Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.647
Peer-review started: August 15, 2021
First decision: December 16, 2021
Revised: February 5, 2022
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: April 27, 2022
Processing time: 250 Days and 2.5 Hours
Chronic hepatitis C virus (HCV) infection is a major global public health problem, particularly in developing part of the world. Significant advances have been made in the early diagnosis and treatment of the disease. Its management has been particularly revolutionized during the past two decades. In this review, we summarize the major advances in the diagnostic and management arma
Core Tip: Chronic hepatitis C virus (HCV) infection is a major public health threat worldwide, particularly in resource-constrained countries. Although significant advances have been made in the early diagnosis and treatment of the disease, many unmet challenges remain to be tackled, particularly the affordability, equitable distribution and access to these methods. The World Health Organization aims to eliminate viral hepatitis including HCV by 2030. This frontier article addresses the burden of chronic HCV infection, delineates the current therapeutic options, and identifies future strategies to tackle this highly prevalent disease.
- Citation: Hanif FM, Majid Z, Luck NH, Tasneem AA, Laeeq SM, Mubarak M. Revolution in the diagnosis and management of hepatitis C virus infection in current era. World J Hepatol 2022; 14(4): 647-669
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/647.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.647
Hepatitis C virus (HCV) accounts for majority of viral hepatitis-related mortality per annum worldwide[1]. Chronic HCV infection persisting for more than 20-30 years causes liver cirrhosis and/or hepatocellular carcinoma (HCC). World Health Organization (WHO) estimates that around 71 million people are suffering from chronic HCV infection with highest incidence in WHO Eastern Mediterranean and European Regions[2]. Globally, around 400000 people die annually due to chronic HCV-associated complications, predominantly as a result of decompensated hepatic cirrhosis or development of HCC. The economic impact of the disease is also enormous, particularly for emerging economies. HCV virus has greater genetic diversity than HBV or human immunodeficiency virus (HIV). HCV has seven genotypes with around 67 subtypes[3]. Spectacular advances have been made in the scientific field in the diagnosis and management of this infection in the past few years. Still, ironically, fewer than 20% of those living with HCV infection globally are aware of their disease, and the immediate challenge is to engage, screen, diagnose and treat everyone in whom treatment is warranted. These undiagnosed HCV cases worldwide represent an important hurdle to achieve the WHO goal of HCV elimination by 2030. Many social, access to healthcare and economic hurdles remain in the path to HCV elimination. However, many success stories are being reported from many high prevalence countries, including Egypt[4]. It is beyond the scope of this review article to cover all aspects of HCV infection; hence, the focus of this review is on the remarkable advances that have taken place in the diagnosis and management of chronic HCV infection, particularly in vulnerable and “difficult-to-treat” groups and on strategies being implemented to eliminate HCV infection.
The story of discovery of HCV is unique in that it was identified by non-conventional means, i.e., molecular biologic techniques rather than direct visualization and cell culture. It is pertinent to briefly revisit this story here to better understand the advances that have taken place in the diagnostic and therapeutic aspects of HCV infection. It all started with the finding a new type of hepatitis in patients who received blood transfusion in early 1970s. As hepatitis A virus and hepatitis B virus (HBV) were not present in such patients, Alter and co-workers in 1975 coined the term “non-A, non-B (NANB) hepatitis” for this type of hepatitis. They collected plasma/serum samples from a blood donor with chronic hepatitis and four people who developed “NANB hepatitis” after receiving blood transfusion, and injected these samples into five chimpanzees. All five chimpanzees developed hepatitis, as evidenced by rise of serum alanine aminotransferase levels as well as liver pathological changes, confirming the presence of a yet unknown transmissible agent in the blood of patients with NANB hepatitis. In 1989, Houghton and co-workers constructed a random-primed complementary DNA (cDNA) library using plasma samples from patients with NANB hepatitis. One clone in this library was not derived from host DNA, and appeared to be from a novel RNA virus belonging to the Flavivirus family (at least 10000 nucleotides and positive-stranded). They named this novel virus as HCV. Later on, Rice and co-workers constructed a full-length clone of HCV cDNA that was able to be transcribed to an infectious RNA variant of HCV. Upon intrahepatic inoculation of this clone, chimpanzees developed chronic hepatitis, with production of antibodies against HCV and viral replication in the blood. Subsequently, Bartenschlager and co-workers developed an in vitro cell culture using a human hepatoma cell line to replicate HCV. This cell-based model was indispensable in highlighting the biological features of HCV as well as developing anti-HCV agents. The 2020 Nobel Prize in Physiology or Medicine was awarded to Drs. Harvey J. Alter, Michael Houghton and Charles M. Rice for the discovery of HCV[5].
An accurate diagnosis and linkage to care is the key to successful treatment and ultimately eradication of any infectious disease. HCV infection is no exception to this rule. Hence, simple, affordable, rapid and high quality diagnostic tests of active infection at the point-of-care (POC) are central to the achievement of HCV elimination goal. While the world has focused its attention over the last decade on the final stages within the cascade of care to develop and increase access to directly acting antiviral agents (DAAs), relatively less attention has been paid to ensure accurate and affordable diagnostic tools to make wide-scale global treatment a reality. The diagnostic armamentarium for HCV infection principally comprises of two approaches: detection of antibodies against the virus in the serum and HCV PCR, the later being the gold standard. More recently, HCV antigen test has also been introduced as an alternative to HCV PCR. Each of the tests has merits and demerits. Ironically, in many settings, prohibitively high costs of HCV diagnostics often now exceed the cost of curative therapy. Thus, improving access to rapid, simple, and affordable HCV diagnostics is critical to achieve global HCV elimination and should be considered a public health priority.
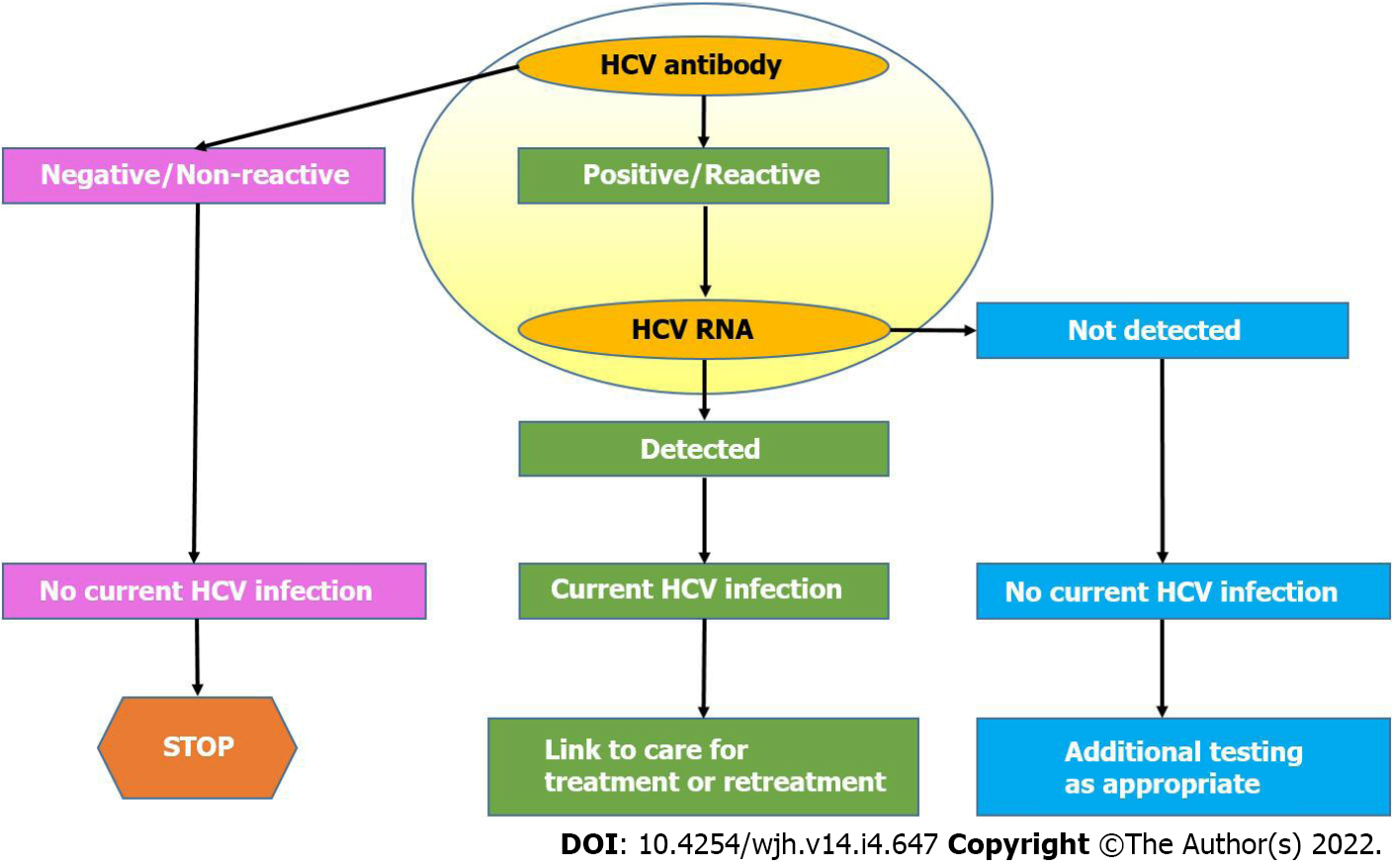
American Association for the Study of Liver Diseases (AASLD) recommends all individuals above 18 years to be screened for HCV for at least one-time owing to treatment benefits and reduction of morbidity and mortality. HCV-antibody tests approved by US Food and Drug Administration (FDA) should be utilized for screening of HCV infection. To detect active viremia and for treatment decisions, HCV RNA with a detection limit of ≤ 25 IU/mL is advised. However, immunocompromised populations or patients exposed to HCV within 6 mo should undergo HCV RNA testing despite negative HCV antibody test. A simple and updated HCV testing algorithm recommended by Center for Disease Control is shown in Figure 1. Such simplification of current hepatitis C diagnostic algorithms and the advent of digital diagnostic devices will play a pivotal role in achieving the WHO’s target goals of hepatitis C elimination by 2030. Over the last decade or so, hepatitis C diagnostics have been revolutionized by the introduction and commissioning of state-of-the-art HCV diagnostic platforms which have been efficiently applied in high-risk HCV populations in developed countries as well as in some low-to-middle income countries (LMICs) to diagnose millions of undiagnosed hepatitis C-infected people. POC rapid diagnostic tests (POC-RDTs), reflexive RNA testing, dried blood spot sample analysis and hepatitis C self-test assays have demonstrated their diagnostic value in real-world clinical experiences, in mass hepatitis C screening campaigns, and disenfranchised native hepatitis C populations in remote areas[6].
The development of successful antiviral chemotherapeutic agents lagged behind the antibiotics and has primarily evolved in past 50 years[7]. This developmental delay has as its cause many hurdles like the delays in the advent of culture system, experimental animal models and a standard method for antiviral drug formulation. Moreover, the challenges encountered in developing a targeted therapy for a specific viral agent included drug toxicity, viral genetic variability and resistance profile, all these lengthened its developmental process[7].
HCV-specific antiviral agents met the same development delay. The discovery of HCV began from 1975 with the identification of new transfusion-related “NANB” hepatitis virus to the isolation of a single cDNA clone named HCV in 1989[5,8-10]. The successful pilot study on NANBH, by Hoofnagle et al[11], in 1986 formed the basis of two randomized trials. Both trials demonstrated on-treatment effectiveness of interferon (IFN) alpha-b in HCV eradication[12]. Thus, in 1991, US FDA approved IFN-alpha for the treatment of chronic HCV infection. Later on, ribavirin (RBV), an oral nucleoside analogue, was utilized as a monotherapy for HCV infection. Due to transient antiviral effect of RBV, the focus of clinical trials shifted to combination therapy[9,12].
By increasing the treatment duration and with the addition of IFN-alpha with RBV, the sustained virological response (SVR) rate escalated from 6% to 42%[13]. However, this treatment option had many caveats, mainly the IFN-associated side effects and intolerability, which were later improved with the advent of once weekly pegylated IFN (PegIFN). Moreover, with the encouraging response in HCV genotypes 2 and 3, having an SVR rate of 70% to 80%, the combination of PegIFN with RBV, thus became the standard of care[12,14]. Although the “golden era of IFN” persisted for over a decade but the large cohort of patients having decompensated chronic liver disease, hemoglobinopathies, pregnancy and organ transplants were deprived of its treatment benefit[6,15].
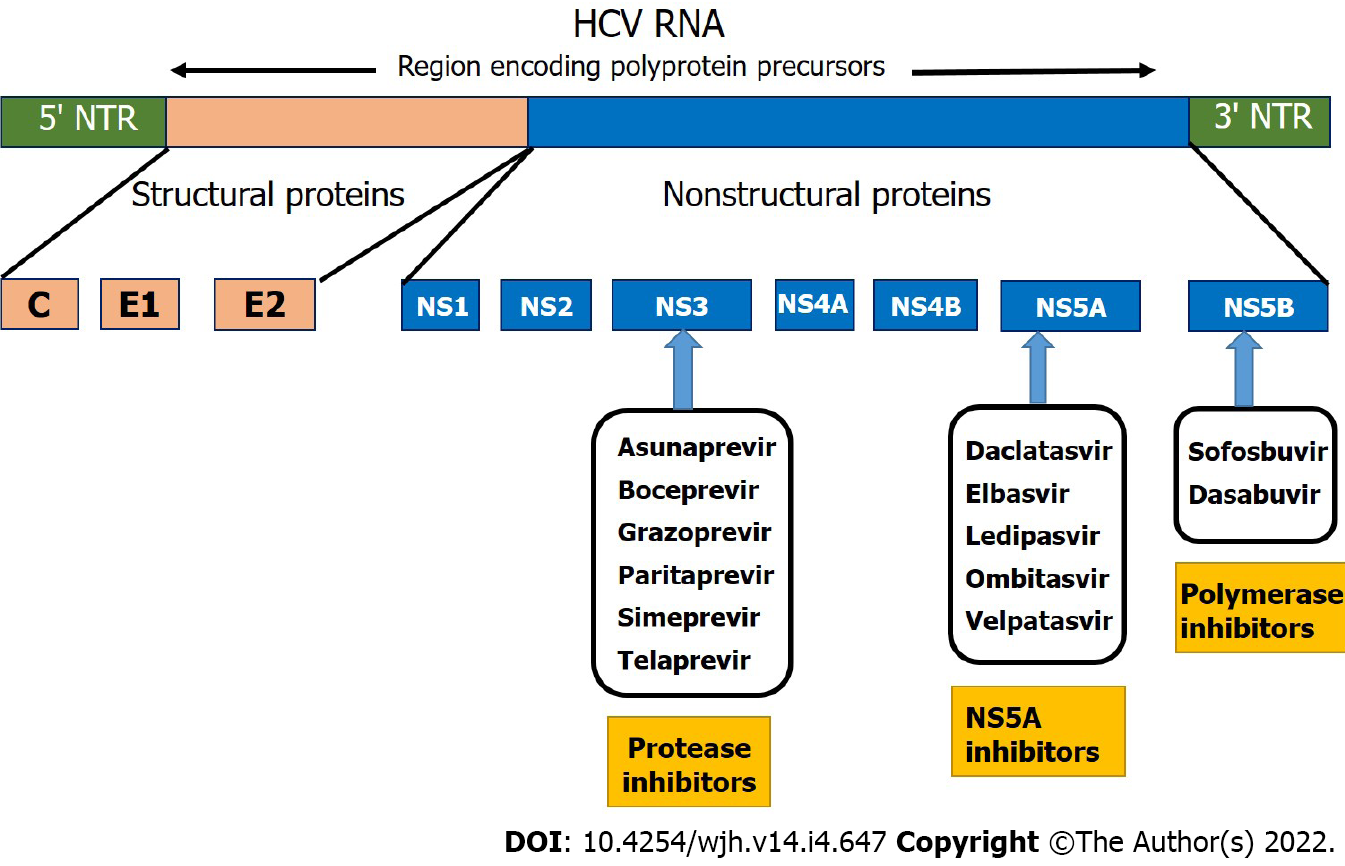
Later on, with the advancement in the molecular virology of HCV and the unraveling of HCV life cycle, three dimensional structure of HCV and its enzymes, led to development of the first generation of NS3-4A protease inhibitors: namely telaprevir and boceprevir[6]. These agents were only approved for treatment of chronic HCV genotype 1 patients in combination with PegIFN and RBV[12]. Due to their unfavorable pharmokinetics, drug- drug interactions, adverse effects and efficacy only for genotype 1, these agents were eventually replaced by second generation directly acting antiviral agents (DAAs); the early agent, simeprevir (SMV), in combination with PegIFN and RBV was effective against only genotypes 1, 2 and 4[16]. It was sofosbuvir (SOF), NS5A polymerase inhibitor, which led to a paradigm shift in the treatment cascade of HCV[6,9]. The discovery of SOF was not only a breakthrough in the advent of all-oral DAAs but was a beacon of light for dealing with the HCV infection in cirrhotics. Figure 2 depicts a simplified classification and major sites of action of various DAAs.
Initially, SOF was used in combination with PegIFN and RBV and was approved for genotypes 1 to 4. Cumulative SVR of various trials documented 87.6%, 95.6%, 91.3% and 92.3% in genotypes 1, 2, 3 and 4, respectively. Phase 4 TARGET trial documented effectiveness of SOF and RBV combination for 12 wk. The trial reported 91.9% and 75% SVR12 in non-cirrhotic population while 71.9% and 55.3% in cirrhotics treatment-naïve population with genotype 2 and genotype 3, respectively[17]. Moreover, ASTRAL 2 and ASTRAL 3 trials reported 80.4% and 73% SVR for HCV infection amongst the non-cirrhotic and cirrhotic populations treated with SOF and RBV combination for 24 wk[18].
In 2014, SOF with ledipasvir (LDV), a single pill combination, was approved for genotypes 1, 4, 5 and 6. ION-1 and ION-2 trials documented high efficacy of SOF/LDV combination in naïve and treatment-experienced population. Additionally, the use of RBV or treatment extension to 24 wk did not provide any significant benefit[19,20]. The pooled SVR12 of various trials was 92.2% and 96.1% in genotype 1 infected patients with and without cirrhosis[17].
Later in the same year, triple DAAs fixed dose combination was approved for genotype 1. The paritaprevir, ombitasvir, ritonavir combination with dasabuvir (PrOD) was evaluated in various trials with or without RBV for 12 wk. The combination showed more than 90% SVR12 in both cirrhotic and non-cirrhotic genotype 1 patients[17]. However, the risk of hepatotoxicity precluded its use in decompensated cirrhosis[4].
The usage of these initial DAAs made the least responsive genotype, genotype 1, safely and effectively treatable as opposed to what was seen with IFN, while genotype 3 became the “difficult to treat” genotype[21]. This scenario was dealt with by NS-5A replication complex inhibitor, daclatasvir (DAC). ALLY-3, a phase III trial included treatment naïve and experienced HCV populations with genotype 3. The trial documented overall 96% SVR12 with 12-week therapy of SOF and DAC combination but with sub-optimum response in cirrhotic patients[22]. However, ALLY-3+ study evaluated response of SOF, DAC and RBV combination for 12 and 16 wk in HCV genotype 3 patients with fibrosis stage > 3. The authors reported 100% SVR 12 in patients with fibrosis stage 4, while 86% in patients with compensated cirrhosis. Moreover, the trial also concluded comparable SVR 12 in 12- and 16-wk groups[23].
ASTRAL 3 study compared the response of 12-wk SOF/Velpatasvir (VEL) with 24-wk SOF with RBV combination in genotype 3 HCV infected patients. In this study population, overall SVR12 rate was statistically significantly higher in SOF/ VEL group (95%) than SOF RBV group (80%) [P < 0.001]. Moreover, in the SOF/VEL group with cirrhosis, 93% SVR12 was achieved in treatment-naïve group and 89% in experienced group with no discontinuation of treatment due to adverse effects[18].
Both the EASL and AASLD have recommended a shorter duration of therapy with the DAAs (8-12 wk) for non-cirrhotics and 12 wk for the cirrhotic patients[24,25].
The POLARIS 2 and 3 trials, which were phase III trials, compared 8 wk of SOF-VEL-voxilaprevir (VOX) with 12 wk SOF-VEL combination in cirrhotic and non-cirrhotic HCV population. POLARIS-2 study group excluded genotype 3 and documented non-inferior 95% SVR12 with SOF-VEL-VOX while SOF-VEL combination showed SVR 12 of 98%. However, the lower efficacy was attributed to higher relapse rate in genotype 1a population. POLARIS 3 trial documented similar efficacy of both regimens in HCV genotype 3 patients with compensated cirrhosis[25].
Thus, it has become possible to treat HCV infection even in the presence of decompensated cirrhosis, which was otherwise quite cumbersome during the IFN era. ASTRAL-4 trial documented response to SOF/VEL with or without RBV in decompensated liver disease. Therapy was associated with improved disease severity as documented by Model for End-stage Liver Disease (MELD) and Child Turcotte Pugh (CTP) scores. The study documented 83% and 86% overall SVR 12 with SOF/VEL for 12 and 24 wk, respectively. Moreover, 94% SVR12 was observed with SOF/VEL for 12 wk with RBV combination. However, study was not powered to detect significant difference between regimens[26]. Moreover, since the HCV protease inhibitors undergo hepatic elimination, many regimens that contain these agents are not recommended in patients with decompensated cirrhosis. FDA has received rare reports of worsening liver function or liver failure when these patients are treated with the following drugs: Elbasvir/grazoprevir (ELB-GRA); glecaprevir/pibrentasvir (GLE-PIB); SOF-VEL-VOX; and PrOD. Hence, these drugs should not be prescribed in patients with a history of prior hepatic decompensation[27].
The treatment of decompensated HCV-associated cirrhosis with DAAs has shown to cause an improvement in CTP as well as MELD scores. The TOSCAR study in which patients with MELD 15 or more were treated with SOF/DAC for 24 wk, showed that three fourth of the patients who achieved SVR had their median MELD and CTP scores decreased by two points[28]. Furthermore, an Italian multicenter study, showed a significant increase in the rate of switch to CTP A, at 24 wk post-SVR[29].The improvement in the CTP and MELD scores has also been shown to result in the delisting of patients who were earlier candidates for liver transplantation[30,31].
Table 1 depicts the summary of the main trials of various DAAs in the treatment of chronic HCV infection.
| Trial Names | Regimens | Treatment experienced /naive | Genotype | Duration | Cirrhotics/non-cirrhotics | SVR12, % |
| TARGET | Sofosbuvir-Ribavirin | Naïve | 2 | 12 wk | Cirrhotics | 91.9 |
| Non-cirrhotics | 71.9 | |||||
| 3 | Cirrhotics | 75 | ||||
| Non-cirrhotics | 55.3 | |||||
| ASTRAL 2 and ASTRAL 3 | Sofosbuvir-Ribavirin | Naïve | 3 | 24 wk | Cirrhotics | 73.3 |
| Non-cirrhotics | 90.4 | |||||
| ION 1 | Sofosbuvir-Ledipasvir | Naïve | 1 | 24 wk | Cirrhotics | 96.9 |
| Non-cirrhotics | 99.5 | |||||
| TURQUOISE-III | Ombitasvir-Paritasprevir- Ritonavir | Naïve | 1b | 12 wk | Cirrhotics | 100 |
| ASTRAL 3 | Sofosbuvir- Velpatasvir | Naïve | 3 | 12 wk | Cirrhotics | 93 |
| Non-cirrhotics | 98.2 | |||||
| Treatment Experienced | 12 wk | Cirrhotics | 89.2 | |||
| Non-cirrhotics | 91.2 | |||||
| ASTRAL 4 | Sofosbuvir- Velpatasvir-Ribavarin | Naïve | 1 | 12 wk | Cirrhotics | 94.4 |
| Treatment Experienced | 90 | |||||
| ASTRAL 4 | Sofosbuvir- Velpatasvir-Ribavarin | Naïve | 3 | 12 wk | Cirrhotics | 84.6 |
| Treatment Experienced | 96.2 | |||||
| POLARIS 2 | Sofosbuvir-Velpatasvir-Voxilaprevir | Naïve | 1-6 | 8 wk | Cirrhotics | 91 |
| Non-cirrhotics | 96 | |||||
| POLARIS 3 | Sofosbuvir-Velpatasvir-Voxilaprevir | Naïve | 3 | 8 wk | Cirrhotics | 96.3 |
| Treatment Experienced | 97 |
It is estimated that 3.26 to 5.0 million children and adolescents worldwide have chronic HCV infection. To date, the global response has focused mainly on the adult population, but DAA regimens are now approved for children aged ≥ 3 years. Transmission routes, disease progression and treatment indications in children differ from those in adults. Globally, vertical transmission accounts for most HCV infections in the pediatric population, but transmission also occurs through unsafe medical interventions, especially in LMICs. Adolescents may acquire infection through injection drug use (IDU), and high-risk sexual practices especially among men who have sex with men (MSM). Although the occurrence of severe disease or cirrhosis in children is low at 2%, progression of liver disease can occur in childhood, and can impact quality of life. Early diagnosis can help timely access to treatment and prevention of long-term morbidity.
There are significant gaps in policies for HCV-infected children and adolescents. Many countries have no national guidance on HCV testing and treatment in children and adolescents. There is an urgent need for advocacy and updated policies and guidelines specific for children and adolescents. According to the joint recommendation by the AASLD and IDSA, children born to HCV infective mothers should be first checked with anti-HCV antibody at 18 mo followed by checking of HCV RNA at 3 years of age to confirm the diagnosis. DAAS are recommended in children aged 3 years and above[25].
Chronic HCV infection is a significant problem in patients with various types of cancer. The prevalence of chronic HCV infection among patients with cancer in the United States has been estimated to range from 1.5% to 10.6%, but this range may be an underestimate because many cancer centers do not routinely screen patients for HCV. The impact of chronic HCV infection on cancer management can be profound but can be mitigated through early diagnosis and treatment. Early diagnosis of HCV infection and virologic cure improve liver and cancer outcomes and survival of patients with various cancers. Chronic HCV is not a contraindication to any cancer regimens but can disrupt liver functions and eventually lead to fibrosis in those on such regimens. Increased HCV replication in cancer patients on immunosuppressive therapy is less common than in HBV. In the DAAs era, it is not acceptable to exclude patients with cancer and chronic HCV infection from oncology trials because of HCV alone; HCV-infected patients facing life-threatening malignancies should have access to investigational chemotherapy. In summary, overall benefits of DAAs in terms of virologic, hepatic, and oncologic outcomes far outweigh the risks of not treating this curable infection[25].
Transfusion-dependent patients (e.g. Thalassemia) are at a higher risk of acquiring blood borne infections even under conditions of safe transfusion. Since, HCV is one of the most common blood borne pathogen, HCV infection is highly prevalent in children with β-thalassemia major in many countries despite strict pre-transfusion blood testing. This should raise the attention to environmental and community acquired factors. Quality management to insure infection control in minor operative procedures and adding more sensitive tests for blood screening are recommended. Patients with acute HCV and thalassemia have low rates of spontaneous resolution of HCV infection, and the majority develop chronic HCV infection. DAAs combinations are associated with high SVR rates and low adverse events in treatment naïve and experienced patients with chronic HCV and thalassemia. Liver fibrosis is accelerated in thalassemia patients with chronic HCV; therefore, early diagnosis, treatment with DAAs, adequate iron chelation, and non-invasive monitoring of liver status are recommended to prevent development of cirrhosis and HCC[25].
Currently, the most common mode of transmission for HCV infection in the United States is through IDU; approximately 54% to 77% of new HCV diagnoses are among people who inject drugs (PWID). According to an estimate, 3.5 million people have injected drugs in the United States during their lifetime, with the prevalence of HCV infection in this population projected to be 73% (range 70%-77%). Because of the high probability of contracting HCV infection through needle-sharing, treating PWID infected with HCV, particularly in early stages of the disease, may reduce transmission. Treatment of people who inject drugs (PWIDs) is a top priority because of both the high burden of infection and the potential to transmit to others. The success of treating PWIDs is well established. In the recent SIMPLIFY trial, 103 persons with recent injection drug use (74% injected in the past month) received treatment with SOF-VEL for 12 wk and 94% achieved HCV cure with no virologic failures. Those with prior and current drug use, those on opiate substitution therapy (OST), and those not on OST had similar rates of cure with DAA therapy. Modeling of treatment in populations of PWIDs highlights the need for prevention measures concurrent with HCV treatment[25].
Global HCV prevalence in MSM varies by region and HIV status. Behavior counseling and regular HCV monitoring are needed in HIV-positive subgroups and high-risk regions. Given the upward trend of HCV incidence and sexual risk behaviors, there is also a continued need to reinforce risk-reduction intervention. Antiviral therapy along with counselling regarding the disease process regarding a high risk of disease recurrence in these patients is advised. Furthermore, these patients should also be told to incooperate measures that reduce the recurrence of HCV infection. Annual checking with HCV RNA is recommended in these patients who are sexually active[25].
According to the EASL guidelines of 2020, those patients who were treated with DAAs and had failed to achieve clearance, are advised to be treated via a multidisciplinary team and should undergo HCV RNA resistance testing before retreatment. EASL HCV guidelines 2020 also recommend that those patients without cirrhosis or with compensated cirrhosis who failed DAAs regimen should be retreated with SOF/VEL/VOX for a duration of 3 mo. Those who fail to achieve SVR even after treatment with SOF/VEL/VOX, should be administered therapy containing the SOF/GLE/PIB for a period of 24 wk with RBV. Those with decompensated CLD who fail DAAs and with contraindications to the use of DAAs are advised to be treated with SOF/VEL/RBV for 24 wk[25].
Patients with decompensated cirrhosis who have achieved an SVR after treatment with DAAs are still at high risk of developing HCC due to the advanced stage of cirrhosis. This is due to the oncogenic property of virus itself along with the interaction of viral with the host factors that cause liver cirrhosis to progress towards HCC[32]. This risk increases in obese patients, those co-infected with hepatitis B virus (HBV) and/or human immunodeficiency virus (HIV), type 2 diabetes mellitus and HCV genotype[4,32,33]. Kanwal et al[34] evaluated Veteran American HCV Clinical Case Registry and documented 80% higher risk of HCC with genotype 3 as compared to genotype 1. Lee et al[35] showed that genotype 6 has higher risk association in South Asian population.
On the contrary, various studies have documented that successful viral eradication, established by SVR, is associated with a decreased risk of HCC and decompensation events; hence, reduced HCV-related morbidity and mortality[36,37]. However, in the past, as PegIFN was contraindicated in decompensated cirrhosis, large cohorts of patients were deprived of treatment benefits[38,39].
Although, treatment with IFN was associated with a low cure rate and higher adverse effects, studies reported that achieving SVR reduced the risk of HCC to 0.5%-1% per annum[40]. The IHIT Study Group also documented decrease in the incidence of HCC in patients with chronic HCV infection treated with IFN and who achieved SVR and biochemical response[41]. Similar response was also documented by Hsu et al[42] in Taiwanese population. Moreover, a few studies also demonstrated beneficial response of IFN therapy in patients with cirrhosis and advanced fibrosis but with a lower response[37,43,44].
Although, the favorable response to oral DAAs revolutionized the treatment armamentarium, this development was marred by reports of higher incidence of HCC, HBV reactivation and drug-drug interactions[45-47]. Kanwal et al[34] reported cumulative incidence of HCC at 2.8% while Tani et al[48] documented 6% at 3 years following treatment with DAAs. However, other studies negated these results. A large prospective observational French cohort documented association of DAAs therapy with decreased all-cause mortality and HCC[49]. Delgado Martínez et al[50] reported lower incidence of HCC with DAAs as compared to untreated patients (2.90 and 4.48 per 100 person-years, respectively). Moreover, Romano et al[51] reported declining trend in the incidence of HCC among HCV-related cirrhotics treated with DAAs.
A meta-analysis of 41 studies compared recurrence of HCC in patients treated with either DAAs or IFN therapy. The authors demonstrated similar rate of HCC recurrence in patients treated with IFN and DAAs’ studies (9.21/100 per year vs 12.16/100 per year), respectively[52]. However, Reig et al[53] reported higher recurrence of HCC (27.6%) in 5.7 mo of follow-up in 103 DAAs treated patients. Even though, Cabibbo et al[54] in a multicenter Italian study reported higher incidence of HCC recurrence in DAAs treated population but concluded that risk is comparable to untreated population. In a recent review by Muzica et al[40] the author concluded that incidence of both HCC occurrence and recurrence is significantly reduced by achieving SVR with DAAs.
To summarize, a vast majority of studies support the use of DAAs therapy in patients with advanced liver disease and successfully treated HCC. Whether or not to treat patients with uncured HCC with DAAs is an issue, which still needs to be studied.
The encouraging response of DAAs in decompensated cirrhosis was refuted by detrimental reports of HBV reactivation. In 2016, FDA had issued black box warning of HBV reactivation based on multiple reports including liver failure and deaths[55]. In HBV-HCV co-infected patients, both HBV and HCV have reciprocal inhibition on each other[56]. In the IFN era, HBV reactivation was a rare occurrence due to its dual antiviral effects on both viruses[57]. In the DAAs era, the reported prevalence of HBV reactivation ranges from 2%-57% in HBsAg-positive while 0%-3% in HBsAg-negative or anti-HBc-positive patients when treated with DAAs[58]. Thus, the pharmacological suppression of HCV with DAAs curbs the inhibitory effect on HBV genome and thus may lead to HBV reactivation[56].
Recently, Mücke et al[59] in a meta-analysis reported 24% and 1.4% risk of HBV reactivation in patients with chronic HBV and HBc-total positive patients, respectively. The authors also highlighted that the risk of reactivation was not related to nadir HBV DNA levels or severity of liver disease. Thus, frequent testing and monitoring is required in this population.
AASLD recommends that patients fulfilling treatment criteria for HBV should be treated in patients with co-infection. On the other hand, patients with a low or undetectable HBV DNA levels, can either be treated prophylactically or monitored regularly. Thus, all HCV patients to be treated with DAAs should be tested for HBsAg, anti-HBc total and anti-HBs titer prior to start of DAAs[4].
The kidney is involved in chronic HCV infection due to immune complex deposition. Epidemiological studies have documented a high prevalence of chronic kidney disease (CKD) in HCV positive patients[60].
Fabrizi et al[61] in a systematic review and meta-analysis documented increased risk of proteinuria and CKD in patients with positive HCV serology. Mendizabal et al[62] retrospectively evaluated large database of HCV infected and non-infected population. The authors documented 27% increased risk of CKD in HCV infected compared to non-infected population. Moreover, the risk of CKD reduced by 30% with effective antiviral therapy. Studies have documented rapid renal deterioration from CKD to end-stage renal disease (ESRD). Thus, there is a high rate of morbidity and mortality in HCV-infected CKD patients[63-65].
On the other hand, dialysis patients are at increased risk of acquiring the virus due to poor hygiene, increased risk of nosocomial infection and lack of proper sterilization techniques along with improper handling of equipment. Hence, testing all dialysis patients at entry and periodically thereafter is recommended[66,67]. However, there is some controversy on the type of testing (serology or NAT) in these patients[68]. These patients have higher prevalence of HCV infection, higher risk of HCC and cirrhosis, and lower survival than the general population[69]. Therefore, withholding HCV treatment till renal transplantation would be detrimental.
During the early era of DAAs, SOF was not recommended in patients with eGFR < 30 mL/min due to the fear that these patients accumulate SOF and its metabolites[70,71].To address this issue, various authors have experimented with SOF dosage in these patients[72,73] Bhamidimarri et al[74] documented virological response with daily 200 mg vs alternate 400 mg SOF dose in patients with ≤ 30 mL/min per 1.73 m2 of eGFR. However, we documented 96.9% SVR12 in 133 hemodialysis patients treated with SOF-based regimen at daily dose of 400 mg[75]. Moreover, Shehadeh et al[76] in a systemic review and meta-analysis reported no statistically significant differences in SVR12 rates amongst dialysis patients treated with 400 mg daily, 400 mg on alternate days or 200 mg daily SOF dose.
A meta-analysis of patients with CKD stage 4 and 5 documented cumulative SVR12 of 89.4% on treatment with SOF-based regimen[77-79]. Borgia et al[80] reported SVR12 of 95% among 59 dialysis-dependent patients treated with SOF-VEL combination. In 2019, considering the published safety and efficacy of SOF in advanced CKD patients, Food and Drug Administration (FDA) permitted the use of SOF-based therapy in patients with eGFR ≤ 30 mL/min including dialysis. AASLD also recommended that no dose adjustment is required in this population[4].
Elbasvir/grazoprevir (EBR-GZR) combination was the first to be approved for hemodialysis patients with HCV infection[81]. The efficacy of EBR-GZR for 12 wk was demonstrated in C-SURFER trial in genotype 1 with CKD stage 4 or 5 (eGFR < 30 mL/min). In the immediate treatment group, SVR12 was achieved in 99.1%[82]. The deferred group population were prescribed EBR/GZR combination after 16 wk of trial inclusion (n = 99). The authors documented 98% SVR 12 in this group[82]. The study by Bruchfeld et al[83] re-inforced the safety and efficacy of EBR/GZR combination in stage 4-5 CKD with HCV genotype 1 infection. AASLD recommends EBR/GZR in genotype 4 infection in stage 4/5 CKD considering encouraging response in general population[4].
EXPEDITION-4 trial assessed pan-genotypic fixed dose combination of GLE-PIB (100/20 mg) in non-cirrhotic stage 4/5 CKD patients. The trial included HCV genotypes 1 to 4 and also treatment-naïve and experienced population. In a total of 104 patients, SVR 12 was achieved in 98% with no virological failures[84]. Subsequently, EXPEDITION-5, a phase 3 trial, evaluated the same fixed dose combination in stage 3b, 4, or 5 CKD in compensated cirrhotic and non-cirrhotic populations. The overall SVR12 was achieved in 97% of the study population[85]. Although trial reported 5% non-serious side effects, Harrison et al[86] reported a case of drug- drug interaction with colchicine. Despite 50% reduction of colchicine dose, patient with stage 4 CKD developed rhabdomyolysis and acute kidney injury (AKI) with GLE-PIB combination. The authors recommended withholding colchicine during treatment with NS5A inhibitor containing DAAs, specifically with renal dysfunction. Similarly, Patel et al[87] also reported rhabdomyolysis in stage 3 CKD patients secondary to interaction with SOF/LDV, atorvastatin and colchicine use.
In summary, various novel DAAs are highly effective and safe in CKD population. However, drug- drug interactions should be considered in case of use of NS5A inhibitor containing DAAs with P-glycoprotein (P-gp) inducers.
Although, HCV can lead to tubulointerstitial nephritis, it is the HCV-associated glomerular disease that is more frequently encountered[81,88]. Nonetheless, its incidence remains fairly low. Other than immune complex deposition in glomeruli, Toll-like receptor 3 has also been postulated to cause renal injury in HCV-infected population[60,89].
Various histological types of HCV-associated renal diseases include cryoglobulinemic membranoproliferative glomerulonephritis (MPGN), mesangial proliferative GN, focal segmental glomerulosclerosis, membranous nephropathy, etc[88]. However, the most frequent glomerulopathy is Type I MPGN associated with type II mixed cryoglobulinemia (MC)[60,89]. Around 20% to 56% of patients with HCV-associated MC type II may develop renal involvement[60]. The clinical presentation may vary and nephrotic syndrome, acute nephritic syndrome and oliguric acute renal failure have been reported in 20%, 30% and 5% of patients, respectively[90,91]. In pre-DAAs era, HCV-associated glomerulopathies were treated with IFN. A systematic review and meta-analysis reviewed response of conventional or PegIFN for HCV-associated MC. The kidney involvement was documented in 11%-74% in analyzed 11 studies. The authors reported excellent association of virological response with clinical remission in majority of patients[92]. Similarly, another meta-analysis documented association of virological and clinical response in patients treated with combination of PegIFN and RBV therapy in HCV-infected MC. The kidney involvement in study population ranged from 4% to 39%[93]. Other studies have reported lesser efficacy and more side effects with IFN-based treatment in HCV-MC as compared to HCV-infected general population[94,95]. Moreover, even with > 70% remission with IFN-based therapy in MC-induced vasculitis, the associated adverse effects discourage it use in this population[96].
Although limited, a few studies have documented good response with DAAs. It has also been observed that clinical and immunological response may not correspond to SVR[97-99]. Fabrizi et al[88], reviewed 9 clinical studies (n = 67) and documented 92% SVR with DAAs though cumulative complete clinical response was low i.e., 38.5%. Furthermore, few case reports have documented new-onset or relapsing glomerular diseases even in patients who achieved SVR with DAAs[100,101].
In view of satisfactory efficacy and lesser side effects, DAAs are advised for viral eradication in patients with HCV-associated MC[88]. Treatment is based on severity of disease involvement. In patients with mild to moderate form of disease (GFR > 30 mL/min/1.73 m2 (with or without non-nephrotic proteinuria), DAAs are the first line of treatment. However, immunosuppressive agents (IS) are advised for non-responsive cases or drug intolerance. In patients with cryoglobulinemic flare or severe glomerular injury, IS agents (rituximab) are in the initial treatment algorithm with or without plasma exchange. The resolution of acute phase is followed by HCV treatment with DAAs. However, IS agents and DAAs can be prescribed as per clinicians’ discretion[88].
In summary, HCV can be found in 85%-95% of patients with MC[102]. However, only 10%-15% will have clinical manifestations including glomerulopathy[103]. Thus, KDIGO Clinical Practice Guidelines recommend annual evaluation for proteinuria, hematuria and eGFR in HCV-infected population with or without renal dysfunction especially with cryoglobulinemia[104]. The mainstay treatment still remains HCV eradication. Studies have documented encouraging response with DAAs, which, albeit, may lead to partial response[88].
Apart from having a favorable response in the decompensated liver disease, the novel DAAs have led to a paradigm shift in the management of HCV-related disease in the post-transplant setting. In this section, we will highlight the important landmark studies and trials for the treatment of HCV in the solid organ transplant recipients.
During the IFN era, majority of patients with end-stage liver disease were deprived of therapy due to its deleterious side effects or contraindications[105,106]. Hence, HCV positive liver transplant recipients experienced universal liver graft reinfection; consequently leading to poor outcome[107]. However, attainment of SVR post liver transplant was associated with improved survival[108,109].
This scenario has been altered with the advent of DAAs. Cholankeril et al[110] in a retrospective study documented 91.9% and 89.8%, one year survival in HCV positive liver recipient transplanted in DAA era vs pre-DAA era, respectively. Similarly , Cotter et al[111] in a prospectively collected cohort of 18,746 documented statistically significant improved 1 and 3 year post transplant survival in HCV positive recipients in DAA era as compared to past. Among various factors, viral genotype is an important determinant of SVR in post liver transplant recipients[112,113]. Campos-Varela et al[112] reported higher risk of advanced fibrosis and lower rate of SVR with PegIFN-based treatment in genotype 1 infected liver transplant recipients. Moreover, the authors also reported statistically significant association of HCV genotypes 2 and 3 with SVR as compared to genotype 1. Similarly, Chen et al[114] concurred that HCV genotype 1 was less likely to achieve SVR than non- genotype 1 infection. Zanaga et al[115] reported higher SVR with genotype 3 and statistically significant association with SVR on a univariate analysis in post-liver transplant population. A systematic review and a meta-analysis reported pooled SVR12 of 90% with simeprevir (SMV) and SOF combination with or without RBV in recurrent genotype 1 in post liver transplant population. However, interaction with Cyclosporine immunosuppression was also documented[116].
SOF, combined with RBV, was used in 40 liver transplant recipients of all genotypes, and achieved an SVR12 rate of 70% in the study population with no graft loss or rejection. However, no genotype-specific response was documented[117]. Subsequently, the phase 3 ALLY-1 trial documented response of SOF and DAC combination with RBV for 12 wk in liver transplant population with recurrent HCV. Although the study population included treatment-experienced recipients, trial documented 95% and 91% SVR12 in patients with genotype 1 and 3 infections, respectively[118].
SOLAR 1, a phase 2 open label study that was conducted in USA, evaluated the response of LDV and SOF with RBV in 223 liver transplant recipients with HCV genotypes 1 and 4 infections. The study participants were randomly assigned 12 and 24 wk of treatment and achieved SVR12 in 96% and 98%, respectively, without cirrhosis. Moreover, lower rate of SVR12 was achieved in participants with CTP B and CTP C cirrhosis[119]. Similarly, SOLAR 2 trial conducted in Europe, Australia, Canada, and New Zealand also reported higher SVR rate of 93% and 100% in post liver transplant non-cirrhotic recipients treated with LDV/SOF combination with RBV for 12 and 24 wk, respectively. However, amongst recipients with CTP class C cirrhosis, SVR was higher with 24-wk treatment[120].
The use of the first pan-genotypic oral agent, SOF/VEL combination for 12 wk was evaluated in liver transplant recipients with genotypes 1 to 4[121-123]. Considering the beneficial effect of SOF/VEL in decompensated cirrhosis, AASLD, recommends SOF/VEL with RBV combination in liver transplant recipient with decompensated cirrhosis for 12 or 24 wk. Extended treatment is considered for recipients with treatment-experienced genotype 3 infection and presence of HCC[4].
Another pan-genotypic fixed dose single pill combination of GLE-PIB (300/120 mg) is also recommended in transplant recipients[4]. MAGELLAN-2 trial evaluated 100 non-cirrhotic post-transplant patients with or without treatment experience. In intention-to-treat analysis, liver and kidney transplant recipients achieved 97.5% and 100% SVR12, respectively. Although, minor reduction in tacrolimus was required in 1st week but the median dose of cyclosporine, everolimus or sirolimus, remained unchanged[124]. Similarly, SVR12 of 98% with 8 wk or 12 wk of GLE/PIB combination was observed in a multicenter trial of 24 liver transplant patients. Study population also included prior DAAs experience, severe renal impairment, hemodialysis and post-liver transplant jaundice[125].
Although, DAAs are safe and highly efficacious in treatment-naïve and experienced recipients, but in general 5% of the population fails to achieve SVR. This is mostly encountered in recipients with associated decompensated cirrhosis or HCC[126]. Despite lack of published data, on the basis of expert consensus, AASLD recommends SOF/VEL/VOX in patients with DAAs experienced post liver transplant patients[4]. Cardona-Gonzalez et al[127] reported successful treatment of recurrent genotype 3 in liver transplant recipients with SOF/VEL/VOX combination in DAAs experienced individuals. Similarly, Higley et al[126] recently published a case series of six HCV liver transplant recipients with DAAs failure. The authors documented successful HCV eradication with 12 wk of treatment with no adverse effect or virological relapse during study period.
EASL recommends to initiate DAAs as early as possible after liver transplant once the recipient’s clinical condition is stabilized. Generally, it is advised to start treatment after 3 mo of transplant. However, exact time frame for starting DAAs in non-hepatic solid organ transplants has not being recommended. We believe that with widespread availability of new DAAs in pre-transplant period, there will be an increase in the number of DAAs-experienced and/or treatment failure patients among transplant recipients. Thus, pan-genotypic, efficacious and safe salvage therapy is warranted in these special scenarios.
With the increasing availability of liver transplant facilities, the growing demand of donor organs has yet to be met worldwide. Historically, HCV positive donors were only accepted for transplantation in recipients with dire complications like fulminant hepatic failure[128]. However, the recurrence of HCV infection and associated morbidity and mortality were added risks. With the advancements in DAAs, the question of utilizing HCV positive donors was addressed by multiple studies[129]. As compared to renal transplants, data on PCR positive donors to PCR negative liver recipients is limited. However, Cholankeril et al[130] and Cotter et al[131] reviewed the OPTN registry from 2015 to 2020 and reported comparable 1 and 2-year post transplant survival of patients transplanted with HCV viremic organs, in NAT negative recipients. Bethea et al[132] reported 100% SVR in 10 liver recipients who received NAT positive donors treated with 12 wk of GLE/PIB combination. Nonetheless, one recipient developed acute cellular rejection. In a real world experience, Jandovitz et al[133] also reported beneficial response of GLE/PIB combination in three HCV negative liver transplant recipients.
HCV-infected renal transplant recipients (RTRs) have a higher survival as compared to being on waiting list despite the complications[134-136]. However, HCV-infected RTRs have reduced graft and patient survival compared to non-infected counterparts. Fabrizi et al[137] documented the presence of anti-HCV antibody as a prognostic factor for patient and allograft survival in RTRs with relative risk of 1.79 (95%CI: 1.57-2.03) and 1.56 (95%CI: 1.35-1.80), respectively. An observational meta-analysis reported higher rate of liver- and cardiovascular-related mortality[138]. Although HCV in RTRs leads to slow progression to chronic liver disease (CLD), the increased risk of HCC cannot be disregarded. Long-term immunosuppression is possible culprit to accelerated liver fibrosis; thus, leading to cirrhosis and HCC[139]. Zylberberg et al[140] reported significantly higher yearly progression of hepatic necroinflammation and fibrosis in HCV infected as compared to non-infected recipients. Moreover, HCV in RTRs also increases the incidence of infection, glomerulopathy, vasculitis and post-transplant diabetes mellitus[141-144].
In the past, IFN was contraindicated in RTRs due to inferior virological response, low tolerance and increased risk of graft rejection[137,145]. A meta-analysis reported 18% SVR and drop-out rate of 35% in recipients treated with IFN-based regimen[146]. Occasional studies, mostly case reports, documented beneficial response of IFN in renal transplant population[147,148]. Early in DAAs era, SOF was not recommended in patients with eGFR < 30 mL/min due to possible accumulation of SOF and its metabolites causing renal dysfunction[71]. However, various studies have reported favorable response of SOF with RBV in RTRs[149,150]. We have observed 89.2% end-of-treatment response (ETR) and 100% SVR12 in our renal transplant population treated exclusively with SOF and RBV combination. Moreover, we also reported resolution of liver-related ascites in two out of four decompensated recipients[151].
Multiple studies reported 90% to 100% SVR 12 in recipients treated with 2 different class of DAAs[152,153-155]. We also treated our 79 treatment-naive and treatment-experienced RTRs. Majority received SOF and RBV (78.5%) while remaining received SOF, DAC and RBV combination. ETR and SVR12 were achieved in 98.7% and 96.2%, respectively[151] Coral-1 study evaluated liver and RTRs. The study population including 12 non-cirrhotic RTRs received PrOD with and without RBV in genotype 1a and genotype 1b, respectively. RTRs achieved lower SVR of 75% with premature treatment discontinuation as compared to liver transplant recipients[156]. However, Scott et al[157] in a multicenter randomized trial documented 98% SVR 12 in 114 RTRs with genotypes 1 and 4, treated with SOF-LDV for 12 or 24 wk.
A multicenter, prospective observational trial, HCV-TARGET, demonstrated efficacy and safety of SOF-based regimen in transplant population. The cohort included 347, 60 and 50 Liver transplant, kidney transplant and dual liver kidney transplant recipients, respectively. The regimen included SOF-LDV, SOF-DAC and PrOD with or without RBV. In RTRs, trial reported 94.5% SVR12 and acute rejection in two recipients[158]. MAGELLAN-II trial documented safety and efficacy of GLE/GDP combination in liver and kidney transplant population. The population included 20 RTRs with genotypes 1, 3 and 4, among which four were treatment-experienced with IFN-based regimen. The study documented 100% SVR12 with no virological relapse[124]. Long-term follow-up documented by Zhang et al[159] reported no virological relapse at 24 and 96 wk post-treatment in eight RTRs treated with SOF-based regimen.
One of the major apprehensions for use of DAAs in transplant population was the drug- drug interaction, thus leading to graft rejection. In a Spanish renal transplant registry, 55.3% of study population required immunosuppression adjustment. Although renal function remained stable during treatment, 2.9% developed acute allograft rejection[160]. Similarly, Scott et al[157] reported immunosuppressive dose alteration in 18% of RTRs treated with SOF-LDV combination. Özer Etik et al[149] reported 100% SVR in RTRs but 45% of transplant recipients required increased dose of calcineurin inhibitors. The authors attributed the increased requirement to improved liver function; thus, enhanced drug metabolism. However, various studies did not report immunosuppressive dose modification with SOF-based regimen. AASLD latest guidance recommends not to co-administer cyclosporine with EBR-GZR combination or with GLE/PIB combination. However, Tacrolimus level may need to be adjusted with GLE-PIB combination[4].
Although, multiple studies have documented effective HCV eradication, no graft rejection and stable renal function during and after DAAs therapy[161,162]. Other authors have documented worsening proteinuria in transplant recipients with higher pretreatment levels[155]. Thus, although DAAs are efficacious and safe in RTRs but caution should be practiced with monitoring of calcineurin inhibitor levels, renal functions and proteinuria.
To counteract the shortage of kidney donors, researches have focused on utilizing HCV positive kidneys in HCV negative RTRs[141]. Even with favorable results, the American Transplant Society (ATS) and KDIGO recommend that HCV infected organs can be transplanted into HCV NAT negative recipients as a research protocol only with an informed consent and approval from ethical committee[163,164]. However, KDIGO guidelines recommend HCV NAT-positive kidney to be transplanted to NAT positive recipients with the aim to decrease the organ wastage. Nevertheless, liver fibrosis stage and availability of effective DAAs prior to transplantation should be ensured[163].
Prior to 2000, various studies suggested increased incidence of hepatitis and subsequently poor graft survival in anti-HCV positive kidney recipients transplanted with anti-HCV positive organs[165,166]. Moreover, negative recipients receiving positive donors were associated with higher liver-related complications[167,168]. The effective response to DAAs in post-transplant period had led to address the issue of discarded HCV-positive organs.
To expand the donor pool, researchers have reported response of HCV viremic donors to HCV-negative recipients. The first prospective trial, THINKER-1 in 2017 followed by THINKER-2 in 2018 reported 100% SVR in aviremic RTRs who received NAT-positive organs. All the recipients received EBR/GZR combination for 12 or 16 wk depending on NS5A resistance-associated substitutions (RASs). Although among a total of 20 recipients, two developed proteinuria due to FSGS but all achieved SVR12[169,170]. Moreover, EXPANDER trial evaluated preemptive treatment regimen in NAT-positive donors to aviremic recipients. All recipients received one dose of EBR/GZR followed by EBR/GZR × 12 wk with or without SOF depending on genotype. In total, 30% of the study population had detectable viremia post-transplant. However, all achieved SVR12[171]. La Hoz et al[172] documented no statistically significant difference in graft survival and acute cellular rejection (ACR) in aviremic recipients receiving HCV-positive or HCV negative kidney.
Recently, Jandovitz et al[133], in a single center prospective study, evaluated the response of GLE-PIB, SOF-LDV and SOF-VEL in 64 RTRs with positive donors and negative recipients. The author reported 95% detectable viremia post-transplant with SVR12 in 41/58 recipients. The result of 17 recipients was awaited at the time of publication. Moreover, two patients developed fibrosing cholestatic hepatitis (FCH), which was successfully treated with DAAs. The study documented 98% patient and graft survival.
In the pre-DAAs era, despite the fact that heart and lung tissue are not reservoirs for HCV, utilization of HCV-positive organs was controversial. Studies have reported lower patient survival as compared to recipients with aviremic donors[173,174]. However, the benefits of procedure outweigh the morbidities associated with no transplants in this special group[128].
Abdelbasit et al[175] reported first case series of five lung recipients transplanted with viremic lungs. The recipients responded to SOF-based regimen with 100% SVR 12 and 100% patient survival 12 mo after transplant. Recently, Cypel et al[176] compared ex vivo lung perfusion with or without ultraviolet C radiation (UVC) in 22 NAT-negative lung recipient transplanted with positive donors. In 20 recipients with detectable viremia, 96% achieved SVR12 with SOF/VEL combination. The relapse in two patients including one with FCH was successfully treated with SOF-VEL-VOX and RBV combination.
Similarly, various studies reported beneficial response of DAAs in heart transplant recipients with NAT-positive donors with > 90% SVR12[133,177,178]. Kilic et al[179] and Reyentovich et al[180] reported no statistically significant differences in 1-year survival in heart recipients transplanted with viremic or aviremic donors. To increase the donor pool, studies have evaluated transplantation of positive donors in NAT-negative recipients, also called HCV aviremic recipients. Bethea et al[132] evaluated 20 HCV non-viremic heart recipients treated with first dose of GLE-PIB combination prior to transplantation followed by 8 wk of therapy after transplantation. The author reported 100% SVR12 and 100% graft and patient survival for median of 10.7 mo.
Schlendorf et al[181] reported favorable response of SOF-VEL and SOF-LDV in 11 HCV aviremic heart recipients transplanted with HCV-viremic donors. Out of which, nine developed post-transplant viremia, among which eight successfully achieved SVR12. Remaining one recipient was under treatment at the time of publication. Similarly, DONATE HCV trial evaluated the response of four weeks of SOF-VEL combination, started within few hours of transplantation, with NAT-positive donors. The trial included 44 HCV aviremic recipients; 36 underwent lung transplants while eight received heart transplants. Till the time of study publication, 35 recipients achieved SVR12 and reported excellent graft survival at follow-up of six mo[182].
To decrease the risk of infection transmission, trials have been focused on preemptive and shortened DAAs course. Feld et al[183] suggested shortest pan-genotypic DAA course in recipients receiving viremic donors. The authors evaluated 30 HCV NAT-negative recipients who received viremic organs which included; 6 hearts, 13 lungs, 10 kidneys and one dual kidney-pancreas. All recipients received one dose of ezetimibe and GLE/PIB followed by only 7 d of treatment course. All recipients achieved SVR12 with genotypes 1-3.
Although, favorable short-term outcomes has been reported for HCV NAT positive and NAT negative donors, the long-term effects of the virus, the infected organs and drug interaction are not known. Hence, during consideration of accepting a HCV viremic donor, the risk of HCV complications including FCH and HCC, insurance policy and availability of pan-genotypic DAAs should be addressed in the informed consent.
Fibrosing Cholestatic Hepatitis (FCH), a dreaded complication of HCV recurrence, has been described in liver[184,185], renal[186] and heart[187] transplant recipients. It is seen in around 2%–15% of liver transplant recipients and causes significant morbidity and mortality[188,189]. This rapidly progressive disease is characterized by cholestatic jaundice with a high HCV viral load[190,191]. A low threshold of suspicion along with histopathological diagnosis is needed for its prompt management. In pre-DAAs era, despite contraindication in RTRs, KDIGO had recommended IFN in FCH considering the risk-to-benefit ratio. However, this treatment was associated with a low tolerance rate and a poor outcome[141]. The standard of care in transplant population is reduction or withholding immunosuppression followed by anti-viral therapy. Historically treatment with IFN was associated with lower success rate and higher side effects[191,192].
Xue et al[193] reported 80% SVR 12 in 10 transplant recipients treated with SOF/RBV combination with PegIFN. The SOLAR 1 and SOLAR 2 trials reported 100% SVR 12 in 6 and 5 transplant recipients, respectively with FCH treated with SOF-LDV and RBV combination[119,120]. Cypel et al[176] reported successful treatment of FCH with SOF-VEL-VOX and RBV combination in a lung transplant recipient. Leroy et al[194] documented 96% SVR12 in 23 Liver transplant recipients treated with either SOF/RBV or SOF/DAC combination. Moreover, 4 recipients in this study population had concomitant HIV infection. Shinzato et al[195] reported a case of post renal transplant FCH treated with GLE/PIB combination. The patient expired due to progressive hepatic failure despite decreased HCV viral load. Jandovitz et al[133] reported successful treatment with DAAs in 2 aviremic renal recipients transplanted with HCV-positive donors. Hence, it is proven that the use of DAAs can be beneficial in FCH in post-solid organ transplant recipients.
From a low virological response to an almost curative treatment for all genotypes, therapy for HCV has evolved markedly in recent years. However, the greatest challenge is yet to be overcome, that is the availability of treatment for everyone. The WHO aims to eliminate viral hepatitis including HCV by 2030 since its poses a major public health threat. In order to implement this, various strategies have been devised to reduce the incidence of viral hepatitis by 90% and decrease liver-related mortality due to these viruses by 65%. To achieve this target, WHO has enlisted five core interventions that need to be focused by all countries globally. These interventions includes vaccination for HBV, prevention of HBV transmission from mother to child, use of screened blood products and safe use of injections, harm reduction in drug users, testing and treatment of HBV and HCV[196]. Despite WHO’s support, only a few countries have been able to develop an effective hepatitis control program while even fewer are currently on track to achieve the elimination goal[197]. Egypt, with highest prevalence of chronic HCV infection in the world few years back, conducted a successful HCV screening program that covered more than 50 million people and treated more than 4 million. It is poised to be the first country in the world to eliminate HCV within its borders. The lessons learned from this experience can inform the elimination plans of other LMICs with high HCV burden[198].
Interestingly, DAAs with their high efficacy and short duration of therapy have provided hope on achieving this target but a higher cost of therapy, lack of insurance coverage and un-availability of therapy in many LMICs have become a major obstacle[199]. Some LMICs have heavily subsidized the DAAs for achieving the ambitious goals of HCV elimination. In addition to this, in several countries, effective diagnostic facilities are expensive. Other challenges include inadequate surveillance data, limited coverage of preventive programs and lack of focused leadership to combat HCV menace. However, the major obstacle seen globally is the lack of financial support in hepatitis programs[200,201].
Therefore, there is an urgent need to strengthen the healthcare system and develop a national plan against hepatitis in low-, middle- and even high-income countries. Moreover, support from civil societies, pharmaceutical and medical companies is also required to help the governments of various countries to combat this deadly disease.
Significant advances have been made in the fields of diagnostics and therapeutics for optimal management of chronic HCV infection. However, the disease still remains a formidable challenge for all stakeholders, particularly in developing countries. Many hurdles remain to be tackled before the disease is eliminated as envisaged by WHO’s goal of eradication of hepatitis by 2030. Concerted and focused global efforts are needed to tackle and eliminate this silent killer effectively.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iannolo G, Italy; Kumar A, India; Mogahed EA, Egypt; Mukhopadhyay A, India; Nie XQ, China; Papadopoulos N, Greece S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (20)] |
| 2. | Warkad SD, Song KS, Pal D, Nimse SB. Developments in the HCV Screening Technologies Based on the Detection of Antigens and Antibodies. Sensors (Basel). 2019;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Jafri SM, Gordon SC. Epidemiology of Hepatitis C. Clin Liver Dis (Hoboken). 2018;12:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti Infect Ther. 2018;16:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Hu W, Zhang C, Shi JJ, Zhang JY, Wang FS. Hepatitis C: milestones from discovery to clinical cure. Mil Med Res. 2020;7:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Applegate TL, Fajardo E, Sacks JA. Hepatitis C Virus Diagnosis and the Holy Grail. Infect Dis Clin North Am. 2018;32:425-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Bryan-Marrugo OL, Ramos-Jiménez J, Barrera-Saldaña H, Rojas-Martínez A, Vidaltamayo R, Rivas-Estilla AM. History and progress of antiviral drugs: from acyclovir to direct-acting antiviral agents (DAAs) for Hepatitis C. Medicina universitari. 2015;17:165-174. |
| 8. | Laugi H. Discovery of Hepatitis C Virus: 2020 Nobel Prize in Medicine. Euroasian J Hepatogastroenterol. 2020;10:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Basyte-Bacevice V, Kupcinskas J. Evolution and Revolution of Hepatitis C Management: From Non-A, Non-B Hepatitis Toward Global Elimination. Dig Dis. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 10. | Houghton M. Discovery of the hepatitis C virus. Liver Int. 2009;29 Suppl 1:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 11. | Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M, Waggoner JG, Park Y, Jones EA. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 646] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Yu ML, Chuang WL. Path from the discovery to the elimination of hepatitis C virus: Honoring the winners of the Nobel Prize in Physiology or Medicine 2020. Kaohsiung J Med Sci. 2021;37:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Umar M, Khan AG, Abbas Z, Arora S, Asifabbas N, Elewaut A, Esmat G, Foster G, Fried M, Goh KL, Hamama TB, Imawari M, Isakov V, Krabshuis J, LaBrecque D, Lemair A, Malfertheiner P, Ryder S, Schiedermaier P, Stimac D, Tandon R, Villamil F, Zapata R, Ferenci P; World Gastroenterology Organisation. World Gastroenterology Organisation global guidelines: diagnosis, management and prevention of hepatitis C April 2013. J Clin Gastroenterol. 2014;48:204-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Yau AH, Yoshida EM. Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review. Can J Gastroenterol Hepatol. 2014;28:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 16. | Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J Transl Int Med. 2017;5:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | Li G, De Clercq E. Current therapy for chronic hepatitis C: The role of direct-acting antivirals. Antiviral Res. 2017;142:83-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 19. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 20. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 21. | Sundaram V, Kowdley KV. Dual daclatasvir and sofosbuvir for treatment of genotype 3 chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2016;10:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA; ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 23. | Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, Pol S, Stuart K, Tse E, McPhee F, Bhore R, Jimenez-Exposito MJ, Thompson AJ. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology. 2016;63:1430-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 25. | AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. [accessed 3rd February 2022]. Available from: http://www.hcvguidelines.org. |
| 26. | Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton M; ASTRAL-4 Investigators. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 27. | Dai CY, Chuang WL, Yu ML. EASL recommendations on treatment of hepatitis C: Final update of the series - Some issues. J Hepatol. 2021;74:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | McCaughan GW, Thwaites PA, Roberts SK, Strasser SI, Mitchell J, Morales B, Mason S, Gow P, Wigg A, Tallis C, Jeffrey G, George J, Thompson AJ, Parker FC, Angus PW; Australian Liver Association Clinical Research Network. Sofosbuvir and daclatasvir therapy in patients with hepatitis C-related advanced decompensated liver disease (MELD ≥ 15). Aliment Pharmacol Ther. 2018;47:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Gentile I, Scotto R, Coppola C, Staiano L, Amoruso DC, De Simone T, Portunato F, De Pascalis S, Martini S, Macera M, Viceconte G, Tosone G, Buonomo AR, Borgia G, Coppola N. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity-LINA cohort). Hepatol Int. 2019;13:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Bou Daher H, Sharara AI. Treatment of Chronic HCV Infection in Patients With Thalassemia. Clin Liver Dis (Hoboken). 2019;14:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Vaziri A, Gimson A, Agarwal K, Aldersley M, Bathgate A, MacDonald D, McPherson S, Mutimer D, Gelson W. Liver transplant listing for hepatitis C-associated cirrhosis and hepatocellular carcinoma has fallen in the United Kingdom since the introduction of direct-acting antiviral therapy. J Viral Hepat. 2019;26:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Axley P, Ahmed Z, Ravi S, Singal AK. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. J Clin Transl Hepatol. 2018;6:79-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr. 2015;68:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Lee MH, Hsiao TI, Subramaniam SR, Le AK, Vu VD, Trinh HN, Zhang J, Jin M, Wong VW, Wong GL, Nguyen MH. HCV Genotype 6 Increased the Risk for Hepatocellular Carcinoma Among Asian Patients With Liver Cirrhosis. Am J Gastroenterol. 2017;112:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, Macías J, Merino D, Márquez M, Ríos-Villegas MJ, Gea I, Merchante N, Rivero A, Torres-Cornejo A, Pineda JA; Grupo Andaluz para el Estudio de las Hepatitis Víricas de la Sociedad Andaluza de Enfermedades Infecciosas. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 653] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 38. | Gurusamy KS, Tsochatzis E, Toon CD, Davidson BR, Burroughs AK. Antiviral prophylaxis for the prevention of chronic hepatitis C virus in patients undergoing liver transplantation. Cochrane Database Syst Rev. 2013;CD006573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 40. | Muzica CM, Stanciu C, Huiban L, Singeap AM, Sfarti C, Zenovia S, Cojocariu C, Trifan A. Hepatocellular carcinoma after direct-acting antiviral hepatitis C virus therapy: A debate near the end. World J Gastroenterol. 2020;26:6770-6781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 780] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 42. | Hsu CS, Huang CJ, Kao JH, Lin HH, Chao YC, Fan YC, Tsai PS. Interferon-based therapy decreases risks of hepatocellular carcinoma and complications of cirrhosis in chronic hepatitis C patients. PLoS One. 2013;8:e70458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Morisco F, Granata R, Stroffolini T, Guarino M, Donnarumma L, Gaeta L, Loperto I, Gentile I, Auriemma F, Caporaso N. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19:2793-2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Poynard T, Massard J, Rudler M, Varaud A, Lebray P, Moussalli J, Munteanu M, Ngo Y, Thabut D, Benhamou Y, Ratziu V. Impact of interferon-alpha treatment on liver fibrosis in patients with chronic hepatitis B: an overview of published trials. Gastroenterol Clin Biol. 2009;33:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, Lin YH, Liang PC, Hsieh MY, Lin ZY, Chen SC, Huang CI, Huang JF, Kuo PL, Dai CY, Yu ML, Chuang WL. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol. 2017;32:1754-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Loggi E, Gitto S, Galli S, Minichiello M, Conti F, Grandini E, Scuteri A, Vitale G, Di Donato R, Cursaro C, Furlini G, Andreone P. Hepatitis B virus reactivation among hepatitis C patients treated with direct-acting antiviral therapies in routine clinical practice. J Clin Virol. 2017;93:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Schulte B, Wübbolding M, Marra F, Port K, Manns MP, Back D, Cornberg M, Stichtenoth DO, Höner Zu Siederdissen C, Maasoumy B. Frequency of Potential Drug-Drug Interactions in the Changing Field of HCV Therapy. Open Forum Infect Dis. 2020;7:ofaa040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Tani J, Morishita A, Sakamoto T, Takuma K, Nakahara M, Fujita K, Oura K, Tadokoro T, Mimura S, Nomura T, Yoneyama H, Kobara H, Himoto T, Tsutsui A, Senoh T, Nagano T, Ogawa C, Moriya A, Deguchi A, Takaguchi K, Masaki T. Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol Lett. 2020;19:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 50. | Delgado Martínez C, Gómez-Rubio M, Gómez-Domínguez C. Is hepatitis C direct-acting antiviral therapy a risk factor for the development and recurrence of hepatocellular carcinoma? Ann Hepatol. 2021;21:100225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Romano A, Angeli P, Piovesan S, Noventa F, Anastassopoulos G, Chemello L, Cavalletto L, Gambato M, Russo FP, Burra P, Vincenzi V, Scotton PG, Panese S, Tempesta D, Bertin T, Carrara M, Carlotto A, Capra F, Carolo G, Scroccaro G, Alberti A. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J Hepatol. 2018;69:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 52. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 53. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 54. | Cabibbo G, Petta S, Calvaruso V, Cacciola I, Cannavò MR, Madonia S, Distefano M, Larocca L, Prestileo T, Tinè F, Bertino G, Giannitrapani L, Benanti F, Licata A, Scalisi I, Mazzola G, Cartabellotta F, Alessi N, Barbàra M, Russello M, Scifo G, Squadrito G, Raimondo G, Craxì A, Di Marco V, Cammà C; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV). Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? Aliment Pharmacol Ther. 2017;46:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 55. | Pockros PJ. Black Box Warning for Possible HBV Reactivation During DAA Therapy for Chronic HCV Infection. Gastroenterol Hepatol (N Y). 2017;13:536-540. [PubMed] |
| 56. | Sagnelli E, Sagnelli C, Macera M, Pisaturo M, Coppola N. An update on the treatment options for HBV/HCV coinfection. Expert Opin Pharmacother. 2017;18:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 57. | Mavilia MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J Clin Transl Hepatol. 2018;6:296-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Pisaturo M, Macera M, Alessio L, Calò F, Coppola N. Hepatitis B Virus (HBV) Reactivation Following Pharmacological Eradication of Hepatitis C Virus (HCV). Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Mücke MM, Backus LI, Mücke VT, Coppola N, Preda CM, Yeh ML, Tang LSY, Belperio PS, Wilson EM, Yu ML, Zeuzem S, Herrmann E, Vermehren J. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 60. | Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20:7544-7554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C Virus Infection Increases the Risk of Developing Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2015;60:3801-3813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Mendizabal M, Reddy KR. Chronic hepatitis C and chronic kidney disease: Advances, limitations and unchartered territories. J Viral Hepat. 2017;24:442-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Fabrizi F, Messa P. The epidemiology of HCV infection in patients with advanced CKD/ESRD: A global perspective. Semin Dial. 2019;32:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2012;6:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O'Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | García-Agudo R, Aoufi-Rabih S, Salgueira-Lazo M, González-Corvillo C, Fabrizi F. 'Real-life' experience with direct-acting antiviral agents for hepatitis C virus in end-stage renal disease. Int J Artif Organs. 2018;41:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008;48:1690-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Papadopoulos N, Griveas I, Sveroni E, Argiana V, Kalliaropoulos A, Martinez-Gonzalez B, Deutsch M. HCV viraemia in anti-HCV-negative haemodialysis patients: Do we need HCV RNA detection test? Int J Artif Organs. 2018;41:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Stamm LM, Brainard DM, McHutchison JG. Sofosbuvir/velpatasvir for patients with chronic genotype 3 HCV infection with compensated cirrhosis: Response to EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2019;70:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Esforzado N, Morales JM. Hepatitis C and kidney transplant: The eradication time of the virus has arrived. Nefrologia (Engl Ed). 2019;39:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014;7:387-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Agarwal SK, Bagchi S, Yadav RK. Hemodialysis Patients Treated for Hepatitis C Using a Sofosbuvir-based Regimen. Kidney Int Rep. 2017;2:831-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, Roth D, Schiff E, O'Brien C, Martin P. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 75. | Mandhwani R, Hanif FM, Lail G, Luck NH, Khalid MA, Ul Haque MM, Laeeq SM, Aziz T. Use of sofosbuvir based regimen in patients with end-stage renal disease and chronic hepatitis C; an open label, non-randomized, single arm, single center study from Pakistan. Gastroenterol Hepatol Bed Bench. 2020;13:141-146. [PubMed] |
| 76. | Shehadeh F, Kalligeros M, Byrd K, Shemin D, Mylonakis E, Martin P, D'Agata EMC. Efficacy and safety of sofosbuvir in the treatment of hep C among patients on hemodialysis: a systematic review and meta-analysis. Sci Rep. 2020;10:14332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Li T, Qu Y, Guo Y, Wang Y, Wang L. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int. 2017;37:974-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Li M, Chen J, Fang Z, Li Y, Lin Q. Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4-5 chronic kidney disease: a systematic review and meta-analysis. Virol J. 2019;16:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Majd Jabbari S, Maajani K, Merat S, Poustchi H, Sepanlou SG. An updated systematic review and meta-analysis on efficacy of Sofosbuvir in treating hepatitis C-infected patients with advanced chronic kidney disease. PLoS One. 2021;16:e0246594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Borgia SM, Dearden J, Yoshida EM, Shafran SD, Brown A, Ben-Ari Z, Cramp ME, Cooper C, Foxton M, Rodriguez CF, Esteban R, Hyland R, Lu S, Kirby BJ, Meng A, Markova S, Dvory-Sobol H, Osinusi AO, Bruck R, Ampuero J, Ryder SD, Agarwal K, Fox R, Shaw D, Haider S, Willems B, Lurie Y, Calleja JL, Gane EJ. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 81. | Latt NL. Update on the Management of Hepatitis C Virus Infection in the Setting of Chronic Kidney Disease and Kidney Transplantation. Gastroenterol Hepatol (N Y). 2018;14:687-705. [PubMed] |
| 82. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 83. | Bruchfeld A, Roth D, Martin P, Nelson DR, Pol S, Londoño MC, Monsour H Jr, Silva M, Hwang P, Arduino JM, Robertson M, Nguyen BY, Wahl J, Barr E, Greaves W. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4-5 chronic kidney disease: clinical, virological, and health-related quality-of-life outcomes from a phase 3, multicentre, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2017;2:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 85. | Lawitz E, Flisiak R, Abunimeh M, Sise ME, Park JY, Kaskas M, Bruchfeld A, Wörns MA, Aglitti A, Zamor PJ, Xue Z, Schnell G, Jalundhwala YJ, Porcalla A, Mensa FJ, Persico M. Efficacy and safety of glecaprevir/pibrentasvir in renally impaired patients with chronic HCV infection. Liver Int. 2020;40:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Harrison DS, Giang J, Darling JM. An Interaction Between Glecaprevir, Pibrentasvir, and Colchicine Causing Rhabdomyolysis in a Patient With Chronic Renal Disease. Clin Liver Dis (Hoboken). 2020;15:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Patel S, Andres J, Qureshi K. An Unexpected Interaction between Sofosbuvir/Ledipasvir and Atorvastatin and Colchicine Causing Rhabdomyolysis in a Patient with Impaired Renal Function. Case Rep Med. 2016;2016:3191089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Fabrizi F, Cerutti R, Porata G, Messa P, Ridruejo E. Direct-Acting Antiviral Agents for HCV-Associated Glomerular Disease and the Current Evidence. Pathogens. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Morales JM, Kamar N, Rostaing L. Hepatitis C and renal disease: epidemiology, diagnosis, pathogenesis and therapy. Contrib Nephrol. 2012;176:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 91. | Fabrizi F, Lunghi G, Messa P, Martin P. Therapy of hepatitis C virus-associated glomerulonephritis: current approaches. J Nephrol. 2008;21:813-825. [PubMed] |
| 92. | Fabrizi F, Dixit V, Messa P. Interferon mono-therapy for symptomatic HCV-associated mixed cryoglobulinemia : meta-analysis of clinical studies. Acta Gastroenterol Belg. 2013;76:363-371. [PubMed] |
| 93. | Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 95. | Alric L, Plaisier E, Thébault S, Péron JM, Rostaing L, Pourrat J, Ronco P, Piette JC, Cacoub P. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis. 2004;43:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Gragnani L, Piluso A, Urraro T, Fabbrizzi A, Fognani E, Petraccia L, Genovesi A, Giubilei L, Ranieri J, Stasi C, Monti M, Zignego AL. Virological and Clinical Response to Interferon-Free Regimens in Patients with HCV-Related Mixed Cryoglobulinemia: Preliminary Results of a Prospective Pilot Study. Curr Drug Targets. 2017;18:772-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Comarmond C, Cacoub P, Saadoun D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Therap Adv Gastroenterol. 2020;13:1756284820942617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, Wong D, Janssen HLA, Feld JJ. Efficacy and Safety of Direct Acting Antivirals for the Treatment of Mixed Cryoglobulinemia. Am J Gastroenterol. 2017;112:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 99. | Montero N, Favà A, Rodriguez E, Barrios C, Cruzado JM, Pascual J, Soler MJ. Treatment for hepatitis C virus-associated mixed cryoglobulinaemia. Cochrane Database Syst Rev. 2018;5:CD011403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Ghosn M, Palmer MB, Najem CE, Haddad D, Merkel PA, Hogan JJ. New-onset hepatitis C virus-associated glomerulonephritis following sustained virologic response with direct-acting antiviral therapy . Clin Nephrol. 2017;87 (2017):261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Barbieri D, García-Prieto A, Torres E, Verde E, Goicoechea M, Luño J. Mixed cryoglobulinaemia vasculitis after sustained hepatitis C virological response with direct-acting antivirals. Clin Kidney J. 2019;12:362-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Rutledge SM, Chung RT, Sise ME. Treatment of hepatitis C virus infection in patients with mixed cryoglobulinemic syndrome and cryoglobulinemic glomerulonephritis. Hemodial Int. 2018;22 Suppl 1:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Nicolau A, Tănăsescu R, Bălănescu E, Bălănescu P, Pătraşcu R, Tănăsescu C. Hepatitis C virus-mixed cryoglobulinemia-lymphoma relationship. Rom J Intern Med. 2011;49:3-10. [PubMed] |
| 104. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Kim SM, Song IH. Hepatitis C virus infection in chronic kidney disease: paradigm shift in management. Korean J Intern Med. 2018;33:670-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Martini S. Hepatitis C and liver transplantation. Minerva Gastroenterol Dietol. 2018;64:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Bhamidimarri KR, Satapathy SK, Martin P. Hepatitis C Virus and Liver Transplantation. Gastroenterol Hepatol (N Y). 2017;13:214-220. [PubMed] |
| 108. | Little EC, Berenguer M. The New Era of Hepatitis C: Therapy in Liver Transplant Recipients. Clin Liver Dis. 2017;21:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Tsoulfas G, Goulis I, Giakoustidis D, Akriviadis E, Agorastou P, Imvrios G, Papanikolaou V. Hepatitis C and liver transplantation. Hippokratia. 2009;13:211-215. [PubMed] |
| 110. | Cholankeril G, Li AA, March KL, Yoo ER, Kim D, Snyder H, Gonzalez SA, Younossi ZM, Ahmed A. Improved Outcomes in HCV Patients Following Liver Transplantation During the Era of Direct-Acting Antiviral Agents. Clin Gastroenterol Hepatol. 2018;16:452-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, Sundaram V, Charlton M. Improved Graft Survival After Liver Transplantation for Recipients With Hepatitis C Virus in the Direct-Acting Antiviral Era. Liver Transpl. 2019;25:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 112. | Campos-Varela I, Lai JC, Verna EC, O'Leary JG, Todd Stravitz R, Forman LM, Trotter JF, Brown RS, Terrault NA; Consortium to Study Health Outcomes in HCV Liver Transplant Recipients (CRUSH-C). Hepatitis C genotype influences post-liver transplant outcomes. Transplantation. 2015;99:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Vinaixa C, Rubín A, Aguilera V, Berenguer M. Recurrence of hepatitis C after liver transplantation. Ann Gastroenterol. 2013;26:304-313. [PubMed] |
| 114. | Chen Y, Wu G, Zhang H, Xu H, Li H, Chen L, Yang Y, Hu P, Zhang D, Ren H, Hu H. Interferon Treatment of Hepatitis C Reinfection after Liver Transplantation: A Meta-Analysis. Gastroenterol Res Pract. 2015;2015:206302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 115. | Zanaga LP, Vigani AG, Angerami RN, Giorgetti A, Escanhoela CA, Ataíde EC, Boin IF, Stucchi RS. Survival benefits of interferon-based therapy in patients with recurrent hepatitis C after orthotopic liver transplantation. Braz J Med Biol Res. 2017;50:e5540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Perumpail RB, Wong RJ, Ha LD, Pham EA, Wang U, Luong H, Kumari R, Daugherty TJ, Higgins JP, Younossi ZM, Kim WR, Glenn JS, Ahmed A. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Charlton M, Gane E, Manns MP, Brown RS Jr, Curry MP, Kwo PY, Fontana RJ, Gilroy R, Teperman L, Muir AJ, McHutchison JG, Symonds WT, Brainard D, Kirby B, Dvory-Sobol H, Denning J, Arterburn S, Samuel D, Forns X, Terrault NA. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 118. | Wellington J, Ma A, Kottilil S, Ravichandran B, Husson J, Bruno D, Wilson E. Outcomes in Hepatitis C Positive Liver Transplantation: Timing of Direct-Acting Antiviral Treatment and Impact on Graft Fibrosis. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O'Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 633] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 120. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, Agarwal K, Angus P, Yoshida EM, Colombo M, Rizzetto M, Dvory-Sobol H, Denning J, Arterburn S, Pang PS, Brainard D, McHutchison JG, Dufour JF, Van Vlierberghe H, van Hoek B, Forns X; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 121. | Chahine EB, Sucher AJ, Hemstreet BA. Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C. Ann Pharmacother. 2017;51:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Zoratti MJ, Siddiqua A, Morassut RE, Zeraatkar D, Chou R, van Holten J, Xie F, Druyts E. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: A systematic literature review and meta-analysis. EClinicalMedicine. 2020;18:100237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 123. | Burra P, De Martin E, Zanetto A, Senzolo M, Russo FP, Zanus G, Fagiuoli S. Hepatitis C virus and liver transplantation: where do we stand? Transpl Int. 2016;29:135-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Reau N, Kwo PY, Rhee S, Brown RS Jr, Agarwal K, Angus P, Gane E, Kao JH, Mantry PS, Mutimer D, Reddy KR, Tran TT, Hu YB, Gulati A, Krishnan P, Dumas EO, Porcalla A, Shulman NS, Liu W, Samanta S, Trinh R, Forns X. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology. 2018;68:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 125. | Ueda Y, Kobayashi T, Ikegami T, Miuma S, Mizuno S, Akamatsu N, Takaki A, Ishigami M, Takatsuki M, Sugawara Y, Maehara Y, Uemoto S, Seno H. Efficacy and safety of glecaprevir and pibrentasvir treatment for 8 or 12 weeks in patients with recurrent hepatitis C after liver transplantation: a Japanese multicenter experience. J Gastroenterol. 2019;54:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Higley C, Hsu CC, Smith C, Nadella S, Lalos AT. Safety and efficacy of sofosbuvir/velpatasvir/voxilaprevir in post-liver transplant patients with previous direct-acting antiviral failure: Six case reports. World J Hepatol. 2020;12:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Cardona-Gonzalez MG, Goldman JD, Narayan L, Brainard DM, Kowdley KV. Sofosbuvir, Velpatasvir, and Voxilaprevir for Treatment of Recurrent Hepatitis C Virus Infection After Liver Transplantation. Hepatol Commun. 2018;2:1446-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 128. | Chacon MM, Adams AJ, Kassel CA, Markin NW. High-Risk and Hepatitis C-Positive Organ Donors: Current Practice in Heart, Lung, and Liver Transplantation. J Cardiothorac Vasc Anesth. 2020;34:2492-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 129. | Kwong AJ, Wall A, Melcher M, Wang U, Ahmed A, Subramanian A, Kwo PY. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 130. | Cholankeril G, Li AA, Dennis BB, Toll AE, Kim D, Bonham CA, Nair S, Ahmed A. Increasing Trends in Transplantation of HCV-Positive Livers Into Uninfected Recipients. Clin Gastroenterol Hepatol. 2019;17:1634-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Cotter TG, Aronsohn A, Reddy KG, Charlton M. Liver Transplantation of HCV-viremic Donors Into HCV-negative Recipients in the United States: Increasing Frequency With Profound Geographic Variation. Transplantation. 2021;105:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 132. | Bethea ED, Gaj K, Gustafson JL, Axtell A, Lebeis T, Schoenike M, Turvey K, Coglianese E, Thomas S, Newton-Cheh C, Ibrahim N, Carlson W, Ho JE, Shah R, Nayor M, Gift T, Shao S, Dugal A, Markmann J, Elias N, Yeh H, Andersson K, Pratt D, Bhan I, Safa K, Fishman J, Kotton C, Myoung P, Villavicencio MA, D'Alessandro D, Chung RT, Lewis GD. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol Hepatol. 2019;4:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 133. | Jandovitz N, Nair V, Grodstein E, Molmenti E, Fahmy A, Abate M, Bhaskaran M, Teperman L. Hepatitis C-positive donor to negative recipient kidney transplantation: A real-world experience. Transpl Infect Dis. 2021;23:e13540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N. Chronic hepatitis C virus infection in renal transplant: treatment and outcome. Clin Transplant. 2006;20:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Manga Sahin G, Sahin S, Kantarci G, Ergin H. Impact of hepatitis C virus infection on patient and graft survival in kidney transplantation. Transplant Proc. 2006;38:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of Hepatitis C virus infection on kidney transplantation outcomes: A systematic review of 18 observational studies: The impact of HCV on renal transplantation. Hepat Mon. 2011;11:247-254. [PubMed] |
| 137. | Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat. 2014;21:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 138. | Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 139. | Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 140. | Zylberberg H, Nalpas B, Carnot F, Skhiri H, Fontaine H, Legendre C, Kreis H, Bréchot C, Pol S. Severe evolution of chronic hepatitis C in renal transplantation: a case control study. Nephrol Dial Transplant. 2002;17:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 141. | Fabrizi F, Cerutti R, Silva M. HCV-infected solid organ donors, direct-acting antivirals and the current challenges. Expert Rev Clin Pharmacol. 2020;13:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Mohammad KG, Idrees MK, Ali T, Akhtar F. Posttransplant diabetes mellitus among live-related kidney transplant recipients: Sindh Institute of Urology and Transplantation experience. Saudi J Kidney Dis Transpl. 2018;29:1320-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 143. | Morales JM, Campistol JM, Andrés A, Rodicio JL. Glomerular diseases in patients with hepatitis C virus infection after renal transplantation. Curr Opin Nephrol Hypertens. 1997;6:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Chen HZ, Ji L, Li L, Wang G, Bai XW, Cheng CD, Sun B. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine (Baltimore). 2017;96:e7487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 145. | Maness DL, Riley E, Studebaker G. Hepatitis C: Diagnosis and Management. Am Fam Physician. 2021;104:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 146. | Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 147. | Pageaux GP, Hilleret MN, Garrigues V, Bismuth M, Audin-Mamlouk H, Zarski JP, Mourad G. Pegylated interferon-alpha-based treatment for chronic hepatitis C in renal transplant recipients: an open pilot study. Transpl Int. 2009;22:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 148. | Caeiro F, Baptista V, Rodrigues N, Carvalho D, Aires I, Remédio F, Nolasco F. Treatment of hepatitis C virus infection in kidney transplant recipients: case report. Transplant Proc. 2011;43:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 149. | Özer Etik D, Suna N, Öcal S, Selçuk H, Dağlı Ü, Çolak T, Hilmioğlu F, Boyacıoğlu AS, Haberal M. Successful Treatment With Direct-Acting Antiviral Agents of Hepatitis C in Patients With End-Stage Renal Disease and Kidney Transplant Recipients. Exp Clin Transplant. 2019;17:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Darema M, Cholongitas E, Filiopoulos V, Marinaki S, Pavlopoulou ID, Tsoubou I, Boletis JN, Papatheodoridis GV. Efficacy and safety of new direct-acting antivirals in kidney transplant recipients with chronic hepatitis C: a single-center study. Ann Gastroenterol. 2020;33:285-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 151. | Hanif FM, Mandhwani R, Lail G, Luck NH, Aziz T. Virological Response to Sofosbuvir-Based Treatment in Renal Transplant Recipients With Hepatitis C in Pakistan. Exp Clin Transplant. 2019;17:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 152. | Muir AJ. The rapid evolution of treatment strategies for hepatitis C. Am J Gastroenterol. 2014;109:628-35; quiz 636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 153. | Kamal S, Abdelhakam S, Ghoraba D, Mohsen MA, Salam AA, Hassan H, Nabeigh L. The Course of Hepatitis C Infection and Response to Anti-viral Therapy in Patients with Thalassemia major and Hepatitis C Infection: A Longitudinal, Prospective Study. Mediterr J Hematol Infect Dis. 2019;11:e2019060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Lin MV, Sise ME, Pavlakis M, Amundsen BM, Chute D, Rutherford AE, Chung RT, Curry MP, Hanifi JM, Gabardi S, Chandraker A, Heher EC, Elias N, Riella LV. Efficacy and Safety of Direct Acting Antivirals in Kidney Transplant Recipients with Chronic Hepatitis C Virus Infection. PLoS One. 2016;11:e0158431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 155. | Lubetzky M, Chun S, Joelson A, Coco M, Kamal L, Ajaimy M, Gaglio P, Akalin E, De Boccardo G. Safety and Efficacy of Treatment of Hepatitis C in Kidney Transplant Recipients With Directly Acting Antiviral Agents. Transplantation. 2017;101:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 156. | Grebley J, Puoti M, Wedemeyer H, Cooper C, Sulkowski M, Foster G, Berg T, Villa E, Rodriguez-Perez F, Wyles D. Safety and efficacy of ombitasvir, paritaprevir/ritonavir and dasabuvir with or without ribavirin in chronic hepatitis C patients receiving opioid substitution therapy: a pooled analysis across 12 clinical trials. J hepatol. 2017;1:S514. |
| 157. | Scott LJ. Ledipasvir/Sofosbuvir: A Review in Chronic Hepatitis C. Drugs. 2018;78:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 158. | Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, Sulkowski MS, O'Leary JG, Koraishy F, Galati JS, Kuo AA, Vainorius M, Akushevich L, Nelson DR, Fried MW, Terrault N, Reddy KR. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 159. | Zhang J, Sun W, Lin J, Tian Y, Ma L, Zhang L, Zhu Y, Qiu W. Long-term follow-up of HCV infected kidney transplant recipients receiving direct-acting antiviral agents: a single-center experience in China. BMC Infect Dis. 2019;19:645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 160. | Taneja S, Duseja A, De A, Kumar V, Ramachandran R, Sharma A, Dhiman RK, Gupta KL, Chawla Y. Successful treatment of chronic hepatitis C infection with directly acting antivirals in renal transplant recipients. Nephrology (Carlton). 2018;23:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Chen K, Lu P, Song R, Zhang J, Tao R, Wang Z, Zhang W, Gu M. Direct-acting antiviral agent efficacy and safety in renal transplant recipients with chronic hepatitis C virus infection: A PRISMA-compliant study. Medicine (Baltimore). 2017;96:e7568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 162. | Fernández-Ruiz M, Polanco N, García-Santiago A, Muñoz R, Hernández AM, González E, Mercado VR, Fernández I, Aguado JM, Praga M, Andrés A. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int. 2018;31:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 163. | Jadoul M, Berenguer MC, Doss W, Fabrizi F, Izopet J, Jha V, Kamar N, Kasiske BL, Lai CL, Morales JM, Patel PR, Pol S, Silva MO, Balk EM, Gordon CE, Earley A, Di M, Martin P. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 164. | Liapakis A, Formica RN, Levitsky J. Solid organ transplantation of viral hepatitis C positive donor organs into viral hepatitis C negative recipients. Curr Opin Organ Transplant. 2018;23:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Burton JR Jr, Terrault NA, Goldberg DS, Bloom RD, Gilroy R, Heimbach JK, Brown RS Jr, Everson GT, Rubin E, Wiesner R, Pomfret EA. Liver and Kidney Recipient Selection of Hepatitis C Virus Viremic Donors: Meeting Consensus Report From the 2019 Controversies in Transplantation. Transplantation. 2020;104:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 166. | Scalea JR, Barth RN, Munivenkatappa R, Philosophe B, Cooper M, Whitlow V, LaMattina JC. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation. 2015;99:1192-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 167. | Englum BR, Ganapathi AM, Speicher PJ, Gulack BC, Snyder LD, Davis RD, Hartwig MG. Impact of donor and recipient hepatitis C status in lung transplantation. J Heart Lung Transplant. 2016;35:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 168. | Flohr TR, Bonatti H, Hranjec T, Keith DS, Lobo PI, Kumer SC, Schmitt TM, Sawyer RG, Pruett TL, Roberts JP, Brayman KL. Elderly recipients of hepatitis C positive renal allografts can quickly develop liver disease. J Surg Res. 2012;176:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 169. | Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, Bloom RD, Nazarian SM, Sawinski D, Porrett P, Naji A, Hasz R, Suplee L, Trofe-Clark J, Sicilia A, McCauley M, Farooqi M, Gentile C, Smith J, Reese PP. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376:2394-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 170. | Fabrizi F, Cerutti R, Alfieri CM, Messa P. Updated View on Kidney Transplant from HCV-Infected Donors and DAAs. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 171. | Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, Wesson R, Reyad A, Naqvi FF, Ostrander D, Sugarman J, Segev DL, Sulkowski M, Desai NM. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 172. | La Hoz RM, Sandıkçı B, Ariyamuthu VK, Tanriover B. Short-term outcomes of deceased donor renal transplants of HCV uninfected recipients from HCV seropositive nonviremic donors and viremic donors in the era of direct-acting antivirals. Am J Transplant. 2019;19:3058-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 173. | Gasink LB, Blumberg EA, Localio AR, Desai SS, Israni AK, Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 174. | Singh N, Neidlinger N, Djamali A, Leverson G, Voss B, Sollinger HW, Pirsch JD. The impact of hepatitis C virus donor and recipient status on long-term kidney transplant outcomes: University of Wisconsin experience. Clin Transplant. 2012;26:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 175. | Abdelbasit A, Hirji A, Halloran K, Weinkauf J, Kapasi A, Lien D, Nagendran J, Doucette K. Lung Transplantation from Hepatitis C Viremic Donors to Uninfected Recipients. Am J Respir Crit Care Med. 2018;197:1492-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 176. | Cypel M, Feld JJ, Galasso M, Pinto Ribeiro RV, Marks N, Kuczynski M, Kumar D, Bahinskaya I, Bagnato VS, Kurachi C, Slutsky AS, Yeung JC, Donahoe L, de Perrot M, Yasufuku K, Pierre A, Binnie M, Chaparro C, Martinu T, Chen M, Tikkanen J, Chow CW, Sidhu A, Waddell TK, Keshavjee S, Singer LG, Humar A. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med. 2020;8:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 177. | Morris KL, Adlam JP, Padanilam M, Patel A, Garcia-Cortes R, Chaudhry SP, Seasor E, Tompkins S, Hoefer C, Zanotti G, Walsh MN, Salerno C, Bochan M, Ravichandran A. Hepatitis C donor viremic cardiac transplantation: A practical approach. Clin Transplant. 2020;34:e13764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 178. | Siddiqi HK, Schlendorf KH. Hepatitis C Positive Organ Donation in Heart Transplantation. Curr Transplant Rep. 2021;8:359-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 179. | Kilic A, Hickey G, Mathier M, Sultan I, Gleason TG, Horn E, Keebler ME. Outcomes of Adult Heart Transplantation Using Hepatitis C-Positive Donors. J Am Heart Assoc. 2020;9:e014495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 180. | Reyentovich A, Gidea CG, Smith D, Lonze B, Kon Z, Fargnoli A, Pavone J, Rao S, Saraon T, Lewis T, Qian Y, Jacobson I, Moazami N. Outcomes of the Treatment with Glecaprevir/Pibrentasvir following heart transplantation utilizing hepatitis C viremic donors. Clin Transplant. 2020;34:e13989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 181. | Schlendorf KH, Zalawadiya S, Shah AS, Wigger M, Chung CY, Smith S, Danter M, Choi CW, Keebler ME, Brinkley DM, Sacks SB, Ooi H, Perri R, Awad JA, Lewis S, Hayes R, O'Dell H, Darragh C, Carver A, Edmonds C, Ruzevich-Scholl S, Lindenfeld J. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 182. | Bruno S, Nicole B, Nila J D, Gail M, James N, Peter S M, Christopher S H. Heart Transplantation From Hepatitis C-Positive Donors in the Era of Direct Acting Antiviral Therapy: A Comprehensive Literature Review. Transplant Direct. 2019;5:e486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 183. | Feld JJ, Cypel M, Kumar D, Dahari H, Pinto Ribeiro RV, Marks N, Kamkar N, Bahinskaya I, Onofrio FQ, Zahoor MA, Cerrochi O, Tinckam K, Kim SJ, Schiff J, Reichman TW, McDonald M, Alba C, Waddell TK, Sapisochin G, Selzner M, Keshavjee S, Janssen HLA, Hansen BE, Singer LG, Humar A. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: a phase 3, single-centre, open-label study. Lancet Gastroenterol Hepatol. 2020;5:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 184. | Tronina O, Ślubowska K, Mikołajczyk-Korniak N, Komuda-Leszek E, Wieczorek-Godlewska R, Łągiewska B, Pacholczyk M, Lisik W, Kosieradzki M, Durlik M. Fibrosing Cholestatic Hepatitis C After Liver Transplantation: Therapeutic Options Before and After Introduction of Direct-Acting Antivirals: Our Experience and Literature Review. Transplant Proc. 2017;49:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 185. | Antonini TM, Sebagh M, Roque-Afonso AM, Teicher E, Roche B, Sobesky R, Coilly A, Vaghefi P, Adam R, Vittecoq D, Castaing D, Samuel D, Duclos-Vallée JC. Fibrosing cholestatic hepatitis in HIV/HCV co-infected transplant patients-usefulness of early markers after liver transplantation. Am J Transplant. 2011;11:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 186. | Delladetsima JK, Boletis JN, Makris F, Psichogiou M, Kostakis A, Hatzakis A. Fibrosing cholestatic hepatitis in renal transplant recipients with hepatitis C virus infection. Liver Transpl Surg. 1999;5:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 187. | Lim HL, Lau GK, Davis GL, Dolson DJ, Lau JY. Cholestatic hepatitis leading to hepatic failure in a patient with organ-transmitted hepatitis C virus infection. Gastroenterology. 1994;106:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 188. | Narang TK, Ahrens W, Russo MW. Post-liver transplant cholestatic hepatitis C: a systematic review of clinical and pathological findings and application of consensus criteria. Liver Transpl. 2010;16:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 189. | Dumortier J, Boillot O, Scoazec JY. Natural history, treatment and prevention of hepatitis C recurrence after liver transplantation: past, present and future. World J Gastroenterol. 2014;20:11069-11079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 190. | Jiménez-Pérez M, González-Grande R, Rando-Muñoz FJ. Management of recurrent hepatitis C virus after liver transplantation. World J Gastroenterol. 2014;20:16409-16417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 191. | Xiao SY, Lu L, Wang HL. Fibrosing cholestatic hepatitis: clinicopathologic spectrum, diagnosis and pathogenesis. Int J Clin Exp Pathol. 2008;1:396-402. [PubMed] |
| 192. | Hori T, Onishi Y, Kamei H, Kurata N, Ishigami M, Ishizu Y, Ogura Y. Fibrosing cholestatic hepatitis C in post-transplant adult recipients of liver transplantation. Ann Gastroenterol. 2016;29:454-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 193. | Xue W, Liu K, Qiu K, Shen Y, Pan Z, Hu P, Peng M, Chen M, Ren H. A systematic review with meta-analysis: Is ribavirin necessary in sofosbuvir-based direct-acting antiviral therapies for patients with HCV recurrence after liver transplantation? Int J Infect Dis. 2019;83:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 194. | Leroy V, Dumortier J, Coilly A, Sebagh M, Fougerou-Leurent C, Radenne S, Botta D, Durand F, Silvain C, Lebray P, Houssel-Debry P, Kamar N, D'Alteroche L, Petrov-Sanchez V, Diallo A, Pageaux GP, Duclos-Vallee JC; Agence Nationale de Recherches sur le SIDA et les Hépatites Virales CO23 Compassionate Use of Protease Inhibitors in Viral C in Liver Transplantation Study Group. Efficacy of Sofosbuvir and Daclatasvir in Patients With Fibrosing Cholestatic Hepatitis C After Liver Transplantation. Clin Gastroenterol Hepatol. 2015;13:1993-2001.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 195. | Shinzato T, Kubo T, Shimizu T, Nanmoku K, Yagisawa T. Fibrosing cholestatic hepatitis in a kidney transplant recipient with hepatitis C virus. CEN Case Rep. 2019;8:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 196. | World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. Geneva: World Health Organization, 2016. Available from: https://apps.who.int/iris/handle/10665/246177. |
| 197. | Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS, Zoulim F. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 198. | Hassanin A, Kamel S, Waked I, Fort M. Egypt's Ambitious Strategy to Eliminate Hepatitis C Virus: A Case Study. Glob Health Sci Pract. 2021;9:187-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 199. | Barth H. Hepatitis C virus: Is it time to say goodbye yet? World J Hepatol. 2015;7:725-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 200. | Waheed Y, Siddiq M, Jamil Z, Najmi MH. Hepatitis elimination by 2030: Progress and challenges. World J Gastroenterol. 2018;24:4959-4961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 201. | Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |