Published online Feb 27, 2022. doi: 10.4254/wjh.v14.i2.386
Peer-review started: July 31, 2021
First decision: September 29, 2021
Revised: October 12, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 27, 2022
Processing time: 206 Days and 7.8 Hours
The role of the hepatic nervous system in liver development remains unclear. We previously created functional human micro-hepatic tissue in mice by co-culturing human hepatic endodermal cells with endothelial and mesenchymal cells. However, they lacked Glisson’s sheath [the portal tract (PT)]. The PT consists of branches of the hepatic artery (HA), portal vein, and intrahepatic bile duct (IHBD), collectively called the portal triad, together with autonomic nerves.
To evaluate the development of the mouse hepatic nervous network in the PT using immunohistochemistry.
Liver samples from C57BL/6J mice were harvested at different developmental time periods, from embryonic day (E) 10.5 to postnatal day (P) 56. Thin sections of the surface cut through the hepatic hilus were examined using protein gene product 9.5 (PGP9.5) and cytokeratin 19 (CK19) antibodies, markers of nerve fibers (NFs), and biliary epithelial cells (BECs), respectively. The numbers of NFs and IHBDs were separately counted in a PT around the hepatic hilus (center) and the peripheral area (periphery) of the liver, comparing the average values between the center and the periphery at each developmental stage. NF-IHBD and NF-HA contacts in a PT were counted, and their relationship was quantified. SRY-related high mobility group-box gene 9 (SOX9), another BEC marker; hepatocyte nuclear factor 4α (HNF4α), a marker of hepatocytes; and Jagged-1, a Notch ligand, were also immunostained to observe the PT development.
HNF4α was expressed in the nucleus, and Jagged-1 was diffusely positive in the primitive liver at E10.5; however, the PGP9.5 and CK19 were negative in the fetal liver. SOX9-positive cells were scattered in the periportal area in the liver at E12.5. The Jagged-1 was mainly expressed in the periportal tissue, and the number of SOX9-positive cells increased at E16.5. SOX9-positive cells constructed the ductal plate and primitive IHBDs mainly at the center, and SOX-9-positive IHBDs partly acquired CK19 positivity at the same period. PGP9.5-positive bodies were first found at E16.5 and HAs were first found at P0 in the periportal tissue of the center. Therefore, primitive PT structures were first constructed at P0 in the center. Along with remodeling of the periportal tissue, the number of CK19-positive IHBDs and PGP9.5-positive NFs gradually increased, and PTs were also formed in the periphery until P5. The numbers of NFs and IHBDs were significantly higher in the center than in the periphery from E16.5 to P5. The numbers of NFs and IHBDs reached the adult level at P28, with decreased differences between the center and periphery. NFs associated more frequently with HAs than IHBDs in PTs at the early phase after birth, after which the number of NF-IHBD contacts gradually increased.
Mouse hepatic NFs first emerge at the center just before birth and extend toward the periphery. The interaction between NFs and IHBDs or HAs plays important roles in the morphogenesis of PT structure.
Core Tip: The portal tract (PT) consists of branches of the hepatic artery (HA), portal vein, intrahepatic bile ducts (IHBD), and autonomic nerves. This study evaluated the mouse hepatic nervous system development using immunohistochemistry. Hepatic nerve fibers (NFs) first emerge at the hepatic hilus just before birth and extend toward the periphery with IHBD in the PT after birth. The hepatic NFs associated more frequently with the HA than the IHBD in the PT after birth. The hepatic NFs may play important roles in the morphogenesis and stabilization of the PT during development of the liver.
- Citation: Koike N, Tadokoro T, Ueno Y, Okamoto S, Kobayashi T, Murata S, Taniguchi H. Development of the nervous system in mouse liver. World J Hepatol 2022; 14(2): 386-399
- URL: https://www.wjgnet.com/1948-5182/full/v14/i2/386.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i2.386
Recently, structures resembling whole organs, termed organoids, have been generated from stem cells through the development of three-dimensional culture systems[1]. The liver is the largest organ in the abdomen and is a pivotal center of metabolism, detoxification, and digestion. The mammalian liver has a structural and functional unit called the liver lobule, and in the periphery of the lobule, Glisson’s sheath, also known as the portal tract (PT) consisting of the portal vein (PV), bile duct (BD), and hepatic artery (HA), develops[2]. We previously created the three-dimensional vascularized functional human micro-hepatic tissue in mice by co-culturing human hepatic endodermal cells with endothelial and mesenchymal cells derived from human-induced pluripotent stem cells (iPSCs)[3]; however, they lacked PTs. The construction of stable blood vessels is a fundamental challenge for tissue engineering in regenerative medicine[4]. During evolution, organs have come to perform complex functions, requiring an increased degree of information processing by neurons and a supply of nutrients by blood vessels[5]. Therefore, neurons, which arose earlier in evolution than vessel, may also should be important to construct functional artificial organs. No current organoid systems contain an integrated peripheral nervous system; however, Workman et al[6] successfully generated human-iPSC-derived intestinal tissue with a functional enteric nervous system[6]. The hepatic nervous system exerts important roles in the overall regulation of the organism, for example, in the glucose and lipid metabolism, circadian rhythm, regeneration, hepatic blood flow, food intake and obesity[7-9]. Development of vessels and BDs in the liver have been thoroughly explored[10]. It has been known for many years that intrahepatic bile duct (IHBD) development is initiated near the hilum of the liver before progressing toward the periphery of the lobes[10]. At the late phase of mouse embryonic development, differentiated cholangiocytes [biliary epithelial cells (BECs)] are discontinuously scattered in the periportal mesenchyme and form ductal plate (DP) and biliary cysts followed by the development of ductal structure and incorporation into the continuous network structure after birth[11,12]. In addition, the angiogenic growth factors produced by periportal mesenchymal cells and BECs seem to provide a molecular link between the developing biliary and arterial structures[13]. However, the role of the hepatic nervous system has not been elucidated in liver development[14]. Recently, Tanimizu et al[15] reported that IHBDs guide the extension of nerve fibers (NFs) by secreting nerve growth factor (NGF) during their development. NFs with IHBDs extend through the PT from the hepatic hilus to the periphery[15]. But it has not been clarified if the hepatic nerve network plays important roles in the morphogenesis and stabilization of the PTs after birth.
In this work, we histologically observed the mouse liver and evaluated the developmental process of PTs, with a special focus on the hepatic nervous system in PTs and the correlation between NFs and BDs or vessels using immunohistochemistry. This study aims to explore the role of nerve cells in the development of the liver and whether the addition of nerve cells is useful for constructing liver organoids with PT structures.
Pregnant female C57BL/6J mice were purchased from Sankyo Labo Service Corporation, Inc. (Tokyo, Japan) to acquire embryos and newborn mice for the experiments. Adult male mice (8-wk-old) were also purchased from Sankyo Labo Service Corporation, Inc. (Tokyo, Japan). The mice were bred and maintained according to the Yokohama City University institutional guidelines for the use of laboratory animals. All experimental procedures were approved by the institutional review board of the Animal Research Center, Yokohama City University School of Medicine (No. 075). Liver samples of C57BL/6J mice were collected at different developmental periods, beginning at embryonic day (E) 10.5 until 8-week-old [adult animals; postnatal day (P) 56]. Specifically, samples were collected at E10.5, E11.5, E12.5, E13.5, E15.5, E16.5, E17.5, P0, P3, P5, P7, P28, and P56 (n = 4 for each stage).
Neonatal and adult liver tissues were fixed in 4% paraformaldehyde and were paraffin-embedded. Then, 3-μm thin sections of cut surface through the hepatic hilus were examined for hematoxylin-eosin (HE) and immunostaining. The primary antibodies used for immunostaining are listed in Table 1. We used protein gene product 9.5 (PGP9.5) as a marker of neurons and cytokeratin 19 (CK19) as a marker of BEC. A preliminary immunohistochemical study was performed to select antibodies appropriate for identifying NFs. Control fetal liver specimens were immunohistochemically stained for a panel of antibodies for PGP9.5 (monoclonal, Abcam, Cambridge, MA, United States), S100 protein (polyclonal, Agilent, Santa Clara, CA, United States), and β tubulin III (monoclonal, Merk. Darmstadt, Germany). As a result, immunostaining of PGP9.5 most clearly and specifically identified NFs. Therefore, we used PGP9.5 for the study. SRY-related high mobility group-box gene 9 (SOX9), another BEC marker; hepatocyte nuclear factor 4α (HNF4α), a marker of hepatocytes; and Jagged-1, a Notch ligand, were also immunostained to observe the PT ontogeny.
| Antibody | Origin | Dilution | Source |
| PGP9.5 (13C4/I3C4) monoclonal | Mouse | × 500 | Abcam |
| CK-19 (EP1580Y) monoclonal | Rabbit | × 200 | Abcam |
| SOX9 polyclonal | Rabbit | × 1000 | Chemicon International |
| HNF4α (K9218) monoclonal | Mouse | × 100 | Thermo Fisher Scientific |
| Jagged-1 (EPR4290) monoclonal | Rabbit | × 300 | Abcam |
Immunohistochemistry was performed using the Envision method according to the manufacturer’s instructions (Agilent). Each thin section was deparaffinized, and antigens were retrieved for immunohistochemical reactions (pH 9.0, 20 min) by PT Link (Agilent). After blocking the endogenous peroxidase with hydrogen peroxide, slides were incubated with primary antibodies overnight at 4 ºC. The sections were then washed, and the antigen was visualized using the DAKO Autostainer using the Envision flex kit (Agilent). The slides were counterstained with hematoxylin for 30 s and dehydrated and sealed with coverslips. The slides were then examined microscopically. Positive and negative control tissues were stained in each run. Images were acquired using a light microscope (Olympus Corporation, Tokyo, Japan).
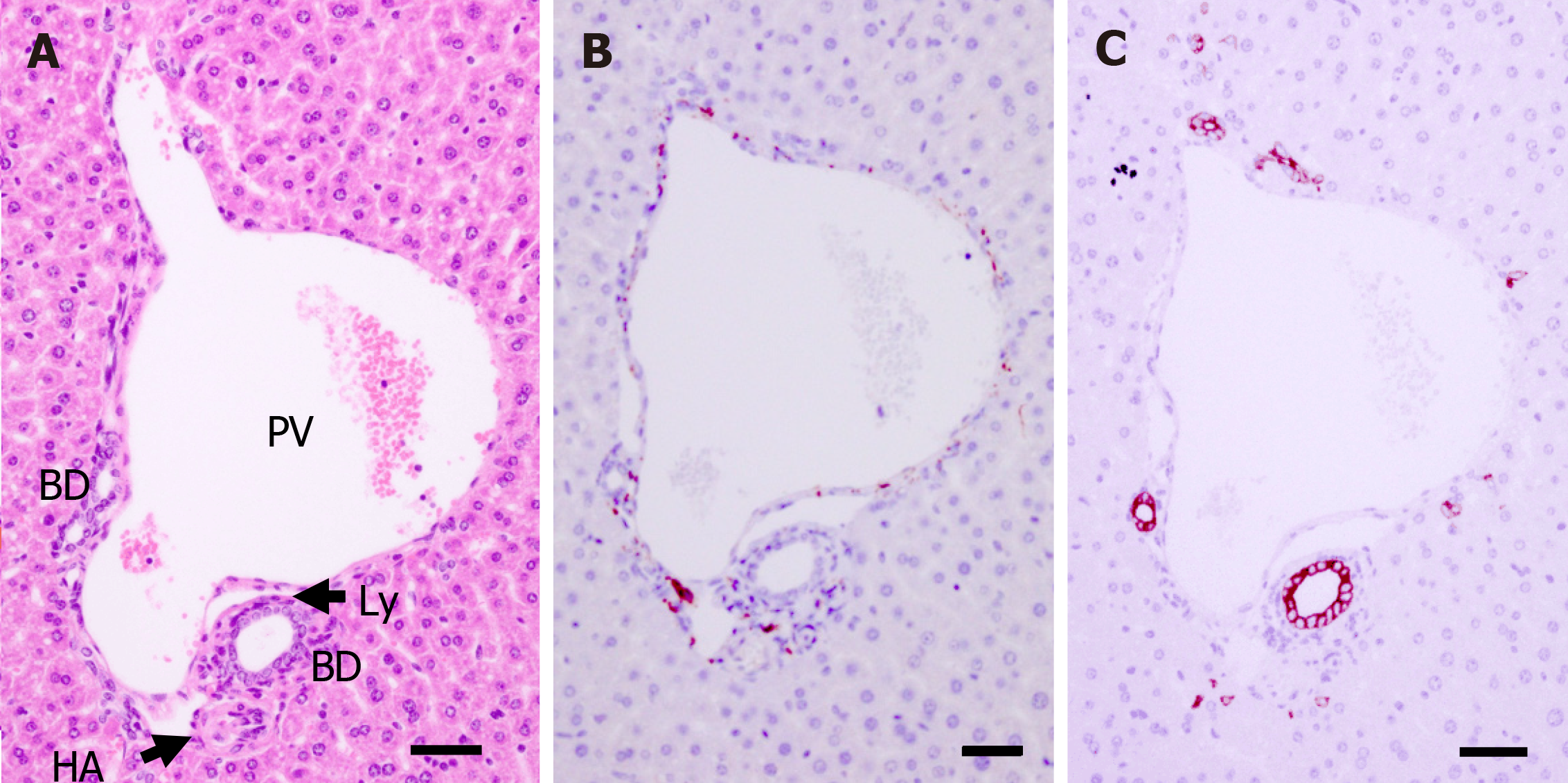
The HAs in the PT were identified under HE staining as vessels with thick walls composed by a tunica media layer that contains concentric rings of smooth muscle.
It has been known for many years that IHBD development is initiated near the hilum of the liver before progressing toward the periphery of the lobes[10]. The development of IHBDs and NFs were examined near the hepatic hilus (center) and in the peripheral region (periphery) separately. Figure 1 indicates the center and the periphery. Inside and outside of the red line were defined as the center (around the hepatic hilus) and periphery, respectively (Figure 1). In addition, thin sections of the surface cut through the hepatic hilus (center) were examined using PGP9.5 and CK19 antibodies, which are markers of NFs and BDs, respectively. Primitive BDs and NFs in the liver have been reported to emerge in the periportal mesenchyme around the newborn period. Then, PVs and periportal mesenchyme evolve into PTs. The numbers of NFs and BDs were separately counted in three randomly selected PVs with periportal tissue or a PT in the center and the periphery of the liver, with the average values being compared between the center and periphery at each developmental time point.
Quantification of the relationship between NFs and IHBDs or NFs and HAs in PTs was made by manually counting the numbers of NFs that contacted IHBDs or HAs in the same three selected PTs in which the number of NFs was counted using a light microscope.
means ± SD are typically reported. Statistical analyses were performed using the an unpaired-t test using GraphPad Prism v9.02 (GraphPad, La Jolla, CA, United States). Differences at P < 0.05 were considered to be statistically significant.
