Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.389
Peer-review started: March 30, 2020
First decision: April 22, 2020
Revised: June 4, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 27, 2020
Processing time: 114 Days and 15.8 Hours
Zinc is an essential trace element integral to many cellular and immune functions. Zinc deficiency is highly prevalent in patients with cirrhosis and related to disease severity.
To evaluate whether zinc supplementation improves clinical outcomes (disease severity and mortality) in patients with cirrhosis.
This prospectively registered systematic review (PROSPERO reference: CRD42018118219) included all studies in Medline, Embase or Cochrane database with inclusion criteria of adult human studies, comparing zinc supplementation of at least 28 d with standard care or placebo in patients with cirrhosis. Mortality and clinical severity score data were extracted. Random effects meta-analyses compared mortality at 6 mo and 2 years. Risk of bias was assessed using the National Institutes of Health quality assessment tool.
Seven hundred and twelve articles were identified of which four were eligible. Zinc formulations and doses varied (elemental zinc 3.4-214 mg daily) for different intervention periods in patients with differing etiology and severity of cirrhosis. Two studies were considered to be at high risk of bias. There was no significant difference in 6-mo mortality between patients treated with zinc versus controls [risk ratio 0.98 (0.90-1.05)]. Changes in severity scores were not reported in any study.
Zinc supplementation is not associated with reduced mortality in patients with cirrhosis. Findings are limited by the small number of eligible studies and significant heterogeneity in intervention and patient population.
Core tip: Zinc deficiency is highly prevalent in patients with cirrhosis and may contribute to disease progression and mortality. This systematic review aimed to determine whether zinc supplementation was associated with clinical outcomes in patients with cirrhosis. Meta-analysis of data from four eligible studies found that zinc supplementation was not associated with reduced mortality at 6 mo. No study reported changes in disease severity or complications. Eligible studies were highly heterogeneous with different zinc formulations, dosage and duration applied to varying patient populations. Further well-designed prospective studies are required to determine whether zinc supplementation improves long-term clinical outcome in patients with cirrhosis.
- Citation: Tan HK, Streeter A, Cramp ME, Dhanda AD. Effect of zinc treatment on clinical outcomes in patients with liver cirrhosis: A systematic review and meta-analysis. World J Hepatol 2020; 12(7): 389-398
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/389.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.389
Chronic liver disease is a serious problem both in the United Kingdom and globally. Approximately 0.1% of the European population is affected by cirrhosis which account for 1.8% of all deaths corresponding to 170000 deaths per year[1].
Liver cirrhosis constitutes the third most common cause of premature death in the United Kingdom[2]. The incidence of liver cirrhosis has been increasing in the United Kingdom at a faster rate than the top four most-commonly diagnosed cancers (lung, breast, bowel and prostate)[2]. It is estimated that over 600000 people in the United Kingdom have serious liver disease, and 60000 of those have cirrhosis. Over 14000 people die of liver disease each year in the United Kingdom. This figure has increased by 400% since the 1970s[3].
Regardless of the underlying etiology of liver disease, malnutrition becomes a significant clinical problem as it progresses[4]. Protein-calorie malnutrition is well documented and accounts for more than 60% of patients with advanced alcohol-related cirrhosis[5]. Micronutrient deficiency including trace element deficiency has also been documented, which may play a role in disease pathogenesis through regulating antioxidant, anti-inflammatory and anti-fibrotic pathways[6].
Zinc is the second most abundant trace element in the body after iron. It forms part of more than 300 enzymes in the body[7]. It plays an indispensable role in cell growth, cell differentiation and human metabolism[7]. Therefore, zinc deficiency can contribute to oxidative stress, growth disorder, cognitive disorder and immune dysfunction[7]. Attesting to zinc’s biological importance is its known association with the activity of proteins, including the enzymes needed for the production and destruction of collagen, thus directly affecting the process of fibrosis[7]. It also possesses anti-inflammatory and antioxidant characteristics that may indirectly affect hepatic stellate cells[8].
The global prevalence of zinc deficiency ranges from 4% in countries rich in animal protein and up to 73% in countries with plant-based diets[8]. However, up to 83% of cirrhotic patients have zinc deficiency which is associated with disease severity[8]. Zinc deficiency in liver disease is multifactorial[9]. Changes in carbohydrate-lipid metabolism precipitates protein calorie and micronutrient malnutrition in patients with chronic liver disease[9]. Zinc is bound to albumin, alpha 2-macroglobulin and acids so the rate of zinc absorption is due largely to albumin concentrations[10]. As liver disease progresses, the level of albumin decreases and this may lead to decreased absorption of zinc, resulting in progression of liver disease and an increased risk of hepatocellular carcinoma[10]. Other factors that are responsible for zinc deficiency in liver cirrhosis include disturbed zinc absorption by the digestive tract as a result of the effects of cytokines, mainly interleukin-6 and endotoxins on gut blood flow[11]. This changes the small bowel intestinal mucosa and decreases zinc absorption[11]. Diuretic therapy also plays a factor as it increases renal zinc excretion and reduces serum albumin and the binding capacity for zinc.
Zinc supplementation has beneficial effects on antioxidant and inflammatory pathways and therefore may delay or prevent progression of cirrhosis[12,13]. We performed a systematic review of the published literature to determine whether zinc supplementation was associated with improved clinical outcomes and long-term survival in patients with cirrhosis. A systematic review of the role of zinc supplementation in the management of chronic liver diseases has been recently published[14]. The review evaluated its effect on response to hepatitis C virus treatment, hepatic encephalopathy and changes in biochemistry but did not assess the effect of zinc supplementation on overall long-term survival[14].
The protocol for this systematic review was prospectively registered on the PROSPERO database (reference: CRD42018118219) including the literature search strategy.
We included all interventional clinical trials in humans including randomized controlled trials and open-label trials or observational cohort studies that compared zinc supplementation of at least 28 d with those of standard intervention, or placebo in patients with cirrhosis. Trials were included irrespective of publication status, year of publication or language. We excluded non-human studies and laboratory studies using non-clinical samples.
All adults (> 18 years old) with liver cirrhosis of any etiology, diagnosed using by liver histology, imaging or non-invasive methods. We excluded studies with patients under 18 years of age and with known solid organ cancer including hepatocellular carcinoma.
Studies that compared more than 28 d of zinc supplementation via any route (oral or parenteral) with placebo or other standard intervention for the management of patients with cirrhosis.
The primary outcome was 1-year mortality. Secondary outcomes were 6-mo mortality, 2-year mortality, change in severity scores [Child Pugh/ model for end-stage liver disease (MELD) score], complication rate from cirrhosis (hepatic encephalopathy, new ascites, variceal bleed, new jaundice or hepatocellular carcinoma). Studies had to report at least one of these outcomes to be considered for inclusions in the systematic review.
Electronic searches via MEDLINE (PubMed) 1961-present, EMBASE (1974-present), the Cochrane library (Cochrane Database of Systematic reviews), Cochrane Central Register of Controlled Trials, conference abstracts from 1980 for the following annual meetings: American Gastroenterology Association, American Association for the Study of Liver Disease, European Association for the Study of the Liver, United European Gastroenterology, British Society for Gastroenterology and British Association for the Study of the Liver.
Comprehensive searches of the following biomedical electronic databases were also conducted: MEDLINE, EMBASE, PubMed and TRIPS. The search strategy included subject headings and keywords related to “alcohol”, “zinc” and “liver”. The full search strategy is presented in Supplementary material. The references in all identified review articles and studies were also inspected to identify other trials. Two authors independently assessed the eligible studies.
Titles and abstracts of studies retrieved using the search strategy were screened independently by two authors (Tan HK and Dhanda AD) to identify eligible studies. For potentially relevant articles or in cases of disagreement between the two reviewers, the full text article was obtained and inspected independently by a third reviewer. Where an eligible study failed to report data on the primary or secondary outcomes, this information was requested from the corresponding author by email. A follow-up email was sent after 2 wk if no response was obtained.
Data were extracted independently by two authors (HT and AD) using a standardized, pre-piloted form for assessment of study quality and evidence synthesis. We studied the following data: Study setting and target population, study methodology, details of intervention, primary and secondary outcomes and method of measurement and information of bias. Extracted data were discussed and this discussion was documented.
Two authors independently assessed risk of bias in the trials without masking the trial names using a standard checklist. Risk of bias was assessed using the National Institutes of Health risk of bias tools for controlled trials or observational studies[15]. Any discrepancies or unusual patterns were checked with an independent reviewer.
Controlled intervention studies were assessed for randomization, allocation method, blinding, and similarity of baseline characteristics, drop-out rate, protocol adherence, outcome measures and method of analysis.
Observational studies were assessed for whether there was a defined population, the participation of eligible population, appropriate outcome measures and analysis methods, loss to follow-up rates and confounding factors.
We provide a narrative synthesis of the findings from the included studies, structured around the target population, timing of intervention, and type of outcome.
The survival (until death) rates from each study contributed to a meta-analysis of the efficacy of zinc supplementation in reducing mortality. If heterogeneity was deemed to be at least moderate as determined by the I2 statistic exceeding 30% detected at least at the 5% level, then the data would be meta-analyzed with a random effects model, otherwise a fixed effects model would be considered, if there were too few studies to detect heterogeneity. If any subset of studies were found to share characteristics that contributed to heterogeneity across all the studies, then further meta-analyses would be conducted on those subsets. Analysis was performed using the meta package[16] with a current installation in R.
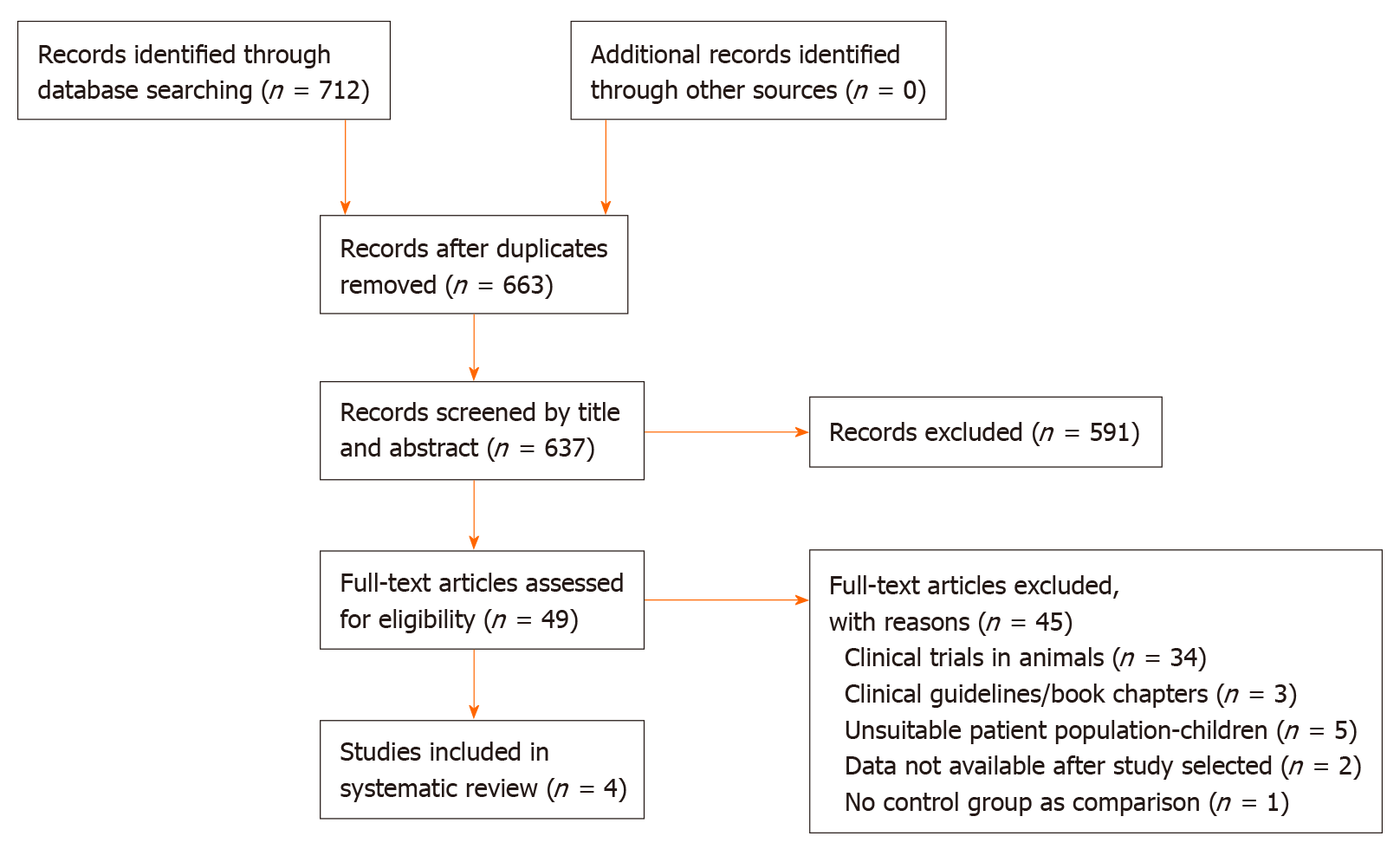
Seven hundred and twelve records were identified; 49 of them were retrieved and assessed for eligibility. Six studies met selection criteria but outcome data could not be obtained from one and another was an uncontrolled cohort study. Therefore, a total of four randomized controlled trials were included in this systematic review (Figure 1)[17-20]. Study characteristics are presented in Table 1.
| Bresci et al[19] | Vilar Gomez et al[18] | Hayashi et al[20] | Takuma et al[17] | |
| Country | Italy | Cuba | Japan | Japan |
| Number of participants | 90 | 100 | 40 | 79 |
| %male (control group) | 23 | 18 | 13 | 40 |
| %male (intervention group) | 33 | 22 | 10 | |
| Age (control group) | 49 | 56.6 (8.4) | 65.1 (11.3) | 66.5 (7.4) |
| Age (intervention group) | 51 | 58.5 (8.9) | 66.0 (9.9) | 66.5 (5.7) |
| Inclusion criteria | Cirrhosis (any aetiology) with encephalopathy | Decompensated HCV cirrhosis | Cirrhosis of any aetiology | Cirrhosis (any aetiology) with encephalopathy |
| MELD (control group) | N/A | 13.3 (4.7) | N/A | 11.8 (3.2) |
| MELD (intervention group) | N/A | 12.5 (3.7) | N/A | 11.8 (3.2) |
| Control group treatment | Placebo | Placebo | BCAAs | BCAAs |
| Intervention group treatment | Zinc acetate | Viusid | Zinc sulfate + BCAAs | Polaprezinc + BCAAs |
| Zinc preparation and dosage | Zinc acetate 600 mg/d | Viusid 3 tablets/d | Zinc sulfate 200-600 mg/d | Polaprezinc |
| Elemental Zinc dosage (mg Zn/d) | 214 | 3.4 | 45-136 | 51 |
| Duration of intervention | 6 mo | 96 wk | 5-6 mo | 6 mo |
| Primary Outcome | Hepatic encephalopathy assessments | Overall survival rate | Change in biochemistry | Hepatic encephalopathy assessments |
| Results | No significant improvement in hepatic encephalopathy assessments | Significant improvement in overall survival, time to disease progression and cumulative incidence of HCV | Significant improvement in blood ammonia levels | Significant improvement in the physical component scale, but not the mental component scale |
| Duration of follow up | 1 yr | 96 wk | 5-6 mo | 6 mo |
All four included studies were randomized controlled trials (RCTs). All but one study documented both gender and age. None of the studies reported ethnicity of their patient cohort. All four studies selected different clinical populations including patients with cirrhosis with hepatic encephalopathy, decompensated hepatitis C cirrhosis, and cirrhosis with etiology not documented. All studies were conducted in community settings. The definition of cirrhosis was based on histological and biochemical confirmation in three studies; the remaining study did not specify the method of diagnosis of cirrhosis. Only one study was designed to test the effect of zinc supplementation on mortality.
Only two studies (Takuma et al[17] and Vilar Gomez et al[18]) reported severity of cirrhosis with mean Child Pugh scores of 6.0 and 6.3 and MELD scores of 11.8 and 12.9.
Bresci et al[19] examined the effect of long-term oral zinc supplementation on recurrent hepatic encephalopathy. Ninety cirrhotic patients with recurrent encephalopathy were treated with 600 mg of oral zinc acetate daily for 30 d in addition to standard therapy. The final values of psychometric tests were better in the zinc group compared to the standard therapy group, but the differences were not statistically significant. Three deaths were reported within 6 mo (one in the placebo and two in the intervention group).
Vilar Gomez et al[18] evaluated the efficacy of Viusid for 96 wk in reducing mortality in 100 patients with hepatitis C-related decompensated cirrhosis. Viusid was chosen because it is a nutritional supplement with recognized anti-inflammatory and antioxidant properties. It contains 11 active compounds including zinc sulphate and glycyrrhizic acid. Glycyrrhizin is the most active ingredient of Viusid and has anti-inflammatory, immune-modulating and antiviral properties. The total amount of daily elemental zinc participants received was 3.4 mg, the lowest zinc content of any of the included studies. The study demonstrated a significant improvement in overall survival, time to disease progression and cumulative incidence of hepatocellular carcinoma. No differences were observed between groups for incidence of liver decompensation (including hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome and gastrointestinal bleeding).
Hayashi et al[20] randomized 40 patients with cirrhosis to receive a combination of branched chain amino acids and zinc sulfate 600 mg daily for 6 mo. No deaths were reported during the study. They demonstrated a significant improvement in blood ammonia levels in the combination group, but there was no further investigation done to determine the mechanism of action and long-term clinical efficacy.
Takuma et al[17] investigated the effectiveness of oral polaprezinc (51 mg zinc and 174 mg of L-carnosine daily) in 79 patients with hepatic encephalopathy. They concluded that oral polaprezinc did significantly improve the physical component scale, but not the mental component scale in patients treated with zinc supplementation. One death occurred in the placebo treated arm only. This study was limited by short-term follow-up (6 mo) and non-blinded treatment allocation.
No studies reported 1-year mortality, which was the primary outcome of this systematic review. Three studies (Bresci et al[19], Hayashi et al[20], and Takuma et al[17]) reported 6-mo survival and one (Vilar Gomez et al[18]) reported 2-year survival. There is a substantial amount of heterogeneity across the selected study trials in terms of zinc formulation, dosage and patient characteristics. The heterogeneity includes dose quantity and frequency, formulation and types of patients.
We used the National Institutes of Health risk of bias tools to assess the quality of included studies[16]. Two studies (Vilar Gomez et al[18] and Hayashi et al[20]) were of low risk of bias and two studies of uncertain risk of bias (Bresci et al[19] and Takuma et al[17]). Risk of bias assessments are presented in Supplementary Table 1. The two studies scored low as a result of lack of blinding and non-similarity in baseline characteristics. Three out of four studies reported information on patient characteristics. Thus, multivariate meta-regression was not appropriate. Selection bias is likely as two studies included only male subjects. Lack of information precluded a proper evaluation of all the risk of bias in the studies.
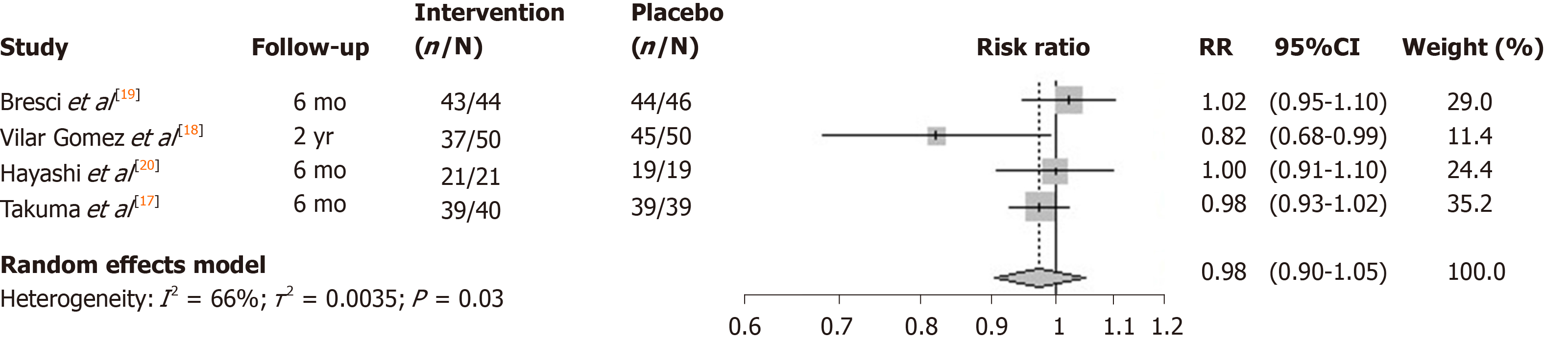
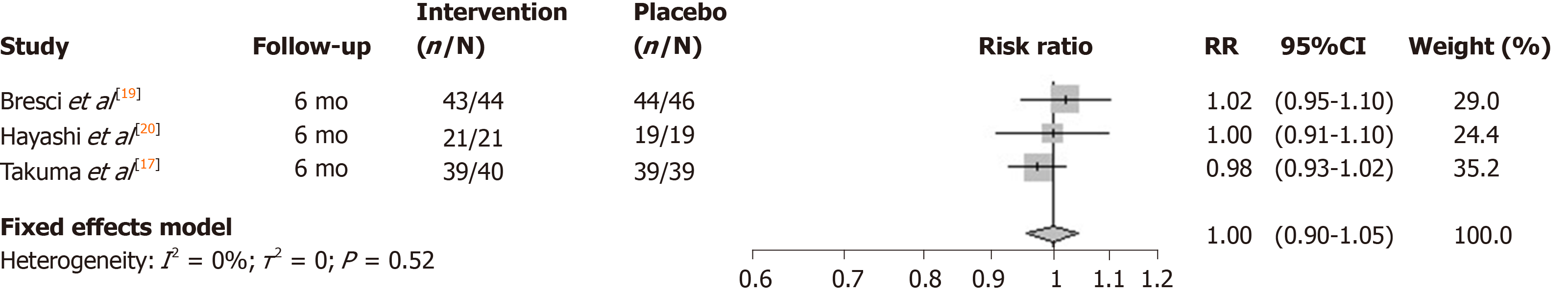
The I2 statistic measuring heterogeneity across all included RCTs was estimated to be 66% and significant at the 5% level (P = 0.03). The overall effect of zinc supplements from the included RCTs was estimated to reduce the risk of mortality by 0.98 [95% confidence interval (CI): 0.90-1.05; Figure 2]. Three out of the four included studies had a 6-mo follow-up. The study by Vilar Gomez et al[18] reported the largest effect (risk ratio = 0.82), although uncertainty around the point estimate was relatively wide (95%CI: 0.68-0.99). Notably it had a longer 2-year follow-up, and so the meta-analysis was repeated without this particular study, which according to its weight, contributed the least to the overall estimated effect. The effect of all the studies measuring mortality within a 6-mo follow-up were similarly located close to, and not significantly different from, the null effect. Repeating the meta-analysis on these resulted in a risk ratio of unity (95%CI: 0.90-1.05) for the effect of zinc supplementation on the risk of mortality over a 6-mo follow-up (Figure 3).
This systematic review identified just four studies that assessed whether zinc supplementation is associated with improved clinical outcomes in patients with cirrhosis. All four eligible studies were highly heterogeneous in terms of patient characteristics, treatment formulation and duration. Four different zinc preparations, with daily elemental zinc doses ranging from 3.4 to 214 mg daily, were tested. Two of these (Viusid, polaprezinc) contained other active compounds, which could have contributed to the positive clinical outcome, for example Viusid contains only 3.4 mg of elemental zinc along with 10 other active ingredients. Three studies tested zinc supplementation in combination with other intervention (branch chain amino acids and nutritional support).
A further limitation is the low mortality rate in all eligible studies. With such a low event rate, any effect of zinc on mortality is difficult to determine. This suggests that either severity of cirrhosis was mild or follow-up duration was insufficient. Only two studies reported severity of cirrhosis with the majority of subjects classified as Child Pugh class A. Two out of four studies were designed to determine the benefit of zinc on hepatic encephalopathy and not powered to detect differences in mortality.
Both Vilar Gomez et al[18] and Takuma et al[17] suggested the most likely beneficiaries from zinc supplementation would be those with portal hypertension and leaky gut with its resultant risk of sepsis. Preclinical and in vivo evidence suggests that zinc may reduce gut permeability[21]. Zinc carnosine (polaprezinc) improved in vitro gut epithelial cell migration and proliferation[21]. In healthy volunteers, zinc carnosine treatment reversed both indomethacin-induced and extreme exercise-induced gut leakiness[22]. To test the benefit of a combination strategy of zinc supplementation and treatment of gut leakiness in advanced cirrhosis, long-term studies in patients with portal hypertension are required.
Given the limited data, we are unable to determine whether zinc supplementation improves survival or reduces disease severity in patients with cirrhosis. Research is still needed to confirm the preclinical benefits seen with zinc supplementation on anti-fibrotic, anti-inflammatory and antioxidant processes. To determine whether zinc supplementation improves clinical outcomes of patients with cirrhosis, further high quality studies are required to ascertain the optimal zinc formulation, dose, duration of treatment and patient population to treat.
In conclusion, this systematic review has highlighted the paucity of high quality studies investigating the effect of zinc supplementation in patients with cirrhosis. Eligible studies were of variable design and quality. The primary analyses all had substantial heterogeneity reflecting the differences in study design, inclusion criteria and primary outcome. The difference in etiology and severity of liver cirrhosis also make the effect of zinc supplementation difficult to interpret. With a plausible rationale for benefit from zinc supplementation, there is a strong argument to develop well designed studies in patients stratified clearly by severity of cirrhosis and presence of portal hypertension to determine the long term outcome of zinc supplementation.
Zinc is an essential trace element integral to many cellular and immune functions. Zinc deficiency is highly prevalent in patients with cirrhosis and related to disease severity.
Zinc supplementation has been used to treat complications of cirrhosis including hepatic encephalopathy. However, it is unknown whether zinc supplementation in patients with cirrhosis results in a change in the risk of progression of cirrhosis or death.
This study aimed to evaluate whether zinc supplementation improves clinical outcomes and long-term survival in patients with cirrhosis.
A systematic review was performed including all studies in Medline, Embase or Cochrane database with inclusion criteria of adult human studies, comparing zinc supplementation of at least 28 d with standard care or placebo in patients with cirrhosis. Mortality and clinical severity score data were extracted. Random effects meta-analyses determined risk of mortality in patients receiving zinc supplementation versus comparator at 6 mo and 2 years. Risk of bias was assessed using the National Institutes of Health quality assessment tool.
Seven hundred and twelve articles were identified of which four were eligible. Zinc formulations and doses varied for different intervention periods in patients with differing etiology and severity of cirrhosis. Two studies were considered to be at high risk of bias. There was no significant difference in 6-mo mortality between patients treated with zinc versus controls. Changes in severity scores were not reported in any study.
Findings are limited by the small number of eligible studies and significant heterogeneity in intervention and patient population. Zinc supplementation is not statistically associated with reduced mortality or improved long term outcome in patients with cirrhosis.
There is substantial heterogeneity in study design, inclusion criteria and primary outcome. The difference in etiology and severity of liver cirrhosis also make the effect of zinc supplementation difficult to interpret. Further well-designed prospective studies are required to determine whether zinc supplementation improves long-term clinical outcome in patients with cirrhosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ruiz-Margáin A S-Editor: Liu M L-Editor: A E-Editor: Ma YJ
| 1. | World Health Organization. Age-standardized death rates of liver cirrhosis 2016. [accessed 9 September 2019]. Available from: https://www.who.int/gho/alcohol/harms_consequences/deaths_liver_cirrhosis/en/. |
| 2. | Williams R, Alexander G, Aspinall R, Batterham R, Bhala N, Bosanquet N, Severi K, Burton A, Burton R, Cramp ME, Day N, Dhawan A, Dillon J, Drummond C, Dyson J, Ferguson J, Foster GR, Gilmore I, Greenberg J, Henn C, Hudson M, Jarvis H, Kelly D, Mann J, McDougall N, McKee M, Moriarty K, Morling J, Newsome P, O'Grady J, Rolfe L, Rice P, Rutter H, Sheron N, Thorburn D, Verne J, Vohra J, Wass J, Yeoman A. Gathering momentum for the way ahead: fifth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;392:2398-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, Ferguson J, Forton D, Foster G, Gilmore I, Hickman M, Hudson M, Kelly D, Langford A, Lombard M, Longworth L, Martin N, Moriarty K, Newsome P, O'Grady J, Pryke R, Rutter H, Ryder S, Sheron N, Smith T. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 5. | Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Nangliya V, Sharma A, Yadav D, Sunder S, Nijhawan S, Mishra S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol Trace Elem Res. 2015;165:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 1800] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 8. | Sengupta S, Wroblewski K, Aronsohn A, Reau N, Reddy KG, Jensen D, Te H. Screening for Zinc Deficiency in Patients with Cirrhosis: When Should We Start? Dig Dis Sci. 2015;60:3130-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Maret W. Zinc and human disease. Met Ions Life Sci. 2013;13:389-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Stamoulis I, Kouraklis G, Theocharis S. Zinc and the liver: an active interaction. Dig Dis Sci. 2007;52:1595-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling. J Am Coll Nutr. 2008;27:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Diglio DC, Fernandes SA, Stein J, Azeredo-da-Silva A, de Mattos AA, Tovo CV. Role of zinc supplementation in the management of chronic liver diseases: A systematic review and meta-analysis. Ann Hepatol. 2020;19:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | National Institutes of Health. Study quality assessment tools. [accessed 1 March 2019]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. |
| 16. | Schwarzer G, Carpenter JR, Rücker G. Small-Study Effects in Meta-Analysis. In: Meta-Analysis with R. Use R! Switzerland: Springer Cham, 2015: 107-141. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32:1080-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Vilar Gomez E, Sanchez Rodriguez Y, Torres Gonzalez A, Calzadilla Bertot L, Arus Soler E, Martinez Perez Y, Yasells Garcia A, Abreu Vazquez Mdel R. Viusid, a nutritional supplement, increases survival and reduces disease progression in HCV-related decompensated cirrhosis: a randomised and controlled trial. BMJ Open. 2011;1:e000140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Bresci G, Parisi G, Banti S. Management of hepatic encephalopathy with oral zinc supplementation: a long-term treatment. Eur J Med. 1993;2:414-416. [PubMed] |
| 20. | Hayashi M, Ikezawa K, Ono A, Okabayashi S, Hayashi Y, Shimizu S, Mizuno T, Maeda K, Akasaka T, Naito M, Michida T, Ueshima D, Nada T, Kawaguchi K, Nakamura T, Katayama K. Evaluation of the effects of combination therapy with branched-chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatol Res. 2007;37:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007;56:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Davison G, Marchbank T, March DS, Thatcher R, Playford RJ. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. 2016;104:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |