Published online Aug 27, 2021. doi: 10.4254/wjh.v13.i8.949
Peer-review started: March 25, 2021
First decision: June 4, 2021
Revised: June 14, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: August 27, 2021
Processing time: 148 Days and 3.3 Hours
Liver fibrosis leads to liver-related events in patients with chronic hepatitis C (CHC) infection. Although non-invasive tests (NITs) are critical to early detection of the development of liver fibrosis, the prognostic role of NITs remains unclear due to the limited types of NITs and liver outcomes explored in previous studies.
To determine the prognostic value of NITs for risk stratification in CHC patients.
The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42019128176). The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Search was performed using MEDLINE and EMBASE databases under a timeframe from the inception of the databases through February 25, 2020. We restricted our search to CHC cohort studies reporting an association between liver fibrosis assessed by NITs and the development of hepatocellular carcinoma, decompensation, or mortality. Pooled hazard ratios (HR) and area under the receiver operating characteristic (AUROC) for each NIT were estimated using a random effects model. Subgroup analyses were performed for NITs assessed at pre-treatment or post-treatment with sustained virologic response (SVR), treatment with either pegylated interferon and ribavirin or direct acting antiviral, Eastern or Western countries, and different cutoff points.
The present meta-analysis included 29 cohort studies, enrolling 69339 CHC patients. Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and liver stiffness measurement (LSM) were found to have hepatocellular carcinoma predictive potential with pooled adjusted HRs of 2.48 [95% confidence interval (CI): 1.91-3.23, I2 = 96%], 4.24 (95%CI: 2.15-8.38, I2 = 20%) and 7.90 (95%CI: 3.98-15.68, I2 = 52%) and AUROCs of 0.81 (95%CI: 0.73-0.89, I2 = 77%), 0.81 (95%CI: 0.75-0.87, I2 = 68%), and 0.79 (95%CI: 0.63-0.96, I2 = 90%), respectively. Pooled adjusted HR with a pre-treatment FIB-4 cutoff of 3.25 was 3.22 (95%CI: 2.32-4.47, I2 = 80%). Pooled adjusted HRs for post-treatment with SVR FIB-4, APRI, and LSM were 3.01 (95%CI: 0.32-28.61, I2 = 89%), 9.88 (95%CI: 2.21-44.17, I2 = 24%), and 6.33 (95%CI: 2.57-15.59, I2 = 17%), respectively. Pooled adjusted HRs for LSM in patients with SVR following direct acting antiviral therapy was 5.55 (95%CI: 1.47-21.02, I2 = 36%). Pooled AUROCs for post-treatment with SVR FIB-4 and LSM were 0.75 (95%CI: 0.55-0.95, I2 = 88%) and 0.84 (95%CI: 0.66-1.03, I2 = 88%), respectively. Additionally, FIB-4 and LSM were associated with overall mortality, with pooled adjusted HRs of 2.07 (95%CI: 1.49-2.88, I2 = 27%) and 4.04 (95%CI: 2.40-6.80, I2 = 63%), respectively.
FIB-4, APRI, and LSM showed potential for risk stratification in CHC patients. Cutoff levels need further validation.
Core Tip: Previous meta-analyses have evidenced the potential of non-invasive tests (NITs) in determining prognosis. However, these syntheses included studies on chronic liver diseases from various etiologies and did not comprehensively explore all liver-related outcomes. We aimed to assess the importance of validated NITs in risk stratification, specifically in chronic hepatitis C (CHC) patients. Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score and liver stiffness mea
- Citation: Yongpisarn T, Thimphitthaya C, Laoveeravat P, Wongjarupong N, Chaiteerakij R. Non-invasive tests for predicting liver outcomes in chronic hepatitis C patients: A systematic review and meta-analysis. World J Hepatol 2021; 13(8): 949-968
- URL: https://www.wjgnet.com/1948-5182/full/v13/i8/949.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i8.949
Chronic hepatitis C (CHC) infection can lead to the development of liver fibrosis and cirrhosis that are commonly associated with hepatocellular carcinoma (HCC), other liver-related events (LREs), and mortality. Liver biopsy is considered the gold standard for evaluating liver fibrosis in patients with chronic liver disease. Since the introduction of non-invasive tests (NITs), biopsy use has substantially declined. Currently available NITs for liver fibrosis assessment include direct and indirect serum markers and radiologic examination such as liver stiffness measurement (LSM). According to the 2018 European Association for the Study of the Liver guidelines, the degree of liver fibrosis should be assessed by NITs in CHC patients prior to any treatment[1]. The degree of liver fibrosis determines optimal treatment regimen and whether the patient requires post-treatment monitoring of HCC development. NITs are also recommended for monitoring untreated CHC patients every 1 to 2 years[2].
Although serum markers and LSM have been shown to identify accurately patients with cirrhosis (F4) and patients without fibrosis (F0), their ability to stage intermediate degrees of fibrosis and post-treatment residual fibrosis is suboptimal[2,3]. The difficulties in the prediction of significant or advanced fibrosis without histologic confirmation has made risk stratification problematic for some CHC patients. For instance, the decision to pursue HCC surveillance following successful treatment of hepatitis C virus (HCV) infection [i.e. sustained virologic response (SVR)] is controversial for patients with advanced fibrosis (F3)[2,4].
Previous meta-analyses have evidenced the potential of NITs in determining prognosis. However, these syntheses included studies on chronic liver diseases from various etiologies and did not comprehensively explore all liver-related outcomes[5,6]. Types of NITs investigated in these meta-analyses were also limited. In this present review, we provided an updated systematic review and meta-analysis to assess the importance of validated NITs in risk stratification specific to CHC patients.
The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42019128176). The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines[7]. Search was performed using MEDLINE and EMBASE databases from the inception of databases to February 25, 2020. The NITs for hepatic fibrosis included in our review were retrieved from the European Association for the Study of the Liver, Asociación Latinoamericana para el Estudio del Hígado Clinical Practice Guidelines[1]. The list of serum biomarkers and respective formulae are provided in Supplemental Table 1. In addition to the list of NITs, the terms prognosis, decompensation, hepatocellular cancer, chronic hepatitis C, and their related terms were selected as keywords. The details of the search strategy are provided in Supplemental Table 2. We restricted our search to cohort studies. Publications in the reference list of our included studies, publications that cited the included studies, and publications that were included in recent meta-analyses[8,9] of NITs and chronic liver diseases were also reviewed.
| Ref. | Country | n | NITs | Outcomes |
| Chun et al[49], 2020 | South Korea | 669 | FIB-4 | HCC |
| Chalouni et al[18], 2019 | France | 998 | APRI, FIB-4, TE | LRE |
| Chen et al[45], 2019 | China | 691 | FIB-4 | OM |
| Hansen et al[20], 2019 | Denmark | 591 | TE | OM, LRD, HD |
| Ioannou et al[13], 2019 | United States | 48135 | FIB-4 | HCC |
| Na et al[33], 2019 | South Korea | 295 | APRI, FIB-4 | HCC |
| Nakagomi et al[34], 2019 | Japan | 1146 | TE | HCC |
| Ogasawara et al[38], 2019 | Japan | 398 | FIB-4, TE | HCC, HD |
| Ogasawara et al[47], 2019 | Japan | 457 | FIB-4 | OM |
| Peleg et al[23], 2019 | Israel | 515 | TE | HCC, OM, HD |
| Pons et al[14], 2019 | Spain | 572 | TE | HCC |
| Rinaldi et al[15], 2019 | Italy | 258 | TE | HCC |
| Shili-Masmoudi et al[28], 2019 | France | 1062 | TE | OM, LRM |
| Sou et al[41], 2019 | China | 1884 | APRI, FIB-4 | HCC |
| Tamaki et al[30], 2019 | Japan | 346 | FIB-4, MRE | HCC |
| Watanabe et al[44], 2019 | Japan | 1174 | APRI, FIB-4 | HCC |
| Bloom et al[17], 2018 | Australia | 780 | TE | LRE |
| Hamada et al[29], 2018 | Japan | 196 | FIB-4, SWE | HCC |
| Munteanu et al[22], 2018 | France | 3449 | Fibrotest | OM, LRM |
| Cepeda et al[25], 2017 | United States | 964 | TE | OM |
| Gomez-Moreno et al[19], 2017 | Spain | 343 | TE | HCC, HD, LRM |
| Merchante et al[26], 2017 | Spain | 446 | TE | HD |
| Thandassery et al[43], 2017 | Qatar | 1605 | APRI, FIB-4 | HCC, HD, LRE |
| Akuta et al[39], 2016 | Japan | 958 | FIB-4 | HCC |
| Lee et al[31], 2016 | South Korea | 598 | APRI | HCC |
| Lee et al[46], 2016 | South Korea | 190 | TE | LRE |
| Ng et al[36], 2016 | China | 105 | APRI | HCC |
| Pérez-Latorre et al[24], 2016 | Spain | 957 | TE | LRE, OM |
| Sato et al[40], 2016 | Japan | 355 | APRI, FIB-4 | HCC |
| Tada et al[48], 2016 | Japan | 1723 | FIB-4 | LRM, OM |
| Berenguer et al[12], 2015 | Spain | 903 | FIB-4 | LRE, OM |
| Macías et al[21], 2015 | Spain | 1046 | TE | HD, OM |
| Narita et al[35], 2014 | Japan | 151 | TE | HCC |
| Nojiri et al[37], 2014 | Japan | 142 | APRI, FIB-4, Forns index | HCC |
| Tamaki et al[42], 2014 | Japan | 1046 | FIB-4 | HCC |
| Bambha et al[16], 2012 | United States | 450 | APRI, FIB-4 | OM |
| Nunes et al[27], 2010 | United States | 303 | APRI, FIB-4 | LRM |
| Masuzaki et al[32], 2009 | Japan | 984 | TE | HCC |
| Yu et al[11], 2006 | China | 1338 | APRI | HCC, OM |
| Analysis | HR | aHR | ||||||
| Pooled HR (95%CI) | I2 (%) | Ref. | No. of cases | Pooled aHR (95%CI) | I2 (%) | Ref. | No. of cases | |
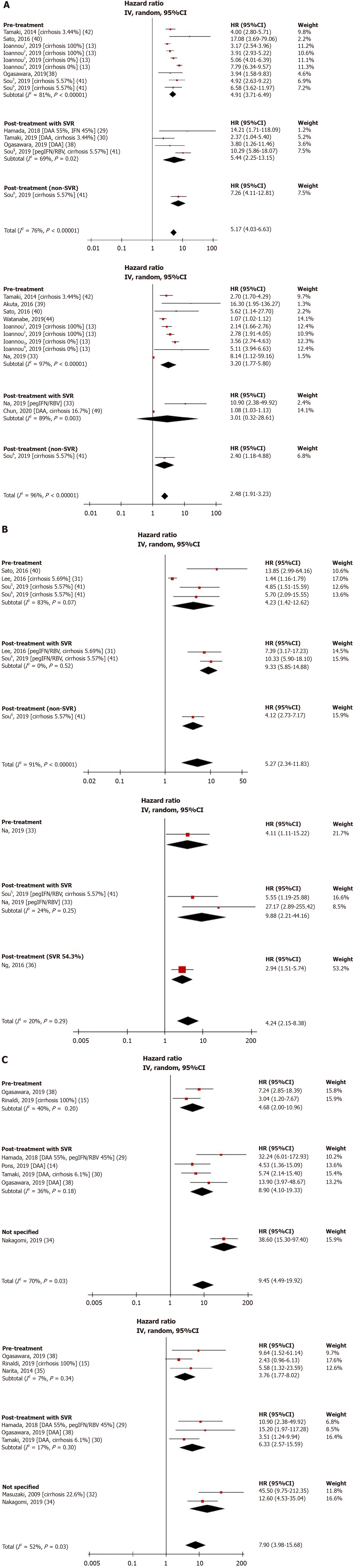
| FIB-4 | 5.17 (4.03-6.63) | 76 | [13,29,30,38,40-42] | 1831 | 2.48 (1.91-3.23) | 96 | [13,33,39-42,44,49] | 1842 |
| pre-Rx | 4.91 (3.71-6.49) | 81 | [13,38,40-42] | 1781 | 3.20 (1.77-5.80) | 97 | [13,33,39-40,42,44] | 1699 |
| post-Rx with SVR | 5.44 (2.25-13.15) | 69 | [29,30,38,41] | 173 | 3.01 (0.32-28.61) | 89 | [33,49] | 21 |
| APRI | 5.27 (2.34-11.83) | 91 | [31,40,41] | 150 | 4.24 (2.15-8.38) | 20 | [33,36,41] | 149 |
| pre-Rx | 4.23 (1.42-12.62) | 83 | [31,40,41] | 142 | - | - | [33] | 12 |
| post-Rx with SVR | 9.33 (5.85-14.88) | 0 | [31,41] | 130 | 9.88 (2.21-44.16) | 24 | [33,41] | 134 |
| LSM | 9.45 (4.49-19.92) | 70 | [14,15,29,30,34,38] | 301 | 7.90 (3.98-15.68) | 52 | [15,29,30,32,34,35,38] | 362 |
| pre-Rx | 4.68 (2.00-10.96) | 40 | [15,38] | 54 | 3.76 (1.77-8.02) | 7 | [15,35,38] | 63 |
| post-Rx with SVR | 8.90 (4.10-19.33) | 36 | [14,29,30,38] | 76 | 6.33 (2.57-15.59) | 17 | [29,30,38] | 51 |
Two reviewers (TY and CT) independently searched for studies on the prognosis of CHC patients based on non-invasive staging of liver fibrosis. Title and abstract of the studies were initially screened. The full-text of these studies were then independently assessed for eligibility by the two reviewers. Cohort studies that met the following criteria were included: (1) NITs documented and used to identify CHC patients who had a risk of developing LREs including hepatic decompensation, HCC, and/or mortality. Hepatic decompensation (HD) was defined as the development of variceal bleeding, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, jaundice, and/or hepatorenal syndrome; (2) Patients were free of HCC and HD at enrollment; (3) Development of HD, HCC and mortality were assessed; and (4) Outcomes of interest were reported by hazard ratio (HR), relative risk, or area under the receiver operating characteristic (AUROC). Whereas studies of any size or language were included, the following studies were excluded: (1) Case-control studies, cross-sectional studies, case series, and conference abstracts; and (2) Trials enrolling patients with no evidence of HCV infection or when more than 10% of the patients were co-infected with HBV. Publications detailing the same patient cohorts but reporting different outcomes of interest were selected for separate analysis. When publications from the same cohort described the same outcomes, the study with the most comprehensive data or with the longest follow-up was selected for each outcome[10]. Any disa
A standardized form was used to extract data from the selected papers. Data included study characteristics (primary author, country, publication year, patient enrollment period, duration of follow-up), patient characteristics (age, sex, co-infection, baseline levels of NITs, fibrosis stages, HCV treatment regimen, response), method of NITs, endpoint (HD, HCC, overall and liver-related mortality), HR and AUROCs with 95% confidence intervals (95%CI), and control variables used for the adjusted analysis. Two reviewers (TY and CT) extracted the data independently, discrepancies were identified and discussed with a third reviewer (PL). Any missing data from the publications were requested from the study authors.
A quality assessment of prognostic studies was performed independently by TY and CT using the Quality In Prognosis Studies tool[10]. Any disagreements between the reviewers over the risk of bias in particular studies were resolved via discussion with a third reviewer (PL).
Primary analysis assessed the performance of NITs in the prediction of LRE development in CHC patients. The analysis of each outcome was computed using a random-effects model. Since relative risk was provided by only one study[11], it was not included in our meta-analysis. Inverse variance method was used to pool the results. Unadjusted and adjusted HRs were pooled separately. Additionally, the significance of each NIT’s prognostic value was assessed vs the random value (mean AUROC of each NIT was compared with 0.50 or the “random” value representing the absence of prognostic value). We then pooled the results, and 0.50 was added back to illustrate the overall prognostic value of each NIT. The AUROCs of different NITs were then compared using t-tests to identify any statistical difference in terms of prognostic ability. Subgroup analyses based on timing of liver fibrosis assessment (before or after HCV treatment) were performed when possible. Heterogeneity between studies was considered when I2value was greater than 50%. Publication bias was first evaluated by constructing funnel plots. Egger's linear regression test was also performed due to possible bias ascertained from funnel plots. All analyses were conducted using Review Manager (RevMan) [Computer program], Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, and ProMeta (Version 3) [Computer software] (Internovi, Cesena, Italy).
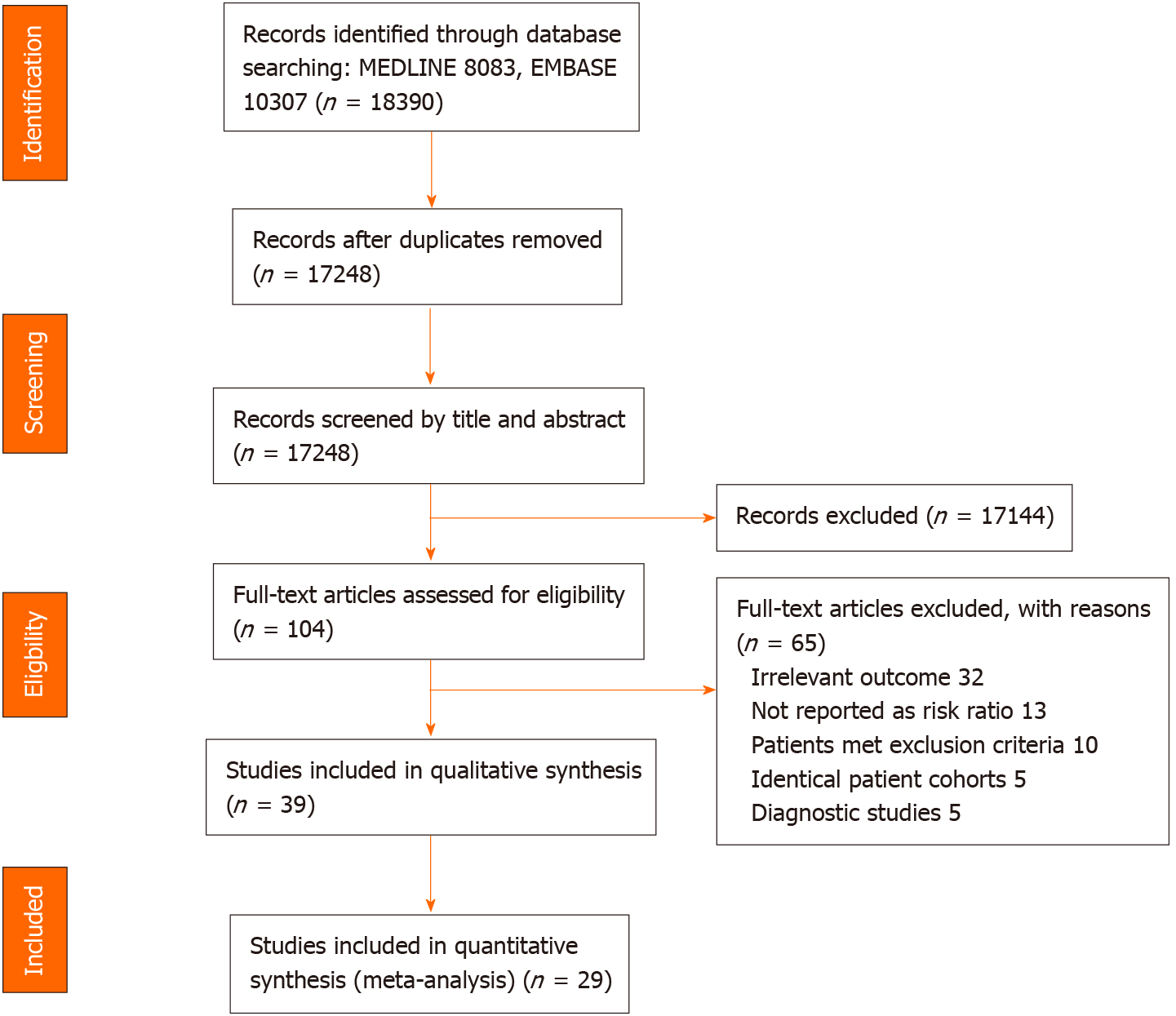
After removing duplicate publications, 17248 papers were identified and screened by title and abstract. Of these, 104 full articles met our predefined selection criteria and were further examined. We further excluded 65 publications due to the following reasons: Non-relevant outcomes (n = 32), outcomes not reported as risk ratio (n = 13), patients meeting our exclusion criteria, e.g., prior history of HCC (n = 10), studies of the same patient cohorts (n = 5), and NITs being used as diagnostic tests for HCC or HD (n = 5) (Figure 1).
Among the 39 cohort studies matching our selection criteria, 29 studies (69339 HCV-infected patients) were selected for quantitative analysis, with the 10 remaining studies slated only for qualitative analysis.
These 39 included studies enrolled a total of 77920 participants between 1990 and 2015. Seventeen and 22 studies were conducted in Western[12-28] and Asian countries[11,29-49], respectively (Table 1, Supplemental Table 3).
| Ref. | NIT1 | Outcome | AUROC (95%CI) |
| Chalouni et al[18], 2019 | APRI | OM | 0.58 (N/A) |
| LRM | 0.80 (N/A) | ||
| LRE | 0.75 (N/A) | ||
| FIB-4 | OM | 0.66 (N/A) | |
| LRM | 0.88 (N/A) | ||
| LRE | 0.78 (N/A) | ||
| TE | OM | 0.69 (N/A) | |
| LRM | 0.88 (N/A) | ||
| LRE | 0.88 (N/A) | ||
| Hansen et al[20], 2019 | TE | OM | 0.70 (0.62–0.78) |
| LRM | 0.93 (0.89–0.98) | ||
| HD (HCC included) | 0.89 (0.82–0.97) | ||
| Munteanu et al[22], 2018 | Fibrotest | OM | 0.74 (0.71-0.77) |
| LRM | 0.88 (0.85-0.90) | ||
| Thandassery et al[43], 2017 | APRI (Pre-Rx) | HD | 0.54 (0.06–0.78) |
| FIB-4 (Pre-Rx) | HD | 0.85 (0.74–0.96) | |
| Pérez-Latorre et al[24], 2016 | TE | OM | Estimation cohort 0.87 (0.84-0.90) |
| Validation cohort 0.88 (0.84-0.91) | |||
| Lee et al[46], 2016 | TE (Post-Rx) | A composite outcome of HD, HCC, and/or LRM | 0.92 (0.84-1.00) |
| Berenguer et al[12], 2015 | FIB-4 (Pre-Rx) | LRE (HD or HCC) | 0.75 (0.72-0.78) |
| Yu et al[11], 2006 | APRI (Pre-Rx) | OM | 0.53 (0.35-0.72) |
| APRI (Post-Rx) | OM | 0.87 (0.81-0.93) |
The performance of the Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and LSM tests for the prediction of LREs and mortality were characterized in 20, 11, and 19 studies, respectively. LSM was mainly performed by ultrasound-based transient elastography (TE), except in two studies that used either magnetic resonance elastography (MRE)[30], or 2D-shear wave elastography (2D-SWE)[29].
The primary outcomes of interest were HCC, overall mortality, and liver-related mortality in 21[11,13-15,29-44,49], 12[3,12,17-24,38,46], and 10[16,18,20,21,25,27,28,45,47,48] studies, respectively. Twelve studies selected HD or a compound of LREs as relevant outcome(s)[12,17-21,23,24,26,38,43,46]. Characteristics of all the studies are summarized in Table 1 and Supplemental Table 3.
Eleven studies enrolled patient cohorts with HCV and human immunodeficiency virus co-infection[12,16,18,21,22,24-28,45]. Fifteen reports included only patients who were successfully treated, i.e. having SVR[13,14,23,29-31,33,36,38-40,44,46,47,49], while two studies enrolled only patients with cirrhosis[13,15]. All studies had a mean or median follow-up time of at least 1 year.
FIB-4, APRI, and LSM were among the most extensively explored NITs (Table 2). We did not conduct quantitative analysis using other NITs due to their very limited usage (n = 1 for Forns index[37] and Fibrotest[22], n = 0 for other NITs).
The included studies were mostly rated as low risk of bias (n = 27)[11-14,16,19-23,25,26,28-30,32,33,35-39,42-44,46,48] (Supplementary Table 4, Supplementary Figure 1). However, five studies were rated as high risk of bias because of concerns about selective reporting of multivariate analysis and other biases[34,40,41,45,49]. Only 13 studies provided the number of patients lost to follow-up[13,14,17,20-22,24,28,32,36,37,44,45]. The agreement between the two reviewers’ assessment was excellent (93%).
Among NITs included in the present analysis, FIB-4 score was the most studied NIT for its role in HCC prediction. Eleven studies including 1891 HCC cases examined the relationship between FIB-4 values and HCC development[13,29,30,33,38-42,44,49]. The FIB-4 cutoffs selected in these studies ranged from 2.5 to 4.5. All these studies reported a significant positive association between high FIB-4 values and risk of HCC development, with pooled unadjusted and adjusted HRs of 5.17 (95%CI: 4.03-6.63, I2 = 76%) and 2.48 (95%CI: 1.91-3.23, I2 = 96%), respectively (Figure 2A).
Five studies totaling 169 HCC cases evaluated the prognostic value of APRI and found a statistically significant positive association between high APRI values and HCC occurrence[31,33,36,40,41]. The APRI cutoffs used in these studies ranged from 0.5 to 2.0. The overall pooled unadjusted and adjusted HRs were 5.27 (95%CI: 2.34-11.83, I2 = 91%) and 4.24 (95%CI: 2.15-8.38, I2 = 20%), respectively (Figure 2B).
Eight studies with 387 HCC cases investigated the association between LSM and HCC risk[14,15,29,30,32,34,35,38]. The LSM cutoffs chosen for each study were all unique and ranged from 3.75 to 30. Consistent with FIB-4 score and APRI results, the overall pooled unadjusted and adjusted HRs were 9.45 (95%CI: 4.49-19.92, I2 = 70%) and 7.90 (95%CI: 3.98-15.68, I2 = 52%), respectively (Figure 2C).
Subgroup analyses were performed for NITs assessed at pre-treatment and post-treatment with SVR. Pooled adjusted HRs for pre-treatment FIB-4 and LSM were 3.20 (95%CI: 1.77-5.80, I2 = 97%) and 3.76 (95%CI: 1.77-8.02, I2 = 7%), respectively. Pooled adjusted HRs for post-treatment with SVR FIB-4, APRI, and LSM were 3.01 (95%CI: 0.32-28.61, I2 = 89%), 9.88 (95%CI: 2.21-44.16, I2 = 24%), and 6.33 (95%CI: 2.57-15.59, I2 = 17%), respectively (Figure 2). The prognostic ability of these NITs remains valid even after the introduction of direct-acting antiviral (DAA) therapy. Pooled unadjusted and adjusted HRs for LSM in patients with SVR following DAA therapy were 6.80 (95%CI: 3.54-13.05, I2 = 0%) and 5.55 (95%CI: 1.47-21.02, I2 = 36%), respectively (Supplementary Figure 2).
To determine the optimal cutoff for HCC prediction, we pooled the results using a pre-treatment FIB-4 cutoff of 3.25 as this cutoff was applied in four studies, accounting for over 51360 CHC patients (Supplementary Figure 3). We found that the pooled, unadjusted and adjusted HRs were 4.79 (95%CI: 3.58-6.42, I2 = 85%) and 3.22 (95%CI: 2.32-4.47, I2 = 80%), respectively, for predicting HCC development.
Given the high heterogeneity of the analysis of pre-treatment FIB-4, we performed subgroup analyses by location of study. We found that, in the subgroup of Asian countries, pooled unadjusted and adjusted HRs of 4.91 (95%CI: 3.60-6.70, I2 = 18%) and 3.12 (95%CI: 1.31-7.42, I2 = 87%) for the pre-treatment FIB-4 and HCC development (Supplementary Figure 4). The I2 of pooled unadjusted HR decreased from 76% to 18%, while the I2 of pooled adjusted HR slightly decreased from 97% to 87%. We hypothesized that the remaining high heterogeneity stemmed from the variety of FIB-4 cutoff used in the different studies.
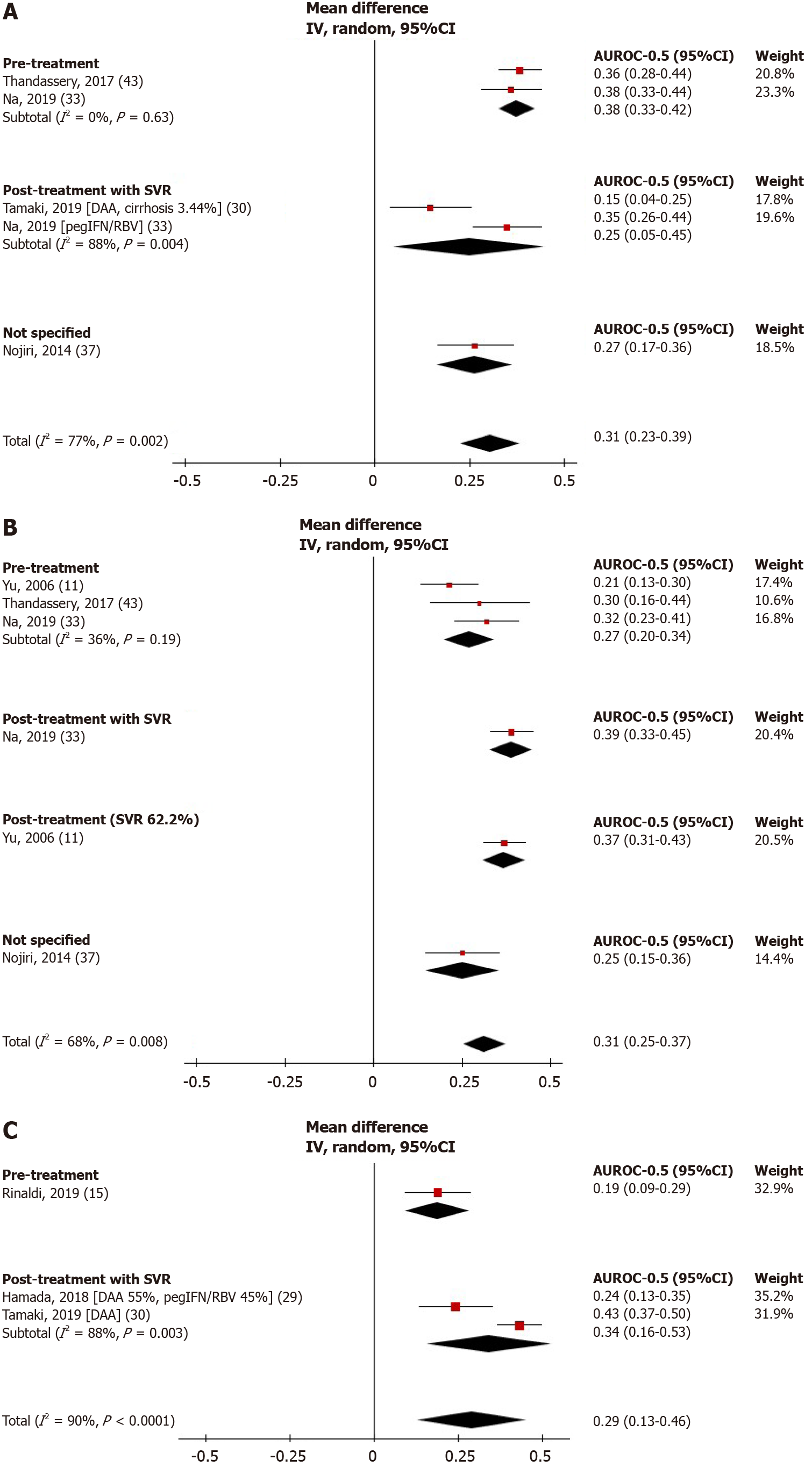
Figure 3 shows the performance of NITs for HCC prediction. FIB-4 score, APRI, and LSM was significantly greater than random (AUROC = 0.5), with pooled AUROCs of 0.81 (95%CI: 0.73-0.89, I2 = 77%), 0.81 (95%CI: 0.75-0.87, I2 = 68%), and 0.79 (95%CI: 0.63-0.96, I2 = 90%), respectively. The pooled AUROCs of FIB-4 and APRI were both statistically higher than that of the LSM, P < 0.0001 for both, respectively.
We further analyzed the prognostic values of NITs before and after HCV treatment. For the pre-treatment period, the pooled AUROC of FIB-4 score was significantly greater compared to APRI (0.88, (95%CI: 0.83-0.92, I2 = 0%) vs 0.77, (95%CI: 0.70-0.84, I2 = 36%), P < 0.0001). For NITs assessed at post-treatment among patients with SVR, the pooled AUROC of LSM was 0.84 (95%CI: 0.66-1.03, I2 = 88%), which was statistically higher than that of FIB-4 (pooled AUROC 0.75, 95%CI: 0.55-0.95, I2 = 88%), P < 0.0001. The pooled AUROC of pre-treatment LSM and post-treatment APRI score was not estimated due to the limited number of studies (n = 1 each).
Four studies identifying 823 deaths among 3321 patients reported a significant positive association between FIB-4 score and overall mortality with pooled unadjusted and adjusted HRs of 3.06 (95%CI: 1.38-6.67, I2 = 90%) and 2.07 (95%CI: 1.49-2.88, I2 = 27%), respectively (Supplementary Figure 5)[16,45,47,48]. Likewise, a significant positive association between LSM and overall mortality was reported from four studies containing 3663 patients with 368 deaths[20,21,25,28], with pooled unadjusted and adjusted HRs of 5.52 (95%CI: 2.81-10.85, I2 = 74%) and 4.04 (95%CI: 2.40-6.80, I2 = 63%), respectively (Supplementary Figure 6).
The pooled HR and AUROC of APRI performance for the prediction of mortality was not estimated because only one study was included in this meta-analysis. The AUROCs for predicting overall mortality reported in individual studies are shown in Table 3.
Due to the broad definitions of HD and LRE outcomes, we did not perform a meta-analysis on these outcomes. However, taken individually, any NIT showed statistically significant positive associations and predictive values for their respective outcomes. The HRs and AUROCs of NITs and liver-related outcomes are summarized in Tables 4 and 5[12,16-21,23-28,38,43,45-48].
| Unadjusted hazard ratio (HR) | ||||
| Ref. | NIT1 | HR (95%CI) | P value | |
| Hansen et al[20], 2019 | TE | 97.00 (13.20–713.00) | < 0.005 | |
| Shili-Masmoudi et al[28], 2019 | TE | 29.65 (8.88–99.01) | < 0.001 | |
| Nunes et al[27], 2010 | APRI | 10.18 (4.86–21.32) | N/A | |
| FIB-4 | 9.45 (4.51–19.79) | N/A | ||
| Adjusted hazard ratio (aHR) | ||||
| Ref. | NIT1 | aHR (95%CI) | P value | Adjustment variables |
| Hansen et al[20], 2019 | TE | 11.00 (1.22–98.60) | 0.018 | SVR |
| Shili-Masmoudi et al[28], 2019 | TE | 20.60 (5.99–70.78) | < 0.001 | Gender, alcohol consumption, drug consumption, CD4 count, HCV genotype, metabolic disorders, previous HCV treatment |
| Macías et al[21], 2015 | TE | 29.90 (4.30–217.00) | 0.001 | Age, gender, platelet counts, AIDS at baseline, alcohol use, treatment against HCV, time-varying CD4 cell counts, undetectable HIV RNA |
| Tada et al[48], 2016 | FIB-4 (Pre-Rx) | 13.02 (4.16–40.77) | < 0.001 | Age, gender, AST concentration, ALT concentration, albumin, total bilirubin concentration, prothrombin time, platelet count, AFP concentration, FIB-4 index |
| Nunes et al[27], 2010 | FIB-4 | 1.19 (1.12–1.27) | < 0.001 | Gender, MELD |
| FIB-4 | 1.13 (1.05–1.21) | 0.001 | Gender, CPT | |
| APRI | 1.11 (1.01–1.22) | 0.035 | Gender, CPT | |
| APRI | 1.25 (1.15–1.35) | < 0.001 | Gender, MELD | |
| Unadjusted hazard ratio (HR) | |||||
| Ref. | Outcomes | NIT1 | HR (95%CI) | P value | |
| Hansen et al[20], 2019 | HD (HCC included) | TE | 59.00 (17.40–200.00) | < 0.005 | |
| Ogasawara et al[38], 2019 | HD | TE (Pre-Rx) | 7.77 (1.29–46.20) | 0.025 | |
| TE (Post-Rx) | 17.80 (1.85–171.30) | 0.013 | |||
| Bloom et al[17], 2018 | LRE (HD, HCC and OM) | TE | 56.00 (7.00–415.00) | < 0.001 | |
| Gomez-Moreno et al[19], 2017 | LRE (HD, HCC or LRM) | TE | 33.27 (7.25–152.63) | < 0.001 | |
| Pérez-Latorre et al[24], 2016 | HD or HCC, whichever occurred first | TE (Post-Rx) | 37.76 (17.87–79.80) | < 0.001 | |
| Macías et al[21], 2015 | HD (HCC included) | TE | 39.90 (5.50–291.00) | < 0.0001 | |
| Adjusted hazard ratio (aHR) | |||||
| Ref. | Outcomes | NIT1 | aHR (95%CI) | P value | Adjustment variables |
| Hansen et al[20], 2019 | HD (HCC included) | TE | 9.00 (2.49-32.20) | 0.001 | Age, SVR, hyaluronic acid |
| Ogasawara et al[38], 2019 | HD | TE (Pre-Rx) | 4.85 (0.80–29.40) | 0.086 | Platelet count, albumin |
| TE (Post-Rx) | 14.90 (1.45-152.10) | 0.023 | Platelet count, albumin | ||
| Peleg et al[23], 2019 | OM or HCC | TE (Post-Rx) | 2.32 (0.97-6.59) | 0.062 | liver steatosis, baseline serum platelets |
| Gomez-Moreno et al[19], 2017 | LRE (HD, HCC and OM) | TE | 30.97 (6.73-142.51) | < 0.001 | Age, gender, time since HCV diagnosis, HCV genotype, injection drug use, high alcohol intake, HCV antiviral therapy |
| Merchante et al[26], 2017 | HD | TE | 1.90 (1.04–3.64) | < 0.001 | Age, gender, SVR during follow-up |
| Lee et al[46], 2016 | HD, HCC, and/or LRM | TE (Post-Rx) | 9.47 (1.02-88.13) | 0.048 | Age, AFP |
| Macías et al[21], 2015 | HD (HCC included) | TE | 59.50 (8.30-427.00) | < 0.001 | Age, gender, platelet counts, AIDS at baseline, alcohol use, treatment against HCV, time-varying CD4 cell counts and undetectable HIV RNA. |
| Berenguer et al[12], 2015 | OM/LRE (HD or HCC), whichever occurred first. | FIB-4 (Pre-Rx) | 3.90 (2.46-6.16) | < 0.001 | Age, gender, HIV transmission category, Centers for Disease Control and Prevention HIV clinical category, CD4 cell nadir, HCV genotype, HCV RNA, alcohol intake, methadone use, SVR |
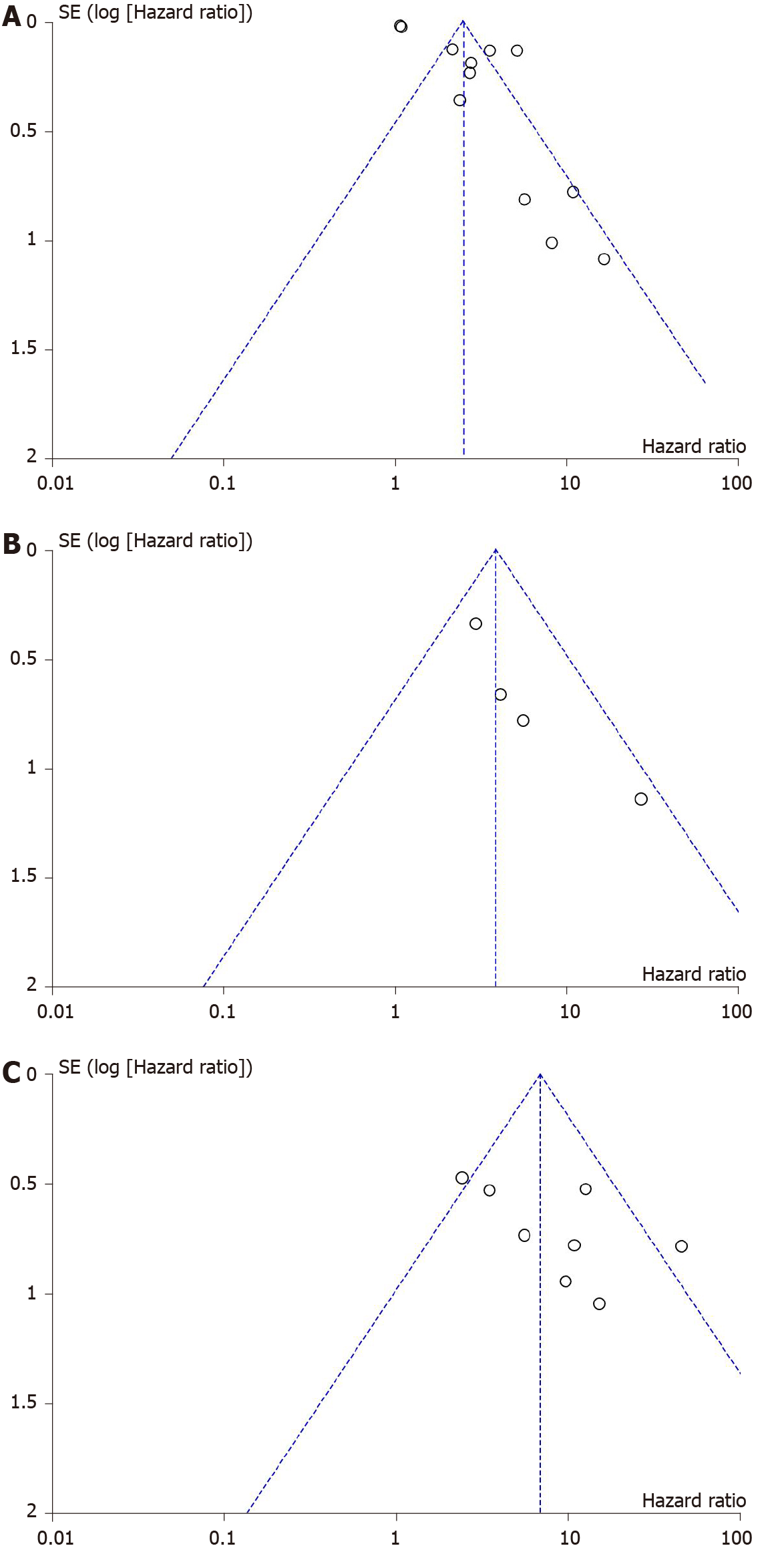
Publication bias was assessed through Deeks funnel plots for unadjusted and adjusted HRs of NITs and LREs. The distribution of studies was symmetrical for all analyses, except for adjusted HRs of FIB-4, APRI, LSM, and HCC development, which showed asymmetry (Figure 4). Egger’s regression asymmetry test detected publication bias in adjusted HRs of FIB-4 (P < 0.001) but not in HRs of APRI or LSM (P = 0.081 and 0.097, respectively). We found that five out of eight studies that reported an adjusted HR for FIB-4 score each had more than 1000 participants[33,39-41,49]. When only studies with > 1000 participants were selected for the subgroup analysis of adjusted HRs of FIB-4 and HCC development, publication bias was no longer detected (P = 0.12), suggesting that bias resulted from the inclusion of small studies.
NITs for liver fibrosis assessment play an important role in the management of HCV infection. Liver fibrosis staging is determinant for treatment prioritization and regimen in low- and middle-income countries as well as HCC surveillance. In addition to fibrosis staging, NITs are increasingly evaluated for their prognostic value. Our systematic review highlighted the potential use of FIB-4, APRI, and LSM to guide risk-stratified strategies in HCV-infected patients.
We found that LSM had a higher pooled HR for HCC development than APRI and FIB-4. TE is the most validated method for LSM as judged by its clinical imple
Although LSM is the most commonly used and validated NIT for liver fibrosis staging, several drawbacks can limit its use in practice such as costly equipment and maintenance, need for frequent calibration and skilled operators, and limited performance in obese patients. Therefore, the use of serologic markers such as APRI or FIB-4 score were recommended by the World Health Organization (WHO)[52] to assess hepatic fibrosis in resource-limited settings. Indeed, these scores can be easily calculated using only patient age and common laboratory data (aspartate aminotransferase, alanine aminotransferase, platelets). Considering the current recommendation to measure the degree of liver fibrosis prior to HCV treatment[2], we found that in a pre-treatment setting APRI and FIB-4 score performed well in terms of HCC prediction, with AUROCs of 0.77 and 0.88, respectively. They could provide similar, if not higher, prognostic value in comparison to LSM.
WHO has committed to eradicate viral hepatitis by 2030. Since the introduction of direct acting antiviral (DAA) therapy, the number of treated CHC patients achieving SVR has greatly increased. SVR is independently associated with improved hepatic function and prognosis[35,36]. Despite achieving SVR, some patients can develop HCC or LREs suggesting that regular follow-up remains necessary[13,30,31,33,39,41,49]. Non-invasive assessment of residual fibrotic burden in post-therapy patients who achieved SVR is currently unreliable[2]. This issue could explain at least partly the decision of international guidelines not to recommend NITs for monitoring of post-treatment residual fibrosis[1,2]. Despite its questionable diagnostic potential, we found that among patients with SVR, APRI and LSM can predict HCC development with AUROC values of 0.75 and 0.84, respectively. This was shown to be helpful even in the DAA era, as shown in our study that the adjusted HR of LSM and HCC risk in patients achieving SVR after DAA era was 5.55.
Large variations in NIT cutoffs were observed in the studies included in our meta-analysis. For example, the cutoff of FIB-4 score recommended by WHO for predicting significant fibrosis (METAVIR ≥F2) is 1.45 for high sensitivity and 3.25 for high specificity[52]. We found that five out of 11 studies included in this meta-analysis chose the cutoff of 3.25[13,33,41,42,49], while no studies used the cutoff of 1.45. Accordingly, we pooled the results for unadjusted and adjusted HRs of pretreatment FIB-4 using the 3.25 cutoff and found that this cutoff had a statistically significant potential to be used clinically for HCC risk stratification, with a pooled adjusted HR of 3.22 (no subgroup analysis of post-treatment SVR population was done due to the lack of studies). Notably, this does not justify excluding patients with FIB-4 below this cutoff from HCC screening, as it is still debatable whether this cutoff adequately identifies the at-risk population. Decisions regarding HCC screening in patients with low FIB-4 should be individualized based on patient risk profile.
The strength of this meta-analysis resides in the inclusion of all recently validated noninvasive fibrosis tests, including both radiological and serological tests, as we aimed to make this review as comprehensive as possible. There are some limitations. Although the present meta-analysis extensively assessed several clinically relevant outcomes including HCC, HD, and overall and liver-related mortality, our analysis was nevertheless narrowed by several unavailable data such as the timing in which NITs were assessed after receiving treatment or achieving SVR. Statistical heterogeneity was found in some of our analyses. However, this could be explained by subgroup-analyses of the following factors: NITs assessed at pre-treatment or post-treatment with SVR, treatment with either pegylated interferon and ribavirin or DAA, Eastern or Western countries, and different cutoff points. For instance, statistical heterogeneity found in the analyses of pre-treatment FIB-4 and HCC development is partially explained by country of study. In the subgroup analysis on Eastern countries, there was a reduction of I2 from 76% to 18% for the unadjusted HR. Since the majority of studies are from Eastern countries with Asian participants, further studies conducted in other ethnicities are needed. Residual statistical heterogeneity seen in some of the analyses could also be explained by factors such as the presence of cirrhotic patients in the study and the type of HCV treatment regimen. Due to the limited number of studies and lack of information provided in some studies, we were unable to perform subgroup analysis on these factors. Instead, we provided this information in the figures, wherever subgroup analysis was not possible. More studies are needed to make it possible for us to explore the remaining statistical heterogeneity, by either subgroup analysis or meta-regression.
The publication bias in adjusted HR for FIB-4 index could be explained by biased selection of outcomes in four studies. Notably, only adjusted HRs for significant variables were reported, while non-significant variables were either omitted or considered as non-significant without providing a numerical adjusted HR[39-41,49]. However, through subgroup analysis, we have concluded that the publication bias detected was due to the inclusion of small studies.
FIB-4, APRI, and LSM showed predictive value in stratifying risk for CHC patients, particularly for pre-cirrhotic patients with significant fibrosis. Patients with a higher degree of fibrosis based on NITs were found to be at increased risk of complications, regardless of treatment regimen and response. Therefore, liver fibrosis measurement by NITs could benefit any HCV patient as it can determine the priority to monitor for the development of HCC and other LREs. The clinical implementation of these NITs does require future studies that can validate their respective cutoff levels.
Non-invasive tests (NITs) have reduced the need for liver biopsy in chronic hepatitis C (CHC) patients. Despite its limited diagnostic performance in patients with an intermediate degree of fibrosis or in post-treatment setting, previous meta-analyses have evidenced the potential of NITs in determining prognosis. However, these studies focused on chronic liver diseases from various etiologies and did not comprehensively explore all liver outcomes.
The authors aimed to explore all validated NITs for liver fibrosis, specifically their ability to predict liver-related outcomes in CHC patients.
The main goal was to determine the prognostic value of NITs for risk stratification in CHC patients.
A literature search was performed to identify CHC cohort studies that reported an association between liver fibrosis assessment by NITs and outcomes such as hepatocellular carcinoma. Hazard ratios (HR) and area under the receiver operating characteristic from those studies were then pooled using the random effects model. Subgroup analyses were performed based on treatment status, treatment regimen, countries, and different cutoff points.
Fibrosis-4 (FIB-4) index, aspartate aminotransferase to platelet ratio (APRI) score, and liver stiffness measurement (LSM) were found to have hepatocellular carcinoma predictive potential with pooled adjusted HR of 2.48 (95%CI: 1.91-3.23, I2 = 96%), 4.24 (95%CI: 2.15-8.38, I2 = 20%) and 7.90 (95%CI: 3.98-15.68, I2 = 52%) and area under the receiver operating characteristic of 0.81 (95%CI: 0.73-0.89, I2 = 77%), 0.81 (95%CI: 0.75-0.87, I2 = 68%) and 0.79 (95%CI: 0.63-0.96, I2 = 90%), respectively.
FIB-4, APRI, and LSM were found to have prognostic value, and can potentially be used to stratify risk for CHC patients, regardless of their treatment status or regimen.
To facilitate clinical implementation, validation of FIB-4, APRI and LSM cutoff levels are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SC S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Poynard T, Ngo Y, Perazzo H, Munteanu M, Lebray P, Moussalli J, Thabut D, Benhamou Y, Ratziu V. Prognostic value of liver fibrosis biomarkers: a meta-analysis. Gastroenterol Hepatol (N Y). 2011;7:445-454. [PubMed] |
| 2. | Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 3. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1212] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 5. | Mendes LC, Stucchi RS, Vigani AG. Diagnosis and staging of fibrosis in patients with chronic hepatitis C: comparison and critical overview of current strategies. Hepat Med. 2018;10:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3243] [Article Influence: 463.3] [Reference Citation Analysis (1)] |
| 7. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47198] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 8. | Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 2284] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 9. | Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY, Chuang WL, Liaw YF. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Berenguer J, Zamora FX, Aldámiz-Echevarría T, Von Wichmann MA, Crespo M, López-Aldeguer J, Carrero A, Montes M, Quereda C, Téllez MJ, Galindo MJ, Sanz J, Santos I, Guardiola JM, Barros C, Ortega E, Pulido F, Rubio R, Mallolas J, Tural C, Jusdado JJ, Pérez G, Díez C, Álvarez-Pellicer J, Esteban H, Bellón JM, González-García J; Grupo de Estudio del SIDA (GESIDA) HIV/HCV Cohort Study Group. Comparison of the prognostic value of liver biopsy and FIB-4 index in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2015;60:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264-1278.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 12. | Pons M, Rodríguez-Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, Buti M, Augustin S, Forns X, Mínguez B, Genescà J. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Rinaldi L, Guarino M, Perrella A, Pafundi PC, Valente G, Fontanella L, Nevola R, Guerrera B, Iuliano N, Imparato M, Trabucco A, Sasso FC, Morisco F, Ascione A, Piai G, Adinolfi LE. Role of Liver Stiffness Measurement in Predicting HCC Occurrence in Direct-Acting Antivirals Setting: A Real-Life Experience. Dig Dis Sci. 2019;64:3013-3019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Bambha K, Pierce C, Cox C, French AL, Tien PC, Sharp GB, Augenbraun M, Glesby MJ, Villacres MC, Plankey M, Strickler HD, Gange SJ, Peters MG. Assessing mortality in women with hepatitis C virus and HIV using indirect markers of fibrosis. AIDS. 2012;26:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Bloom S, Kemp W, Nicoll A, Roberts SK, Gow P, Dev A, Bell S, Sood S, Kronborg I, Knight V, Lewis D, Lubel J. Liver stiffness measurement in the primary care setting detects high rates of advanced fibrosis and predicts liver-related events in hepatitis C. J Hepatol. 2018;69:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Chalouni M, Sogni P, Miailhes P, Lacombe K, Poizot-Martin I, Chas J, Vittecoq D, Neau D, Aumaitre H, Alric L, Piroth L, Bouchaud O, Katlama C, Morlat P, Lascoux-Combe C, Gervais A, Naqvi A, Rosenthal E, Garipuy D, Barange K, Esterle L, Salmon D, Wittkop L; ANRS CO13 HEPAVIH study group. Liver stiffness and fibrosis-4 alone better predict liver events compared with aspartate aminotransferase to platelet ratio index in a cohort of human immunodeficiency virus and hepatitis C virus co-infected patients from ANRS CO13 HEPAVIH cohort. Eur J Gastroenterol Hepatol. 2019;31:1387-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Gomez-Moreno AZ, Pineda-Tenor D, Jimenez-Sousa MA, Sánchez-Ruano JJ, Artaza-Varasa T, Saura-Montalban J, Ryan P, Resino S. Liver stiffness measurement predicts liver-related events in patients with chronic hepatitis C: A retrospective study. PLoS One. 2017;12:e0184404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Hansen JF, Christiansen KM, Staugaard B, Moessner BK, Lillevang S, Krag A, Christensen PB. Combining liver stiffness with hyaluronic acid provides superior prognostic performance in chronic hepatitis C. PLoS One. 2019;14:e0212036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Macías J, Mancebo M, Márquez M, Merino D, Téllez F, Rivero A, von Wichmann MA, López-Cortés LF, Merchante N, Santos J, Raffo M, Pérez-Pérez M, Camacho Á, Iribarren JA, Pineda JA. Low risk of liver decompensation among human immunodeficiency virus/hepatitis C virus-coinfected patients with mild fibrosis in the short term. Hepatology. 2015;61:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Munteanu M, Pais R, Peta V, Deckmyn O, Moussalli J, Ngo Y, Rudler M, Lebray P, Charlotte F, Thibault V, Lucidarme O, Ngo A, Imbert-Bismut F, Housset C, Thabut D, Ratziu V, Poynard T; FibroFrance Group. Long-term prognostic value of the FibroTest in patients with non-alcoholic fatty liver disease, compared to chronic hepatitis C, B, and alcoholic liver disease. Aliment Pharmacol Ther. 2018;48:1117-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Harif Y, Oxtrud E, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Pérez-Latorre L, Rivero-Juárez A, Hontañón V, Díez C, Cuenca F, Martín-Carbonero ML, Montes ML, Bellón JM, Aldámiz-Echevarría T, Carrero A, Rivero A, González-García J, Berenguer J. Prognostic Value of Transient Elastography in Human Immunodeficiency Virus-Infected Patients With Chronic Hepatitis C. Open Forum Infect Dis. 2016;3:ofw212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Cepeda JA, Thomas DL, Astemborski J, Sulkowski MS, Kirk GD, Mehta SH. Increased Mortality Among Persons With Chronic Hepatitis C With Moderate or Severe Liver Disease: A Cohort Study. Clin Infect Dis. 2017;65:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Merchante N, Rivero-Juárez A, Téllez F, Merino D, Ríos-Villegas MJ, Ojeda-Burgos G, Omar M, Macías J, Rivero A, Pérez-Pérez M, Raffo M, López-Montesinos I, Márquez-Solero M, Gómez-Vidal MA, Pineda JA; Grupo Andaluz para el Estudio de las Hepatitis Víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Liver stiffness predicts variceal bleeding in HIV/HCV-coinfected patients with compensated cirrhosis. AIDS. 2017;31:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Nunes D, Fleming C, Offner G, Craven D, Fix O, Heeren T, Koziel MJ, Graham C, Tumilty S, Skolnik P, Stuver S, Horsburgh CR Jr, Cotton D. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Shili-Masmoudi S, Sogni P, de Ledinghen V, Esterle L, Valantin MA, Poizot-Martin I, Simon A, Rosenthal E, Lacombe K, Pialoux G, Bouchaud O, Gervais-Hasenknoff A, Goujard C, Piroth L, Zucman D, Dominguez S, Raffi F, Alric L, Bani-Sadr F, Lascoux-Combe C, Garipuy D, Miailhes P, Vittecoq D, Duvivier C, Aumaître H, Neau D, Morlat P, Dabis F, Salmon D, Wittkop L; ANRS CO13 HEPAVIH study group. Increased liver stiffness is associated with mortality in HIV/HCV coinfected subjects: The French nationwide ANRS CO13 HEPAVIH cohort study. PLoS One. 2019;14:e0211286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Hamada K, Saitoh S, Nishino N, Fukushima D, Horikawa Y, Nishida S, Honda M. Shear wave elastography predicts hepatocellular carcinoma risk in hepatitis C patients after sustained virological response. PLoS One. 2018;13:e0195173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Tamaki N, Higuchi M, Kurosaki M, Kirino S, Osawa L, Watakabe K, Wang W, Okada M, Shimizu T, Takaura K, Takada H, Kaneko S, Yasui Y, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Enomoto N, Izumi N. Risk assessment of hepatocellular carcinoma development by magnetic resonance elastography in chronic hepatitis C patients who achieved sustained virological responses by direct-acting antivirals. J Viral Hepat. 2019;26:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Lee K, Sinn DH, Gwak GY, Cho HC, Jung SH, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Prediction of the Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Patients after Sustained Virological Response by Aspartate Aminotransferase to Platelet Ratio Index. Gut Liver. 2016;10:796-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N, Ikeda H, Shiina S, Kawabe T, Omata M. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 31. | Na SK, Lee SJ, Cho YK, Kim YN, Choi EK, Song BC. Aspartate Aminotransferase-to-Platelet Ratio or Fibros-4 Index Predicts the Development of Hepatocellular Carcinoma in Chronic Hepatitis C Patients with Sustained Virologic Response to Interferon Therapy. J Interferon Cytokine Res. 2019;39:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Nakagomi R, Tateishi R, Masuzaki R, Soroida Y, Iwai T, Kondo M, Fujiwara N, Sato M, Minami T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Kondo Y, Tanaka Y, Otsuka M, Kato N, Moriya K, Ikeda H, Koike K. Liver stiffness measurements in chronic hepatitis C: Treatment evaluation and risk assessment. J Gastroenterol Hepatol. 2019;34:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Narita Y, Genda T, Tsuzura H, Sato S, Kanemitsu Y, Ishikawa S, Kikuchi T, Hirano K, Iijima K, Wada R, Ichida T. Prediction of liver stiffness hepatocellular carcinoma in chronic hepatitis C patients on interferon-based anti-viral therapy. J Gastroenterol Hepatol. 2014;29:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Ng KJ, Tseng CW, Chang TT, Tzeng SJ, Hsieh YH, Hung TH, Huang HT, Wu SF, Tseng KC. Aspartate aminotransferase to platelet ratio index and sustained virologic response are associated with progression from hepatitis C associated liver cirrhosis to hepatocellular carcinoma after treatment with pegylated interferon plus ribavirin. Clin Interv Aging. 2016;11:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Nojiri S, Fujiwara K, Shinkai N, Endo M, Joh T. Evaluation of hepatocellular carcinoma development in patients with chronic hepatitis C by EOB-MRI. World J Hepatol. 2014;6:930-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Ogasawara N, Saitoh S, Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, Kobayashi M, Suzuki Y, Arase Y, Ikeda K, Kumada H. Advantage of liver stiffness measurement before and after direct-acting antiviral therapy to predict hepatocellular carcinoma and exacerbation of esophageal varices in chronic hepatitis C. Hepatol Res. 2020;50:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Akuta N, Kobayashi M, Suzuki F, Sezaki H, Fujiyama S, Kawamura Y, Hosaka T, Saitoh S, Suzuki Y, Arase Y, Ikeda K, Kumada H. Liver Fibrosis and Body Mass Index Predict Hepatocarcinogenesis following Eradication of Hepatitis C Virus RNA by Direct-Acting Antivirals. Oncology. 2016;91:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Sato S, Genda T, Ichida T, Amano N, Sato S, Murata A, Tsuzura H, Narita Y, Kanemitsu Y, Hirano K, Shimada Y, Iijima K, Wada R, Nagahara A, Watanabe S. Prediction of Hepatocellular Carcinoma Development after Hepatitis C Virus Eradication Using Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Sou FM, Wu CK, Chang KC, Lu SN, Wang JH, Hung CH, Chen CH, Kee KM, Yen YH, Lin MT, Tsai MC, Hu TH. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Tamaki N, Kurosaki M, Matsuda S, Muraoka M, Yasui Y, Suzuki S, Hosokawa T, Ueda K, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Asahina Y, Izumi N. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol. 2014;49:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Thandassery RB, Kaabi SA, Soofi ME, Tharian B, Singh R. Noninvasive serum models to predict significant liver related events in chronic hepatitis C. Hepatol Int. 2017;11:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K, Yukimoto A, Hirooka M, Abe M, Hiasa Y. Predictors of hepatocellular carcinoma occurrence after direct-acting antiviral therapy in patients with hepatitis C virus infection. Hepatol Res. 2019;49:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Chen X, Liu X, Tang R, Ye R, Yang Y, Yao S, Wang J, Ding Y, Duan S, He N. Fibrosis-4 index predicts mortality in HIV/HCV co-infected patients receiving combination antiretroviral therapy in rural China. Biosci Trends. 2019;13:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Lee HW, Chon YE, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Jung KS, Park YN, Han KH. Predicting Liver-Related Events Using Transient Elastography in Chronic Hepatitis C Patients with Sustained Virological Response. Gut Liver. 2016;10:429-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Ogasawara N, Saitoh S, Akuta N, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Kobayashi M, Suzuki F, Suzuki Y, Arase Y, Ikeda K, Kumada H. Long-term outcome of hepatocellular carcinoma occurrence, esophageal varices exacerbation, and mortality in hepatitis C virus-related liver cirrhosis after interferon-based therapy. Hepatol Res. 2019;49:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T, Tanaka J. Long-term prognosis of patients with chronic hepatitis C who did not receive interferon-based therapy: causes of death and analysis based on the FIB-4 index. J Gastroenterol. 2016;51:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Chun HS, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Lee CH, Lee YB, Cho EJ, Yu SJ, Kim YJ, Yoon JH, Lee JH, Kim SU. Design and validation of risk prediction model for hepatocellular carcinoma development after sustained virological response in patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2020;32:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M, Pol S. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 49. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 50. | WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version. Geneva: World Health Organization, 2016. |
| 51. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 52. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |