Published online Aug 27, 2021. doi: 10.4254/wjh.v13.i8.904
Peer-review started: March 7, 2021
First decision: March 29, 2021
Revised: April 2, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 27, 2021
Processing time: 165 Days and 16.7 Hours
The multi-organ failure syndrome associated with acute and acute-on-chronic liver failure (ACLF) is thought to be mediated by overwhelming systemic inflammation triggered by both microbial and non-microbial factors. Therapeutic plasma exchange (TPE) has been proven to be an efficacious therapy in autoimmune conditions and altered immunity, with more recent data supporting its use in the management of liver failure. Few therapies have been shown to improve survival in critically ill patients with liver failure who are not expected to survive until liver transplantation (LT), who are ineligible for LT or who have no access to LT. TPE has been shown to reduce the levels of inflammatory cytokines, modulate adaptive immunity with the potential to lessen the susceptibility to infections, and reduce the levels of albumin-bound and water-bound toxins in liver failure. In patients with acute liver failure, high volume TPE has been shown to reduce the vasopressor requirement and improve survival, particularly in patients not eligible for LT. Standard volume TPE has also been shown to reduce mortality in certain sub-populations of patients with ACLF. TPE may be most favorably employed as a bridge to LT in patients with ACLF. In this review, we discuss the efficacy and technical considerations of TPE in both acute and acute-on-chronic liver failure.
Core Tip: Multi-organ failure accompanying liver failure is mediated by overwhelming systemic inflammation and altered host immunity. Therapeutic plasma exchange has been proven to be an efficacious therapy in autoimmune conditions and altered immunity. We review the efficacy and technical considerations of therapeutic plasma exchange in both acute and acute-on-chronic liver failure.
- Citation: Chris-Olaiya A, Kapoor A, Ricci KS, Lindenmeyer CC. Therapeutic plasma exchange in liver failure. World J Hepatol 2021; 13(8): 904-915
- URL: https://www.wjgnet.com/1948-5182/full/v13/i8/904.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i8.904
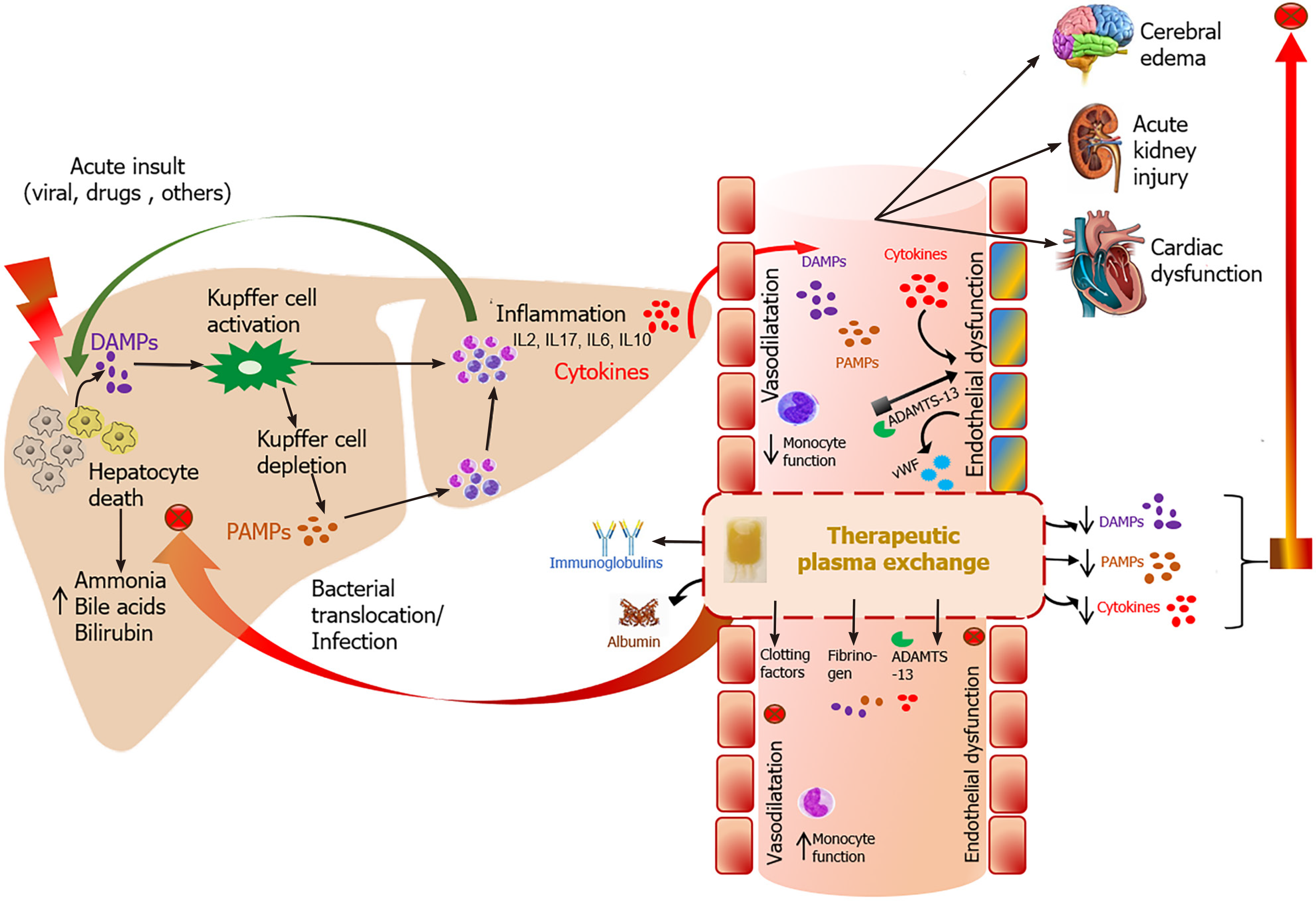
Acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) are two distinct classifications of severe hepatic dysfunction associated with secondary multi-organ failures (MOFs), both of which effect significant morbidity and mortality[1-4]. The exact mechanisms by which MOFs are mediated have not been definitively established but are thought to be driven by excessive systemic inflammation and dysregulated immune activation triggered by both microbial and non-microbial factors, and less so by the primary insult to the liver[3,5-7].
The pathogenesis of MOFs in ALF has been attributed to the release of damage-associated molecular patterns (DAMPs) from injured hepatic cells and microbial pathogen-associated molecular patterns (PAMPs) in the presence of superimposed infection or bacterial translocation[7]. The innate immune cells activated by PAMPs and DAMPs produce proinflammatory cytokines [interleukin (IL)-6, IL-1ß, IL-8, tumor necrosis factor-alpha (TNF-α)] that mediate systemic inflammation and further hepatocyte injury[7,8]. In support of this hypothesis, levels of TNF-α and IL-6 have been shown to be significantly higher in patients with fulminant hepatitis when compared to patients with acute liver injury[9].
Similarly, the hallmark of the ACLF clinical syndrome is excessive systemic inflammation and bacterial translocation mediated by PAMPs and DAMPs[1,6,10]. ACLF patients have been shown to manifest elevated levels of pro- and anti-inflammatory cytokines, as well as white blood cell count and C reactive protein. Moreover, there is a proven correlation between cytokine levels and number of organ failures in ACLF[6,11].
Despite advances in the supportive medical management of patients with liver failure, significant morbidity and mortality persist[12,13]. Urgent liver transplantation (LT) remains the definitive treatment in patients with high likelihood of death; however, access to transplant remains limited. In addition, eligibility for transplant can be hampered by psychosocial factors, active substance use, and progressive MOFs that may preclude safe LT or contribute to mortality while awaiting LT[14,15]. Expanded treatment options are needed to bridge critically ill patients to LT or to preserve liver function when LT is either contra-indicated or unavailable. Therapeutic plasma exchange (TPE) has been proposed as a beneficial treatment modality in these patients. The practice of exchange transfusion in patients with cirrhosis dates back to the 1960s when exchange blood transfusion was employed for the treatment of hepatic coma[16]. Therapies were later modified to TPE as apheresis equipment became more widely available and as a means to reduce the risks associated with whole blood transfusion[17,18]. Historically, TPE in liver failure has been primarily described in case series and cohort studies. The first randomized control trial (RCT) describing the utility of TPE in ALF patients was reported in 2016 by Larsen et al[19].
TPE in liver failure requires the extracorporeal removal of large compounds from the blood, including albumin-bound and water-soluble toxins and replacement with plasma and/or albumin. As shown in Figure 1, these toxins include cytokines, endotoxins, bilirubin, bile acids, ammonia, and aromatic amino acids[20,21]. These substances have been proposed as important mediators of both hepatic encephalopathy (HE) and MOFs in ALF and ACLF[8,22-24]. By comparison, extracorporeal albumin dialysis (ECAD) systems remove albumin-bound and water-soluble toxins via hemodialysis augmented by an albumin-infused dialysate with or without the addition of adsorption columns (charcoal filter and anion exchange resins). These ECAD systems include the molecular adsorbent recirculation system (MARS), single pass albumin dialysis, and fractionated plasma separation and adsorption[25-27].
When considering the therapeutic differences between TPE and ECAD, MARS in particular has been recognized to be more costly than TPE and can entail a more logistically complex initiation. Furthermore, the MARS filter-membrane dictates a size selection threshold of approximately 50 KDa[28], whereas TPE is capable of removing larger molecular proteins, including antibodies, immune complexes, and lipoproteins[29]. To date, no head-to-head adult clinical trial has directly compared TPE with MARS or any of the ECAD systems. However, in a retrospective single center pediatric study comparing MARS with the combination of TPE and hemodialysis, TPE and hemodialysis effected a greater reduction in bilirubin, ammonia, and international normalized ratio[30]. Another theoretical advantage of TPE over ECAD hinges on the exchange of plasma, which replaces plasma proteins, including clotting factors, that may be decreased as a result of impaired hepatic synthetic function in both ALF and ACLF.
TPE has been shown to reduce levels of circulating inflammatory cytokines, improve hemodynamics, and improve transplant-free survival in ALF[9,19,31-33]. While encouraging, head-to-head comparisons between the studies supporting these findings have been challenging due to the broad variation in treatment protocols. Often the volume of exchange, treatment frequency and duration of therapy vary between studies.
Specifically, TPE has been shown to moderate TNF-α, histone-associated DNA (member of the DAMP family), IL-6, IL-8, endotoxins, bilirubin, ammonia, and to improve coagulopathy[9,19,34]. In addition, TPE modulates adaptive immunity in ALF through the reduction of soluble B7 molecules, particularly sCD86[35]. Soluble B7 molecules are produced by injured hepatocytes and increase the expression of cytotoxic T-lymphocyte-associated protein 4 on CD4+ T cells, resulting in impaired antimicrobial responses and increased susceptibility to infections[35].
In the only RCT designed to study outcomes associated with high volume TPE (HV-TPE) in ALF, patients who received HV-TPE manifested significantly improved mean arterial blood pressure (MAP) with associated reduction in vasopressor requirement when compared to patients who received standard medical therapy (SMT) only[19]. In the same study, plasma creatinine remained stable in the HV-TPE group but increased significantly in the SMT group. Accordingly, fewer HV-TPE patients required renal replacement therapy when compared to those who received SMT. In contrast, Wiersema et al[31] reported no significant reduction in vasopressor requirement in ALF patients receiving TPE, despite reporting significantly improved MAP on therapy. Notably, this single arm, single centered study employed standard volume TPE as opposed to HV-TPE.
In addition to hemodynamic benefits, TPE has been shown to reduce ammonia level, improve HE grades and cerebral hemodynamics independent of simultaneous filtration or dialysis[33,36]. However, TPE has not been shown to effect significant differences in intracranial pressure (ICP) in ALF, though few patients in Larsen’s study underwent invasive ICP assessment (32 of the randomized 182 patients)[19]. On the contrary, a retrospective review of 43 patients with Wilsonian-ALF who received HV-TPE manifested no improvement in ammonia or creatinine levels, but did demonstrate improved transplant-free survival at 90 d[37].
Finally, Larsen’s RCT in ALF demonstrated a significant improvement in transplant-free survival in patients who received HV-TPE when compared to SMT [hazard ratio (HR) 0.56, 95% confidence interval (CI) 0.36-0.86, P = 0.0083], with no difference in outcomes between paracetamol and non-paracetamol etiology of liver failure[19]. In subgroup analysis of the same study, HV-TPE was shown to specifically improve survival among patients not listed for LT due to contraindications. By contrast, no survival benefit was identified in patients who received HV-TPE as a bridge to LT. Other non-randomized studies in ALF have reported improvement in survival days with TPE in non-transplanted patients[38,39]. There have been no studies to date that have examined the combination of TPE with any of the ECAD systems in ALF patients.
Patients with ACLF have been shown to manifest significantly higher levels of cytokines (TNF- alpha, IL-10, IL-2, IL-4, and IFN-Y) compared to healthy controls. These same cytokines are also effectively reduced after TPE[40]. In the same study by Mao et al[40], higher cytokine levels predicted poor prognosis irrespective of the treatment received. Moreover, bilirubin levels, coagulopathy, and ammonia levels have been shown to improve after TPE-based therapy[41-43]. The effect of TPE on blood pressure and vasopressor requirement in ACLF patients has not been reported. In their single center and small sample size study, Stahl et. al. reported no difference in vasopressor requirement between patients who underwent TPE vs SMT[44].
TPE has been shown in limited series to improve survival in ACLF; however, this data is limited by protocol variation. Many of these studies have been performed in Asia among patients with hepatitis B virus- (HBV) related ACLF, used different definitions for ACLF, combined TPE with other liver support systems, and were single center retrospective studies[42,45-47]. Tan et al[48] reported improved survival with TPE-based therapies (combined with other extracorporeal therapy) compared to SMT in non-transplanted patients at 30 d and 90 d with a pooled odds ratio (OR) of 0.60 [95%CI: 0.46-0.77]. In the only RCT of TPE in ACLF, patients with HBV ineligible for LT who received TPE-based therapies manifested significantly improved survival rates when compared to patients who received SMT (60% vs 47%, P < 0.05) at 90 d[47]. In addition, Mao et al[45] demonstrated improved survival with TPE among patients with HBV-ACLF and model for end-stage liver disease (MELD) scores between 20-30 (50%) when compared to patients with MELD scores above 30 (31.7%)[45]. Whether the results of these studies can be extrapolated and generalized to the ACLF patient population at large remains uncertain. Stahl et al[44] retrospectively studied the differences in outcomes between ACLF patients bridged to LT vs patients bridged to spontaneous recovery. In this study, the risk of 30-d mortality was significantly lower in LT candidates (bridge to transplant group) than in non-transplant candidates (recovery strategy group) treated with TPE (HR 0.35, 95%CI 0.14-0.87, P = 0.024).
As described above, TPE is commonly combined with another dialysis modality depending on the individual patient profile (coagulopathy, renal function, HE, or water and/or electrolyte imbalance). Although continuous renal replacement therapy (CRRT), without TPE, is commonly employed in liver failure-induced severe hyperammonemia to reduce the risk of cerebral edema and intracranial hypertension (ICH)[49,50], no head-to-head comparison study has yet been done to compare ammonia clearance in TPE vs CRRT. Among patients with HBV-ACLF, Yao et al[43] compared TPE with double plasma molecular adsorption (DPMAS) therapy, a special broad-spectrum adsorption column that binds inflammatory mediators and bilirubin. Their group found a significantly higher rate of 28-d survival in the TPE with DPMAS group compared with TPE alone (57.4% vs 41.7%, P = 0.043) only among patients with intermediate and advanced stage ACLF (defined as prothrombin activity less than 30%)[43]. Separate studies have shown that DPMAS alone or in combination with TPE in ACLF does not confer survival benefits despite increasing the clearance of bilirubin[42,43].
Severe acute alcohol-associated hepatitis (SAH) is recognized to be a common precipitant of ACLF[5]; however, TPE has not been specifically studied in this important patient population. Moreover, sub-group analysis of the limited number of patients with alcohol-associated liver disease included in the available trials has not been described. Case reports suggest that TPE with standard medical therapy may lead to clinical improvement in patients with SAH[51,52]. Randomized, controlled trials in patients with SAH are needed to better define the therapeutic effect of TPE for this indication.
TPE can be performed by either centrifugation or filtration-based mechanisms. Centrifugation separates the blood into its components using density, whereas filtration uses a hollow fiber design to separate the plasma from the cellular components. Both centrifugation and filtration-based systems are similar in safety, efficiency, therapeutic effects[53,54], and are approved by the Food and Drug Administration for use in the United States. TPE is usually provided in collaboration with nephrologists or hematologists depending on the center’s preference.
Typical TPE treatments exchange 1 to 1.5 times the patient’s estimated plasma volume, approximately 3 L in an average sized adult. For reference, a plasma volume is an estimate of the total volume of plasma in an individual and is a common unit of measurement in therapeutic apheresis procedures. Plasma volume can be calculated from estimated total blood volume using common physiological variables, including an individual’s sex, height, weight, body muscle composition, and hematocrit[55]. The removal of substances using TPE follows the formula: y/yo = e-x, where y and yo are the concentration of the removed substance after and before plasma exchange and x is the number of plasma volumes processed[56]. A 1 to 1.5 plasma volume exchange will remove approximately 70% of the substances in the intravascular space[56].
The only RCT comparing TPE and SMT in ALF patients studied HV-TPE, defined as plasma replacement at 15% of ideal body weight or 8 to 12 L per session[19]. HV-TPE should remove approximately 90%-98% of the toxins in the intravascular space. The majority of studies on TPE in ALF patients before this RCT treated one plasma volume (2 L to 4 L) during each exchange[38,57-59]. Recently, Stahl et al[60] in their single center study compared 20 patients with ALF who received low volume TPE and SMT with 20 matched historical controls who received SMT only. TPE volume exchange was employed using 3 L to 4L per session daily until clinical improvement or LT. No head-to-head comparison of standard volume and HV-TPE in ALF has been performed, but the current evidence favors HV-TPE for ALF[61,62].
There is also no consensus or evidence-based strategy for the frequency and duration of treatment. A small single center study showed that one treatment session of TPE is associated with improvement in biochemical parameters and survival in patients with Wilsonian ALF[37]. The RCT by Larsen et. al performed HV-TPE for 3 consecutive days[19]. Other studies employed either the same regimen or every other day treatments, and continued until the patient improved clinically, died, or underwent LT[63-65]. The most commonly used replacement fluid is plasma, although albumin or plasma substitute is sometimes used in conjunction with plasma[66-69]. However, no studies have used albumin alone as a replacement fluid. Plasma is typically chosen as a replacement fluid as it contains coagulation factors and is thought to replenish those missing as a consequence of the underlying liver dysfunction.
All studies in the ACLF population have used standard volume replacement ranging from 2 L to 4.5L exchange per session. Most studies utilized plasma as replacement fluid and performed TPE sessions 2 to 3 times per week and continued until clinical improvement, transplant, or death[41,70-72]. Only one study reported daily plasma exchange, but the proportion of the study population that received daily exchanges was not described[41].
Sodium citrate and heparin are the two common anticoagulants employed to prevent clotting of the extracorporeal circuits. The patient’s clinical condition and physician’s preferences guide selection; both agents can be used if a single agent is inadequate for anticoagulation. Citrate is preferred because of its shorter half-life of 30-60 min, favorable safety profile, rapid reversibility with intravenous calcium, and its minimal systemic anticoagulation effect[73]. Sodium citrate undergoes hepatic and renal metabolism. Patients with liver failure are particularly susceptible to citrate toxicity as a consequence of impaired hepatic metabolism, often exacerbated by concomitant renal impairment. Citrated plasma replacement fluid can further worsen the risk of procedural hypocalcemia. Citrate is partly cleared by the kidney and can be safely utilized in acute kidney injury as long as the acid-base balance is closely monitored[74,75]. In a single study, tandem procedure with dialysis reduced the risk of citrate toxicity in ACLF patients undergoing TPE[76].
Common adverse effects of citrate include hypocalcemia (with or without symptoms) and metabolic alkalosis. Symptomatic hypocalcemia is not uncommon and occurs in 1.5% to 9% of all patients undergoing TPE[74]. Notably patients receiving TPE for liver failure are at increased risk of hypocalcemia due to the associated metabolic impairment. Prophylactic calcium replacement based on citrate load and continuous ionized calcium monitoring is recommended[29]. Supplementation with Calcium gluconate or Calcium chloride can reduce the risk of symptomatic hypo
Some physicians favor heparin because of the associated risks with citrate as described above. The application of both unfractionated and low molecular weight heparin have been reported[78,79]. Nevertheless, most patients can undergo filtration-based TPE without the need for anticoagulation similar to anticoagulation-free hemodialysis and hemofiltration[80-82].
Acute kidney injury requiring CRRT is a common manifestation of MOF in both ALF and ACLF[83-85]. In addition, CRRT is commonly utilized in patients with severe hyperammonemia to reduce the risk of ICH and cerebral edema[49,50,86]. CRRT is usually delivered over 24 h and the interruption of CRRT for TPE may compromise the duration of CRRT. Moreover, additional vascular access for TPE exposes the patient to the otherwise avoidable risk of catheter related complications. Simultaneous dialysis and TPE was first introduced in 1999; descriptions of the safety and feasibility of the combined therapies are limited to case reports and case series[21,87-90]. There are no defined standards for connection; tandem procedures connected in series or parallel have been reported in the literature[21,80,87,75,91]. These tandem connections have the advantage of minimizing vascular access procedures.
The combination of TPE with other extracorporeal therapies aside from CRRT in adults is not well described. In a randomized controlled study from Huang et al[92], MARS in combination with TPE was shown to reduce serum total bilirubin more effectively when compared with MARS monotherapy. There was no significant difference in survival between the two groups. However, the theoretical benefit of MARS therapy combined with TPE is unclear, as both therapies rely on the removal of albumin-bound toxins. TPE employed simultaneously with extracorporeal membrane oxygenation (ECMO) in adults with liver failure has not been reported. However, tandem ECMO, TPE, and CRRT combination therapy has been described in the pediatric population with sepsis-induced multiorgan failure[93].
The common complications associated with TPE are related to the choice of anticoagulation, replacement fluid, and vascular access. This includes citrate-induced hypocalcemia, hemodynamic instability, and transfusion reactions. In their RCT of HV-TPE in ALF patients, Larsen et. al found no significant differences in cardiac arrhythmias, pancreatitis, transfusion related acute lung injury, acute respiratory distress syndrome, hemorrhage, and infection between patients who received HV-TPE vs SMT[19]. A prospective study comparing HV-TPE with SMT in Wilsonian ALF similarly demonstrated no significant difference in the incidence of complications[37]. In addition, TPE has been shown to be safe and tolerable in ACLF patients; severe procedure-related adverse effects have not been reported[44,47]. An open label RCT in ACLF patients reported a higher rate of hypotension in patients who received TPE-based therapy compared to SMT (20.2% vs 9.2%, P = 0.02)[46]. Moreover, there were no significant differences in the rates of bleeding, infection, and respiratory failure between groups[47].
The 2019 American Society for Apheresis (ASFA) has recommended HV-TPE as a first line therapy for ALF and fulminant Wilson disease. In ALF, ASFA recommends performing at least 3 HV-TPE procedures daily and to consider performing daily treatments until LT or liver recovery. In fulminant Wilson disease, daily standard volume plasma exchange treatments until LT or liver recovery is recommended[61]. The 2016 European Association for the study of liver disease recommended HV-TPE as a level I, grade I evidence in ALF, but no recommendation has been made for ACLF[62]. The 2011 American Association for the Study of Liver Disease guidelines suggested plasma exchange as a means to acutely lower serum copper and limit copper-mediated kidney damage in Wilsonian ALF while waiting LT. However, no recommendation was made for the general use of TPE in ALF and ACLF patients[94].
Advanced therapies aimed at improving survival in liver failure rely on the removal of toxins and inflammatory mediators while simultaneously supporting the synthetic and metabolic function of the liver while awaiting either LT or spontaneous hepatic regeneration. Although no ideal extracorporeal liver replacement therapy yet exists, TPE remains a safe, reliable, and feasible treatment. Future studies should replicate the survival benefit demonstrated by Larsen et al[19], examine the role of combination therapies with ECADs, identify which etiologies of ALF and ACLF are best served by TPE, and confirm the optimal exchange volume, frequency, and duration of treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ince V, Janicko M, Meng Q S-Editor: Wu YXJ L-Editor: A P-Editor: Li X
| 1. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2172] [Article Influence: 181.0] [Reference Citation Analysis (5)] |
| 2. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 3. | Dong V, Nanchal R, Karvellas CJ. Pathophysiology of Acute Liver Failure. Nutr Clin Pract. 2020;35:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, Durkalski V, Larson AM, Liou I, Fix O, Schilsky M, McCashland T, Hay JE, Murray N, Shaikh OS, Ganger D, Zaman A, Han SB, Chung RT, Smith A, Brown R, Crippin J, Harrison ME, Koch D, Munoz S, Reddy KR, Rossaro L, Satyanarayana R, Hassanein T, Hanje AJ, Olson J, Subramanian R, Karvellas C, Hameed B, Sherker AH, Robuck P, Lee WM. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 5. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 6. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 7. | Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM. Pathogenesis of liver injury in acute liver failure. Gastroenterology. 2012;143:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Iwai H, Nagaki M, Naito T, Ishiki Y, Murakami N, Sugihara J, Muto Y, Moriwaki H. Removal of endotoxin and cytokines by plasma exchange in patients with acute hepatic failure. Crit Care Med. 1998;26:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 1389] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 11. | Solé C, Solà E, Morales-Ruiz M, Fernàndez G, Huelin P, Graupera I, Moreira R, de Prada G, Ariza X, Pose E, Fabrellas N, Kalko SG, Jiménez W, Ginès P. Characterization of Inflammatory Response in Acute-on-Chronic Liver Failure and Relationship with Prognosis. Sci Rep. 2016;6:32341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 574] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 13. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 431] [Article Influence: 86.2] [Reference Citation Analysis (2)] |
| 14. | Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, Vogel W. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 16. | Demeulenaere L, Barbier F, Vermeire P. Plasmapheresis in hepatic coma. Lancet. 1969;1:152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Sabin S, Merritt JA. Treatment of hepatic coma in cirrhosis by plasmapheresis and plasma infusion (plasma exchange). Ann Intern Med. 1968;68:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Graw RG Jr, Buckner CD, Eisel R. Plasma exchange transfusion for hepatic coma. New technic. Transfusion. 1970;10:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W, Auzinger G, Shawcross D, Eefsen M, Bjerring PN, Clemmesen JO, Hockerstedt K, Frederiksen HJ, Hansen BA, Antoniades CG, Wendon J. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016;64:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (4)] |
| 20. | Cohen J, Aslam M, Pusey CD, Ryan CJ. Protection from endotoxemia: a rat model of plasmapheresis and specific adsorption with polymyxin B. J Infect Dis. 1987;155:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Nagaki M, Hughes RD, Keane HM, Lau JY, Williams R. In vitro plasma perfusion through adsorbents and plasma ultrafiltration to remove endotoxin and cytokines. Circ Shock. 1992;38:182-188. [PubMed] |
| 22. | Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Atluri DK, Prakash R, Mullen KD. Pathogenesis, diagnosis, and treatment of hepatic encephalopathy. J Clin Exp Hepatol. 2011;1:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 25. | Krisper P, Haditsch B, Stauber R, Jung A, Stadlbauer V, Trauner M, Holzer H, Schneditz D. In vivo quantification of liver dialysis: comparison of albumin dialysis and fractionated plasma separation. J Hepatol. 2005;43:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Stadlbauer V, Krisper P, Beuers U, Haditsch B, Schneditz D, Jung A, Putz-Bankuti C, Holzer H, Trauner M, Stauber RE. Removal of bile acids by two different extracorporeal liver support systems in acute-on-chronic liver failure. ASAIO J. 2007;53:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Evenepoel P, Laleman W, Wilmer A, Claes K, Maes B, Kuypers D, Bammens B, Nevens F, Vanrenterghem Y. Detoxifying capacity and kinetics of prometheus--a new extracorporeal system for the treatment of liver failure. Blood Purif. 2005;23:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Kapoor D. Molecular adsorbent recirculating system: albumin dialysis-based extracorporeal liver assist device. J Gastroenterol Hepatol. 2002;17 Suppl 3:S280-S286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Gashti CN. Membrane-based Therapeutic Plasma Exchange: A New Frontier for Nephrologists. Semin Dial. 2016;29:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Schaefer B, Schaefer F, Engelmann G, Meyburg J, Heckert KH, Zorn M, Schmitt CP. Comparison of Molecular Adsorbents Recirculating System (MARS) dialysis with combined plasma exchange and haemodialysis in children with acute liver failure. Nephrol Dial Transplant. 2011;26:3633-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Wiersema UF, Kim SW, Roxby D, Holt A. Therapeutic plasma exchange does not reduce vasopressor requirement in severe acute liver failure: a retrospective case series. BMC Anesthesiol. 2015;15:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Kwon YD, Lee HS, Park CH, Jeen YT, Chun HJ, Lee SW, Choi JH, Kim CD, Ryu HS, Hyun JH. [A case of auto-immune hepatitis associated with primary Sjogren's syndrome]. Taehan Kan Hakhoe Chi. 2003;9:25-30. [PubMed] |
| 33. | Larsen FS, Hansen BA, Ejlersen E, Secher NH, Clemmesen JO, Tygstrup N, Knudsen GM. Cerebral blood flow, oxygen metabolism and transcranial Doppler sonography during high-volume plasmapheresis in fulminant hepatic failure. Eur J Gastroenterol Hepatol. 1996;8:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Clemmesen JO, Kondrup J, Nielsen LB, Larsen FS, Ott P. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure. Am J Gastroenterol. 2001;96:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Khamri W, Abeles RD, Hou TZ, Anderson AE, El-Masry A, Triantafyllou E, Bernsmeier C, Larsen FS, Singanayagam A, Kudo N, Possamai LA, Lebosse F, Auzinger G, Bernal W, Willars C, Weston CJ, Lombardi G, Wendon J, Thursz M, Antoniades CG. Increased Expression of Cytotoxic T-Lymphocyte-Associated Protein 4 by T Cells, Induced by B7 in Sera, Reduces Adaptive Immunity in Patients With Acute Liver Failure. Gastroenterology. 2017;153:263-276.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Kim JE, Chun S, Sinn DH, Kim NJ, Kim S, Kang W, Kim JM, Choi GS, Joh JW, Cho D. Initial experience with high-volume plasma exchange in patients with acute liver failure. J Clin Apher. 2021;36:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Pawaria A, Sood V, Lal BB, Khanna R, Bajpai M, Alam S. Ninety days transplant free survival with high volume plasma exchange in Wilson disease presenting as acute liver failure. J Clin Apher. 2021;36:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Hung YM, Hung GC, Hsu PI, Hung SY, Chou KJ, Chung HM. Short-term survival advantage after plasma exchange in the treatment of acute on chronic liver failure or acute liver failure. Clin Intens Care. 2004;15:93-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Li M, Sun J, Li J, Shi Z, Xu J, Lu B, Cheng S, Xu Y, Wang X, Zhang X. Clinical observation on the treatment of acute liver failure by combined non-biological artificial liver. Exp Ther Med. 2016;12:3873-3876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Mao WL, Chen Y, Chen YM, Li LJ. Changes of serum cytokine levels in patients with acute on chronic liver failure treated by plasma exchange. J Clin Gastroenterol. 2011;45:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Cheng YL, Chang CH, Chen WT, Tsai MH, Lee WC, Tu KH, Tian YC, Chen YC, Hung CC, Fang JT, Yang CW, Chang MY. Prognostic factors and treatment effect of standard-volume plasma exchange for acute and acute-on-chronic liver failure: A single-center retrospective study. Transfus Apher Sci. 2018;57:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Yue-Meng W, Yang LH, Yang JH, Xu Y, Yang J, Song GB. The effect of plasma exchange on entecavir-treated chronic hepatitis B patients with hepatic de-compensation and acute-on-chronic liver failure. Hepatol Int. 2016;10:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Yao J, Li S, Zhou L, Luo L, Yuan L, Duan Z, Xu J, Chen Y. Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure. J Clin Apher. 2019;34:392-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 44. | Stahl K, Busch M, Fuge J, Schneider A, Manns MP, Seeliger B, Schmidt JJ, Wiesner O, Schmidt BMW, Taubert R, Vondran FWR, Hoeper MM, David S. Therapeutic plasma exchange in acute on chronic liver failure. J Clin Apher. 2020;35:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Mao W, Ye B, Lin S, Fu Y, Chen Y. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J. 2010;56:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Xia Q, Dai X, Huang J, Xu X, Yang Q, Liu X, Chen Y, Li L. A single-center experience of non-bioartificial liver support systems among Chinese patients with liver failure. Int J Artif Organs. 2014;37:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Qin G, Shao JG, Wang B, Shen Y, Zheng J, Liu XJ, Zhang YY, Liu YM, Qin Y, Wang LJ. Artificial liver support system improves short- and long-term outcomes of patients with HBV-associated acute-on-chronic liver failure: a single-center experience. Medicine (Baltimore). 2014;93:e338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol. 2020;26:219-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (4)] |
| 49. | Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ; US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 50. | Warrillow S, Fisher C, Tibballs H, Bailey M, McArthur C, Lawson-Smith P, Prasad B, Anstey M, Venkatesh B, Dashwood G, Walsham J, Holt A, Wiersema U, Gattas D, Zoeller M, García Álvarez M, Bellomo R; Australasian Management of Acute Liver Failure Investigators (AMALFI). Continuous renal replacement therapy and its impact on hyperammonaemia in acute liver failure. Crit Care Resusc. 2020;22:158-165. [PubMed] |
| 51. | Horie Y. Granulocytapheresis and plasma exchange for severe alcoholic hepatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Hirano T, Yoshioka K, Teramoto K, Morikawa T, Okada N, Konishi Y. Successful treatment of severe alcoholic hepatitis with plasma exchange and steroid therapy. Nihon Toseki Igakkai Zasshi. 2003;36:285-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 53. | Lyu RK, Chen WH, Hsieh ST. Plasma exchange vs double filtration plasmapheresis in the treatment of Guillain-Barré syndrome. Ther Apher. 2002;6:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Gurland HJ, Lysaght MJ, Samtleben W, Schmidt B. A comparison of centrifugal and membrane-based apheresis formats. Int J Artif Organs. 1984;7:35-38. [PubMed] |
| 55. | Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224-232. [PubMed] |
| 56. | Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 57. | Lin S, Li Y, Long J, Liu Q, Yang F, He Y. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults: A case report and review of the literature. Medicine (Baltimore). 2016;95:e5431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Holt EW, Guy J, Gordon SM, Hofmann JC, Garcia-Kennedy R, Steady SL, Bzowej NH, Frederick RT. Acute liver failure caused by herpes simplex virus in a pregnant patient: is there a potential role for therapeutic plasma exchange? J Clin Apher. 2013;28:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Freeman JG, Matthewson K, Record CO. Plasmapheresis in acute liver failure. Int J Artif Organs. 1986;9:433-438. [PubMed] |
| 60. | Stahl K, Hadem J, Schneider A, Manns MP, Wiesner O, Schmidt BMW, Hoeper MM, Busch M, David S. Therapeutic plasma exchange in acute liver failure. J Clin Apher. 2019;34:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 61. | Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM, Schwartz GEJ. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34:171-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 870] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 62. | European Association for the Study of the Liver. Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 63. | Göpel W, Schnetzke U, Hochhaus A, Scholl S. Functional acute liver failure after treatment with pegylated asparaginase in a patient with acute lymphoblastic leukemia: potential impact of plasmapheresis. Ann Hematol. 2016;95:1899-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Shen C, Zhao CY, Liu F, Wang YD, Wang W. Acute liver failure associated with occupational exposure to tetrachloroethylene. J Korean Med Sci. 2011;26:138-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Kondrup J, Almdal T, Vilstrup H, Tygstrup N. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1992;15:669-676. [PubMed] |
| 66. | Chen KJ, Chen TH, Sue YM, Chen TJ, Cheng CY. High-volume plasma exchange in a patient with acute liver failure due to non-exertional heat stroke in a sauna. J Clin Apher. 2014;29:281-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Riveiro-Barciela M, Muñoz-Couselo E, Fernandez-Sojo J, Diaz-Mejia N, Parra-López R, Buti M. Acute liver failure due to immune-mediated hepatitis successfully managed with plasma exchange: New settings call for new treatment strategies? J Hepatol. 2019;70:564-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Nakae H, Eguchi Y, Saotome T, Yoshioka T, Yoshimura N, Kishi Y, Naka T, Furuya T. Multicenter study of plasma diafiltration in patients with acute liver failure. Ther Apher Dial. 2010;14:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (3)] |
| 69. | Pham HP, Schwartz J, Cooling L, Hofmann JC, Kim HC, Morgan S, Pagano MB, Schneiderman J, Winters JL, Yamada C, Wong EC, Wu Y. Report of the ASFA apheresis registry study on Wilson's disease. J Clin Apher. 2016;31:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 70. | Zhou PQ, Zheng SP, Yu M, He SS, Weng ZH. Prognosis of acute-on-chronic liver failure patients treated with artificial liver support system. World J Gastroenterol. 2015;21:9614-9622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange vs double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Chen JJ, Huang JR, Yang Q, Xu XW, Liu XL, Hao SR, Wang HF, Han T, Zhang J, Gan JH, Gao ZL, Wang YM, Lin SM, Xie Q, Pan C, Li LJ. Plasma exchange-centered artificial liver support system in hepatitis B virus-related acute-on-chronic liver failure: a nationwide prospective multicenter study in China. Hepatobiliary Pancreat Dis Int. 2016;15:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 73. | Shunkwiler SM, Pham HP, Wool G, Ipe TS, Fang DC, Biller E, Treml A, Weiss J, Baron BW, Berg M; Therapeutic Apheresis Subsection of the AABB. The management of anticoagulation in patients undergoing therapeutic plasma exchange: A concise review. J Clin Apher. 2018;33:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Mokrzycki MH, Balogun RA. Therapeutic apheresis: a review of complications and recommendations for prevention and management. J Clin Apher. 2011;26:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Kramer L, Bauer E, Joukhadar C, Strobl W, Gendo A, Madl C, Gangl A. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Ma Y, Xu Y, Chen F, Wang Y, Bai L, Tang H. Good Tolerance of Citrate Accumulation due to Plasma Exchange among Patients with Acute-on-Chronic Liver Failure: A Prospective, Observational Study. Can J Gastroenterol Hepatol. 2018;2018:4909742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Kankirawatana S, Huang ST, Marques MB. Continuous infusion of calcium gluconate in 5% albumin is safe and prevents most hypocalcemic reactions during therapeutic plasma exchange. J Clin Apher. 2007;22:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Reimann PM, Mason PD. Plasmapheresis: technique and complications. Intensive Care Med. 1990;16:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Schinzel H, Berghoff K, Beuermann I, Sauer O, von Mach MA, Weilemann LS. Anticoagulation with low-molecular-weight heparin (dalteparin) in plasmapheresis therapy: initial experience. Transfusion. 2006;46:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Kaushik M, Liew ZH, Sewa DW, Phua GC, Cao L, Krishnamoorthy TL, Ng SY, Lim AEL, Ng LC, Koniman R, Teo SH, Tan HK; S. I.N:G.A.P.O.R.E Initiative (Science In Nephrology: Growth And Progress Of Research & Education Initiative). Description of parallel and sequential configurations for concurrent therapeutic plasma exchange and continuous kidney replacement therapy in adults. J Clin Apher. 2021;36:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Sahota S, Rodby R. Inpatient hemodialysis without anticoagulation in adults. Clin Kidney J. 2014;7:552-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Yuan S, Qian Y, Tan D, Mo D, Li X. Therapeutic plasma exchange: A prospective randomized trial to evaluate 2 strategies in patients with liver failure. Transfus Apher Sci. 2018;57:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Jindal A, Bhadoria AS, Maiwall R, Sarin SK. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int. 2016;36:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Zang H, Liu F, Liu H, You S, Zhu B, Wan Z, Xin S. Incidence, risk factors and outcomes of acute kidney injury (AKI) in patients with acute-on-chronic liver failure (ACLF) of underlying cirrhosis. Hepatol Int. 2016;10:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Tujios SR, Hynan LS, Vazquez MA, Larson AM, Seremba E, Sanders CM, Lee WM; Acute Liver Failure Study Group. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015;13:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 86. | Naorungroj T, Yanase F, Eastwood GM, Baldwin I, Bellomo R. Extracorporeal Ammonia Clearance for Hyperammonemia in Critically Ill Patients: A Scoping Review. Blood Purif. 2020;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Siami GA, Siami FS. Intensive tandem cryofiltration apheresis and hemodialysis to treat a patient with severe calciphylaxis, cryoglobulinemia, and end-stage renal disease. ASAIO J. 1999;45:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Filler G, Clark WF, Huang SH. Tandem hemodialysis and plasma exchange. Pediatr Nephrol. 2014;29:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Nakae H, Yonekawa C, Moon S, Tajimi K. The series-parallel circuit in the treatment of fulminant hepatitis. Ther Apher Dial. 2004;8:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Pérez-Sáez MJ, Toledo K, Ojeda R, Crespo R, Soriano S, Alvarez de Lara MA, Martín-Malo A, Aljama P. Tandem plasmapheresis and hemodialysis: efficacy and safety. Ren Fail. 2011;33:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Nakae H, Asanuma Y, Tajimi K. Cytokine removal by plasma exchange with continuous hemodiafiltration in critically ill patients. Ther Apher. 2002;6:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 92. | Huang YK, Tan DM, Xie YT, Fan XG, Huang Y, Liu ZB, Li SL. Randomized controlled study of plasma exchange combined with molecular adsorbent re-circulating system for the treatment of liver failure complicated with hepatic encephalopathy. Hepatogastroenterology. 2012;59:1323-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 93. | Kawai Y, Cornell TT, Cooley EG, Beckman CN, Baldridge PK, Mottes TA, Luckritz KE, Plomaritas KS, Meade JM, Odetola FO, Han YY, Blatt NB, Annich GM. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med. 2015;16:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (35)] |