella, and ECMO during their hospitalization.
We conducted a retrospective study using the 2017 National Inpatient Sample (NIS) database, created by The Agency for Healthcare Research and Quality. The NIS is designed as a stratified probability sample to represent all non-federal acute care inpatient hospitalizations in the United States. The population depicted in the database is a randomly selected 20% of the total population from all participating hospitals. Both patient and hospital-level information are collected from these patients and entered into NIS. Information about patients, including demographics, diagnoses, and resource utilization, including length of hospital stay, procedures, and total hospitalization charges, is utilized. NIS does not include unique patient identifiers. Hospitals categorize by bed size, geographical location, teaching status, and urban/rural location. Each hospital discharge is weighted (weight is equal to the total number of patient discharges from all acute care hospitals in the United States divided by the number of discharges included in the 20% sample) to depict a nationally representative sample. Up to 25 discharge diagnoses and 25 procedures are collected using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) coding system. NIS has previously been used to provide reliable estimates of the burden of gastrointestinal diseases[1,9,10].
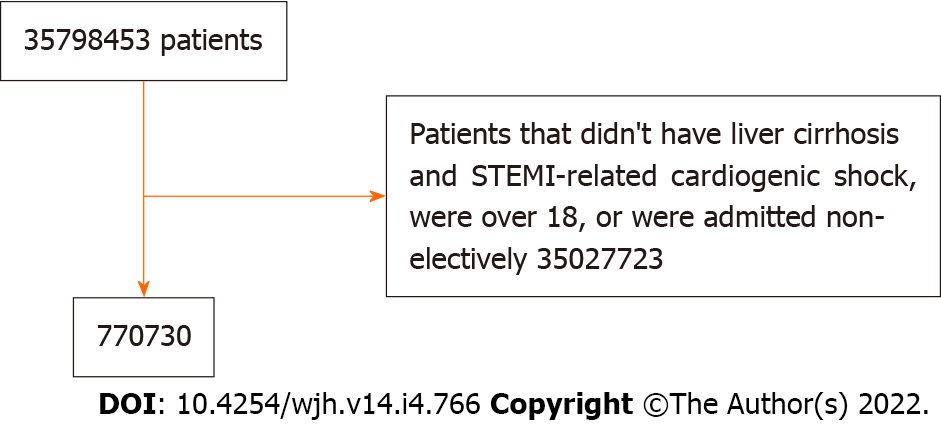
Patients are included in the study if they had a primary ICD-10 diagnosis code indicating STEMI or STEMI related cardiogenic shock and liver cirrhosis. Inclusion criteria were patients above the age of 18 and non-elective admission. In the 2017 NIS, there are a total of 35798453 weighted discharges.
The exposure of interest was the presence of STEMI or STEMI related cardiogenic shock in patients with liver cirrhosis. The variable STEMI or STEMI-related cardiogenic shock is a principal diagnosis. The variable LC is a principal or secondary diagnosis. Other variables, including factors to assess the severity of LC (ascites, gastrointestinal bleed, etc.), and inpatient outcome measures are also secondary diagnoses.
Patient demographics are provided directly in NIS and consist of age (assessed as a continuous variable), sex, race (Caucasian, African American, Hispanic, Asian or Pacific Islander, Native American, and other), median income in the patient's ZIP code (quartile 1: $1-38999; quartile 2: $39000-47999; quartile 3: $48000-63999; quartile 4: $640001), primary insurance (Medicare, Medicaid, private insurance, and uninsured), and comorbidities measured by the Charlson Comorbidity Index (CCI; categorized as 0, 1 to 2, or over 2), Elixhauser comorbidity index, as well as hospital location (rural vs urban), region (Northeast, Midwest, West, or South), teaching status, and size (small, medium, or large).
The primary outcome in this study is inpatient mortality in LC patients that developed STEMI or SRCS and underwent an interventional procedure including PCI, Impella placement, or ECMO during the hospitalization. PCI and Impella were defined as "early" if performed within the first 24 h of admission. ECMO was defined as early if it occurred within the first days of admission. Inpatient mortality was compared between the liver cirrhosis patients who received the procedures before and those that received the procedures after the period defined as early. The secondary outcomes included length of stay and total hospital charges between the two cohorts.
Predictors of inpatient mortality [sepsis, acute kidney injury (AKI), AKI on chronic kidney injury, cardiac arrest, any bleed/hemorrhage, electrolyte, and fluid disturbances, all gastrointestinal bleeds, respiratory failure requiring mechanical ventilation] and markers of decompensated cirrhosis [hypoalbuminemia, hepatic encephalopathy (HE), portal vein thrombosis, variceal upper gastrointestinal bleed, ascites, coagulopathy, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (HRS)] were generated using secondary diagnosis codes. We cannot use Child-Pugh and model for end-stage-liver-disease (MELD) scoring to stratify the severity of liver cirrhosis as they do not exist as ICD-10 codes. Therefore, previously generated variables by the CCI were utilized (mild liver disease and moderate-severe liver disease) as a placeholder for previously validated scoring systems but cannot be a replacement.
Continuous variables were compared using the Student t-test, and categorical variables were compared using the chi-square test. Univariate analysis was conducted using logistic regression to identify confounders. Confounders were adjusted for using multivariate logistic regression analysis. The model was constructed by including all statistically significant variables associated with the outcome on univariate analysis with a cutoff P value of 0.2 to construct a more accurate model. In addition, all variables considered to be clinically meaningful predictors of the outcomes (based on prior studies' findings) were included in the regression analysis regardless of the P value.
All statistical analysis was performed using STATA 16 (StataCorp LP, College Station, TX, United States). Survey (svy) commands were used to account for the stratified sampling design of the NIS. A two-tailed P value of 0.05 marks the threshold for significance for all tests. Two different methods control confounders in this study: Propensity matched scoring and multivariate logistic regression. Propensity scores match patients with SRCS with and without liver cirrhosis. A non-parsimonious multivariate logistic regression model was generated using the following variables: Age, sex, race, income in patient's zip code, Charlson's comorbidity index, insurance status, hospital location, hospital teaching status, hospital bed size, and hospital region. This robust double method for controlling confounders was then used to calculate treatment weights. The inverse probability of treatment weights was used to match cases with controls using a generalized linear model. Secondary analysis was computed using multivariate logistic regression using all variables significantly associated with the outcome on univariate analysis (with a P value cutoff of 0.2). Any variable believed to be a critical confounder identified from previous literature was added to optimize the model. Logistic regression was utilized to analyze binary outcomes, while linear regression was adopted for continuous variables. Proportions were compared using the Fisher exact test, and continuous variables were compared using the Student t-test.
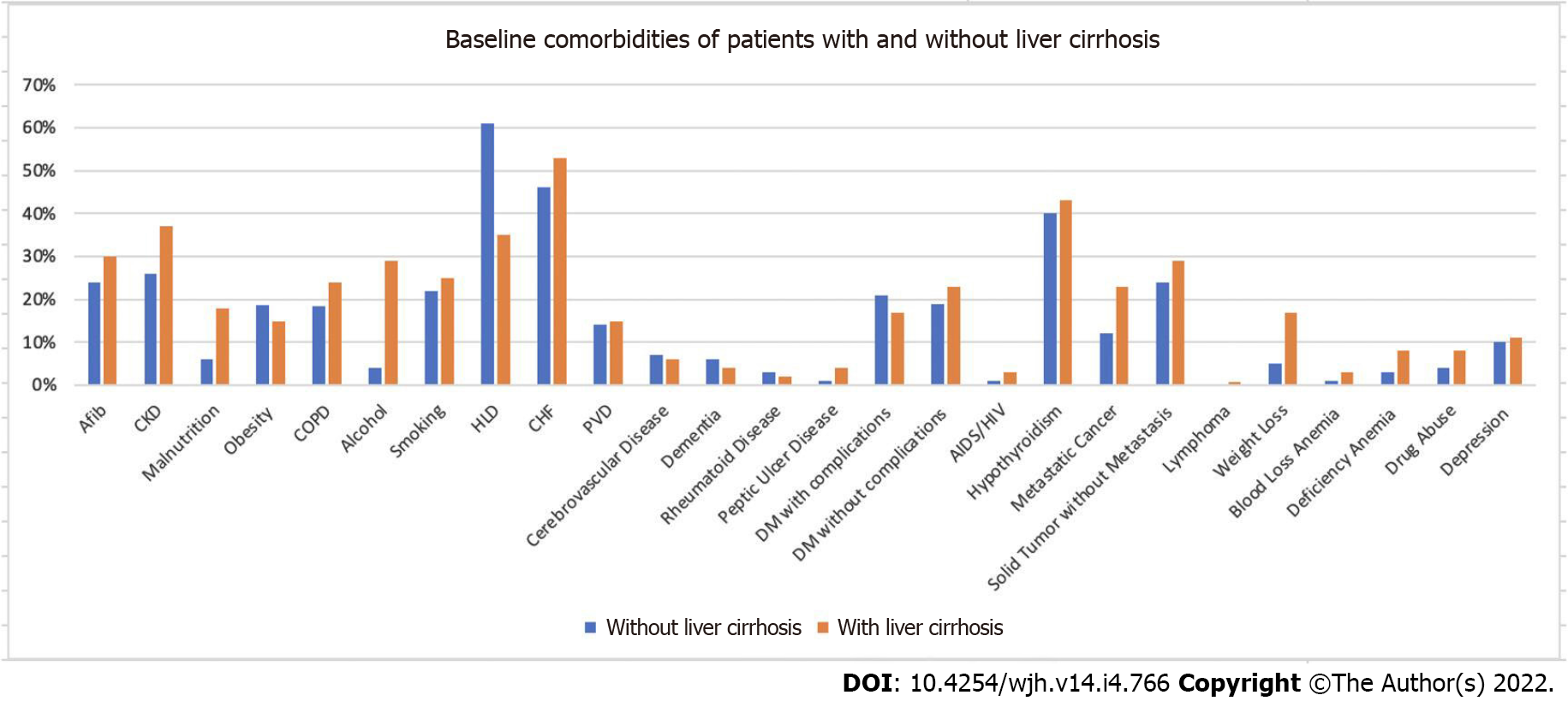
Figure 1 illustrates a flow diagram for study inclusion. From the 35798453 weighted hospitalizations in the United States during 2017, 770730 patients met the inclusion criteria. Table 1 demonstrates the baseline patient characteristics. Patients with liver cirrhosis were younger, with a mean age of 62.7 compared to 66.7 in those without liver cirrhosis. LC patients have a higher percentage of Hispanic patients (13% vs 9% P < 0.05). The two groups have a similar percentage of females, hospital bed size distribution, hospital region, hospital location, and median household income. Patients with LC have a higher proportion with Medicaid insurance (21% vs 10% P < 0.05) and a lower percentage of patients with Medicare (56% vs 60%). The LC cohort also had more comorbidities reflected by a higher Charlson Comorbidity Index (CCI) (90% vs 53%. P < 0.05). Figure 2 illustrates baseline comorbidities. Patients with LC have higher percentages of almost all comorbidities listed.
| Characteristics | SRCS without liver cirrhosis | SRCS with liver cirrhosis | P value |
| N = 758810 (%) | N = 11920 (%) | ||
| Patient age, mean (SD) | 66.7 (66.6-66.8) | 62.7 (62.2-63.2) | < 0.05 |
| Sex | < 0.05 | ||
| Female | 293963 (39) | 11920 (34) | |
| Male | 464847 (61) | 7901(66) | |
| Race N (%) | < 0.05 | ||
| White | 546570 (72) | 7996 (67) | |
| Black | 96975.918 (13) | 1676 (14) | |
| Hispanic | 66092 (9) | 1525 (13) | |
| Asian or Pacific Islander | 21930 (3) | 273 (2) | |
| Native American | 4325 (1) | 118 (1) | |
| Other | 22840 (3) | 330 (3) | |
| Insurance, N (%) | < 0.05 | ||
| Medicare | 456880 (60) | 6728 (56) | |
| Medicaid | 79068 (10) | 2495 (21) | |
| Private | 188716 (25) | 2145 (18) | |
| Uninsured | 34146 (5) | 552 (5) | |
| Household median income, N (%) | < 0.05 | ||
| 1-38999 | 232348 (31) | 4271 (36) | |
| 39000-47999 | 210114 (28) | 3055 (26) | |
| 48000-62900 | 175513 (23) | 2595 (22) | |
| > 63000 | 140835 (18) | 1998 (17) | |
| Bed size, N (%) | < 0.05 | ||
| Small | 124065 (16) | 1665 (14) | |
| Medium | 230602 (30) | 3454 (29) | |
| Large | 404218 (53) | 6800 (57) | |
| Hospital region | < 0.05 | ||
| Northeast | 134309 (18) | 1860 (16) | |
| Midwest | 170201 (22) | 2360 (20) | |
| South | 306180 (40) | 4915 (41) | |
| West | 148196 (20) | 2785 (23) | |
| Hospital location | < 0.05 | ||
| Rural | 51220 (7) | 465 (4) | |
| Urban | 707590 (93) | 11455 (96) | |
| Charlson comorbidity index, mean (SD) | < 0.05 | ||
| 0 | 10016 (1) | 0 | |
| 1 | 166938 (22) | 335 (3) | |
| 2 | 176043 (23) | 830 (7) | |
| 3 | 405736 (53) | 10755 (90) |
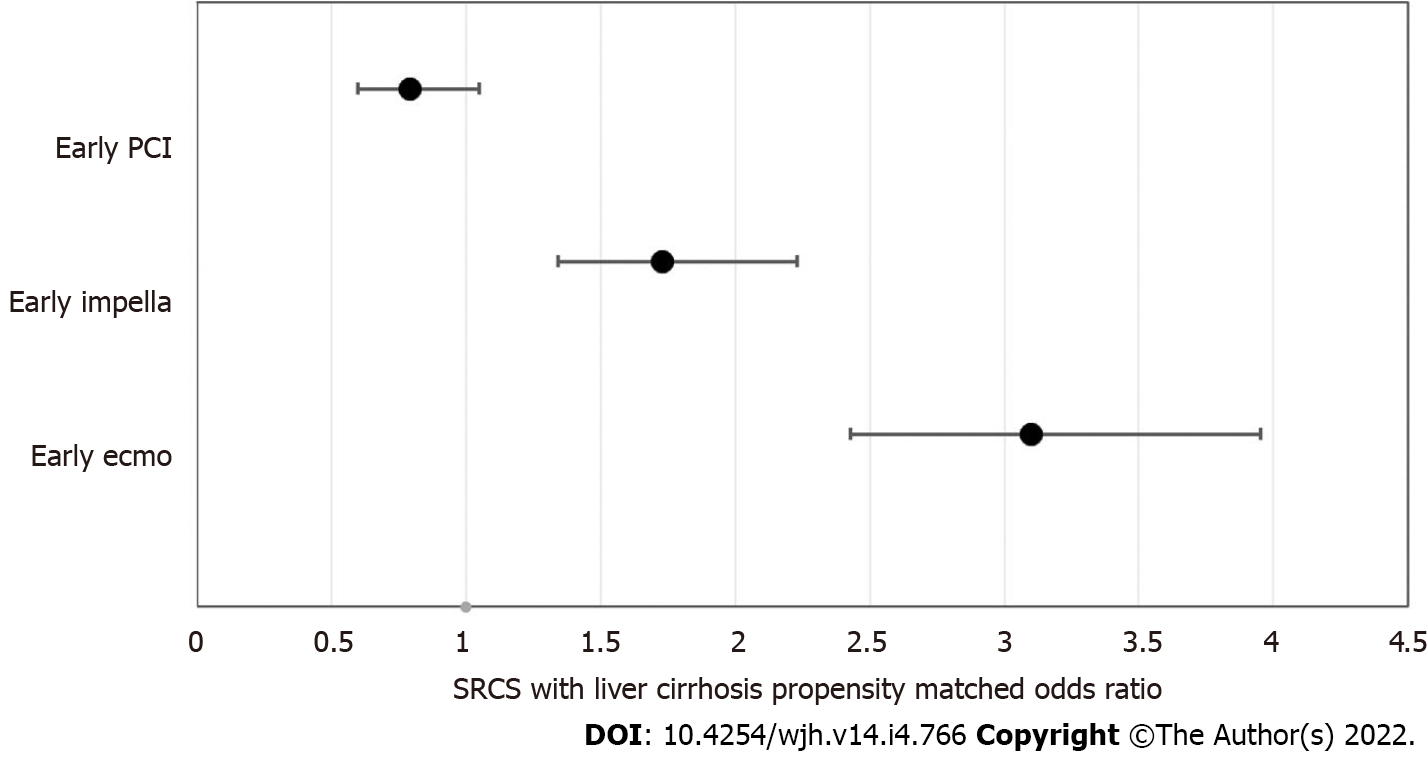
