Published online Jun 27, 2021. doi: 10.4254/wjh.v13.i6.709
Peer-review started: February 17, 2021
First decision: March 16, 2021
Revised: March 29, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: June 27, 2021
Processing time: 125 Days and 21.9 Hours
Metastasis occurs as a late event in the natural history of hepatocellular carcinoma (HCC), and most patients die of liver failure attributed to the tumor supplanting the liver. Conversely, the brain is a less common metastatic site.
We describe a rare case of hepatitis C virus-related multiple HCC metastasizing to the cavernous sinus, Meckel’s cave, and the petrous bone involving multiple cranial nerves in an 82-year-old woman. At admission imaging studies including Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI) revealed multiple HCC nodules in both right and left lobes. Ultrasound guided biopsy of the left lobe revealed moderately differentiated HCC. Molecular targeted therapy with Lenvatinib (8 mg/d for 94 d, per os) and Ramucirumab (340 mg/d and 320 mg/d, two times by intravenous injection) were administered for 4 mo, resulting in progression of the disease. Three months after the start of molecular target therapy, the patient presented with symptoms of hyperalgesia of the right face and limited abduction of the right eye, indicating disturbances in the right trigeminal and abducens nerves. Brain MRI disclosed a mass involving the cavernous sinus, Meckel’s cave and the petrous bone. Contrast-enhanced MRI with gadolinium-chelated contrast medium revealed a well-defined mass with abnormal enhancement around the right cavernous sinus and the right Meckel’s cave.
The diagnosis of metastatic HCC to the cavernous sinus, Meckel’s cave, and the petrous bone was made based on neurological findings and imaging studies including MRI, but not on histological examinations. Further studies may provide insights into various methods for diagnosing HCC metastasizing to the craniospinal area.
Core Tip: We describe a case of hyperalgesia of the right side of the face and limited abduction of the right eye caused by hepatocellular carcinoma (HCC) metastasizing to the right cavernous sinus, the right Meckel’s cave, and the right petrous bone diagnosed through neurological findings and imaging studies. Although HCC metastasizing to the cavernous sinus, Meckel’s cave and the petrous bone is rare, clinicians need to be vigilant when the patients show neurological dysfunction, especially cranial nerve involvement.
- Citation: Kim SK, Fujii T, Komaki R, Kobayashi H, Okuda T, Fujii Y, Hayakumo T, Yuasa K, Takami M, Ohtani A, Saijo Y, Koma YI, Kim SR. Distant metastasis of hepatocellular carcinoma to Meckel’s cave and cranial nerves: A case report and review of literature. World J Hepatol 2021; 13(6): 709-716
- URL: https://www.wjgnet.com/1948-5182/full/v13/i6/709.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i6.709
Hepatocellular carcinoma (HCC), the most common liver cancer, is considered to bring more than 25 hundred thousand deaths worldwide every year. Metastasis is one of the most major points influencing prognosis. HCC often involves metastasis in the liver, but metastasis out of the liver to the lung, bone, and adrenal glands is less frequent, whereas the brain is commonly not connected. The authors report a case of hyperalgesia of the right side of the face and limited abduction of the right eye caused by HCC metastasizing to the right cavernous sinus, the right Meckel’s cave, and the right petrous bone diagnosed through neurological findings and radiological studies.
An 82-year-old woman was in November 2019 admitted to Kobe Asahi Hospital for the treatment of HCC with molecular targeted therapy such as Lenvatinib (LEN) (8 mg/d).
She had overcome hepatitis C virus infection (HCV) 10 years earlier with interferon treatment, but still retained Child A liver cirrhosis.
She has suffered from chronic obstructive pulmonary disease for 20 years.
Nothing particular.
She had no hepatomegaly and no splenomegaly.
Laboratory examinations at admission revealed the following: Total protein 7.3 g/dL (normal 6.5-8.3), albumin 3.6 g/dL (3.8-5.3), aspartate aminotransferase 92 IU/L (10-40), alanine aminotransferase 172 IU/L (5-40), gamma-glutamyl transpeptidase 90 IU/L (< 35), alkaline phosphatase 422 IU/L (115-359), T-bil 1.3 mg/dL (0.2-1.2), NH3 163 μg/dL (< 130), pertussis toxin 88.3% (70-130), white blood cell 67 × 103/μL (36-90), Hb 13.6 g/dL (11.5-15.0), platelets 32.0 × 104/μL (13.4-34.9), hepatitis B surface antigen (-), HCVAb (+), HCV RNA (-), tumor markers were as follows: Alpha-fetoprotein (AFP) 30332.7 ng/mL (< 10.0), PIVKA-Ⅱ 1395 mAU/mL (< 40) (Table 1).
| Parameters | Results | Parameters | Results |
| WBC | 67 × 103/μL | ALP | 422 IU/L |
| Hb | 13.6 g/dL | γ-GTP | 90 IU/L |
| Platelets | 32.0 × 104/μL | NH3 | 163 μg/dL |
| PT | 88.3% | HBsAg | (-) |
| TP | 7.3 g/dL | HCVAb | (+) |
| ALB | 3.6 g/dL | HCV RNA | (-) |
| T-bil | 1.3 mg/dL | AFP | 30332.7 ng/mL |
| AST | 92 IU/L | PIVKA-II | 1395 mAU/mL |
| ALT | 172 IU/L |
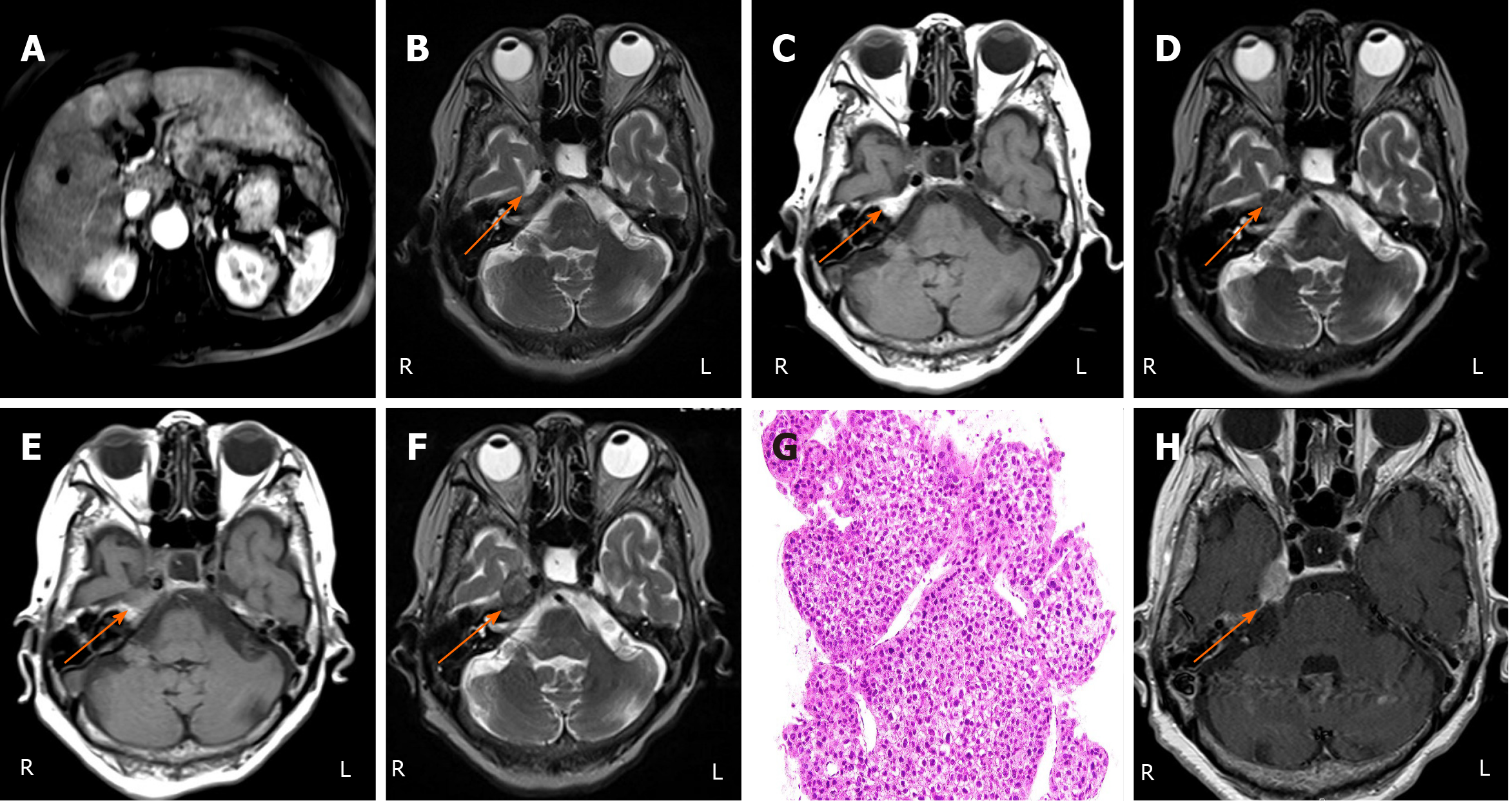
Imaging examination 1: At admission imaging studies including Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI) showed multiple HCC nodules in both right and left lobes (Figure 1A). Gastrointestinal fiberscope revealed atrophic gastritis.
Imaging examination 2: Brain MRI revealed high intensity in the bilateral globus pallidus on T2-weighted images (T2WI), ascribed to elevated serum ammonia (163 μg/dL), but no findings in the cavernous sinus or Meckel’s cave (Figure 1B), and marrow in the petrous bone was intact (Figure 1C).
Imaging examination 3: Brain MRI revealed a low intensity mass around the right Meckel’s cave on T2WI (Figure 1D) and loss of normal fatty bone marrow signal intensity in the right petrous bone on T1-weighted images (T1WI) (Figure 1E).
Imaging examination 4: MRI revealed a low intensity mass around the right cavernous node, the right Meckel's cave, and the right petrous bone on T2WI (Figure 1F). Based on MRI findings, the rapid increase in the size of the lesions over 1 mo and the onset of neurologic dysfunction, such as impairment of right trigeminal and abducens nerves, were most likely due to the metastasizing HCC.
Ultrasound guided biopsy of the left lobe revealed moderately differentiated HCC (Figure 1G).
Contrast-enhanced MRI with gadolinium-chelated contrast medium revealed a well-defined mass with abnormal enhancement around the right cavernous sinus and the right Meckel’s cave (Figure 1H).
Molecular targeted therapy with LEN (8 mg/d for 94 d, per os) and Ramucirumab (340 mg/d and 320 mg/d, two times by intravenous injection) were administered for 4 mo, resulting in progression of the disease. Two months after the start of molecular targeted therapy, tumor markers were as follows: AFP 3830 ng/mL, PIVKA-Ⅱ 3782 mAU/mL.
Three months after the start of molecular targeted therapy, tumor markers were as follows: AFP 25761 ng/mL, PIVKA-Ⅱ 13045 mAU/mL. The patient demonstrated hyperalgesia of the right side of the face and limited abduction of the right eye.
Four months after the start of molecular targeted therapy, tumor markers were as follows: AFP 226112 ng/mL, PIVKA-Ⅱ 268638 mAU/mL, carcinoma embryonic antigen 3.7 ng/mL (< 5.0), CA19-9 126.8 U/mL (< 37.0), interleukin-2R 824 U/mL (122-496).
Five months after the start of molecular targeted therapy, tumor markers were as follows: AFP 26795 mg/dL, PIVKA-Ⅱ 258061 mAU/mL.
Based on the diagnosis, γ knife treatment was performed resulting in relief of the right side of the hyperalgesia. Fourteen days after γ knife treatment, the patient died due to the worsening of general condition.
Metastasis occurs as an advanced incident in the clinical course of liver cancer, and most patients expire because of hepatic insufficiency due to the cancer supplanting the liver. Distant metastases are routinely discovered at autopsy in over 50% of the cases[1-3]. On the contrary, the brain is an uncommon metastatic location. Accidental distant lesions at such more unusual locations are less a considered as possible metastases when metastatic HCC is not discovered at the more usual locations (the lungs, lymph nodes, and bone)[1-3].
The central nervous system is an uncommon location of metastatic HCC[4-8]. Before 1990, the diagnosis of HCC metastasizing to the craniospinal place was evidenced by histopathological findings of biopsy, operative and post-mortem tissues. Lately diagnosis is confirmed by neurologic tests and radiological findings, including computed tomography (CT) and MRI due to advances in such examinations[9-13]. In the 20th century, seven cases of HCC presenting as brain metastasis with no overt liver connection have been reported: Distant metastasis of liver cancer to the cerebrum in one case, and to the cranium in 6 cases[8]. Each showing slightly unusual hepatic examination early assessed, led to the diagnosis that in brain metastasis of obscure origin in a place where it is a usual illness, liver cancer should be viewed in differential diagnoses[8]. In Japan as in Taiwan, the place where liver cancer is a usual illness, HCC metastasizing to the cranium base relating to plural cranial nerves has not been described until now, but one case of cranium metastasis related to emergent epidural HCC[9].
After the 20th century, several cases of metastatic HCC to the cranial nerves have been reported: A 50-year-old female with HCV-associated recurrent multiple HCC metastasizing to the skull base involving multiple cranial nerves shows with conditions drop of eyelid, settlement of the right eyeball, and left abducens paralysis, suggesting disabilities of the right oculomotor and trochlear nerves, and both side abducens nerves. Contrast-enhanced CT of the brain shows an indistinct tumor with unusual increase surrounding the sella turcica. Brain MRI reveals that the tumor involves the clivus, the cavernous sinus, and the petrous apex. On contrast-enhanced MRI with gadolinium-chelated contrast medium, the tumor shows imbalanced middle increase. The diagnosis of metastatic liver cancer to the skull base is done based on of neurologic studies and radiological findings such as CT and MRI, but not on histopathological findings[13].
Two patients with HCC metastasizing to the skull base, the pituitary gland, the sphenoid sinus, and the cavernous sinus present with diplopia, retro-orbital headache, and multiple cranial nerve palsies. One is diagnosed with HCC prior to transsphenoidal operation of the pituitary metastasis. The second patient is, with histopathological examination, diagnosed to have HCC signs and symptoms associating with the primary tumor[14].
Two cases of HCC metastasizing to the cavernous cavity and the sphenoid cavity presenting with double vision and back eye socket headache, are performed operation for primary pituitary gland tumors. After operation, both cases are diagnosed as metastases from HCC[15].
A 73-year-old woman with HCV-related HCC shows a slightly limited abduction, more focused on the left eye with horizontal double vision. MRI of the face and paranasal cavity reveals a tumor in the left sphenoid cavity (22 mm × 16 mm × 16 mm) that invades the cavernous cavity and the forward slope of Meckel’s cave[16,17]. HCC cases of metastasis to the brain from literature were summarized in Table 2 [Age: 56 (25-82), male: 16, female: 7]. Meckel’s cave, a natural mouth-shaped aperture measuring 4 mm × 9mm wide at its opening and 15 mm in length within petrous apex’s meningeal dura propria and periosteal layers, is the central part of the mid cranial fossa; it plays as a main route for the biggest cranial nerve (the fifth)[18,19]. The cavernous sinus is an important element of the cranial vascular organization, having immediate or indirect relations with the cerebrum, cerebellum, brainstem, face, eye, eye socket, nasopharynx, mastoid, and middle ear[20,21].
| No | Age | Sex | Presenting symptoms | Site of metastasis | Survival (from the onset of symptoms) | Ref. |
| 1 | 25 | M | Headache and left weakness | Right temporoparietal brain | 1 d | Chang and Chen[5], 1979 |
| 2 | 50 | M | Weakness of right leg, focal seizure of right leg | Calvarium of the skull, dura, brain | 3 mo | Chang and Chen[5], 1979 |
| 3 | 51 | F | Epistaxis, ptosis, diplopia, facial weakness in the left side | Skull base | 6 mo | Chang and Chen[5], 1979 |
| 4 | 64 | M | Loss of vision in the left eye, anorexia, weight loss | Lateral aspect of the temporal fossa and in the anterior portion of the middle cranial cavity | 3 mo | Zubler et al[7], 1981 |
| 5 | 59 | M | Left arm weakness and numbness, headache with left weakness, disturbed consciousness | Brain parenchyma (right frontotemporal parietal) with intracranial haemorrhage | 2 mo | Lee[8], 1992 |
| 6 | 58 | F | Progressive enlarging scalp mass over vertex for 4 mo | Calvarium, dura, brain parenchyma | 10 mo | Lee[8], 1992 |
| 7 | 48 | F | Progressive enlarging scalp mass over the left parietal and right frontal region for 6 mo | Calvarium | 8 mo | Lee[8], 1992 |
| 8 | 36 | M | Progressive enlarging scalp mass in right occipital region for 2 mo | Calvarium | 3 mo | Lee[8], 1992 |
| 9 | 60 | M | Diplopia and proptosis for 2 mo. Ophthalmoplegia for 1 mo | Skull base (retrobulbar) | 7 mo | Lee[8], 1992 |
| 10 | 54 | M | Progressive dysarthria and atrophy of left tongue for 2 mo | Skull base (jugular fossa hypoglossal canal) | 4 mo | Lee[8], 1992 |
| 11 | 47 | M | Right hemicrania for 3 mo blurred vision with ptosis and limitation of eye movement (OD) numbness on the right forehead for one month | Skull base (parasellar) | 6 mo | Lee[8], 1992 |
| 12 | 70 | M | Left-sided weakness | Acute epidural hematoma adjacent to the right parietal bone | 2 mo | Hayashi et al[9], 2000 |
| 13 | 58 | F | Progressive weakness of her right leg, right hemianesthesia and weakness | Left parietal region, left high parietal area | 6 mo | Lee and Lee[11], 1988 |
| 14 | 50 | M | Hemiparesis and numbness of left upper arm, explosive headache and vomiting, disturbance of consciousness | Right frontotemporoparietal area | 2 mo | Lee and Lee[11], 1988 |
| 15 | 65 | M | Progressive painful right sided proptosis and ptosis, intermittent right temporal and facial pain, loss of sensation on the right side of the face | Right orbital apex | 9 d | Phadke and Hughes[12], 1981 |
| 16 | 55 | M | Mild right weakness | Left fronto-parietal cerebral hemisphere | 11 d | Phadke and Hughes[12], 1981 |
| 17 | 50 | F | Ptosis, diplopia, left abducens palsy | Clivus, cavernous sinus, petrous apex | Not described | Kim et al[13], 2006 |
| 18 | 40 | M | Diplopia, retro-orbital headache, and occasional vomiting | Pituitary fossa, clivus, sphenoid sinus, and right petrous apex | 3 mo | Aung et al[14], 2002 |
| 19 | 71 | M | Headache, diplopia, ptosis of the right eye | Pituitary gland, optic chiasma, cavernous sinus | 1 yr | Aung et al[14], 2002 |
| 20 | 67 | M | Diplopia, left retro-orbital headache | Sphenoid sinus, pituitary gland, clivus | 15 mo | Tamura et al[15], 2013 |
| 21 | 58 | M | Headache, visual disturbance, general fatigue, diplopia, oculomotor nerve palsy | Pituitary fossa, cavernous sinus | 3 wk | Tamura et al[15], 2013 |
| 22 | 73 | F | Frontotemporal and left periorbital headache with associated photophobia | Left sphenoid sinus, cavernous sinus | Not described | Morais et al[16], 2018 |
| 23 | 82 | F | Hyperalgesia of the right face and limited abduction of the right eye | Cavernous sinus, Meckel's cave, petrous bone | 5.5 mo | Our case |
The neural components inside the cavernous sinus contain the sympathetic carotid plexus and 4 cranial nerves. The sites of these nerves, in superior to inferior turn, are the oculomotor (the third), trochlear (the fourth), abducens (the sixth), and ophthalmic divisions of the trigeminal (the fifth)[20].
Differential diagnosis of Meckel’s cave lesions includes neoplastic and non-neoplastic ones.
Meckel’s cave tumors account for only 0.5% of all intracranial tumors. Neoplastic lesions are trigeminal schwannoma (the most common with -33% of cases)[22], meningioma[22,23], pituitary macroadenoma, metastases: Including retrograde spread of head and neck tumors[24-27], epidermoid cysts[28], lipoma, base of skull tumors. All these tumors should be differentiated from Meckel’s cave tumors.
Non-neoplastic lesions include internal carotid artery aneurysms/vascular malformation[29,30], and petrous apex cephalocele.
In our case, benign neoplasms such as schwannoma, meningioma, pituitary macroadenoma, epidermoid cyst, lipoma, base of skull tumors, as well as internal carotid artery aneurysms, vascular malformation and petrous apex cephalocele were ruled out in differential diagnosis.
In our case, brain MRI (T1WI and T2WI) disclosed a mass involving the right cavernous sinus, the right Meckel’s cave and the right petrous bone; MRI with contrast medium revealed abnormal enhancement around the right cavernous sinus, and the right Meckel’s cave.
Moreover, no other malignancies, or lymphoma, have been observed clinically; metastasis from HCC is most likely, irrespective of the absence of histological findings.
Taken together with neurological and imaging findings, our case was diagnosed as metastatic HCC to the right cavernous sinus, the right Meckel’s cave and the right petrous bone involving multiple cranial nerves including the right fifth, and sixth.
The diagnosis of HCC metastasizing to this area is difficult to confirm by histopathological examination because of the deep-seated location and the neurovascular structures; nevertheless, histopathological diagnosis of HCC metastases to the pituitary gland bone has been reported[13,14].
In a previous study, the reason for HCC metastasis to the skull base was explained by the long survival of 15 years with various treatment regimens of chemotherapy and chemoembolization[13]. In our case, HCC metastasis may be due to the biological behavior of HCC such as being moderately differentiated and the failure of molecular targeted therapy, resulting in disease progression.
To our knowledge, our case is the second case of HCC metastasizing to the cavernous sinus, and Meckel’s cave.
Although HCC metastasizing to the cavernous sinus, Meckel’s cave and the petrous bone complicating multiple cranial nerves is very exceptional, medical professionals should be careful and good at managing radiological examinations including CT and MRI, when the patients show neurologic dysfunction, especially cranial nerve connection.
The authors thank Ms. Mika Matsui for excellent technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elpek GO, Ferreira GSA, Gelmini R S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Kojiro M, Nakashima T. Pathology of hepatocellular carcinoma. In: Okuda, Ishak, editors. Neoplasms of the liver. Tokyo: Springer-Verlag, 1987: 81-104. |
| 3. | Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Ihde DC, Sherlock P, Winawer SJ, Fortner JG. Clinical manifestations of hepatoma. A review of 6 years' experience at a cancer hospital. Am J Med. 1974;56:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Chang YC, Chen RC. Craniospinal and cerebral metastasis of primary hepatomas: a report of 7 cases. Taiwan Yi Xue Hui Za Zhi. 1979;78:594-604. [PubMed] |
| 6. | Collomb H, Sankalé M, Dumas M, Ancelle JP. [Neurologic forms of primary liver cancer (clinical and electroencephalographic study)]. Bull Soc Med Afr Noire Lang Fr. 1968;13:577-585. [PubMed] |
| 7. | Zubler MA, Rivera R, Lane M. Hepatoma presenting as a retro-orbital metastasis. Cancer. 1981;48:1883-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Lee JP. Hepatoma presenting as craniospinal metastasis: analysis of sixteen cases. J Neurol Neurosurg Psychiatry. 1992;55:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hayashi K, Matsuo T, Kurihara M, Daikoku M, Kitange G, Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma: a case report. Surg Neurol. 2000;53:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Asahara T, Yano M, Fukuda S, Fukuda T, Nakahara H, Katayama K, Itamoto T, Dohi K, Nakanishi T, Kitamoto M, Azuma K, Ito K, Moriwaki K, Yuge O, Shimamoto F. Brain metastasis from hepatocellular carcinoma after radical hepatectomy. Hiroshima J Med Sci. 1999;48:91-94. [PubMed] |
| 11. | Lee JP, Lee ST. Hepatocellular carcinoma presenting as intracranial metastasis. Surg Neurol. 1988;30:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Phadke JG, Hughes RC. Hepatocellular carcinoma with cranial metastasis and hyperglobulinaemia. J Neurol Neurosurg Psychiatry. 1981;44:1171-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kim SR, Kanda F, Kobessho H, Sugimoto K, Matsuoka T, Kudo M, Hayashi Y. Hepatocellular carcinoma metastasizing to the skull base involving multiple cranial nerves. World J Gastroenterol. 2006;12:6727-6729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Aung TH, Po YC, Wong WK. Hepatocellular carcinoma with metastasis to the skull base, pituitary gland, sphenoid sinus, and cavernous sinus. Hong Kong Med J. 2002;8:48-51. [PubMed] |
| 15. | Tamura T, Kawamura Y, Ikeda K, Seko Y, Fukushima T, Kumada H, Yamada S, Matumaru Y. Hepatocellular carcinoma metastasis to the brain mimicking primary pituitary tumor around the sella turcica. Clin J Gastroenterol. 2013;6:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Morais R, Cardoso H, Silva M, Macedo G. Hepatocellular carcinoma metastasis to sphenoid and cavernous sinus: An unexpected cause of ptosis. Dig Liver Dis. 2018;50:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Sabancı PA, Batay F, Civelek E, Al Mefty O, Husain M, Abdulrauf SI, Karasu A. Meckel's cave. World Neurosurg. 2011;76:335-41; discussion 266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Malhotra A, Tu L, Kalra VB, Wu X, Mian A, Mangla R, Michaelides E, Sanelli P, Gandhi D. Neuroimaging of Meckel's cave in normal and disease conditions. Insights Imaging. 2018;9:499-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Razek AA, Castillo M. Imaging appearance of granulomatous lesions of head and neck. Eur J Radiol. 2010;76:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Kline LB, Acker JD, Post MJ, Vitek JJ. Department of Ophthalmology, University of Alabama, School of Medicine, Birmingham. AJNR Am J Neuroradiol. 1981;2:299-305. [PubMed] |
| 21. | Henderson WR. A note on the relationship of the human maxillary nerve to the cavernous sinus and to an emissary sinus passing through the foramen ovale. J Anat. 1966;100:905-908. [PubMed] |
| 22. | Soni CR, Kumar G, Sahota P, Miller DC, Litofsky NS. Metastases to Meckel's cave: report of two cases and comparative analysis of malignant tumors with meningioma and schwannoma of Meckel's cave. Clin Neurol Neurosurg. 2010;112:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Fujimoto Y, Kato A, Taniguchi M, Maruno M, Yoshimine T. Meningioma arising from the trigeminal nerve: a case report and literature review. J Neurooncol. 2004;68:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Abdel Aziz KM, van Loveren HR. Primary lymphoma of Meckel's cave mimicking trigeminal schwannoma: case report. Neurosurgery. 1999;44:859-62; discussion 862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 1049] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 26. | Moonis G, Cunnane MB, Emerick K, Curtin H. Patterns of perineural tumor spread in head and neck cancer. Magn Reson Imaging Clin N Am. 2012;20:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Paes FM, Singer AD, Checkver AN, Palmquist RA, De La Vega G, Sidani C. Perineural spread in head and neck malignancies: clinical significance and evaluation with 18F-FDG PET/CT. Radiographics. 2013;33:1717-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Miyazawa N, Yamazaki H, Wakao T, Nukui H. Epidermoid tumors of Meckel's cave: case report and review of the literature. Neurosurgery. 1989;25:951-955. [PubMed] |
| 29. | Hughes MA, Frederickson AM, Branstetter BF, Zhu X, Sekula RF Jr. MRI of the Trigeminal Nerve in Patients With Trigeminal Neuralgia Secondary to Vascular Compression. AJR Am J Roentgenol. 2016;206:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Karibe H, Shirane R, Jokura H, Yoshimoto T. Intrinsic arteriovenous malformation of the trigeminal nerve in a patient with trigeminal neuralgia: case report. Neurosurgery. 2004;55:1433. [PubMed] |