Published online May 27, 2021. doi: 10.4254/wjh.v13.i5.533
Peer-review started: February 17, 2021
First decision: March 16, 2021
Revised: March 28, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: May 27, 2021
Processing time: 92 Days and 5.3 Hours
Liver ischemia-reperfusion injury is a major cause of postoperative liver dysfunction, morbidity and mortality following liver resection and transplantation. Ischemic conditioning has been shown to ameliorate ischemia-reperfusion injury in small animal models. It can be applied directly or remotely when cycles of ischemia and reperfusion are applied to a distant site or organ. Considering timing of the procedure, different protocols are available. Ischemic preconditioning refers to that performed before the duration of ischemia of the target organ. Ischemic perconditioning is performed over the duration of ischemia of the target organ. Ischemic postconditioning applies brief episodes of ischemia at the onset of reperfusion following a prolonged ischemia. Animal studies pointed towards suppressing cytokine release, enhancing the production of hepatoprotective adenosine and reducing liver apoptotic response as the potential mechanisms responsible for the protective effect of direct tissue conditioning. Interactions between neural, humoral and systemic pathways all lead to the protective effect of remote ischemic preconditioning. Despite promising animal studies, none of the aforementioned protocols proved to be clinically effective in liver surgery with the exception of morbidity reduction in cirrhotic patients undergoing liver resection. Further human clinical trials with application of novel conditioning protocols and combination of methods are warranted before implementation of ischemic conditioning in day-to-day clinical practice.
Core Tip: The concept of ischemic conditioning seems easy to apply and is an inexpensive method with the potential to protect the liver during hepatic surgery. It covers a wide spectrum of techniques and allows adjustment of the method to the particular patient. Unfortunately, despite promising animal studies in preventing ischemia-reperfusion injury by ischemic conditioning, currently there is a lack of sufficient data on its clinical efficacy in humans.
- Citation: Stankiewicz R, Grąt M. Direct, remote and combined ischemic conditioning in liver surgery. World J Hepatol 2021; 13(5): 533-542
- URL: https://www.wjgnet.com/1948-5182/full/v13/i5/533.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i5.533
Ischemia-reperfusion injury (IRI) remains an important issue in hepatic surgery. IRI is a pathophysiological phenomenon where cellular damage is caused by reperfusion and reoxygenation following an ischemic period[1]. It is the most important pathogenetic factor occurring during the surgical procedure that impairs both functional reserve through loss of remaining hepatocytes and compromising liver capacity to regenerate. Thus, IRI is a major contributor to increased morbidity and mortality following liver resection and transplantation[2,3].
Ischemic preconditioning (IPC) is an adaptive pathophysiological mechanism based on a concept of preparation of the target organ for ischemic conditions in order to decrease the magnitude of IRI[4]. It was first described by Murry et al[5] in 1986. In a canine model, the authors demonstrated that short repetitive ischemic episodes protected the heart from subsequent sustained ischemic insult.
IPC can be either applied directly[5] or remotely[6]. Remote IPC (RIPC) is based on a concept of brief cycles of ischemia and reperfusion applied to a distant site or organ in order to exert a protective effect on another organ or site. Considering timing of the procedure, remote ischemic perconditioning (RIPer) refers to that performed over the duration of ischemia of the target organ[7].
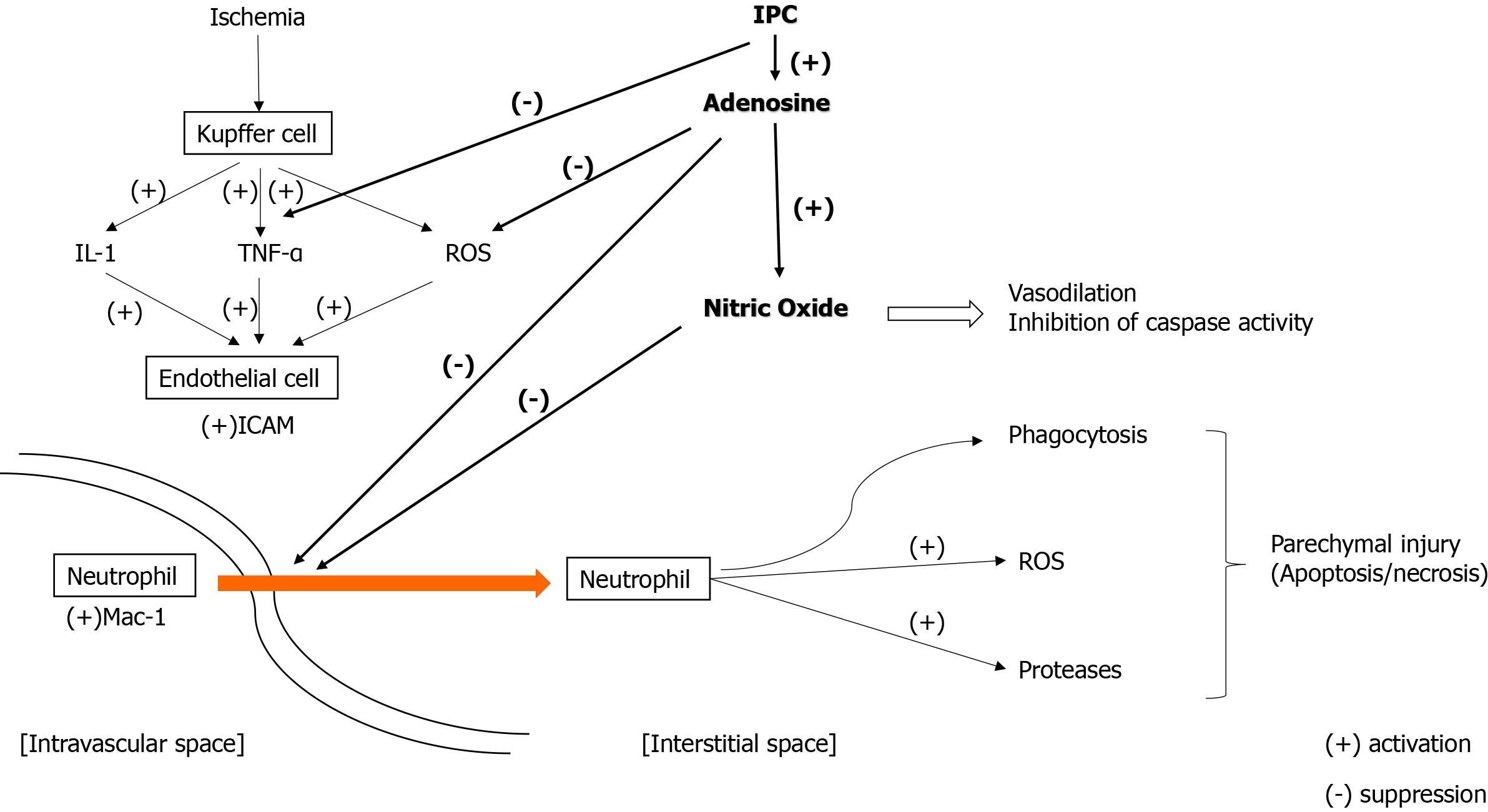
Potential mechanisms responsible for the protective effect of tissue conditioning remain poorly understood. Regarding direct conditioning strategies, it is postulated that IPC suppresses cytokine release, enhances the production of hepatoprotective adenosine and nitric oxide and increases ATP availability by slowing the rate of ATP depletion, thus leading to upregulation of the process of cellular ATP production and liver regeneration and reduction of the liver apoptotic response[8,9]. The summary of IRI mechanism and pathways of IPC is illustrated in Figure 1[10]. In remote ischemic conditioning, reduction of hepatocellular injury in the early phase of IRI is achieved by improvement of parenchymal perfusion and oxygenation[11,12]. Interactions between neural, humoral and systemic pathways all lead to the protective effect of RIPC. In particular, these result in inhibition of the inflammatory response and activation of various hepatoprotective subcellular cascades[13].
In this review, we focus on clinical application of both, direct and remote, ischemic conditioning methods in hepatic surgery in humans. In the discussed papers we highlight clinical endpoints related to mortality, morbidity, intensive care unit (ICU) stay, hospital stay or intraoperative blood loss (in case of parenchymal resection). Postulated mechanisms of hepatocellular protection diminishing IRI are detailed in the referenced studies.
Hepatic steatosis has been associated with worse outcomes in liver surgery, and it is hypothesized that this is caused by a lower tolerance of steatotic livers to IRI[14,15]. Therefore special emphasis is put on outcomes achieved in patients undergoing liver resection and liver transplantation in humans with steatotic livers.
In 2000, Clavien et al[16] published the first non-randomized study on IPC in human liver[16]. Patients were subjected to IPC consisting of 10 min of clamping of the portal triad (Pringle maneuver) followed by 10 min of reperfusion before anatomical left or right hemihepatectomy. Liver cirrhosis, wedge or segmental resections were considered as exclusion criteria. The authors observed lower serum aminotransferase activities and reduced endothelial cell injury in the IPC group. No differences in mortality, hospital stay or blood loss were detected. These findings were followed by another study by Clavien et al[17]. In the randomized controlled trial (RCT), they confirmed previous results and highlighted younger patients and those with liver steatosis as subgroups who derived the most benefits from IPC. Nevertheless, no differences in mortality, hospital stay or blood loss were found. These promising results were followed by a number of studies exploring this field.
Cochrane meta-analysis included four RCTs published until 2008[18]. It assessed IPC followed by continuous clamping (CC) of the portal triad (135 patients) compared with CC alone (136 patients). All the included trials excluded liver resections performed in cirrhotic patients. IPC was achieved by 10 min of clamping followed by 10 min of unclamping, followed by CC in three trials[17,19-21]. In the fourth trial, the duration of initial clamping is likely to be 10 min, although it was not clearly stated. This was followed by 10 min of unclamping followed by CC[22]. The proportion of patients requiring blood transfusion was significantly lower in the IPC group, with no differences in mortality, posthepatectomy liver failure, morbidity, hospital stay or operative time.
Another meta-analysis, conducted by O’Neill et al[23], was published in 2013[23]. It comprised all the aforementioned studies and seven RCTs not included in the Cochrane Hepato-Biliary Group study, of which only one included patients with liver cirrhosis[24]. Ten minutes of the Pringle maneuver for IPC with 10 min of reperfusion was the most frequent strategy. In one study, IPC lasted 5 min with 5 min of reperfusion[24] and in another, IPC lasted 10 min with 15 min of reperfusion[25]. CC was used for parenchymal transection in seven studies[17,20-22,24-26], whereas intermittent clamping was used in the remaining four[27-30]. Eight studies that reported blood loss during liver resection found it to be nonsignificantly lower in the IPC group both in intermittent and CC. No differences in mortality, posthepatectomy liver failure, morbidity, operating time, hospital stay, prothrombin time, bilirubin concentration, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) activities were detected (with and without patients with cirrhosis).
Another meta-analysis was published in 2017[31]. The authors focused only on RCTs investigating the role of IPC before CC. Pooled data were analyzed by combining the results of the 13 RCTs. Five trials enrolled both cirrhotic and noncirrhotic patients (91 in the IPC group and 90 in the control group)[21,32-35]. In three trials, IPC was performed through 5 min of inflow occlusion followed by 5 min of reperfusion[32,34,35]. In one study, IPC was done by inflow occlusion for 10 min followed by reperfusion for 15 min before CC[25]. Ten minutes of the Pringle maneuver for IPC with 10 min of reperfusion was used in nine studies[17,19,22,27-30]. In the case of underlying cirrhosis, IPC reduced postoperative morbidity. However, in patients without cirrhosis, the analysis revealed no significant association between IPC and postoperative morbidity. There were also no differences in morbidity considering ischemia-reperfusion timing (10 + 10 vs 5 + 5). Mortality, operative time, total bilirubin concentration, AST or ALT concentration after postoperative day 1, and hospital and ICU stay were similar regardless of IPC.
Three studies focused on patients with steatotic livers in subgroup analyses. Two studies were RCTs[17,25], and one was a prospective nonrandomized study[16]. A total of 29 patients were analyzed as a subgroup (16 in IPC group and 13 in control group). Cutoff for liver steatosis was set as ≥ 30%, but the type of steatosis (micro- or macrovesicular) was not described. The protocol of IPC was 10 + 10 min in two studies[16,17] and 10 + 15 min in one study[25]. Only peak AST levels were measured as an endpoint in this subgroup comparison. IPC was associated with lower activity of AST after resections in steatotic livers[16,17,25], yet no results on clinical outcomes were provided.
In conclusion, there is currently no evidence supporting direct IPC as a protective strategy against mortality in patients undergoing liver resection, although it may be beneficial for patients with liver cirrhosis with respect to postoperative morbidity. Further investigation of applicability of direct IPC in cirrhotic and steatotic livers is warranted.
In 2016, a meta-analysis on IPC in liver transplantation was published by Robertson et al[36]. Data from ten studies were analyzed (286 patients in IPC group and 307 patients in control group), four nonrandomized[37-40] and six RCTs[41-46]. Only transplantations of grafts procured from donors after brain death were included in these studies, and no grafts underwent machine perfusion. Grafts were preconditioned in the donor by portal triad clamping for 10 min in all but one study. In one study, IPC lasted for 5 min[46]. Time of reperfusion varied among studies from 10 to 39 min. Authors reported that IPC was associated with lower postoperative mortality, lower incidence of primary graft nonfunction and lower rate of retransplantation. None of these findings were statistically significant. Additionally, AST activity on the third postoperative day, length of ICU stay, length of hospital stay and incidence of acute rejection were all nonsignificantly lower in transplantations with IPC.
In living related liver transplantation, two prospective nonrandomized studies were published[47,48]. The protocol of IPC was 10 + 10 min in both studies. Only right lobes were procured from the donors (32 in IPC group and 32 in control group). There were no differences in graft survival, patient survival, morbidity, hospital stay, histological findings and liver function tests between recipients of IPC and non-IPC liver grafts.
Three studies focused on patients with steatotic donor livers in subgroup analyses. All donors were after brain death (25 in IPC group and 29 in control group). Two studies were RCTs[43,46], and one was a retrospective study[39]. The protocol of IPC was 10 + 10 min in one study[39], 10 + 30 min in second study[43], and in the remaining study IPC lasted for 5 min with ongoing reperfusion[46]. Definitions of significant steatosis varied among studies and comprised presence of any steatosis[39], > 15% of macrovesicular steatosis[43] and no specific definition[46]. None of the studies reported results on patient mortality. Clear conclusions cannot be drawn from these studies in terms of impact of IPC on steatotic liver grafts. Morbidity, graft survival, hospital stay, ICU stay and liver function tests seemed to be similar between IPC and non-IPC groups. However, there was a lack of uniform description of severity of hepatic steatosis, and the analyses were limited by small numbers.
In conclusion, there is currently no evidence that direct IPC decreases mortality after deceased and living donor liver transplantation. However, no trial provided data on recipient outcomes after more than 1 year postoperatively, and as such, the long-term effect of IPC on post-transplant outcomes remains to be elucidated. Also, there is insufficient data on IPC impact on steatotic grafts. Therefore, further analysis of this subgroup is warranted.
Only scarce data on remote IPC in liver resection in humans are available (Table 1). In five studies, the total number of 155 patients underwent RIPC with 160 patients serving as controls. Two studies had a third arm, direct IPC, including a total 52 patients. In two studies, liver resection was performed due to colorectal metastases[49,50] and due to primary liver cancers in the others[51-53]. The most common protocol for ischemia-reperfusion was 5 min of upper limb ischemia followed by 5 min of reperfusion in 3 cycles in three studies[50,52,53] and 4 cycles in one study[51]. In the first published pilot randomized feasibility trial, authors applied 2 cycles of 10 min of the lower limb ischemia followed by 10 min of reperfusion[49]. Primary endpoints varied, with serum transaminase activities being the most common. Two studies found significant differences in the early postoperative ALT and AST activities in favor of RIPC[49] and IPC/RIPC over control[50]. In one study, significant differences in postoperative ALT and AST activities on days 1 and 3 in favor of ischemia group (either remote or direct) over control group were observed, but these were absent on postoperative day 7[53]. Analysis of the subgroup of patients with liver cirrhosis was performed in a single study pointing towards no effect of RIPC on ALT activity 24 h posthepatectomy[51]. Mortality, morbidity, blood loss and hospital stay were assessed in three trials, and no differences were found between groups[49,51,52].
| Ref. | Intervention (patients, n) | Ischemia-reperfusion | Place of ischemia | Cirrhosis, n | Pringle maneuver | Primary endpoint |
| Kanoria et al[49], 2017 | RIPC (8) | 2 × 10 + 10 | Lower limb | - | No | Feasibility, safety |
| Control (8) | - | - | - | |||
| Rakić et al[50], 2018 | RIPC (20) | 3 × 5 + 5 | Upper limb | - | Yes | Liver function tests |
| IPC (20) | 15 + 10 | Portal triad | - | |||
| Control (20) | - | - | - | |||
| Teo et al[51], 2020 | RIPC (24) | 4 × 5 + 5 | Upper limb | 13 | Selectively | Serum ALT |
| Control (26) | - | - | 19 | |||
| Liu et al[52], 2019 | RIPC (69) | 3 × 5 + 5 | Upper limb | 56 | Yes (in 20 min cycles) | Peak level of total bilirubin |
| Control (67) | - | - | 51 | |||
| Wu et al[53], 2020 | RIPC (34) | 3 × 5 + 5 | Upper limb | 23 | Yes | Serum ALT and AST |
| IPC (32) | 10 + 10 | Portal triad | 26 | |||
| Control (39) | - | - | 25 |
Data on hepatic steatosis were provided in only two studies. In one trial, all specimens were evaluated for degree of steatosis[49], with minimal liver steatosis found in both groups. In the second study, etiology of liver cirrhosis was nonalcoholic fatty liver disease in 4 patients (2 in the study group and 2 in the control group)[51]. No further information was given.
In conclusion, there is still insufficient data supporting the use of RIPC in liver resection as protection against IRI in order to improve clinical outcomes.
To the authors knowledge, only two studies addressed remote IPC in liver transplantation. In 2017, Robertson et al[54] published a pilot randomized controlled feasibility study on orthotopic liver transplantation from deceased donors (after either brain or cardiac death)[54]. Forty patients were randomized to a sham control group (20 patients) or an RIPC group (20 patients). The protocol for ischemia-reperfusion was 5 min of donor lower limb ischemia followed by 5 min of reperfusion in three cycles. Implantation of the liver graft was performed by standard piggy-back and caval replacement techniques. No differences in 90-d mortality, 90-d graft loss, complications, AST activity on the third postoperative day and hospital and ICU stay were detected.
In 2020, Jung et al[55] published an RCT on the application of RIPC in living donor liver transplantation[55]. In total, 148 donors were randomized to a sham control group (73 donors) or an RIPC group (75 donors). The protocol for ischemia-reperfusion was 5 min of donor upper limb ischemia followed by 5 min of reperfusion in 3 cycles. For the recipients, the medical records were retrospectively analyzed. In the donors, no differences in complications, AST, ALT, total bilirubin and international normalized ratio within 7 postoperative days, incidence of delayed recovery of hepatic function and liver regeneration index depending on the use of RIPC were found. However, recipients who received preconditioned grafts had lower AST activity on postoperative day 7 and the maximal AST activity during the first postoperative week. No differences in other laboratory variables, early graft dysfunction, acute kidney injury, graft failure after 12 mo post-transplantation or hospital and ICU stay were detected.
In conclusion, there is no evidence supporting the use of RIPC in deceased and living donor liver transplantations as protection against IRI in order to improve clinical outcomes.
In search of effective protection against liver IRI, novel concepts are being adapted from experience with other organs. Ischemic postconditioning (IPOS) applies brief episodes of ischemia at the onset of reperfusion following a prolonged ischemia and was first introduced in a rodent heart model[56]. Advantage of IPOS over IPC is that it can be easily applied with precisely controlled timing. Modification of the RIPC technique is RIPer, first applied by Schmidt et al[7] in the context of myocardial ischemia[7]. In a porcine model, alternating periods of occlusion and perfusion of the limb while the myocardium was under ischemia was examined. Little data exists on the efficacy of these methods alone or in combination in hepatoprotection against IRI.
In 2012, a mice liver resection study by Song et al[57] compared IPC, RIPC (hind limb), IPOS and the combination of IPC with IPOS[57]. The authors found that the combination of direct IPC with IPOS offered additional protection over the solo treatment. In contrast, no additive protection of IPOS was found when applied with RIPer in rat liver resection model[58]. In this study, the authors identified RIPer as the most promising technique to avoid hepatic IRI, in comparison with IPOS and combination of RIPer with IPOS. This was in accordance with other studies on rodent liver resection or transplantation, which confirmed a protective effect of RIPer against IRI[59-61]. Combination of different ischemic conditioning techniques in a mouse liver transplantation model was reported by Li et al[62]. By comparing IPC and RIPC with a combination of both methods, they found both techniques effective in hepatic IRI protection but no synergistic and additive effect of IPC and RIPC. Another study designed by this group assigned mice to direct IPC (donor), RIPer (recipients) and IPC + RIPer (donors and recipients were subjected to IPC and RIPer, respectively)[63]. By double protection of the graft, first by IPC in donor then by RIPer before reperfusion in recipient, they showed that combined treatment brought enhanced attenuation in IRI through additive effects on antioxidation, antiapoptosis, modulation of microcirculation disturbance and inhibition of innate immune response.
The aforementioned protocols have only been tested in animal models. No studies on humans have been published researching the possible application of IPOS, RIPer or combined ischemic conditioning. There are currently no ongoing clinical trials on that subject[64].
Direct IPC was not found effective in terms of decreasing mortality after liver resection or transplantation. Its role in specific subgroups of patients remains to be elucidated. Studies on remote IPC in liver resection pointed toward either no beneficial effects or effects limited to moderate reduction of IRI as indicated by serum transaminases and bilirubin concentration. Most studies used protocols with 5 min ischemic periods, which may indicate that this is an insufficient period.
In terms of liver transplantation, RIPC was found to be beneficial only in early graft function from living donors. Those were young, nonsteatotic grafts with relatively short periods of cold and warm ischemia. Other techniques of ischemic conditioning are yet to be assessed in human clinical trials.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SW S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | de Rougemont O, Dutkowski P, Clavien PA. Biological modulation of liver ischemia-reperfusion injury. Curr Opin Organ Transplant. 2010;15:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Robertson FP, Fuller BJ, Davidson BR. An Evaluation of Ischaemic Preconditioning as a Method of Reducing Ischaemia Reperfusion Injury in Liver Surgery and Transplantation. J Clin Med. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5539] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 6. | Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1002] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 7. | Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883-H1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Gomez D, Homer-Vanniasinkam S, Graham AM, Prasad KR. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:657-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Montalvo-Jave EE, Piña E, Montalvo-Arenas C, Urrutia R, Benavente-Chenhalls L, Peña-Sanchez J, Geller DA. Role of ischemic preconditioning in liver surgery and hepatic transplantation. J Gastrointest Surg. 2009;13:2074-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tapuria N, Junnarkar SP, Dutt N, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford). 2009;11:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Szijártó A, Czigány Z, Turóczi Z, Harsányi L. Remote ischemic perconditioning--a simple, low-risk method to decrease ischemic reperfusion injury: models, protocols and mechanistic background. A review. J Surg Res. 2012;178:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with vs without ischemic preconditioning. Ann Surg. 2003;238:843-50; discussion 851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Gurusamy KS, Kumar Y, Pamecha V, Sharma D, Davidson BR. Ischaemic pre-conditioning for elective liver resections performed under vascular occlusion. Cochrane Database Syst Rev. 2009;CD007629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, Pollwein B, Niklas M, Rau HG, Jauch KW, Peter K, Thiel M. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Choukèr A, Martignoni A, Schauer RJ, Rau HG, Volk A, Heizmann O, Dugas M, Messmer K, Peter K, Thiel M. Ischemic preconditioning attenuates portal venous plasma concentrations of purines following warm liver ischemia in man. Eur Surg Res. 2005;37:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, Lemoine A, Adam R, Castaing D. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Heizmann O, Loehe F, Volk A, Schauer RJ. Ischemic preconditioning improves postoperative outcome after liver resections: a randomized controlled study. Eur J Med Res. 2008;13:79-86. [PubMed] |
| 23. | O'Neill S, Leuschner S, McNally SJ, Garden OJ, Wigmore SJ, Harrison EM. Meta-analysis of ischaemic preconditioning for liver resections. Br J Surg. 2013;100:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Li SQ, Liang LJ, Huang JF, Li Z. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2004;10:2580-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Arkadopoulos N, Kostopanagiotou G, Theodoraki K, Farantos C, Theodosopoulos T, Stafyla V, Vassiliou J, Voros D, Pafiti A, Smyrniotis V. Ischemic preconditioning confers antiapoptotic protection during major hepatectomies performed under combined inflow and outflow exclusion of the liver. A randomized clinical trial. World J Surg. 2009;33:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Nuzzo G, Giuliante F, Vellone M, De Cosmo G, Ardito F, Murazio M, D'Acapito F, Giovannini I. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl. 2004;10:S53-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping vs ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921-8; discussion 928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Smyrniotis V, Theodoraki K, Arkadopoulos N, Fragulidis G, Condi-Pafiti A, Plemenou-Fragou M, Voros D, Vassiliou J, Dimakakos P. Ischemic preconditioning vs intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. Am J Surg. 2006;192:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Scatton O, Zalinski S, Jegou D, Compagnon P, Lesurtel M, Belghiti J, Boudjema K, Lentschener C, Soubrane O. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg. 2011;98:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Winbladh A, Björnsson B, Trulsson L, Offenbartl K, Gullstrand P, Sandström P. Ischemic preconditioning prior to intermittent Pringle maneuver in liver resections. J Hepatobiliary Pancreat Sci. 2012;19:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Guo X, Liu G, Zhang X. Meta-analysis of ischemic preconditioning (IP) on postoperative outcomes after liver resections. Medicine (Baltimore). 2017;96:e8217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Hou H, Geng XP, Zhu LX, Ye BG. [The value of hepatic ischemic preconditioning in hepatectomy with a prospective randomized controlled study]. Zhonghua Wai Ke Za Zhi. 2009;47:586-589. [PubMed] |
| 33. | Hahn O, Blázovics A, Váli L, Kupcsulik PK. The effect of ischemic preconditioning on redox status during liver resections--randomized controlled trial. J Surg Oncol. 2011;104:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Liang L, Li S, Huang J. [The protective effect and mechanism of ischemic preconditioning for hepatic resection under hepatic blood inflow occlusion in hepatocellular carcinoma patients with cirrhosis]. Zhonghua Wai Ke Za Zhi. 2002;40:265-267. [PubMed] |
| 35. | Ye B, Zhao H, Hou H, Wang G, Liu F, Zhao Y, Zhang Z, Xie K, Zhu L, Geng X. Ischemic preconditioning provides no additive clinical value in liver resection of cirrhotic and non-cirrhotic patients under portal triad clamping: a prospective randomized controlled trial. Clin Res Hepatol Gastroenterol. 2014;38:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Robertson FP, Magill LJ, Wright GP, Fuller B, Davidson BR. A systematic review and meta-analysis of donor ischaemic preconditioning in liver transplantation. Transpl Int. 2016;29:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Cescon M, Carini R, Grazi G, Caraceni P, Alchera E, Gasloli G, Ravaioli M, Tuci F, Imarisio C, Dal Ponte C, Pertosa AM, Bernardi M, Pinna AD, Albano E. Variable activation of phosphoinositide 3-kinase influences the response of liver grafts to ischemic preconditioning. J Hepatol. 2009;50:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Degli Esposti D, Sebagh M, Pham P, Reffas M, Poüs C, Brenner C, Azoulay D, Lemoine A. Ischemic preconditioning induces autophagy and limits necrosis in human recipients of fatty liver grafts, decreasing the incidence of rejection episodes. Cell Death Dis. 2011;2:e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Azoulay D, Del Gaudio M, Andreani P, Ichai P, Sebag M, Adam R, Scatton O, Min BY, Delvard V, Lemoine A, Bismuth H, Castaing D. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft's preservation and function: the ying and the yang. Ann Surg. 2005;242:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, Wachsberg RH, de la Torre AN, Debroy M, Fisher A, Wilson DJ, Samanta AK. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Jassem W, Fuggle S, Thompson R, Arno M, Taylor J, Byrne J, Heaton N, Rela M. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Franchello A, Gilbo N, David E, Ricchiuti A, Romagnoli R, Cerutti E, Salizzoni M. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI). Am J Transplant. 2009;9:1629-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, Varotti G, D'Errico A, Ballardini G, Pinna AD. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006;12:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R, Fuster J, Hotter G, García-Valdecasas JC. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, de la Torre AN, Merchant A, Arora R, Samanta AK. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Andreani P, Hoti E, de la Serna S, degli Esposti D, Sebagh M, Lemoine A, Ichai P, Saliba F, Castaing D, Azoulay D. Ischaemic preconditioning of the graft in adult living related right lobe liver transplantation: impact on ischaemia-reperfusion injury and clinical relevance. HPB (Oxford). 2010;12:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Testa G, Angelova V, Laricchia-Robbio L, Rondelli D, Chejfec G, Anthony T, Benedetti E. Unilateral ischemic preconditioning and heterologous preconditioning in living donor liver transplantation. Clin Transplant. 2010;24:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Kanoria S, Robertson FP, Mehta NN, Fusai G, Sharma D, Davidson BR. Effect of Remote Ischaemic Preconditioning on Liver Injury in Patients Undergoing Major Hepatectomy for Colorectal Liver Metastasis: A Pilot Randomised Controlled Feasibility Trial. World J Surg. 2017;41:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Rakić M, Patrlj L, Amić F, Aralica G, Grgurević I. Comparison of hepatoprotective effect from ischemia-reperfusion injury of remote ischemic preconditioning of the liver vs local ischemic preconditioning of the liver during human liver resections. Int J Surg. 2018;54:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Teo JY, Ho AFW, Bulluck H, Gao F, Chong J, Koh YX, Tan EK, Abdul Latiff JB, Chua SH, Goh BKP, Chan CY, Chung AYF, Lee SY, Cheow PC, Ooi LLPJ, Davidson BR, Jevaraj PR, Hausenloy DJ. Effect of remote ischemic preConditioning on liver injury in patients undergoing liver resection: the ERIC-LIVER trial. HPB (Oxford). 2020;22:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Liu X, Cao L, Zhang T, Guo R, Lin W. Effect of Remote Ischemic Preconditioning in Patients Undergoing Hepatectomy With Portal Triad Clamping: A Randomized Controlled Trial. Anesth Analg. 2019;129:1742-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Wu G, Chen M, Wang X, Kong E, Yu W, Sun Y, Wu F. Effect of remote ischemic preconditioning on hepatic ischemia-reperfusion injury in patients undergoing liver resection: a randomized controlled trial. Minerva Anestesiol. 2020;86:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Robertson FP, Goswami R, Wright GP, Imber C, Sharma D, Malago M, Fuller BJ, Davidson BR. Remote ischaemic preconditioning in orthotopic liver transplantation (RIPCOLT trial): a pilot randomized controlled feasibility study. HPB (Oxford). 2017;19:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Jung KW, Kang J, Kwon HM, Moon YJ, Jun IG, Song JG, Hwang GS. Effect of Remote Ischemic Preconditioning Conducted in Living Liver Donors on Postoperative Liver Function in Donors and Recipients Following Liver Transplantation: A Randomized Clinical Trial. Ann Surg. 2020;271:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1466] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 57. | Song X, Zhang N, Xu H, Cao L, Zhang H. Combined preconditioning and postconditioning provides synergistic protection against liver ischemic reperfusion injury. Int J Biol Sci. 2012;8:707-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Costa FL, Yamaki VN, Gonçalves TB, Coelho JV, Percário S, Brito MV. Combined remote ischemic perconditioning and local postconditioning on liver ischemia-reperfusion injury. J Surg Res. 2014;192:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Czigány Z, Turóczi Z, Ónody P, Harsányi L, Lotz G, Hegedüs V, Szijártó A. Remote ischemic perconditioning protects the liver from ischemia-reperfusion injury. J Surg Res. 2013;185:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Jia J, Li J, Jiang L, Zhang J, Chen S, Wang L, Zhou Y, Xie H, Zhou L, Zheng S. Protective effect of remote limb ischemic perconditioning on the liver grafts of rats with a novel model. PLoS One. 2015;10:e0121972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | He N, Jia JJ, Li JH, Zhou YF, Lin BY, Peng YF, Chen JJ, Chen TC, Tong RL, Jiang L, Xie HY, Zhou L, Zheng SS. Remote ischemic perconditioning prevents liver transplantation-induced ischemia/reperfusion injury in rats: Role of ROS/RNS and eNOS. World J Gastroenterol. 2017;23:830-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Li DY, Shi XJ, Li W, Sun XD, Wang GY. Ischemic preconditioning and remote ischemic preconditioning provide combined protective effect against ischemia/reperfusion injury. Life Sci. 2016;150:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Li DY, Liu WT, Wang GY, Shi XJ. Impact of combined ischemic preconditioning and remote ischemic perconditioning on ischemia-reperfusion injury after liver transplantation. Sci Rep. 2018;8:17979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | NIH. ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. [cited 26 January 2021]. Available from: https://clinicaltrials.gov/. |