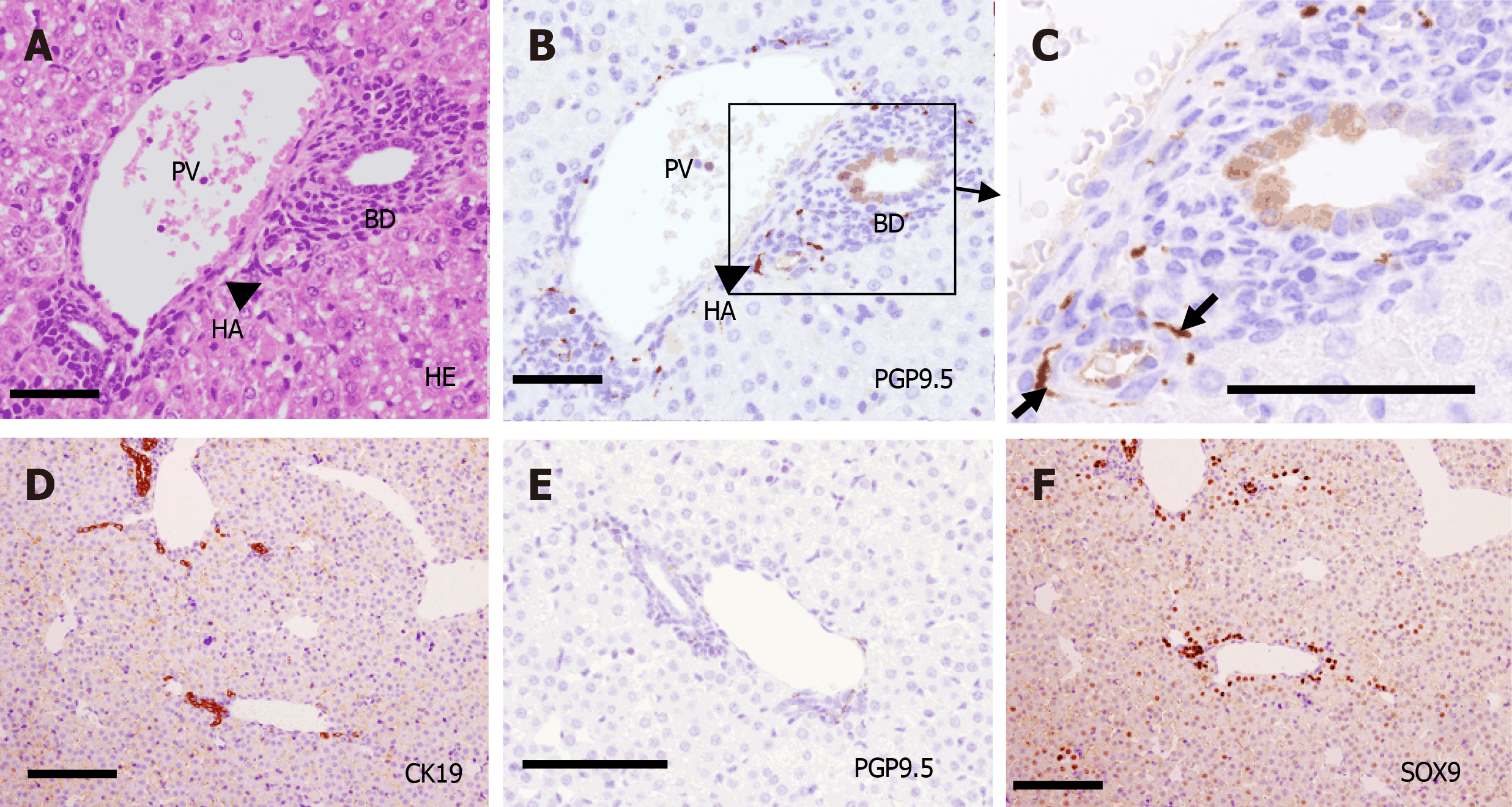
PTs of adult mice (P56) consist of PV, HA, BD, lymphatic vessels, and autonomic nerve branches. These components are also found in human PTs. Many small NFs were observed (as PGP9.5-positive staining) around the PV, HA, and BD, and they produced plexuses (Figure 2).
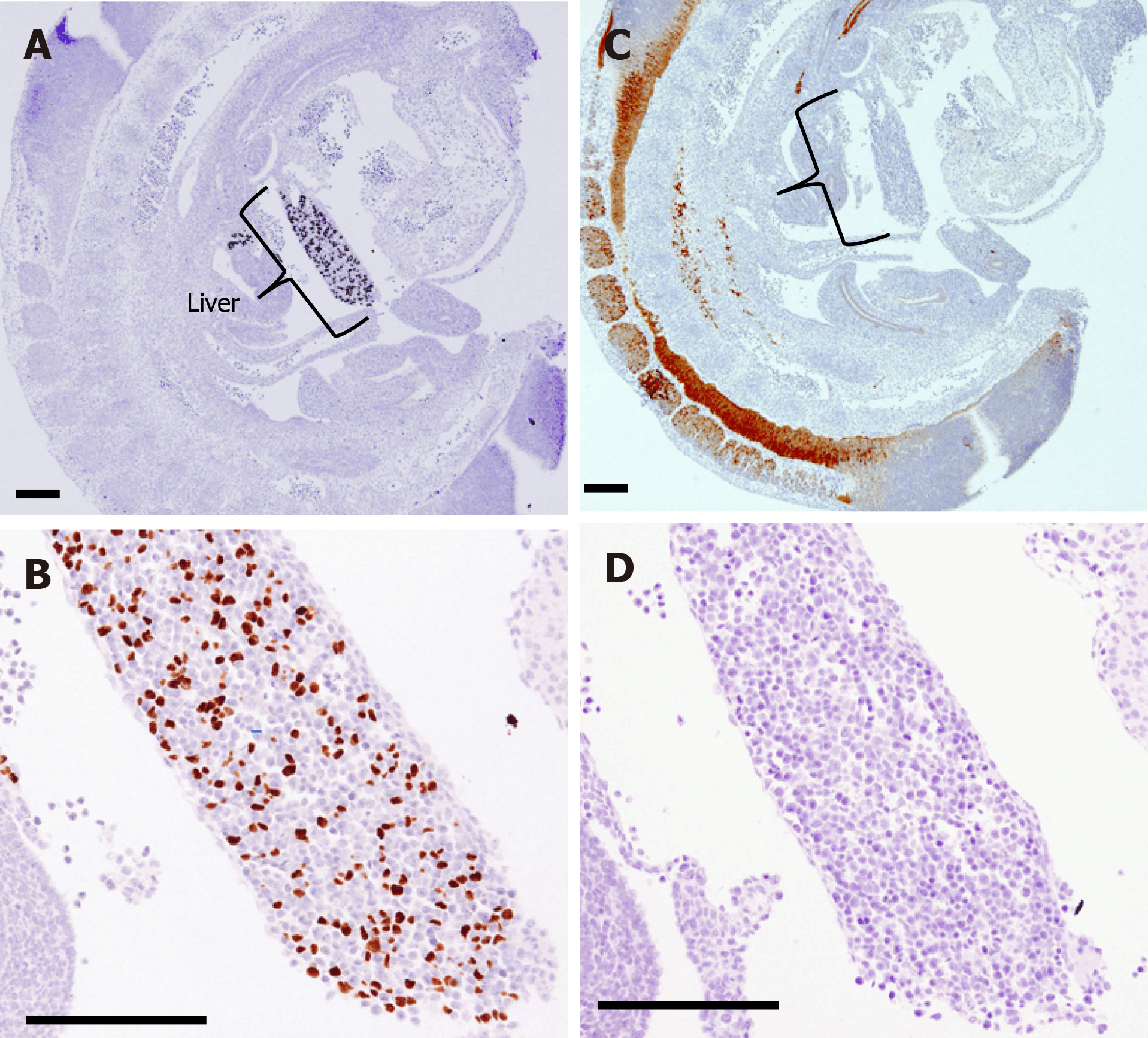
The hepatocyte maker HNF4α is already expressed in the nuclei of many cells in the primitive liver at E10.5; however, PGP9.5 expression was mainly found in the neural tube at the dorsal area of the embryo. PGP9.5 and CK19 were not found in the liver from E10.5 to E11.5, indicating that there is no evidence for the formation of NFs and BDs at this stage (Figure 3).
