Published online Jun 27, 2021. doi: 10.4254/wjh.v13.i6.686
Peer-review started: February 18, 2021
First decision: March 16, 2021
Revised: March 27, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 27, 2021
Processing time: 124 Days and 3.3 Hours
The Budd Chiari syndrome (BCS) is a rare and potentially fatal disease, but there is a paucity of data on the in- hospital mortality as well its economic burden on the health care system.
To evaluate trends in mortality, length of hospital stays and resource utilization among inpatients with BCS.
Data on all adult patients with a diagnosis of BCS were extracted from the National Inpatient Sample (NIS) from 1998 to 2017. To make inferences regarding the national estimates for the total number of BCS discharges across the study period, sample weights were applied to each admission per recommendations from the NIS.
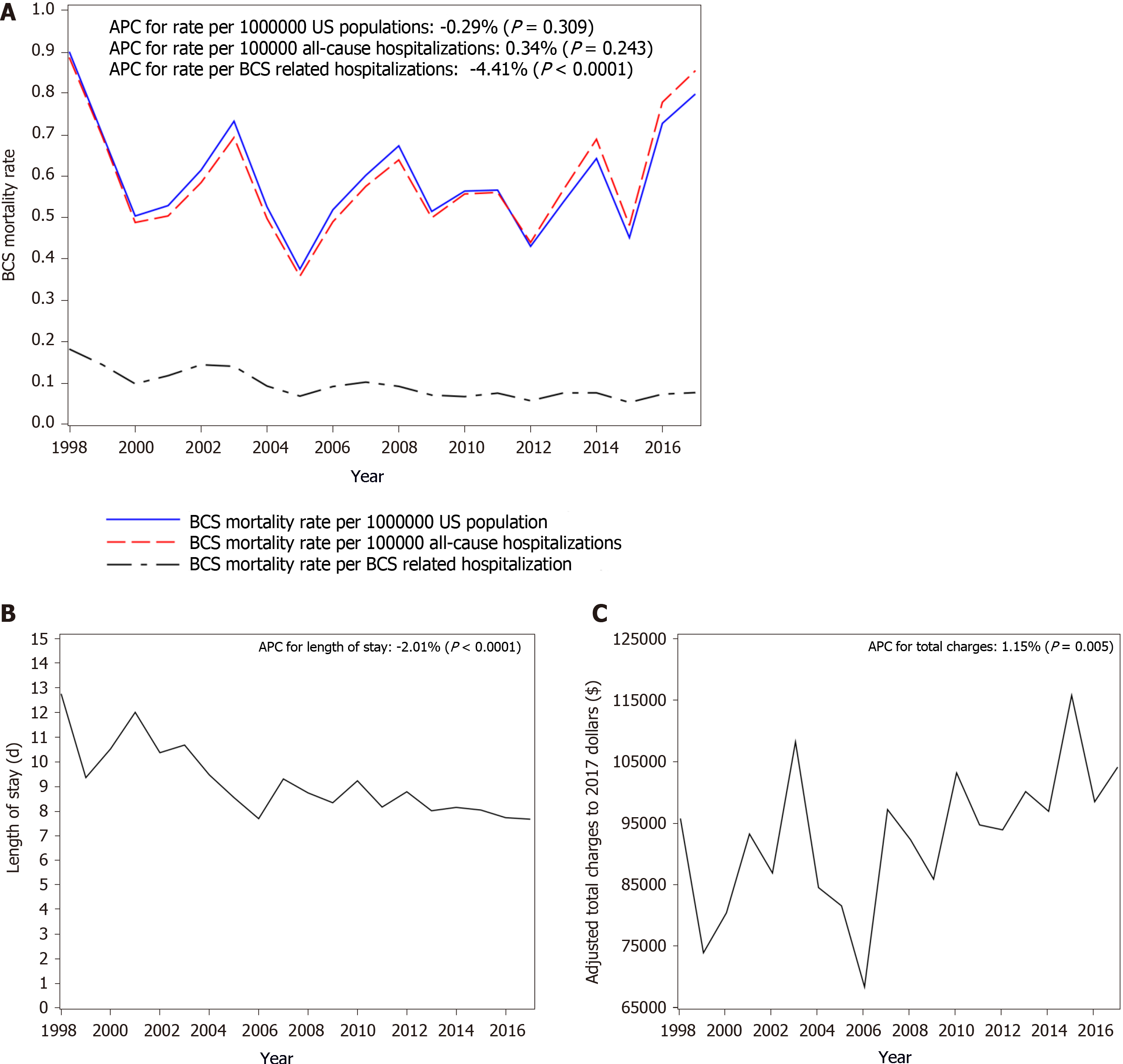
During the study period, there were 3591 (8.73%) in-patient deaths. The overall in-hospital mortality rates among BCS patients decreased from 18% in 1998 to 8% in 2017; the mortality decreased by 4.41% (P < 0.0001) every year. On multivariate analysis, older age, higher comorbidity score, acute liver failure, acute kidney injury, acute respiratory failure, hepatic encephalopathy, hepatorenal syndrome, inferior vena cava thrombosis, intestinal infarct, sepsis/septic shock and cancer were associated increased risk of mortality. The average of length of stay was 8.8 d and it consistently decreased by 2.04% (95%CI: -2.67%, -1.41%, P < 0.001) from 12.7 d in 1998 to 7.6 d in 2017.The average total charges after adjusted for Medical Care Consumers Price Index to 2017 dollars during the time period was $94440 and the annual percentage change increased by 1.15% (95%CI: 0.35%, 1.96%, P = 0.005) from $95515 in 1998 to $103850 in 2017.
The in-hospital mortality rate for patients admitted with BCS in the United States has reduced between 1998 and 2017 and this may a reflection of better management of these patients.
Core Tip: Using a large administrative database, we were able to analyze the mortality and socioeconomic impact of Budd Chiari syndrome hospitalizations in the United States over a 19-year period with a high degree of granularity. We were able to show that while the mortality rate and length of stay has declined significantly, total charges continue to show an upward trend.
- Citation: Alukal JJ, Zhang T, Thuluvath PJ. Mortality and health care burden of Budd Chiari syndrome in the United States: A nationwide analysis (1998-2017). World J Hepatol 2021; 13(6): 686-698
- URL: https://www.wjgnet.com/1948-5182/full/v13/i6/686.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i6.686
The Budd Chiari syndrome (BCS) is a rare but potentially fatal disorder that results from partial or complete obstruction of the hepatic venous outflow in the absence of right heart failure. Unlike Asian countries, the incidence and prevalence of BCS in Western countries is thought to be lower, but there are no large epidemiological studies[1]. BCS is a heterogeneous disease with a protean clinical presentation ranging from asymptomatic or chronic forms to fulminant liver failure[2,3]. Prognosis of BCS is highly variable and studies from large academic centers have reported mortality rates ranging anywhere between 13%-36%[4-9]. These wide ranges of mortality are more likely related to small sample size, variability in follow up period and publication selection bias. Risk factors such as ascites, hepatic encephalopathy, coagulopathy, elevated creatinine or bilirubin are considered to be independent risk factors for mortality[4-8]. A stepwise management approach consisting of anticoagulation, endovascular venoplasty, transjugular intrahepatic portosytemic shunts (TIPS) and liver transplantation (LT) has been proposed for the management of BCS[3,9-12]. However, this approach may not be applicable to all patients because of varying severity of presentation, the extent of venous occlusion and other serious comorbidities.
There are multiple studies that had investigated the mortality and economic burden of decompensated liver cirrhosis in the United States, but there is a paucity of data regarding the mortality burden and health care utilization for patients with BCS. The primary objective of our study was to assess the trends in in-hospital mortality, length of stay (LOS) and resource utilization among inpatients with BCS using the National Inpatient Sample (NIS) database.
This was a retrospective study where data were extracted from the NIS from 1998 to 2017. The NIS is the largest publicly available all-payer inpatient administrative database developed by the Agency for Healthcare Research and Quality (AHRQ) for the Health Care cost and Utilization Project (HCUP). It represents approximately 20% stratified sample of discharges from community hospitals, but excludes long term acute care hospitals and rehabilitation facilities and contains information of more than 7 million hospital discharges annually. The number of states participating in the NIS grew from 8 in 1988 to 48 in 2017. The database captures information about primary and secondary diagnoses during each hospital stay as well as information about procedures. NIS also contains other valuable information such as severity and comorbidity measures, hospital characteristics (size, region, bed size, teaching/non-teaching), payment source (Medicare/Medicaid/private), total charges and length of hospital stay. In 2012, NIS revised the sample design so as to represent a sample of discharges rather than a sample of hospitals. This new strategy is expected to make the estimates more precise by reducing the sampling error. Starting October 1, 2015 all hospitals in the United States adopted International Classification of Diseases (ICD) 10 codes for disease classification as well as for procedures. The calendar year for 2016 and 2017 which is included in this study uses ICD 10 CM/PCS codes.
Data were extracted from the NIS to identify patients ≥ 18 years of age using all listed diagnosis (primary or secondary diagnosis) of BCS from 1998 to 2017. The diagnosis of BCS was captured using the codes 453.0 (ICD-9) and I82.0 (ICD-10).
We obtained information on patient demographics (age, sex, race) and hospital characteristics (region of the country, bed-size, teaching status), patient disposition and insurance status (Medicare, Medicaid and private insurance). Study outcome included changes in inpatient mortality, LOS and total charges with time. We investigated if important complications such as acute liver failure, acute kidney injury, cirrhosis, ascites, hepatic encephalopathy, esophageal varices, portal vein thrombosis, inferior vena cava (IVC) thrombosis and spontaneous bacterial peritonitis had an impact on outcome. We also analyzed inpatient procedures such as liver biopsy, upper gastrointestinal endoscopy, paracentesis, TIPS and LT using appropriate ICD codes (Supplementary Table 1 shows the list of ICD-9 and ICD-10 codes). Severity of illness was measured using the Elixhauser comorbidity index after excluding liver diseases and this included 29 major Elixhauser comorbidity conditions[13].
Descriptive statistics are used to summarize patients’ characteristics, hospital characteristics and utilization, comorbidities, complications, procedures and the outcome by using the weighted survey methods. Data are presented as mean and standard error for continuous variables, percentage and standard error for categorical variables. Standard errors of percentage or mean were estimated using Taylor series linearization method. To make inferences regarding the national estimates for the total number of BCS discharges across the study period, sample weights were applied to each admission per recommendations from the NIS. For the years from 1993-2011, AHRQ had developed discharge trend weights, specifically the NIS Trend Weight Files. Therefore, in our study for trend analyses spanning 2012 and earlier, NIS data trend weights were used to make estimates comparable to the new 2012 NIS design. We used the trend weight in place of the original discharge weights to create national estimates for trend analysis to make the data similar for the entire study period. For 2012 or later data, no trend weights were necessary and the discharge weight supplied on the NIS files were used directly[14]. We calculated BCS discharges rate per 1000000 US populations by dividing the estimated total BCS discharges by projected US population from the Census Bureau.
The annual percentage change (APC) was derived to compare the patients’ characteristics, hospitals’ characteristics and outcomes over time by using Poisson regression for categorical variables and linear regression with natural logarithm transformation for continuous variables. P value for APC was used to determine if the trends in the annual percentage change was significantly different from zero, the change was considered as statistically significant with P value of 0.05 or less.
The hierarchical generalized linear mixed model with hospitals as random effects was performed to evaluate the effects of potential associations between outcomes (mortality, length of stay and total charges) and patients’ demographics (age, gender, and race), patient-level hospitalization variables (primary payer, disposition of patient), hospital-level variables (hospital region, bed size, location and teaching status), comorbidities, complications and procedures separately. Since race was not available in some states, a dummy variable was created for missing data in the models to prevent the observation from being dropped. For mortality, binomial distribution and logit link was used. For length of stay, negative binomial distribution and log links were used. When analyzing the total charges, final total charges were adjusted to 2017 dollars based on medical care Consumer Price Index in US city average provided by the Bureau of Labor Statistics. We specified the models using gamma distributions and log links. A variable with P value of 0.05 or less was retained in the model and considered as statistically significantly associated with outcomes. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, United States)
Between 1998 and 2017, we identified a total of 8435 hospitalizations related to BCS. The mean age of the cohort was 50.5 years, 55% were women and 56 % were white. Nearly half (52%) the patients were covered by government funded health insurance (Medicare and Medicaid) (Table 1). A majority of the patients (59%) were discharged home, and an additional 13.5% were discharged with home health services. While the number of routine home discharges remained the same, there was a 3.31% increase in utilization of home health services (P < 0.0001) (Table 2).
| Study time period | 1998-2017 | Individual effect (Type III test, P value) | ||
| Mortality | Length of stay | Total charges | ||
| BCS patients' characteristics | ||||
| Age | 50.50 (0.19) | < 0.001 | 0.052 | < 0.001 |
| Female | 55.19 (0.54) | 0.003 | 0.001 | < 0.001 |
| Race | 0.138 | 0.013 | < 0.001 | |
| 1: White | 56.03 (0.54) | |||
| 2: Black | 13.26 (0.37) | |||
| 3: Hispanic | 9.56 (0.32) | |||
| 4: Asian/Pacific Islander | 2.56 (0.17) | |||
| 6: Other | 3.65 (0.2) | |||
| 9: Unknown | 14.93 (0.39) | |||
| Primary payer | < 0.001 | 0.002 | < 0.001 | |
| 1: Medicare | 33.17 (0.52) | |||
| 2: Medicaid | 18.42 (0.42) | |||
| 3: Private insurance | 40.20 (0.54) | |||
| 6: Other | 8.21 (0.3) | |||
| Hospital characteristics | ||||
| Hospital size | 0.014 | < 0.001 | < 0.001 | |
| 1: Small | 9.81 (0.32) | |||
| 2: Medium | 18.92 (0.43) | |||
| 3: Large | 71.27 (0.49) | |||
| Hospital location and teaching status | 0.195 | < 0.001 | < 0.001 | |
| 1: Rural | 6.89 (0.28) | |||
| 2: Urban nonteaching | 24.24 (0.47) | |||
| 3: Urban teaching | 68.87 (0.51) | |||
| Hospital region | 0.533 | 0.010 | < 0.001 | |
| 1: Northeast | 21.84 (0.45) | |||
| 2: Midwest | 22.15 (0.46) | |||
| 3: South | 33.45 (0.52) | |||
| 4: West | 22.57 (0.46) | |||
| Clinical characteristics | ||||
| Ascites | 29.93 (0.5) | < 0.001 | < 0.001 | < 0.001 |
| Acute kidney injury | 18.84 (0.43) | < 0.001 | < 0.001 | < 0.001 |
| Hepatic cirrhosis with no mention of alcohol | 18.65 (0.43) | 0.901 | 0.031 | 0.838 |
| Cancer | 17.26 (0.41) | < 0.001 | 0.002 | 0.010 |
| Portal hypertension | 16.57 (0.41) | 0.029 | 0.898 | 0.000 |
| Hepatic encephalopathy | 9.59 (0.32) | < 0.001 | < 0.001 | < 0.001 |
| Portal vein thrombosis | 7.92 (0.3) | 0.006 | 0.372 | 0.073 |
| Esophageal varices without bleeding | 7.44 (0.29) | 0.002 | 0.324 | 0.091 |
| Acute respiratory Failure | 7.03 (0.28) | < 0.001 | < 0.001 | < 0.001 |
| HCC | 6.93 (0.28) | < 0.001 | < 0.001 | 0.543 |
| Acute blood loss anemia/hemorrhagic | 6.62 (0.27) | 0.008 | < 0.001 | < 0.001 |
| IVC thrombosis | 6.39 (0.27) | < 0.001 | < 0.001 | < 0.001 |
| Sepsis | 6.10 (0.26) | < 0.001 | < 0.001 | < 0.001 |
| Alcoholic cirrhosis | 5.73 (0.25) | 0.113 | 0.731 | 0.140 |
| Acute liver failure | 5.60 (0.25) | < 0.001 | < 0.001 | < 0.001 |
| Hepatorenal syndrome | 3.29 (0.2) | < 0.001 | < 0.001 | < 0.001 |
| Variceal bleeding | 3.20 (0.19) | 0.107 | 0.160 | 0.001 |
| Spontaneous bacterial peritonitis | 2.83 (0.18) | < 0.001 | < 0.001 | < 0.001 |
| Intestinal infarct/acute vascular insufficiency | 2.11 (0.16) | < 0.001 | < 0.001 | < 0.001 |
| Elixhauser Comoridity Score excluding liver disease | 9.38 (0.12) | < 0.001 | < 0.001 | < 0.001 |
| 1998-2017 (unweighted: 8435, weighted: 41119) | 1998 (unweighted: 262, weighted: 1367) | 2017 (unweighted: 680, weighted: 3400) | APC (95%CI) | P value for APC | |
| Procedures | |||||
| Number of procedures | 2.64 (0.03) | 3.09 (0.20) | 2.42 (0.13) | -0.51% (-1.09%, 0.06%) | 0.082 |
| Paracentesis | 18.41 (0.42) | 28.56 (2.82) | 16.47 (1.42) | -1.67% (-2.53%, -0.81%) | 0.000 |
| Upper endoscopy | 10.94 (0.34) | 13.08 (2.06) | 11.91 (1.24) | -0.17% (-1.31%, 0.97%) | 0.766 |
| Liver biopsy | 6.24 (0.26) | 10.35 (1.9) | 5.15 (0.85) | -4.01% (-5.42%, -2.58%) | < 0.0001 |
| Portosystemic shunt/TIPS | 3.63 (0.2) | 6.12 (1.54) | 2.94 (0.65) | -4.95% (-6.78%, -3.09%) | < 0.0001 |
| Liver transplantation | 1.9 (0.15) | 1.29 (0.75) | 2.06 (0.54) | -2.68% (-5.26%, -0.02%) | 0.048 |
| Disposition of patient | |||||
| 1: Discharged to home or selfcare | 58.8(0.54) | 50.71 (3.12) | 55.96 (1.91) | -0.28% (-0.77%, 0.21%) | 0.262 |
| 6: Home health care | 13.49 (0.37) | 12.04 (2.04) | 17.23 (1.45) | 3.31% (2.20%, 4.43%) | < 0.0001 |
| 5: Transfer: other type of facility | 11.12 (0.34) | 10.21 (1.89) | 12.08 (1.25) | 1.87% (0.70%, 3.06%) | 0.002 |
| 20: Died in hospital | 8.74 (0.31) | 18.17 (2.44) | 7.66 (1.02) | -4.31% (-5.50%, -3.10%) | < 0.0001 |
| 2: Transfer: short-term hospital | 6.8 (0.28) | 8.25 (1.71) | 5.6 (0.88) | -1.26% (-2.66%, 0.17%) | 0.084 |
| 7: Against medical advice | 1 (0.11) | 0.61 (0.43) | 1.47 (0.46) | 2.94% (-1.02%, 7.07%) | 0.148 |
| Outcomes | |||||
| Number of deaths | 737 (Unweighted); 3591 (Weighted) | 46 (Unweighted); 249 (Weighted) | 52 (Unweighted); 260 (Weighted) | ||
| Mortality rate per 1000000 United States populations | 0.9 | 0.8 | -0.29% (-0.86%, 0.27%) | 0.309 | |
| Mortality rate per 1000000 inpatients | 8.87 | 8.55 | 0.34% (-0.23%, 0.92%) | 0.243 | |
| Mortality rate among BCS inpatients | 0.09 | 0.18 | 0.08 | -4.41% (-4.95%, -3.88%) | < 0.0001 |
| Length of stay (d) | 8.84 (0.13) | 12.73 (1.01) | 7.64 (0.36) | -2.04% (-2.67%, -1.41%) | < 0.0001 |
| Average total charges in 2017 dollars | 94440.04 (1996.06) | 95515.01 (9483.24) | 103850.98 (8183.79) | 1.15% (0.35%, 1.96%) | 0.005 |
Between 1998 and 2017, the in-hospital mortality was 8.74% (n = 737). Using the sample weights provided by HCUP, this corresponded to 3591 deaths (Table 2). Despite a significant increase in the comorbidity score during the time period, overall, in-hospital mortality rate among BCS patients decreased significantly by 4.41% per year (P < 0.0001) from 18% in 1998 to 8% in 2017, with the mortality rate being the lowest in 2015 (5%) (Figure 1A). There were no gender differences in mortality, but those who died were older than those were discharged from the hospital (mean age 58.7 years vs 49.7 years, P < 0.001). Of the patients who died, 53% were Caucasians, 13% were African Americans and 10% were Hispanics. Most deaths occurred in large hospitals (73%) or urban teaching hospitals (71%) (Supplementary Table 2). On multivariate analysis, older age, higher comorbidity score, acute liver failure, acute kidney injury (AKI), acute respiratory failure, hepatic encephalopathy, hepatorenal syndrome, intestinal infarct, IVC thrombosis, sepsis/septic shock and cancer were associated increased risk of mortality (Table 3).
| Response | Beta estimate | Standard error | P value for beta | Odds ratio (95%CI) | |
| Age | 0.024 | 0.003 | < 0.0001 | 1.024 (1.019, 1.029) | |
| Acute respiratory Failure | Yes (reference = No) | 1.652 | 0.109 | < 0.0001 | 5.219 (4.211, 6.468) |
| Intestinal infarct/acute vascular insufficiency | Yes (reference = No) | 1.422 | 0.201 | < 0.0001 | 4.143 (2.795, 6.142) |
| Acute liver failure | Yes (reference = No) | 1.286 | 0.119 | < 0.0001 | 3.617 (2.864, 4.567) |
| Hepatorenal syndrome | Yes (reference = No) | 1.123 | 0.147 | < 0.0001 | 3.072 (2.302, 4.101) |
| Cancer | Yes (reference = No) | 0.882 | 0.098 | < 0.0001 | 2.415 (1.993, 2.927) |
| Acute kidney injury | Yes (reference = No) | 0.803 | 0.092 | < 0.0001 | 2.232 (1.862, 2.675) |
| Sepsis/severe sepsis/septic shock | Yes (reference = No) | 0.635 | 0.122 | < 0.0001 | 1.886 (1.484, 2.398) |
| Hepatic encephalopathy | Yes (reference = No) | 0.280 | 0.117 | 0.020 | 1.323 (1.052, 1.662) |
| Elixhauser Comorbidity Score excluding liver disease | 0.026 | 0.004 | < 0.0001 | 1.027 (1.019, 1.034) |
The average of LOS was 8.8 days and it consistently decreased by 2% (95%CI: -2.67%, -1.41%, P < 0.001) per year from 12.7 d in 1998 to 7.6 d in 2017 (Figure 1B). The LOS in patients who died was longer compared to those who survived (13.54 d vs 8.38 d, P < 0.0001). On multivariate analysis primary payer, and hospital characteristics had impact on LOS. Important complications that had impact on LOS included AKI, acute liver failure, acute respiratory failure, ascites, spontaneous bacterial peritonitis, IVC thrombosis, comorbidity score and cancer (Table 4). Compared to the West, hospitals in the North East, Midwest and South had longer inpatient stays. LOS in urban teaching hospitals was significantly higher than urban non-teaching hospitals (P < 0.0001) (Supplementary Table 3).
| Response | Beta estimate | Standard error | P value for Beta | P value for type 3 test | |
| Primary payer | 1: Medicare (reference) | 0.000 | - | - | 0.022 |
| 2: Medicaid | 0.053 | 0.037 | 0.144 | ||
| 3: Private insurance | 0.084 | 0.030 | 0.005 | ||
| Hospital bed size | 1: Small (reference) | 0.000 | - | - | < 0.0001 |
| 2: Medium | 0.113 | 0.049 | 0.021 | ||
| 3: Large | 0.293 | 0.042 | <.0001 | ||
| Hospital location and teaching status | 1: Rural (reference) | 0.000 | - | - | < 0.0001 |
| 2: Urban nonteaching | 0.206 | 0.054 | <.0001 | ||
| 3: Urban teaching | 0.433 | 0.050 | <.0001 | ||
| Hospital region | 1: Northeast | 0.171 | 0.038 | <.0001 | < 0.0001 |
| 2: Midwest | 0.017 | 0.038 | <.0001 | ||
| 3: South | 0.055 | 0.034 | <.0001 | ||
| 4: West (reference) | 0.000 | ||||
| Complications | |||||
| Acute liver failure | Yes (reference = No) | 0.223 | 0.057 | < 0.0001 | < 0.0001 |
| Acute respiratory Failure | Yes (reference = No) | 0.380 | 0.052 | < 0.0001 | < 0.0001 |
| Acute kidney injury | Yes (reference = No) | 0.255 | 0.035 | < 0.0001 | < 0.0001 |
| Ascites | Yes (reference = No) | 0.118 | 0.028 | < 0.0001 | < 0.0001 |
| Spontaneous bacterial peritonitis | Yes (reference = No) | 0.480 | 0.076 | < 0.0001 | < 0.0001 |
| IVC thrombosis | Yes (reference = No) | 0.138 | 0.052 | 0.008 | 0.008 |
| Intestinal infarct/acute vascular insufficiency | Yes (reference = No) | 0.383 | 0.088 | < 0.0001 | < 0.0001 |
| cancer | Yes (reference = No) | -0.278 | 0.036 | < 0.0001 | < 0.0001 |
| Elixhauser Comorbidity Score excluding liver disease | 0.019 | 0.001 | < 0.0001 | < 0.0001 | |
The average total charges after adjusted for Medical Care Consumers Price Index to 2017 dollars during the time period was $94440, and the APC increased by 1.15% (95%CI: 0.35%, 1.96%, P = 0.005) per year from $95515 in 1998 to $103850 in 2017 (Figure 1C). The hospital charge was higher in patients who died compared to those who survived ($190724 vs $85071, P < 0.0001). The charge was also higher in urban teaching hospitals than urban non-teaching hospitals (P < 0.0001). When stratified by different regions of the country, the charges were higher in the West compared to every other region in the country (P < 0.001, Supplementary Table 4). On multivariate analysis, race, hospital characteristics, number of procedures, length of stay, and comorbidity score were associated with total charges. Important complications that had an effect on total charges included AKI, acute respiratory failure, HRS, IVC thrombosis, cancer, and anemia due to acute blood loss (Table 5).
| Response | Estimate | Standard error | P value for beta | P value for type 3 test | |
| Race | 1: White (reference) | 0.000 | - | - | < 0.0001 |
| 2: Black | -0.037 | 0.026 | 0.162 | ||
| 3: Hispanic | 0.015 | 0.031 | 0.613 | ||
| 4: Asian/Pacific Islander | 0.136 | 0.058 | 0.019 | ||
| 6: Other | 0.082 | 0.046 | 0.077 | ||
| 9: Unknown | -0.185 | 0.025 | < 0.0001 | ||
| Hospital bed size | 1: Small (reference) | 0.000 | - | - | < 0.0001 |
| 2: Medium | 0.080 | 0.034 | 0.018 | ||
| 3: Large | 0.169 | 0.029 | < 0.0001 | ||
| Hospital location and teaching status | 1: Rural (reference) | 0.000 | - | - | < 0.0001 |
| 2: Urban nonteaching | 0.428 | 0.037 | < 0.0001 | ||
| 3: Urban teaching | 0.552 | 0.034 | < 0.0001 | ||
| Hospital region | 1: Northeast | -0.195 | 0.027 | < 0.0001 | < 0.0001 |
| 2: Midwest | -0.333 | 0.027 | < 0.0001 | ||
| 3: South | -0.330 | 0.024 | < 0.0001 | ||
| 4: West (reference) | 0.000 | - | - | ||
| Complications | |||||
| Acute liver failure | Yes (reference = No) | 0.078 | 0.039 | 0.044 | 0.044 |
| Acute respiratory Failure | Yes (reference = No) | 0.204 | 0.036 | < 0.0001 | < 0.0001 |
| Acute kidney injury | Yes (reference = No) | 0.146 | 0.025 | < 0.0001 | < 0.0001 |
| Hepatorenal syndrome | Yes (reference = No) | -0.132 | 0.050 | 0.008 | 0.009 |
| IVC thrombosis | Yes (reference = No) | 0.075 | 0.035 | 0.035 | 0.035 |
| Acute blood loss anemia/ hemorrhagic | Yes (reference = No) | 0.155 | 0.035 | < 0.0001 | < 0.0001 |
| Cancer | Yes (reference = No) | -0.052 | 0.025 | 0.037 | 0.037 |
| Elixhauser Comoridity Score excluding liver disease | 0.005 | 0.001 | < 0.0001 | < 0.0001 | |
| Other variables | |||||
| Number of procedures | 0.118 | 0.004 | < 0.0001 | < 0.0001 | |
| Length of stay | 0.054 | 0.001 | < 0.0001 | < 0.0001 | |
During their in-patient stay, patients underwent an average of 2.64 procedures per hospitalization. Paracentesis was the most frequent procedure (18.4%) followed by upper gastrointestinal endoscopy (10.9%), liver biopsy (6.2%), TIPS (3.6%) and LT (1.9%) (Table 2). Subgroup analysis showed that out of the 307 patients who underwent TIPS, 145 (47%) had LT.
While total number of procedures performed remained stable during the study period, there was a significant and notable reduction in the number of liver biopsies (APC: -4.01%, 95%CI: -5.42%, -2.58%, P < 0.0001), TIPS (APC: -4.95%, 95%CI: -6.78%, -3.09%, P < 0.0001) and LT (APC: -2.68%, 95%CI: -5.26%, -0.02%, P = 0.05). Hispanics underwent more procedures than Caucasians (P < 0.001) and Blacks (P < 0.001). Patients admitted to urban teaching hospitals underwent more procedures than urban non-teaching hospitals (P < 0.0001) and rural hospitals (P < 0.0001) (Supplementary Table 5).
In this large population-based study from the United States, we found that the overall in-patient mortality rate for an unselected group of patients with BCS was 8%. The mortality rates and LOS reduced significantly from 1998 to 2017, but total hospital charges, however, increased during the study period. The patients who survived hospitalization were younger than those who died (49.7 years vs 58.6 years), but race, hospital teaching status and hospital region did not impact survival. The reduction in mortality was multifactorial and possibly could be related to earlier detection of BCS, advances in therapeutic options and a better overall inpatient care.
To our knowledge, there are no prior studies that have exclusively analyzed inpatient mortality secondary to BCS, but multicenter studies in the recent era that investigated prognosis of BCS have reported improvement in survival rates with time[9,10]. A European study that consisted of 157 BCS patients, who were managed using a stepwise treatment algorithm over a median duration of 50 mo reported a mortality of 23%[9]. A majority of these patients succumbed to liver failure (33%) and the median time to death for the cohort was 10 mo. The study found that age, bilirubin and creatinine were independent risk factors for survival. Most patients (88.5%) in their study were on long term anticoagulation and those who did not respond to medical management were treated with percutaneous angioplasty/thrombolysis (n = 22), TIPS (n = 62) and LT (n = 20) in a step wise manner. Due to inherent limitations of the NIS dataset we were unable to determine how many patients in our study were on anticoagulation.
Overall, less than 5% of the patients underwent invasive procedures such as TIPS and LT. There were no significant differences in mortality between patients who underwent these procedures and those who did not. However, 89% of patients who underwent TIPS and 92% who had LT during their inpatient stay survived hospitalization. We also noticed a downward trend in the number of TIPS and LT in hospitalized BCS patients, perhaps because these procedures were done after patients were discharged and hence was not captured by the NIS database. Nearly half (47%) the patients who had TIPS underwent LT, and it possible that TIPS was done in these patients as a bridge to LT, or perhaps they had more complications such as variceal bleeding or refractory ascites.
A management strategy that consists of a stepwise invasive treatment algorithm guided by response to prior treatment have resulted in better short- and long-term outcome in BCS patients[3,9-12,15]. This consists of early and prompt initiation of anticoagulation with low molecular weight heparin to prevent extension of thrombosis, referral to a hematologist for treatment of specific underlying clotting disorders and treatment of portal hypertension related complications. Patients who deteriorate despite optimal medical management are considered for percutaneous or transhepatic angioplasty, TIPS and/or LT. The NIS data set did not include data on venoplasty or stenting perhaps because many of these procedures are done in the outpatient setting. Several studies have reported excellent outcome following LT in patients with BCS. In a previous study, using United Network of Organ Sharing (UNOS) datasets, we had reported 85% 3-year survival in patients with BCS who underwent LT in the United States[16]. Our group recently analyzed outcome of LT in 55 BCS patients who presented with fulminant hepatic failure using the UNOS database and found that expeditious LT in this subset of patients was associated with excellent long-term patient and graft survival. We also found that despite the presence of 3 or more organ failures, LT in these patients was associated with good outcome. They also achieved excellent post LT functional status as determined by the Karnofsky performance status scores[17]. A European series that investigated outcome of LT in 248 patients report actuarial survival of 76% at 1 year, 71% at 5 years and 68% at 10 years, with majority of the deaths occurring in the first 3 mo[18].
In our study we found that the average LOS was 9 d and this reduced consistently with an APC of 2% during the 19-year period. The reduction is consistent with nationwide efforts to reduce LOS for hospitalized patients. Multivariate analysis showed significant association between LOS and complications such as AKI, acute liver failure, acute respiratory failure, SBP and IVC thrombosis. The LOS was longer in medium and large sized hospitals compared to smaller hospitals probably because these hospitals were tertiary care centers and BCS patients admitted in those hospitals were perhaps more sicker requiring prolonged inpatient stay. This would also explain why urban hospitals had a longer LOS compared to hospitals in rural areas. Longer LOS in such hospitals was associated with higher total charges as expected. We also noticed a geographical variation in the LOS, as hospitals in the North East, Midwest and South had longer inpatient stays compared to the West. Although it is difficult to explain this particular finding, a similar observation was made by the HCUP report on US hospital LOS variation by region in 2016 and could be related to physician practice patterns, access to health care services, treatment preferences and cost of living that varies by geographic location in a diverse country like United States[19].
The average total costs for BCS hospitalizations between 1998 and 2017 was $94440 and this continued to show a significant upward trend. We found that compared to the West, hospitals in the Northeast, Midwest and South of United States had lower total charges. We do not have a good explanation for this finding. The increasing financial burden of BCS hospitalizations to the US health care system in our study, despite a reduction in the average LOS, is consistent with other studies that have analyzed the economic impact of hospitalizations related to decompensated cirrhosis and can be attributed to the increasing hospitalization rate as well as increasing severity of disease burden as indicated by comorbidity score[20,21].
Our study has a few limitations most of which are inherent to the use of a large administrative database. The use of ICD codes to capture the diagnosis of BCS could result in coding errors potentially resulting in misclassification. We could not perform a sensitivity analysis because of the absence of patient identifiers in the datasets. Another major shortcoming is that the NIS reports every hospitalization as a separate encounter and not as a unique patient. It is possible that many of these patients were readmitted and were counted more than once. We were also unable to obtain information regarding therapeutic data with respect to anticoagulation and specific pharmacological agents used to treat underlying thrombophilia. Nonetheless, the NIS database is considered to be a powerful research tool providing robust clinical data about real world scenarios and its reliability has been extensively validated[22].
In conclusion, this is the first study from the United States to illustrate reducing mortality related to BCS hospitalizations as well as a reduction in the average LOS. While these findings are reassuring, BCS continues to have a significant economic impact as indicated by the rising healthcare costs.
The Budd Chiari syndrome (BCS) is a rare disorder that results from partial or complete obstruction of the hepatic venous outflow in the absence of right heart failure.
There is a paucity of data on the in-hospital mortality of BCS as well its economic impact on the United States health care system.
This study aimed to evaluate trends in mortality, length of hospital stays and resource utilization among inpatients with BCS.
Retrospective study where data were extracted from the National Inpatient Sample (NIS) from 1998 to 2017. To make inferences regarding the national estimates for the total number of BCS discharges across the study period, sample weights were applied to each admission per recommendations from the NIS.
During the study period, there were 3591 (8.73%) in-patient deaths. The overall in-hospital mortality rate among BCS patients decreased from 18% in 1998 to 8% in 2017; the mortality decreased by 4.41% every year. The average of length of stay was 8.8 d and it consistently decreased by 2.04% from 12.7 d in 1998 to 7.6 d in 2017.The average total charges during the time period was $94440 and the annual percentage change increased by 1.15%
The in-hospital mortality rate for patients admitted with BCS in the United States has reduced between 1998 and 2017 while total charges continued to increase.
Using a large national database, we analyzed the mortality and socioeconomic impact of BCS hospitalizations in the United States with a high degree of granularity.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alvarez-Bañuelos MT S-Editor: Wang JL L-Editor: A P-Editor: Wang LL
| 1. | Valla DC. Hepatic venous outflow tract obstruction etiopathogenesis: Asia vs the West. J Gastroenterol Hepatol. 2004;19:S204-S211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Menon KV, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med. 2004;350:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Zeitoun G, Escolano S, Hadengue A, Azar N, El Younsi M, Mallet A, Boudet MJ, Hay JM, Erlinger S, Benhamou JP, Belghiti J, Valla D. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Langlet P, Escolano S, Valla D, Coste-Zeitoun D, Denie C, Mallet A, Levy VG, Franco D, Vinel JP, Belghiti J, Lebrec D, Hay JM, Zeitoun G. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J Hepatol. 2003;39:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Tang TJ, Batts KP, de Groen PC, van Hoek B, Haagsma EB, Hop WC, Janssen HL. The prognostic value of histology in the assessment of patients with Budd-Chiari syndrome. J Hepatol. 2001;35:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Pavri TM, Herbst A, Reddy R, Forde KA. Budd-Chiari syndrome: a single-center experience. World J Gastroenterol. 2014;20:16236-16244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Garcia-Pagán JC, Heydtmann M, Raffa S, Plessier A, Murad S, Fabris F, Vizzini G, Gonzales Abraldes J, Olliff S, Nicolini A, Luca A, Primignani M, Janssen HL, Valla D, Elias E, Bosch J; Budd-Chiari Syndrome-Transjugular Intrahepatic Portosystemic Shunt Group. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 11. | Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical Guideline: Disorders of the Hepatic and Mesenteric Circulation. Am J Gastroenterol. 2020;115:18-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Tripathi D, Sunderraj L, Vemala V, Mehrzad H, Zia Z, Mangat K, West R, Chen F, Elias E, Olliff SP. Long-term outcomes following percutaneous hepatic vein recanalization for Budd-Chiari syndrome. Liver Int. 2017;37:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6122] [Cited by in RCA: 8316] [Article Influence: 415.8] [Reference Citation Analysis (0)] |
| 14. | Seijo S, Plessier A, Hoekstra J, Dell'era A, Mandair D, Rifai K, Trebicka J, Morard I, Lasser L, Abraldes JG, Darwish Murad S, Heller J, Hadengue A, Primignani M, Elias E, Janssen HL, Valla DC, Garcia-Pagan JC; European Network for Vascular Disorders of the Liver. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Rosenqvist K, Sheikhi R, Eriksson LG, Rajani R, Rorsman F, Sangfelt P, Nyman R. Endovascular treatment of symptomatic Budd-Chiari syndrome - in favour of early transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2016;28:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Segev DL, Nguyen GC, Locke JE, Simpkins CE, Montgomery RA, Maley WR, Thuluvath PJ. Twenty years of liver transplantation for Budd-Chiari syndrome: a national registry analysis. Liver Transpl. 2007;13:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Alukal JJ, Zhang T, Thuluvath PJ. Outcomes of status 1 liver transplantation for Budd-Chiari Syndrome with fulminant hepatic failure. Am J Transplant. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mentha G, Giostra E, Majno PE, Bechstein WO, Neuhaus P, O'Grady J, Praseedom RK, Burroughs AK, Le Treut YP, Kirkegaard P, Rogiers X, Ericzon BG, Hockerstedt K, Adam R, Klempnauer J. Liver transplantation for Budd-Chiari syndrome: A European study on 248 patients from 51 centres. J Hepatol. 2006;44:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Freeman WJ, Weiss AJ, Heslin KC. Overview of U.S. Hospital Stays in 2016: Variation by Geographic Region: Statistical Brief #246 2006. [PubMed] |
| 20. | Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012; 10: 1034-41. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Niu B, Kim B, Limketkai BN, Sun J, Li Z, Woreta T, Chen PH. Mortality from Spontaneous Bacterial Peritonitis Among Hospitalized Patients in the USA. Dig Dis Sci. 2018;63:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Whalen D, Houchens R, Elixhauser A. 2002 HCUP nationwide inpatient sample (NIS) comparison report - HCUP Method Series Report # 2005-03 - ONLINE June 24, 2005 - U.S. Agency for Healthcare Research and Quality. Available from: http://www.hcup-us.ahrq.gov. |