Figure 3 visualizes the odds of mortality of each intervention. The overall inpatient mortality rate was higher for SRCS patients with liver cirrhosis (28.7% vs 10.63%. P < 0.05). After adjusting for potential confounders, the odds of inpatient mortality were higher at 2.25 (CI 1.98-2.55, P < 0.05) for patients with LC compared to those without LC. Patients who had an early PCI had lower odds of mortality (OR: 0.79, CI: 0.6-1.05, P < 0.11) than those who had it later, but this was not statistically significant. Patients who had an early Impella (OR: 2.30, CI: 1.73, CI: 1.34-2.23, P < 0.05) and early ECMO (3.10: CI 2.43-3.95, P < 0.05) had higher odds of mortality compared to those who had it later than the defined period.
Through propensity-matched multivariate regression, moderate-severe liver disease (OR: 3.19, CI: 2.83-3.58, P < 0.05) shows higher odds of mortality compared to mild-liver disease (OR: 1.38, CI: 1.23-1.57, P < 0.05). Table 2 depicts individual markers of decompensated cirrhosis and their respective odds of mortality.
| Markers of decompensated cirrhosis | N (%) | Adjusted odds | Lower limit | Upper limit | P value |
| Hypoalbuminemia | 460 (4) | 1.38 | 1.04 | 1.82 | < 0.05 |
| Hepatic encephalopathy | 1855 (16) | 3.06 | 2.41 | 3.9 | < 0.05 |
| Portal vein thrombosis | 310 (3) | 1.13 | 0.72 | 1.77 | 0.6 |
| Variceal upper GI bleed | 825 (7) | 1.81 | 1.03 | 3.19 | < 0.05 |
| Ascites | 2155 (18) | 1.91 | 1.62 | 2.27 | < 0.05 |
| Coagulopathy | 1290 (11) | 2.18 | 1.86 | 2.56 | < 0.05 |
| SBP | 295 (2) | 2.38 | 0.69 | 8.32 | 0.17 |
| Hepatorenal syndrome | 945 (8) | 1.69 | 1.22 | 2.34 | < 0.05 |
Table 3 depicts propensity-matched adjusted. Sepsis (OR: 2.12, CI: 1.66-2.72, P < 0.05), AKI (OR: 2.72, CI: 2.01-3.68, P < 0.05), cardiac arrest (OR: 4.87, CI: 3.85-6.67, P < 0.05), and any bleed or hemorrhage (OR: 2.44, CI: 1.45-4.10, P < 0.05) were associated with statistically significant higher odds of mortality. Interestingly, the cohort of patients with any GIB was associated with lower mortality odds.
| In-hospital outcomes | Adjusted odds of mortality propensity matched- needs update | Lower limit | Upper limit | P value |
| Sepsis | 2.12 | 1.66 | 2.72 | < 0.05 |
| AKI on CKD | 0.65 | 0.4 | 1.06 | 0.08 |
| AKI | 2.72 | 2.01 | 3.68 | < 0.05 |
| AKI on CKD | 2.24 | 1.84 | 2.74 | < 0.05 |
| Cardiac arrest | 4.87 | 3.85 | 6.67 | < 0.05 |
| Stroke | 1.06 | 0.45 | 2.5 | 0.89 |
| Any bleed/hemorrhage | 2.44 | 1.45 | 4.1 | < 0.05 |
| Electrolyte and fluid disturbance | 0.95 | 0.45 | 1.99 | 0.89 |
| All GI bleed | 0.42 | 0.25 | 0.73 | < 0.05 |
| Respiratory failure requiring mechanical ventilation | 1.14 | 0.74 | 1.78 | 0.55 |
LOS and total hospital charges are markers of hospital resource utilization. LC patients presenting with STEMI or SRCS had a mean LOS of 9 d (CI: 5.5-5.6), while patients without LC had a mean LOS of 5.6 d (CI: 0.1-11.2). Mean LOS of patients with LC who underwent early PCI (6.4 vs 9.8 P < 0.05) and early Impella (7.9 vs 9.2 P < 0.05) had a shorter LOS compared to those without LC. After propensity-matched multivariate logistic regression, while adjusting for all confounders, early PCI (-2.57 < 0.05) and early Impella (-3.25 < 0.05) had a mean average shorter LOS compared to the population without liver cirrhosis. Mean LOS of SRCS patients between patients with and without LC who had an early ECMO is not statistically significant (9 vs 8.9, P = 0.85) and remains so even after adjusting for confounders (-0.79 < 0.20). Early PCI has lower total hospital charges ($144360.30 vs $148362.40), but the difference was not statistically significant (-$11215.00, P = 0.22). Early Impella has higher total hospital charges ($152088.30 vs $146418.70), with a statistically significant adjusted mean difference (-$26103.57, P = 0.05). However, early ECMO increases inpatient total hospital charges ($171334.80 vs $133881.8), and after adjustment for confounders with multivariate logistic regression, SRCS patients with LC when compared to those without LC were found to have an average of $24717.85 (P < 0.05) higher total hospital charges than those without liver cirrhosis.
In this retrospective study, we evaluate the predictors of mortality and the outcomes of patients with LC who develop SRCS compared to a propensity-matched cohort without LC. We note increased rates of inpatient complications, longer lengths of hospital stay, higher total hospital charges, and an overall higher mortality rate in the cohort of patients with LC.
We find sepsis, AKI, cardiac arrest, any bleed or hemorrhage as predictors of mortality. Despite significantly higher bleeding rates in SRCS patients with LC (23% vs 11%), bleeding does not increase the odds of mortality. Not surprisingly, having a cardiac arrest has the highest odds of mortality (OR: 6.67, P < 0.05). This relationship has been seen in previous studies portraying patients with LC who undergo cardiac arrest have a significantly higher mortality rate than those without LC[11]. This is likely multifactorial and may in part be due to their higher rates of comorbidities, non-shockable rhythms, and non-cardiac causes of cardiac arrest, including severe electrolyte disturbances, hemorrhage, or infection[11-14]. Patients with LC are particularly prone to infection and sepsis due to cirrhosis-associated immune dysfunction, disruption in gut permeability, increased bacterial translocation, and upregulation of systemic inflammatory mediators, infection, and sepsis[12,15,16]. Moreover, external factors, including the overuse of proton-pump inhibitors, corticosteroids, broad-spectrum antibiotics, and repeated hospital admissions, place these patients at further risk of infections from invasive and multidrug-resistant organisms. In a recent study done in North America, nosocomial infections developed in 15% of hospitalized patients with cirrhosis and were associated with an increased risk of death[16].
Acute kidney injury is also associated with increased mortality and length of hospital stay amongst all hospitalized patients[17,18]. A recent meta-analysis finds that AKI increases the risk of acute coronary syndrome, suggesting that AKI increases the risk of early mortality[19]. Our study suggests AKI, one of the most common complications of cirrhosis, is one of the highest predictors of mortality in the LC cohort, second only to cardiac arrest[1,20]. This is likely due to the underlying disease-specific physiology of cirrhosis. Portal hypertension causes splanchnic and systemic vasodilation leads to a compensatory increase in systemic vasoconstrictors, renal water and salt retention, and ultimately inadequate renal perfusion flow[1]. The underlying physiology behind portal hypertension coupled with intravascular volume depletion secondary to gastrointestinal bleeding, diuretic use, and lactulose-induced diarrhea leads to AKI development in about 20% of hospitalized patients with LC[17,20,21]. These patients develop AKI predominantly secondary to HRS and acute tubular necrosis, both of which tend to occur more commonly in more decompensated LC[19]. A recent systems biologic analysis suggests that the inflammatory state of HRS is similar to that of a chronic non-hepatic inflammatory disease, like systemic lupus erythematosus[22]. Our study's findings align with previous literature highlighting increased morbidity and mortality with the onset of AKI and HRS[17,21].
Patients with LC are also known to have an increased risk of bleeding. Frequent readmissions for gastrointestinal bleeding, chronic thrombocytopenia, and the elevated international normalized ratio of prothrombin time support this notion[23,24]. Recent evidence suggests that cirrhotic patients may have prothrombotic tendencies as well, rather than solely bleeding diathesis[23,24]. Unfortunately, it cannot be temporally determined when bleeding or thrombosis occurred during the hospital admission. Nevertheless, our findings suggest that both coagulopathy and bleeding directly relate to mortality. As of yet, there is no defined correlation between the MELD score and bleeding complications[24,25]. There is limited data on the benefits of transfusion of blood products for a goal INR or platelet count. One study concluded that an elevated INR (> 1.5) was not predictive of post-procedural bleeding in the LC group[26,27]. Another study found that transfusion of fresh frozen plasma (FFP) did not provide any clear benefit in preventing bleeding risk[27]. Platelet transfusions also offer little help in patients with LC due to the short half-life from splenic sequestration[26,27]. Our study indicates that bleeding and coagulopathy increased mortality in patients with SRCS. The dilemma then reverts to using these corrective measures to prevent bleeding and coagulopathic events in this population. Our study results indicate that it may be beneficial to correct red blood cell and platelet counts before interventional procedures; however, further randomized controlled trials are vital to defining what the best method of correction would be and to what extent.
We also found that patients with markers of decompensated cirrhosis had increased odds of mortality. Liver cirrhosis results in a myriad of microvascular structural changes and functional derangements of synthetic liver function, leading to hemostasis abnormalities, portal hypertension, and circulatory dysfunction develop[28,29]. As the severity of the disease progresses, the intensity of the splanchnic vasodilation ultimately contributes to the characteristic hyperdynamic circulation of cirrhosis and its sequelae of decompensation-defining variable (i.e., HRS, HE, ascites)[28,30]. Our study finds that the decompensation-defining factors hypoalbuminemia, hepatic encephalopathy, variceal upper gastrointestinal bleeds, coagulopathy, hepatorenal syndrome, and ascites are associated with the higher odds of mortality of all. At the same time, SBP and portal vein thrombosis do not increase the odds of mortality. Our findings reflect the critical variables utilized in the multiorgan assessment to predict mortality of patients with underlying liver cirrhosis (i.e., Childs-Pugh) CP[31-33]. This study cannot assess disease severity using these scoring systems; however, we can presume that mortality rises as the severity of the disease progresses[24,34,35].
A previous nationwide study identified higher odds of mortality in patients with LC who develop STEMI than those without LC[36]. Another study also identified increased all cause-mortality of liver cirrhosis patients who develop STEMI compared to those without liver cirrhosis[9]. Some studies debate that liver cirrhosis may protect against the development of thrombosis by the shifted balance of coagulation and altered hemostasis[9]. While this remains a debated topic, our study suggests that if a patient with LC progressed to cardiogenic shock after developing a STEMI, they have significantly higher rates and odds of mortality. Although the odds of mortality were lower if PCI was performed within 24 h, it was not statistically significant. However, we find that patients with LC had higher odds of mortality if both Impella and ECMO were performed early (within five days). This finding may be because both Impella and ECMO carry risks of hemolysis, bleeding, and increased need for transfusion. Literature on using percutaneous mechanical circulatory support in patients with LC is scarce; however, our findings are consistent with a previous study that suggests that inpatient and 1-year mortality rates were higher in LC if patients had two or more risk factors (age ≥ 65, respiratory indications for ECMO, hypoalbuminemia, or liver transplantation)[37]. Patients with LC are likely more vulnerable to the effects of ECMO due to the combination of prothrombotic and anti-thrombotic changes that occur in LC[38]. Known complications of ECMO include thrombosis and bleeding from consumptive coagulation factor deficiency, excessive fibrinolysis, thrombocytopenia, and platelet dysfunction[23,39]. It has been seen in previous studies that multiorgan dysfunction can occur after ECMO, but it is unclear how it alters liver function[40]. Nevertheless, the use of ECMO in LC patients should be done after careful consideration of risks and benefits. More research is vital to evaluate whether hemodynamic and hemostatic optimization lowers mortality rates[34,41,42].
We also noted that the length of hospital stays and total hospital charges were higher amongst patients with liver cirrhosis. The economic burden associated with cirrhosis is significant. In 2004, the US's total direct cost of cirrhosis was approximately $2.5 billion, and the indirect cost was $10.6 billion[10]. Patients with LC had higher odds of adverse inpatient outcomes, which may lead to further intervention, delay in discharge, and higher cost of inpatient stay. In previous studies, the presence of AKI suggests significantly higher inpatient LOS and resource utilization costs[21,43]. Previous studies have also found that infected cirrhotic patients had a higher risk of mortality and associated higher LOS and TOS than those that did not have their hospital course complicated by infection[44]. Interestingly, amongst all patients who received early ECMO, those with LC had significantly higher total hospital charges despite having similar durations of hospital stay. The need for more blood product transfusion and albumin infusion in LC may explain this financial discordance.
Over the years, it has become evident that patients with LC have worse outcomes. The severity of their disease correlates with their risk of mortality, inpatient LOS, and total hospital charges[32]. Our study illustrates which underlying comorbidities, hospital complications, and interventions may increase these odds. Our findings suggest that patients with SRCS and decompensated LC who develop AKI or sepsis are at significantly increased odds of mortality and face further increased odds of mortality if they require early invasive mechanical circulatory support with Impella or ECMO than those without LC. Further research is needed amongst this population to establish a standardized assessment criterion for acute risk stratification in SRCS for patients with LC. More research is essential to evaluate whether hemodynamic and hemostatic optimization lowers mortality rates. Lack of standardization and reliance on gestalt poses a significant risk of mortality for patients, contributes to existing enormous financial burdens, and poses a fundamental dilemma to clinicians.
Our study has some limitations inherent to its retrospective database analysis design. First, the data was obtained from an administrative database. This database uses ICD10 codes to identify the clinical diagnosis rather than clinical parameters. Thus, there is a possibility of misclassification or under-coding of a diagnosis. There is no way to code for the severity of liver cirrhosis using Child-Pugh classification or MELD scoring systems. The CCI has a cohort of ICD codes used to define mild liver disease and moderate-severe liver disease, used as a makeshift placeholder for the scoring systems in this study but cannot be used as a replacement. We can assume that increasingly severe liver disease would have worse clinical outcomes, but we cannot definitively assess this in this study. This idea limits further understanding of how the severity of liver cirrhosis may affect clinical outcomes. We expect any potential misclassification to be equal between the LC and the non-LC groups. This study also uses propensity-matched scoring to reduce this bias. Errors do not change the direction or relationship of the variables. Instead, they make it more challenging to establish a clinical significance. Second, the NIS database does not contain variables that allow for information such as medications, including types and dosages, radiological testing, or laboratory values. Instead, similar to previous studies, also utilizing the NIS database, we provided healthcare resource utilization as reflected by the length of stay and total hospital charges. Third, because NIS only captures in-hospital mortality, the rate we report may be slightly underestimated compared to this population's actual calendar year mortality rate. NIS does not include patients who die at home, en route to the hospital, or even in the emergency department. For our particular study group, this may be a small number as most of these patients, if at all, are likely to die at home if they are safely discharged.
Despite these limitations, our study has many strengths. To our knowledge, this is the first study to identify the impact of LC on interventional management of cardiogenic shock and the effect of its timings during the admission on the mortality rate. We use the largest publicly available all-payer inpatient database in the United States, minimizing the likelihood of beta error. Most importantly, the NIS is a nationally representative sample including small, medium, large, teaching, non-teaching, rural, urban, for-profit, non-profit, publicly owned, or private hospitals across 47 states allowing it to encompass 97% of the United States population. This fact makes the study easily generalizable to all parts of the United States. Due to the United States' diverse population, this also allows for a unique perspective on the global population. The impressive sample size allows ascertaining certain risk factors, which would otherwise be difficult in a smaller single-center study. Furthermore, the unique variables in the database allow for exploring hospitalization costs, household income estimates, and other patient and hospital factors that may not be available in single-center studies.
ST-Elevation myocardial infarction (STEMI) remains a significant cause of morbidity and mortality globally. A particularly susceptible population are patients with liver cirrhosis.
This study aims to find what factors predicted morbidity and mortality in patients with liver cirrhosis that may need to undergo interventional management for STEMI related cardiogenic shock.
We aim to identify predictors of morbidity and mortality in patient with liver cirrhosis that undergo interventional management for STEMI related cardiogenic shock. We aim to find the effect of liver cirrhosis on mortality, length of stay, and hospital costs in patients with STEMI related cardiogenic shock.
We conducted a retrospective review on the national inpatient sample 2017. Using the student t-test and propensity-matched multivariate logistic regression, we were able to find the P value and odds of mortality.
We find that patients with liver cirrhosis have significantly higher morbidity and mortality rates than those without liver cirrhosis. They are also susceptible to adverse outcomes when undergoing interventional management.
Physicians must optimize patients with liver cirrhosis before any interventional procedure. Patients with mild cirrhosis seemed to have better outcomes than patients with moderate-severe liver cirrhosis.
This research will help build the framework for future studies to study this topic further. The goal would be to identify a scoring system that would allow physicians to ascertain which patients would be safely able to undergo interventional management and which would not. As of now, it is mostly under clinical judgment.
Dr. Rosario Ligresti for helping guide through this topic.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng M, China; Sira AM, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Howell WL, Manion WC. The low incidence of myocardial infarction in patients with portal cirrhosis of the liver: A review of 639 cases of cirrhosis of the liver from 17,731 autopsies. Am Heart J. 1960;60:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Vanĕcek R. Atherosclerosis and cirrhosis of the liver. Bull World Health Organ. 1976;53:567-570. [PubMed] |
| 3. | Tiukinhoy-Laing SD, Rossi JS, Bayram M, De Luca L, Gafoor S, Blei A, Flamm S, Davidson CJ, Gheorghiade M. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 5. | Mirbagheri SA, Rashidi A, Abdi S, Saedi D, Abouzari M. Liver: an alarm for the heart? Liver Int. 2007;27:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 2013;12:713-724. [PubMed] |
| 7. | Stephens D. ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction. Published online. 2004;212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Hillerson D, Ogunbayo GO, Salih M, Misumida N, Abdel-Latif A, Smyth SS, Messerli AW. Outcomes and Characteristics of Myocardial Infarction in Patients With Cirrhosis. J Invasive Cardiol. 2019;31:E162-E169. [PubMed] |
| 9. | Wu VC, Chen SW, Chou AH, Wu M, Ting PC, Chang SH, Wang CY, Lin MS, Hung KC, Hsieh IC, Chu PH, Wu CS, Lin YS. Nationwide cohort study of outcomes of acute myocardial infarction in patients with liver cirrhosis: A nationwide cohort study. Medicine (Baltimore). 2020;99:e19575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 11. | Piton G, Chaignat C, Giabicani M, Cervoni JP, Tamion F, Weiss E, Paugam-Burtz C, Capellier G, Di Martino V. Prognosis of cirrhotic patients admitted to the general ICU. Ann Intensive Care. 2016;6:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Roedl K, Wallmüller C, Drolz A, Horvatits T, Rutter K, Spiel A, Ortbauer J, Stratil P, Hubner P, Weiser C, Motaabbed JK, Jarczak D, Herkner H, Sterz F, Fuhrmann V. Outcome of in- and out-of-hospital cardiac arrest survivors with liver cirrhosis. Ann Intensive Care. 2017;7:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 14. | Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS; American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 671] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 15. | Ascione T, Di Flumeri G, Boccia G, De Caro F. Infections in patients affected by liver cirrhosis: an update. Infez Med. 2017;25:91-97. [PubMed] |
| 16. | Bajaj JS, OʼLeary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, Biggins SW, Lai JC, Vargas HE, Maliakkal B, Fallon MB, Thuluvath PJ, Subramanian RM, Thacker LR, Reddy KR. Nosocomial Infections Are Frequent and Negatively Impact Outcomes in Hospitalized Patients With Cirrhosis. Am J Gastroenterol. 2019;114:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol. 2019;25:3684-3703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (4)] |
| 18. | Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 366] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 19. | Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 902] [Cited by in RCA: 855] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 20. | Tariq R, Hadi Y, Chahal K, Reddy S, Salameh H, Singal AK. Incidence, Mortality and Predictors of Acute Kidney Injury in Patients with Cirrhosis: A Systematic Review and Meta-analysis. J Clin Transl Hepatol. 2020;8:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019;39:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Vetrovec G, Stravitz RT. Bleeding in Patients With Cirrhosis Undergoing Invasive Cardiovascular Procedures: Do We Overestimate Risk? Circulation. 2020;141:1279-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Drolz A, Horvatits T, Roedl K, Rutter K, Staufer K, Kneidinger N, Holzinger U, Zauner C, Schellongowski P, Heinz G, Perkmann T, Kluge S, Trauner M, Fuhrmann V. Coagulation parameters and major bleeding in critically ill patients with cirrhosis. Hepatology. 2016;64:556-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2171] [Article Influence: 180.9] [Reference Citation Analysis (5)] |
| 26. | Townsend JC, Heard R, Powers ER, Reuben A. Usefulness of international normalized ratio to predict bleeding complications in patients with end-stage liver disease who undergo cardiac catheterization. Am J Cardiol. 2012;110:1062-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Rassi AB, d'Amico EA, Tripodi A, da Rocha TRF, Migita BY, Ferreira CM, Carrilho FJ, Farias AQ. Fresh frozen plasma transfusion in patients with cirrhosis and coagulopathy: Effect on conventional coagulation tests and thrombomodulin-modified thrombin generation. J Hepatol. 2020;72:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 29. | Arabi Y, Ahmed QA, Haddad S, Aljumah A, Al-Shimemeri A. Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol. 2004;16:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 31. | Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2004;2:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 33. | Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, Shaw S, Burroughs AK. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | McPhail MJ, Shawcross DL, Abeles RD, Chang A, Patel V, Lee GH, Abdulla M, Sizer E, Willars C, Auzinger G, Bernal W, Wendon JA. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol. 2015;13:1353-1360.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Saliba F, Ichai P, Levesque E, Samuel D. Cirrhotic patients in the ICU: prognostic markers and outcome. Curr Opin Crit Care. 2013;19:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 36. | Abougergi MS, Karagozian R, Grace ND, Saltzman JR, Qamar AA. ST Elevation Myocardial Infarction Mortality Among Patients With Liver Cirrhosis: A Nationwide Analysis Across a Decade. J Clin Gastroenterol. 2015;49:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Chou AH, Wu VC, Chen DY, Hung KC, Chang SH, Chu PH, Chen SW. Corrigendum to 'Outcome of extracorporeal membrane oxygenation support in patients with liver cirrhosis: a nationwide population-based cohort study'. Eur J Cardiothorac Surg. 2020;58:665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Zermatten MG, Fraga M, Moradpour D, Bertaggia Calderara D, Aliotta A, Stirnimann G, De Gottardi A, Alberio L. Hemostatic Alterations in Patients With Cirrhosis: From Primary Hemostasis to Fibrinolysis. Hepatology. 2020;71:2135-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Thomas J, Kostousov V, Teruya J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin Thromb Hemost. 2018;44:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 40. | Klimesova K, Kverka M, Zakostelska Z, Hudcovic T, Hrncir T, Stepankova R, Rossmann P, Ridl J, Kostovcik M, Mrazek J, Kopecny J, Kobayashi KS, Tlaskalova-Hogenova H. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm Bowel Dis. 2013;19:1266-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | McPhail MJ, Auzinger G, Bernal W, Wendon JA. Decisions on futility in patients with cirrhosis and organ failure. Hepatology. 2016;64:986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Arroyo V, Moreau R, Jalan R, Ginès P; EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 43. | Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2366] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 44. | Saleem S, Katragadda R, Weissman S, Bleibel W. Morbidity and mortality of infections in the cirrhotic patients: a US population-based study. Gastroenterol Hepatol Bed Bench. 2019;12:233-238. [PubMed] |