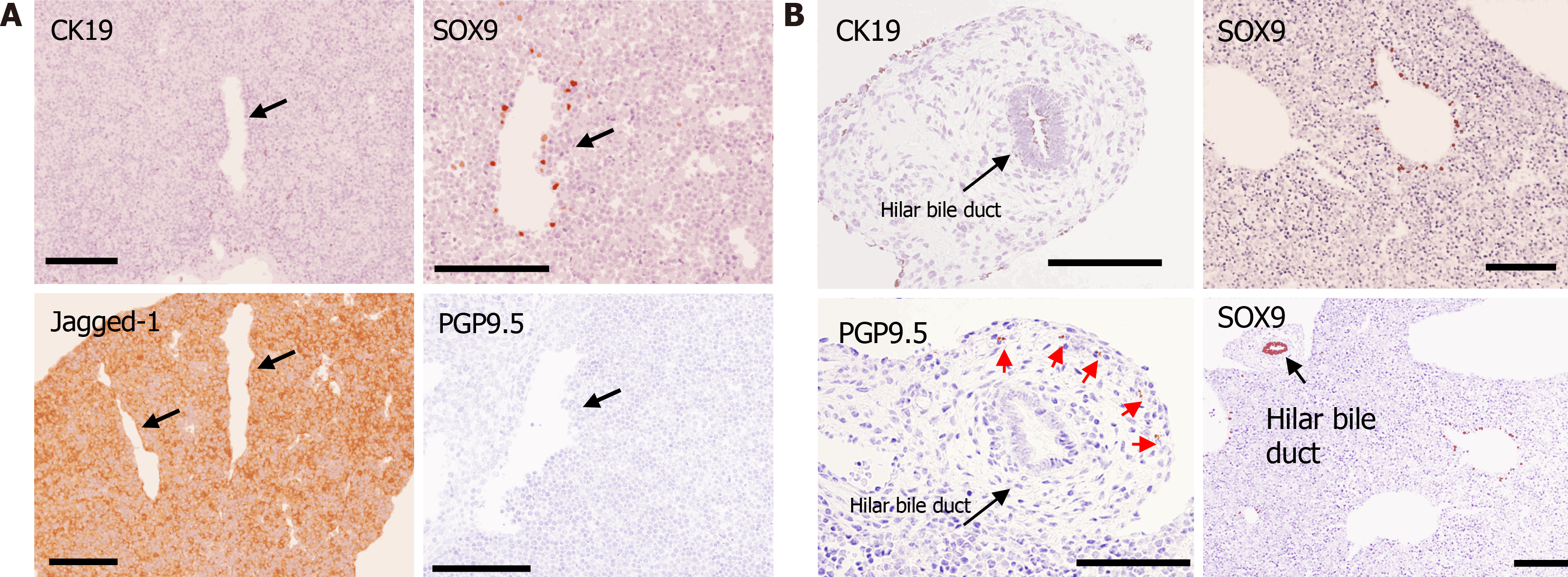
At E12.5, the Notch ligand Jagged-1 was diffusely positive in the liver, and SOX9-positive cells (BECs) were scattered in the periportal area; however, CK19- and PGP9.5-positive cells were not found in the fetal liver from E12.5 to E15.5 (Figure 4). Hilar BD (extrahepatic BD) strongly expressed SOX9 and weakly expressed CK19. PGP9.5-positive bodies were found in the mesenchyme around the hilar BD (Figure 4B).
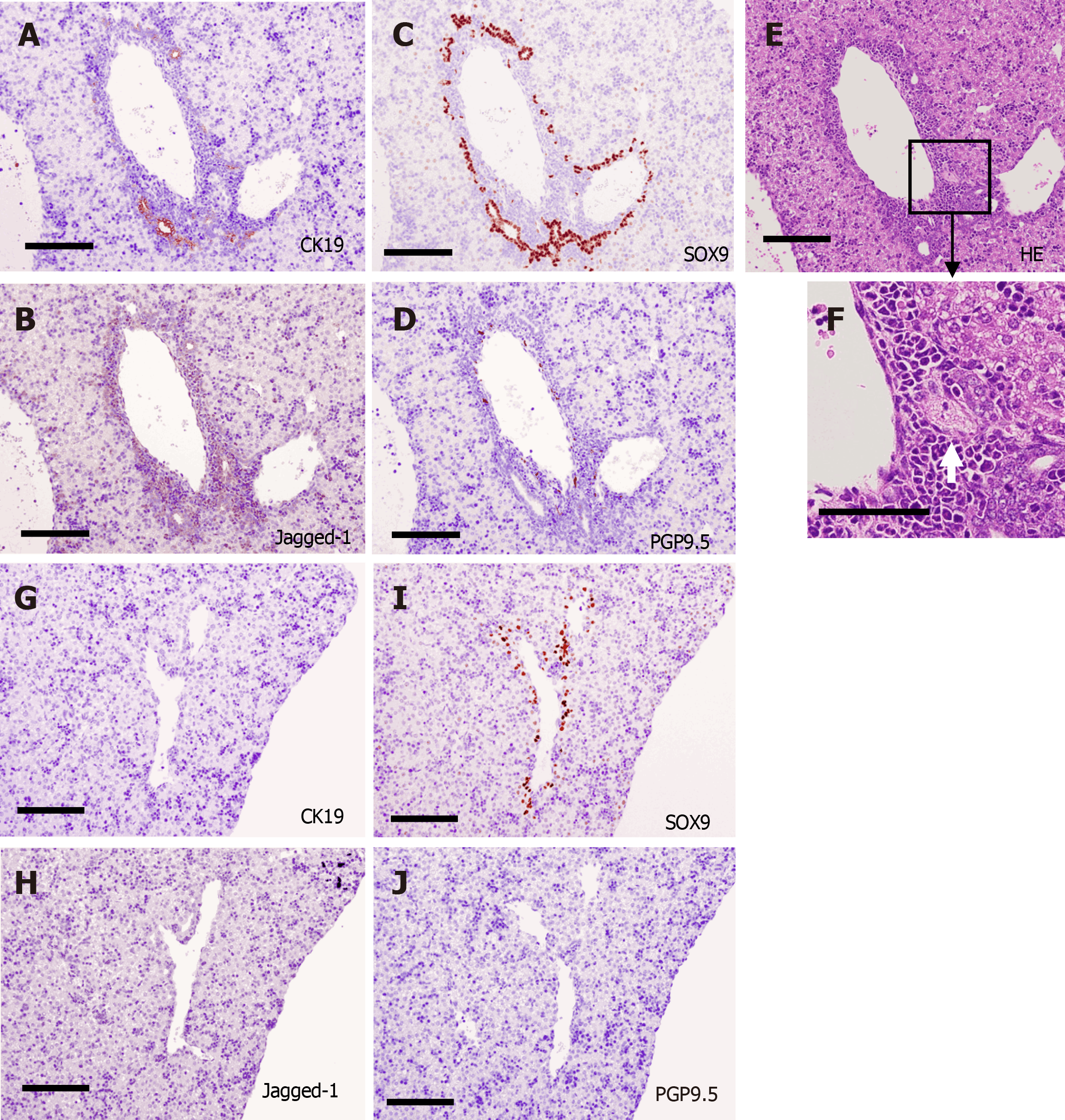
Subsequently, DPs and primitive IHBDs were formed around PVs from E16.5 to P3 (Figures 5 and 6). Jagged-1 was mainly expressed in the periportal tissue, and no cells in the periportal tissue expressed HNF4α in this phase. SOX9-positive cells formed DPs and primitive IHBDs, and CK19-positive cells were first expressed at E16.5 in several SOX9-positive ductal structures, especially around large PVs in the center. PGP9.5-positive bodies were also first found in the periportal tissue in the center at E16.5 (Figure 5). HA emerged at P0, and thus primitive PTs were constructed at first in the center. The numbers of NFs and CK19-positive IHBDs initially increased in the center and then increased in the periphery as well. Jagged-1-positive periportal tissue was thinner in the periphery than in the center, and the number of NFs and IHBDs was smaller in the periphery than in the center in this phase (Figure 6).
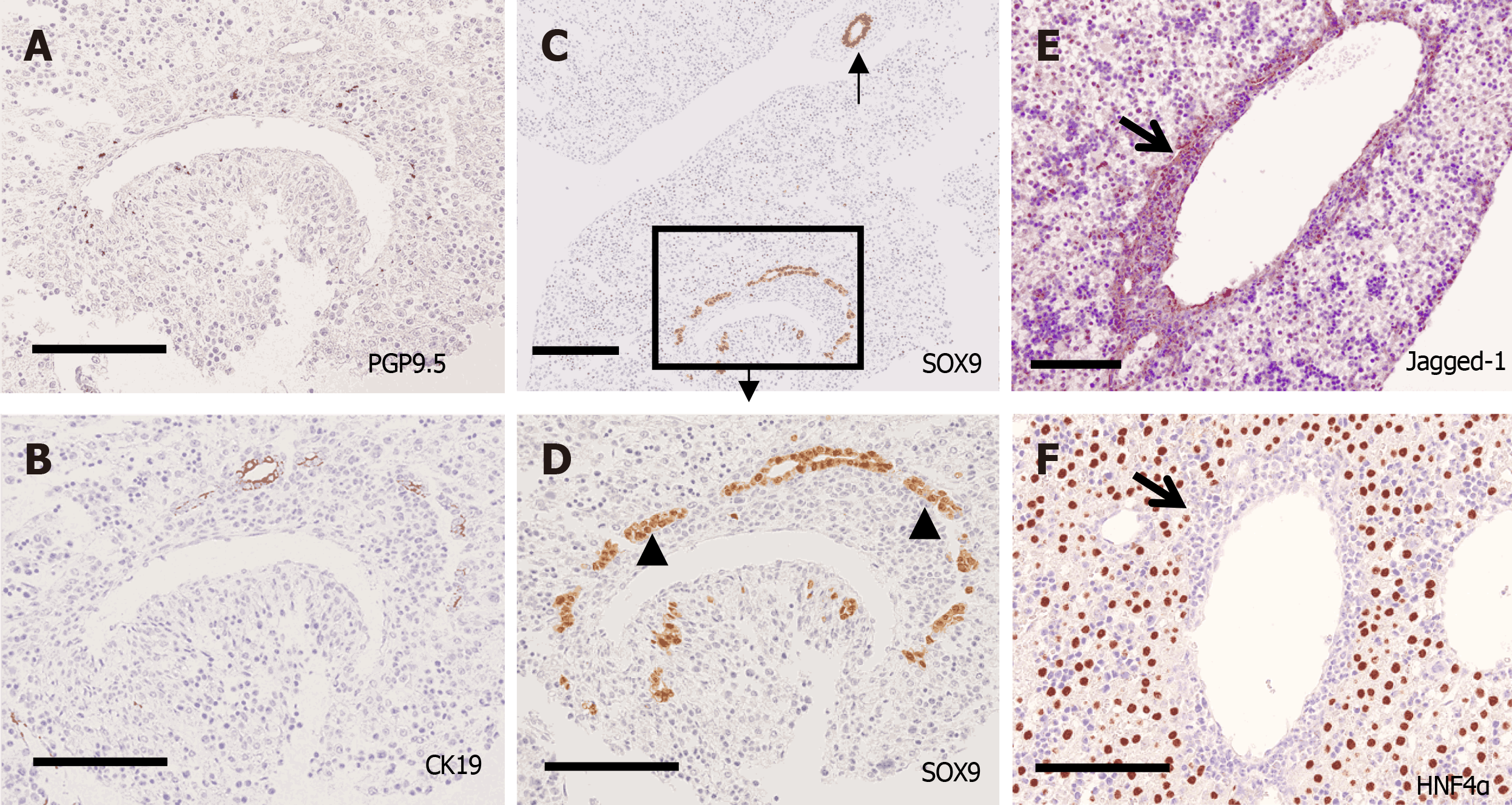
Along with periportal tissue remodeling, excess periportal cells underwent regression, and CK19-positive cells constructed IHBDs not only in the center but also in the periphery from P5 to P7. The distribution of PGP9.5-positive NFs was primarily found in contact with the HA and PV branches and less in contact with IHBD (Figure 7).
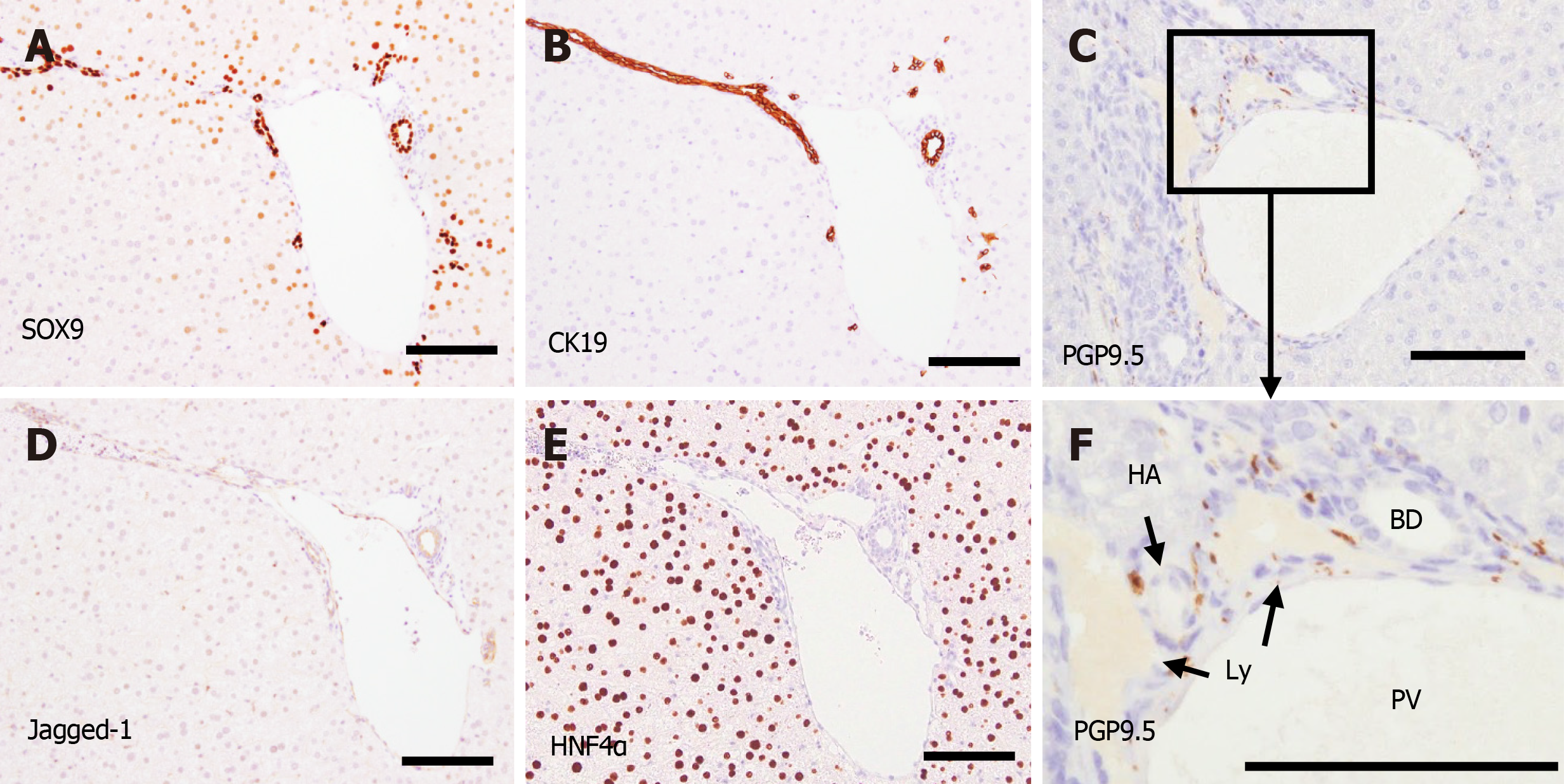
PT formation reached the adult level at P28. PV, BD, NF, and HA were found in PTs both in the center and periphery. According to PGP9.5-positive staining, many small NFs were found around the PVs, and they seemed to produce nerve plexuses surrounding the PVs. However, NFs were found only in the PTs and not in the liver parenchyma. Jagged-1 expression was mainly found in the BD and PV (Figure 8). HNF4α was clearly expressed in the nuclei of hepatocytes, and SOX9 was expressed in the nuclei of BEC during the entire period of this experiment.
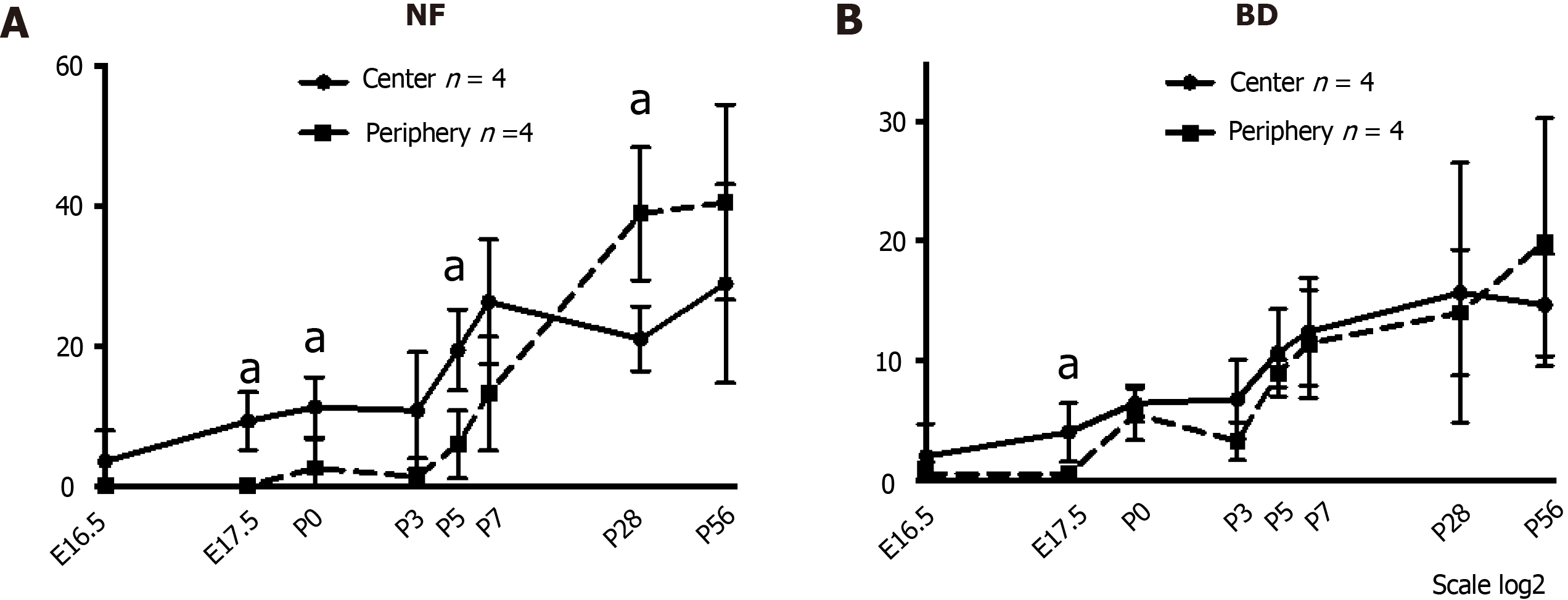
NFs and BDs were first found in the periportal tissue of the center at E16.5. Figure 9 shows quantification of the NF and IHBD in a PT during mouse liver development. In our results, the entire process of PGP9.5-positive NFs and CK19-positive IHBD development progressed from around the hepatic hilus to the peripheral PTs. The numbers of NFs and IHBDs in the PT were larger in the center than in the periphery at the early phase of NF and IHBD development, and the differences were statistically significant at E17.5, P0, and P5 for NFs and at E17.5 for IHBDs. Their numbers gradually increased in the PTs until P7 and then plateaued in the center. On the other hand, their numbers increased even after P7 in the periphery. These results indicate that NFs first emerge at the center just before birth, extend toward the periphery with BDs after birth.
Quantification of NF-IHBD and NF-HA interactions in a PT was made by counting the numbers of NFs that contacted with IHBDs or HAs after birth (Figure 10). NF contacts were observed earlier with HA than with IHBD. The number of NF-HA contacts was larger than the number of NF-IHBD contacts in the center, and the difference was statistically significant from P0 to P7. NF contacts also emerged in the periphery after P3. The number of NF-HA contacts was larger than the number of NF-IHBD contacts in the periphery as well. These results indicate that NF-HA interactions may begin earlier than NF-IHBD interactions.
In-depth studies of both the differentiation and morphogenesis of the liver are prerequisites for in vitro and in vivo reconstitution of hepatic tissue for regenerative medicine. Our study describes morphological and immunohistochemical analyses mainly focused on the development of mouse intrahepatic nerve networks. Organogenesis of the liver and biliary tract occurs from the ventral posterior foregut endoderm near the cardiac mesenchyme as the hepatic diverticulum. The liver is largely composed of hepatocytes and cholangiocytes, which are differentiated from bipotent liver progenitors, the hepatoblasts[10]. It has been known for many years that BD development is initiated near the hepatic hilum before progressing toward the periphery of the lobes. Our understanding of the BD morphogenesis has recently improved with advanced three-dimensional imaging and computer-assisted analysis and with retrograde ink injection enabling visualization of the BD lumina in the whole liver[11,12,16,17]. However, the studies have not sufficiently investigated the embryological development of liver innervation.
Tanimizu et al[15] demonstrated that morphogenesis of IHBDs in the mouse liver gradually spread along the periportal tissue from the hepatic hilus toward the periphery, and the formation of nerve networks followed IHBD development after birth[15]. In addition, they suspected that IHBDs mainly guide the extension of NFs by secreting NGF during NF development in the mouse liver. We previously prepared total RNA from CD45- and Ter119-negative murine liver cells at various developmental stages (E10.5, E11.5, E13.5, E15.5, E17.5, E19.5, P0, P3, and postnatal week 8) using an RNeasy Mini Kit (Qiagen, Venlo, Netherlands). RNA for gene expression profiling was hybridized to the Whole Mouse Genome Agilent 4 × 44K v2 Oligonucleotide Microarray (Agilent) according to the manufacturer’s instructions[3]. To analyze gene expression changes associated with liver development based on microarray data, the processed raw signal intensity of each probe was subjected to 75th percentile normalization. The microarray data also showed an elevated Ngf RNA level in the liver just before birth (Supplemen
The primary function of the fetal liver is hematopoiesis, and the liver receives little innervation during early development. After birth, the role of the liver changes to bile production, metabolism, and protein synthesis, and many different nerve types modulate these functions[18]. The innervation of the liver is different from that of the gastrointestinal tract, which consists of intrinsic ganglionated plexuses situated between the muscle layers of the gut wall. The liver is thought not to contain neural crest-derived intrinsic neurons[18]. For example, experimental studies using a quail/ chick interspecies grafting technique in the chicken embryos supported evidence for this apparent lack of intrinsic neurons in the liver[19]. An ontogenetic study of neuropeptide Y (Npy), a marker of sympathetic nerves showed that sympathetic NFs were not apparent for most of the embryonic period of development until E19. After birth, the density of sympathetic NFs increases to reach a maximum level at 1 wk. In conclusion, the authors suggested that sympathetic NFs derive from extrinsic sources because no neuronal somata were positive for Npy in the fetal liver. In addition, they suspected that sympathetic NFs might enter the liver via the hepatic hilus with HA and PV. Intriguingly, the density of Npy-positive NFs decreased after 1 wk and reached adult level at 2 wk postnatally. Therefore, they thought that transient Npy expression might play an important role in the developing liver[20,21]. In our experiment, neuronal marker-positive bodies were not found during most of the embryonic period in the liver and were only found in the parenchyma of the hilar BD until E15.5. Neuronal marker-positive bodies first emerged around the hepatic hilus at E16.5 and spread toward the periphery thereafter. These results are consistent with results of previous studies.
The NFs are classified by neurotransmitters into aminergic, cholinergic, peptidergic, and nitrergic NFs[22]. The distribution and role of each type of NF have been extensively studied. In addition, in the normal human adult liver, many types of immunoreactive peptidergic nerves have been detected[23-26]. Tiniakos et al[14] reported that Npy-positive NFs, which were examined closely in mouse liver by Ding et al[20,21], were the most abundant peptidergic NFs in the human adult liver and were distributed in PTs and in the acinus along sinusoids. However, no Npy-positive NFs were found in the human fetal liver[14]. On the other hand, during the third trimester, other peptidergic NFs were identified within PTs with a transient expression of galanin, somatostatin and calcitonin-gene-related peptide (CGRP) in the human fetal liver, but these NFs were not observed in the human adult liver. Therefore, the authors suspected that the intrahepatic peptidergic network might play an important role in liver morphogenesis, as Ding et al[20,21] suggested. Our previous microarray data in mice also showed transient elevation of Npy and Cgrp RNA levels around birth; however, galanin and somatostatin RNA was kept at a high level even after birth, different from human studies (Supple
The innervation of the mammalian liver is largely classified as either PT innervation or parenchymal innervation. Parenchymal innervation is composed of NFs present in the hepatic parenchyma. There are no significant differences in PT innervation among species. In contrast, parenchymal innervation is found in humans and guinea pigs but not found in mice and rats[27-30]. Our observations in mice also showed no NFs in the liver parenchyma even in the adult stage. Similarly, the timing of hepatic innervation is different among species. In some human histological studies, NFs emerge before birth in the human liver, much earlier than in the mouse liver. IHBD morphogenesis is thought to start in the fetal liver with the alignment of BEC, which constitute the double-layered cylindrical DP in the PT[17,31]. The DP and primitive IHBD in humans also emerge earlier than in the mouse, and the DP is first found at weeks of gestation (GW) 7 in the hepatic hilus[32,33]. However, many NFs were already found in the hepatic hilus at GW 7, and direct innervation into the DP has also been reported[22]. Therefore, the process of development of the intrahepatic nerve network in humans may be different from in the mouse liver. As already mentioned, many studies have reported that the liver does not appear to be colonized by intrinsic neurons[14,18-20]. To the contrary, Terada reported in a human study that a few neural marker-positive bodies emerged in large PTs at GW 8 and small PTs in the periphery at GW 11; then, the number increased thereafter. Therefore, the neural marker-positive bodies were thought to arise from the intrinsic portal mesenchyme to develop the nerve network in the human liver. However, how intrahepatic NFs connect with the extrinsic nervous system remains unclear[22].
In our study, during PT development, the PV was initially found as a large vessel and constituted a periportal tissue for the development of the IHBDs, HA branching, and NFs. Fabris et al[13] reported that the periportal mesenchyme instructs IHBD development, and VEGF secreted from BECs promotes HA morphogenesis. The interaction between developing BDs and HAs may slightly differ between humans and mice, since arterial morphogenesis in humans occurs along the DP in the fetal liver[34], whereas in mice, HAs emerged around birth in the periportal tissue in our study. Tiniakos et al[30] reported that adrenergic nerves form an intrinsic plexus around the walls of blood vessels but less frequently in relation to BD radicles in PTs in their rodent study. They also showed in their human study a rich neural supply of PTs, mainly around HA and PV branches with less in relation to intralobular BDs[14]. Our study also indicated that NF-HA contacts occur more frequently than NF-BD contacts in the early period of PT development. In adult peripheral tissues, nerves often run along larger blood vessels, reflecting their need for oxygen and nutrients, as well as their physiological control of vasoconstriction and dilation[35]. Similarly, NFs in PTs also regulate hemodynamics in the liver[26]. Arterial vessels are reported to secrete neurotrophic factors such as NGF, neurotrophin 3 (NTF3), and brain-derived neurotrophic factor in some organs[5,36]. A study by Mukouyama et al[37] suggested that VEGF secretion from peripheral nerves provides a template that determines the organotypic pattern of blood vessel branching and arterial differentiation in the skin. Brunet et al[38] indicated that the sympathetic innervation of arteries was facilitated by secretion of the axon guidance molecule netrin-1 by arterial vascular smooth muscle cells in the mesentery. As already mentioned, several key molecules which guide NFs and HA development have already been determined in the BECs[15,17]. On the other hand, the molecules that co-regulate NF and HA development still have not been found. However, the present study showed NF-HA contacts found in an earlier phase than NF-BD contacts and our previous microarray data indicated elevation of Ntf3 and Netrin1 RNA level around birth (Supplemen
Finally, hepatic autonomic nerves are thought to play a role in the liver regeneration. Denervation of the liver NF by vagotomy, operations, or transplantation causes no significant problems in liver functions. This indicates that the autonomous nervous system is not very important regarding lifestyle and life expectancy. However, Hamada et al[39] reported that total hepatic denervation enhanced liver regeneration after a partial hepatectomy. On the contrary, Ikeda et al[40] reported that the parasympathetic system (vagus nerve) contributed to liver regeneration after hepatectomy by stimulating interleukin-6 release from Kupffer cells followed by signal transducer and activator of transcription 3 activation in hepatocytes using mouse experiments. Izumi et al[41] also showed that a vagus-macrophage-hepatocyte link regulates acute liver regeneration after liver injury in mice. Histologically, the DP is directory innervated in a report of Terada. The neural cell adhesion molecule, chromogranin, and synaptophysin are among the markers of hepatic stem/precursor cells (HSPCs). In that study, DP cells were labeled by these molecules, indicating that the DPs contained many HSPCs; therefore, the development of HSPCs might be controlled by NF in the fetus life[22]. Zanchi et al[42] reported the possibility of a widespread interface between nerves and the smallest branches of the proximal biliary tree in human. HSPCs are also thought to be located in the canal of Hering, which is included in the proximal biliary tree in human adult liver. These results suggest that hepatic autonomic nerves impact the development of the liver.
In conclusion, hepatic NFs first emerge at the center just before birth and extend toward the periphery with the IHBDs mainly after birth in mice. Our work and previous reports provide the possibility that the nerve network in the liver plays an important role not only in liver function but also in liver morphogenesis and stabilization of PT structures by interaction between NFs, BDs, and HAs. Furthermore, NFs in the liver may regulate liver progenitor cells. Therefore, nerve progenitor cells may be an additional cell source, along with hepatic, endothelial, and mesenchymal progenitor cells, when liver organoids are constructed.
The hepatic nervous system plays important roles in organisms. However, the role of the hepatic nervous system in liver development remains unclear.
We previously created functional human micro-hepatic tissue in mice by co-culturing human hepatic endodermal cells with endothelial and mesenchymal cells. However, they lacked Glisson’s sheath [the portal tract (PT)]. The PT consists of branches of the hepatic artery (HA), portal vein, and intrahepatic bile duct (IHBD), collectively called the portal triad, together with autonomic nerves. In-depth studies of both the differentiation and morphogenesis of the liver are prerequisites for in vitro and in vivo reconstitution of hepatic tissue for regenerative medicine.
This study describes morphological and immunohistochemical analyses, mainly focusing on the development of mouse intrahepatic nerve networks.
Liver samples from C57BL/6J mice were harvested at different developmental time periods, from embryonic day (E) 10.5 to postnatal day (P) 56. Thin sections of the surface cut through the hepatic hilus were examined using protein gene product 9.5 (PGP9.5) and cytokeratin 19 (CK19) antibodies, markers of nerve fibers (NFs), and biliary epithelial cells. The numbers of NFs and IHBDs were separately counted in a PT around the hepatic hilus (center) and the peripheral area (periphery) of the liver, comparing the average values between the center and the periphery at each developmental stage. NF-IHBD and NF-HA contacts in a PT were also counted, and their relationship was quantified.
Primitive IHBDs at the center partly acquired CK19 positivity at E16.5. PGP9.5-positive bodies were first observed at this time point, and HAs were first detected at P0 in the periportal tissue of the center. Therefore, primitive PT structures were first constructed at P0 in the center. Along with remodeling of the periportal tissue, the number of CK19-positive IHBDs and PGP9.5-positive NFs gradually increased, and PTs also formed in the periphery until P5. The numbers of NFs and IHBDs were significantly higher in the center than in the periphery from E16.5 to P5. The numbers of NFs and IHBDs reached the adult level at P28, with fewer differences between the center and periphery. NFs were more frequently associated with HAs than IHBDs in PTs at the early phase after birth, after which the number of NF-IHBD contacts gradually increased.
Mouse hepatic NFs first emerge at the center just before birth and extend toward the periphery. The interaction between NFs and IHBDs or HAs plays important roles in the morphogenesis and stabilization of the PT structure by interaction between NFs, BDs, and HAs.
Nerve progenitor cells may be an additional cell source, along with hepatic, endothelial, and mesenchymal progenitor cells, when liver organoids are constructed.
We thank Drs. Takanori Takebe, Daisuke Sasai, and Makio Kawakami for their insightful advice and comments and Mr. Hirosuke Nakajima for technical assistance. We are also grateful to Dr. Yasunori Sato for statistical review.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1912] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 2. | Shiojiri N, Kametani H, Ota N, Akai Y, Fukuchi T, Abo T, Tanaka S, Sekiguchi J, Matsubara S, Kawakami H. Phylogenetic analyses of the hepatic architecture in vertebrates. J Anat. 2018;232:200-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1515] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 4. | Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 790] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 6. | Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 7. | Streba LA, Vere CC, Ionescu AG, Streba CT, Rogoveanu I. Role of intrahepatic innervation in regulating the activity of liver cells. World J Hepatol. 2014;6:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kandilis AN, Papadopoulou IP, Koskinas J, Sotiropoulos G, Tiniakos DG. Liver innervation and hepatic function: new insights. J Surg Res. 2015;194:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kamimura K, Inoue R, Nagoya T, Sakai N, Goto R, Ko M, Niwa Y, Terai S. Autonomic nervous system network and liver regeneration. World J Gastroenterol. 2018;24:1616-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Ober EA, Lemaigre FP. Development of the liver: Insights into organ and tissue morphogenesis. J Hepatol. 2018;68:1049-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Takashima Y, Terada M, Kawabata M, Suzuki A. Dynamic three-dimensional morphogenesis of intrahepatic bile ducts in mouse liver development. Hepatology. 2015;61:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Tanimizu N, Mitaka T. Epithelial Morphogenesis during Liver Development. Cold Spring Harb Perspect Biol. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Fabris L, Cadamuro M, Libbrecht L, Raynaud P, Spirlì C, Fiorotto R, Okolicsanyi L, Lemaigre F, Strazzabosco M, Roskams T. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Tiniakos DG, Mathew J, Kittas C, Burt AD. Ontogeny of human intrahepatic innervation. Virchows Arch. 2008;452:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Tanimizu N, Ichinohe N, Mitaka T. Intrahepatic bile ducts guide establishment of the intrahepatic nerve network in developing and regenerating mouse liver. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Kok CY, Miyajima A, Itoh T. Adaptive remodeling of the biliary tree: the essence of liver progenitor cell expansion. J Hepatobiliary Pancreat Sci. 2015;22:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Delalande JM, Milla PJ, Burns AJ. Hepatic nervous system development. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Ding WG, Tooyama I, Kitasato H, Fujimura M, Kimura H. Phylogenetic and ontogenetic study of neuropeptide Y-containing nerves in the liver. Histochem J. 1994;26:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ding WG, Kitasato H, Kimura H. Development of neuropeptide Y innervation in the liver. Microsc Res Tech. 1997;39:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Terada T. Ontogenic development of nerve fibers in human fetal livers: an immunohistochemical study using neural cell adhesion molecule (NCAM) and neuron-specific enolase (NSE). Histochem Cell Biol. 2015;143:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Akiyoshi H, Gonda T, Terada T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver. 1998;18:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Ueno T, Inuzuka S, Torimura T, Sakata R, Sakamoto M, Gondo K, Aoki T, Tanikawa K, Tsutsumi V. Distribution of substance P and vasoactive intestinal peptide in the human liver: light and electron immunoperoxidase methods of observation. Am J Gastroenterol. 1991;86:1633-1637. [PubMed] |
| 25. | el-Salhy M, Stenling R, Grimelius L. Peptidergic innervation and endocrine cells in the human liver. Scand J Gastroenterol. 1993;28:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | McCuskey RS. Anatomy of efferent hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Tsuneki K, Ichihara K. Electron microscope study of vertebrate liver innervation. Arch Histol Jpn. 1981;44:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Ohata M. Electron microscope study on the innervation of guinea pig liver--proposal of sensory nerve terminals in the hepatic parenchyme. Arch Histol Jpn. 1984;47:149-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Miyazawa Y, Fukuda Y, Imoto M, Koyama Y, Nagura H. Immunohistochemical studies on the distribution of nerve fibers in chronic liver diseases. Am J Gastroenterol. 1988;83:1108-1114. [PubMed] |
| 30. | Tiniakos DG, Lee JA, Burt AD. Innervation of the liver: morphology and function. Liver. 1996;16:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432-1438, 1438.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 32. | Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology. 1988;8:1586-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Terada T. Human fetal ductal plate revisited. I. ductal plate expresses NCAM, KIT, MET, PDGFRA, and neuroendocrine antigens (NSE, chromogranin, synaptophysin, and CD56). Microsc Res Tech. 2014;77:814-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Terada T. Development of extrahepatic bile duct excluding gall bladder in human fetuses: histological, histochemical, and immunohistochemical analysis. Microsc Res Tech. 2014;77:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Mukouyama YS. Vessel-dependent recruitment of sympathetic axons: looking for innervation in all the right places. J Clin Invest. 2014;124:2855-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 495] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 38. | Brunet I, Gordon E, Han J, Cristofaro B, Broqueres-You D, Liu C, Bouvrée K, Zhang J, del Toro R, Mathivet T, Larrivée B, Jagu J, Pibouin-Fragner L, Pardanaud L, Machado MJ, Kennedy TE, Zhuang Z, Simons M, Levy BI, Tessier-Lavigne M, Grenz A, Eltzschig H, Eichmann A. Netrin-1 controls sympathetic arterial innervation. J Clin Invest. 2014;124:3230-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Hamada T, Eguchi S, Yanaga K, Inuo H, Yamanouchi K, Kamohara Y, Okudaira S, Tajima Y, Kanematsu T. The effect of denervation on liver regeneration in partially hepatectomized rats. J Surg Res. 2007;142:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Ikeda O, Ozaki M, Murata S, Matsuo R, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kawasaki T, Kohno K, Ohkohchi N. Autonomic regulation of liver regeneration after partial hepatectomy in mice. J Surg Res. 2009;152:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Izumi T, Imai J, Yamamoto J, Kawana Y, Endo A, Sugawara H, Kohata M, Asai Y, Takahashi K, Kodama S, Kaneko K, Gao J, Uno K, Sawada S, Kalinichenko VV, Ishigaki Y, Yamada T, Katagiri H. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat Commun. 2018;9:5300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 42. | Zanchi A, Reidy J, Feldman HJ, Qualter J, Gouw AS, Osbeck J, Kofman A, Balabaud C, Bioulac-Sage P, Tiniakos DG, Theise ND. Innervation of the proximal human biliary tree. Virchows Arch. 2020;477:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |