Published online May 27, 2020. doi: 10.4254/wjh.v12.i5.207
Peer-review started: December 18, 2019
First decision: January 5, 2020
Revised: March 26, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: May 27, 2020
Processing time: 160 Days and 17.9 Hours
Drug-induced liver injury (DILI) and herbal/dietary supplements (HDS) related liver injury present unique diagnostic challenges. Collaboration between the clinician and the pathologist is required for an accurate diagnosis and management.
To report our experience on the clinical-pathological findings of hepatic injury caused by drugs/HDS.
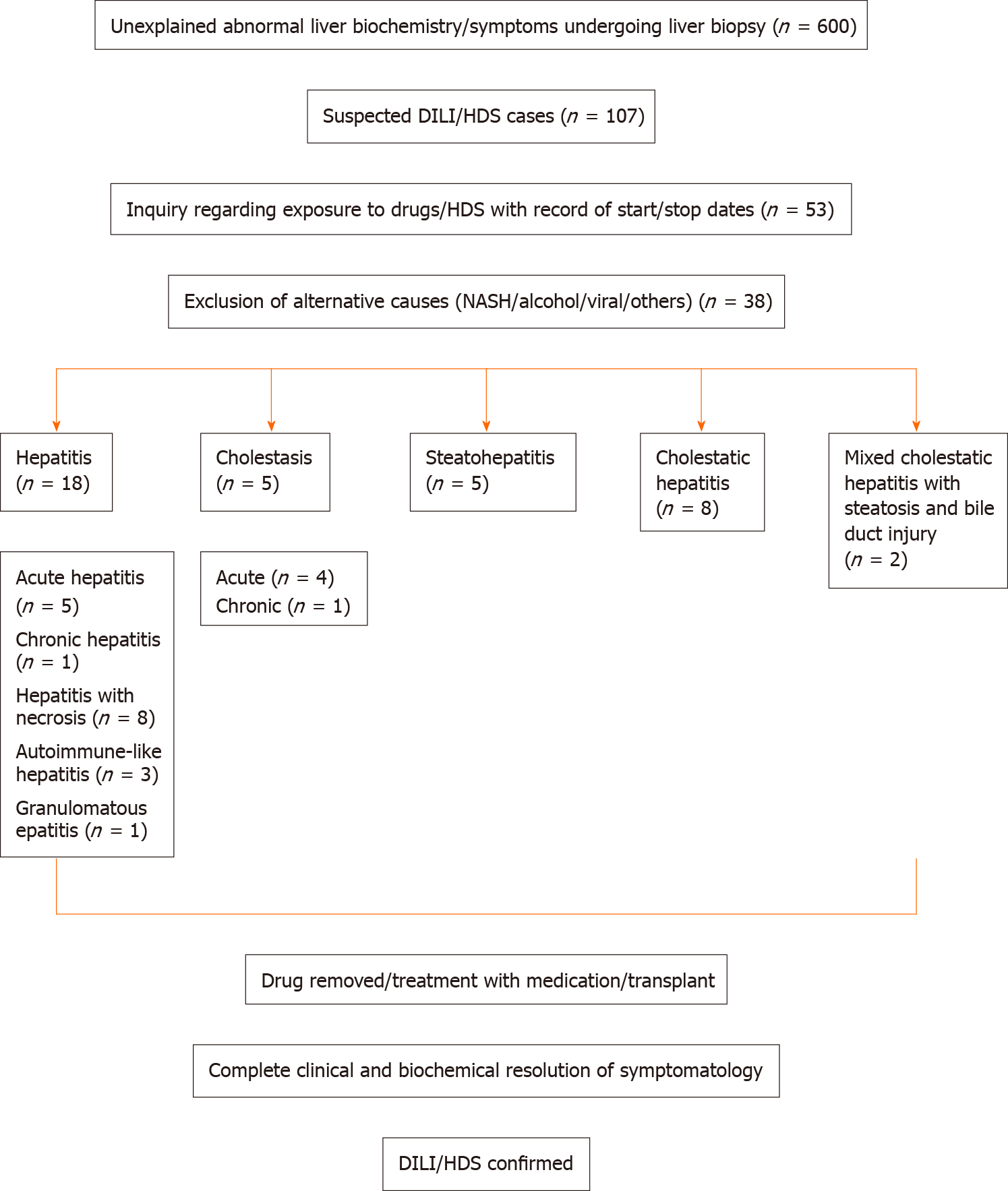
A retrospective review of clinically proven cases of DILI/HDS who presented to our institution from January 1, 2013 to December 31, 2017 was performed. Slides were reviewed for histopathological patterns of injury and correlated with the causative agent. Out of 600 patients presenting with unexplained rise in liver enzymes undergoing biopsy, 107 were suspected to have DILI/HDS. Of these, 53 had a directly linked exposure to drug/herbal supplements. Fifteen patients were excluded for concurrent known liver disease. Thirty-eight patients with clinically proven DILI/HDS were finally included.
Thirty-eight cases of DILI/HDS with a male:female of 1:1.5 and mean age of 51 ± 3 years were identified. DILI was identified in 84.2% cases while HDS injury in 15.8%. Acute hepatitis (42.1%) was the most common pattern of injury while granulomatous hepatitis (2.6%) was the least common. We found one case of acute-cholestasis due to rivaroxaban and two cases of cholestatic-hepatitis due to rizatriptan and trimethobenzamide-hydrochloride that, to the best of our knowledge, have not been previously reported. One case of steatohepatitis due to trimethoprim-sulfamethoxazole and three unusual cases of cholestatic-hepatitis with bile duct injury and steatosis due to dronedarone, C4-Extreme and hydroxycut, were also seen. Of our cohort, 81.6% of the patients fared well with discontinuation of drug and 18.4% underwent transplant; of which 42.9% were deceased.
We describe the clinical findings, histopathological patterns of injury and clinical outcomes caused by drugs. In particular, we report a few previously unreported/ rarely observed clinical and histopathological patterns of hepatic injury.
Core tip: This a retrospective study to evaluate the clinicopathological patterns of drug and herbal/dietary supplement related injury with only reports of proven cases from a large cohort of patients. We describe many unusual patterns of injury as well as newer drugs/herbals causing previously unreported patterns of injury. Drug/Herbal dietary supplements related liver injury is a well-reported topic, but it is important to report newer patterns in this changing era of medicine.
- Citation: Siddique AS, Siddique O, Einstein M, Urtasun-Sotil E, Ligato S. Drug and herbal/dietary supplements-induced liver injury: A tertiary care center experience. World J Hepatol 2020; 12(5): 207-219
- URL: https://www.wjgnet.com/1948-5182/full/v12/i5/207.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i5.207
Drug-induced liver injury (DILI) and herbal/dietary supplements (HDS) related liver injury (DILI/HDS) represent a rare idiosyncratic reaction with potentially devastating clinical outcomes[1]. It may present unique diagnostic challenges, and require a close collaboration between the clinician and the pathologist for an accurate diagnosis and management[1,2]. The true incidence of DILI/HDS is challenging to discern due to difficulties in gathering and arbitrating cases in a quantitative way. However, Icelandic data from 2013 suggests an estimated annual incidence of 19.1 per 100000[3]. The DILI network of the United States reported 300 idiosyncratic DILI cases in 2007 of which 8% died, 2% required a transplant and 14% had continued liver test abnormalities at 6 mo[4]. Additionally idiosyncratic DILI/HDS accounts for 11% of the cases of acute liver failure in the United States[5]. The United States Food and Drug Administration have issued a black box warning and safety alerts for a number of drugs that have been implicated in serious liver injury[6]. Despite these data, new reports on DILI/HDS keep emerging contributing to the annual 23 billion dollar expenditure on hepatitis alone in the United States[7].
For the clinician, the assessment of the presence, pattern and degree of hepatic injury starts by determining the liver function tests (LFTs), and the markers of hepatocellular function (albumin, prothrombin time and bilirubin). The R ratio helps to classify the liver injury into hepatocellular, cholestatic and mixed patterns of injury[8,9]. On the other end, the pathologist by examining the liver biopsy provides information on the histopathological pattern of injury and its severity. Finally and ideally, by a multidisciplinary approach, the clinician and pathologist correlate all the clinical and pathological data, and after exclusion of other causes of liver injury (such as viral hepatitis or autoimmune diseases), the determination of the likelihood of DILI/HDS and the possible drugs or HDS responsible for the damage is made.
The purpose of this study was to report our experience on the clinical-pathological findings including patterns of hepatic injury and its severity caused by drugs and HDS identified in our tertiary care center during the last five years. Additionally, we report a few previously unreported/rarely observed cases of DILI/HDS describing their clinico-pathologic findings at presentation and clinical outcomes.
A retrospective review was done of all patients who presented at our institution with unexplained rise in liver enzymes and subsequently underwent a biopsy from January 1, 2013 to December 31, 2017. Patients who were suspected to have DILI/HDS were included. Medical records were reviewed for a rigorous evaluation of exposure to drug/herbal supplements and only those patients who had a directly linked exposure were accepted for the study. The age, gender and liver enzyme results were obtained, and R ratio scoring was calculated on each case. Strict exclusion criteria were used after review of medical records, and patients with concurrent history of known liver disease such as viral hepatitis, autoimmune hepatitis (AIH), Wilson’s disease, alcoholic liver disease, non-alcoholic fatty liver disease were excluded. Figure 1 summarizes the inclusion criteria of patients for this study.
The biochemical classification of the DILI/HDS was performed using the R ratio[8,9] defined as [Alanine aminotransferase (ALT)/upper limit of normal]/[Alkaline phosphatase (ALP)/upper limit of normal]. An R > 5.0 was considered as an expression of hepatocellular injury, R < 2.0 was consistent with cholestatic injury and R 2.0-5.0 was considered as an expression of a mixed pattern of injury.
The causal factors of the patterns of injury were subdivided into anti-infective agents, analgesics, psychotropic medications, antineoplastic, antilipidemic, immunomodulatory agents, HDS and others. The drugs and/or HDS were then analyzed according to the pattern of injury.
In each case, slides were independently reviewed by two pathologists (Siddique AS and Ligato S) for assessment of the histopathological pattern of injury and the results subsequently correlated with the clinical findings and the potential drug or HDS responsible for the hepatic injury. The histopathological patterns of injury were divided into necroinflammatory pattern (hepatitis), cholestatic injury pattern, combined cholestatic hepatitic pattern, steatotic injury pattern and mixed injury pattern.
Necroinflammatory pattern was subdivided into acute hepatitis (AH), acute hepatitis with extensive necrosis, AIH, chronic hepatitis (CH) and granulomatous hepatitis (GH). AH and CH patterns were similar to viral hepatitic patterns consisting of lobular-dominant inflammation in AH and portal predominant inflammation in CH cases. Acute hepatitis with extensive necrosis was characterized by confluent or coagulative necrosis of hepatocytes, with variable amounts of inflammation. These cases often revealed massive necrosis with collapse of parenchyma between the portal areas. Areas of regeneration or ongoing injury patterns were also observed in some of these cases. AIH cases displayed moderate to severe portal inflammation with interphase necroinflammatory activity with abundant plasma cells and eosinophils along with hepatocyte rosettes and emperipolesis. GH was characterized by non-necrotizing epithelioid granulomas in the parenchyma and portal areas.
Cholestatic injury pattern was subdivided into acute cholestasis (AC) and chronic cholestasis (CC). AC cases displayed canalicular or hepatocellular bile accumulation while CC cases showed cholate stasis along with copper accumulation. Duct injury was often seen in these cases with secondary biliary ductular proliferation. Mixed cholestatic hepatitis (MCH) demonstrated a combination of various patterns of hepatocellular injury, inflammation and cholestasis.
Steatotic injury pattern included small and large droplet macrovesicular steatosis with varying degrees of hepatocellular injury and inflammation. Mixed pattern of injury was a mixture of various degrees of cholestatic pattern, steatotic pattern and hepatitic patterns.
Histochemical stains included trichrome, Periodic Acid–Schiff–diastase, iron and reticulin stain. Trichrome stain helped establish fibrosis. Periodic Acid–Schiff–diastase highlighted the multiple aggregates of pigmented laden macrophages with ceroid material within the cytoplasm suggestive of ongoing necroinflammatory activity. Iron stain demonstrated accumulation of iron in the Kupffer cells and/or hepatocytes. Reticulin displayed the architecture of liver plates such as expansion in regenerative conditions, compression in nodular regenerative hyperplasia and collapse in necrosis. Immunohistochemical stain for cytokeratin 7 helped highlight bile duct loss/damage and secondary biliary ductular proliferation.
The mean, median, ranges, percentages of the age, gender, liver function enzymes, patterns of injury, causative agents were done on Microsoft Excel 2010 (Seattle, WA, United States) and so was the R ratio.
The statistical methods were reviewed by the Biostatistics Department at Hartford Hospital.
This study was performed under a protocol approved by the Institutional Review Board of Hartford Hospital.
Out of 600 patients presenting with unexplained rise in liver enzymes from January 1, 2013 to December 31, 2017, 107 patients were suspected to have DILI/HDS due to polypharmacy. Of these, 53 patients had a recent change in use of drug/herbal supplement explaining a directly linked exposure. Fifteen patients were excluded since they had a history of known liver disease alongside the possible concurrent drug injury. Thirty-eight cases of DILI/HDS were identified with the help of a liver biopsy with a male:female ratio of 1:1.5; and mean age of 51 ± 3 years. The initial ALT at the time of presentation ranged from 12 to 3555 (median 432) U/L. The initial ALP ranged from 66 to 802 (median 178) U/L. The initial aspartate aminotransferase levels ranged from 27 to 1925 (median 310) U/L. The total bilirubin at presentation ranged from 0.4 to 36.7 (median 5.7) mg/dL. The mean platelet count at presentation was 230000 ± 170000. The clinical features of the group subdivided by the histopathological pattern of injury are summarized in Table 1. As displayed in Table 2, a variety of drug categories were seen as causative agents of each pattern of injury including anti-infective agents, analgesics, psychotropic agents, antineoplastic, antilipidemic, immunomodulatory agents, HDS and others. Analgesics were found to be the most common (38.9%) causative agents of hepatocellular injury while anti-infective agents (26.7%) were the most common causal factors of mixed pattern of injury.
| Hepatocellular | Cholestatic | Mixed | |
| n (%) | 18 (49) | 5 (14) | 15 (39) |
| Age (mean ± SD) | 50 ± 8 | 65 ± 5 | 49 ± 18 |
| Gender (M:F) | 5:13 | 3:2 | 7:8 |
| Initial ALT (mean ± SD) | 870 ± 280 | 37 ± 32 | 470 ± 190 |
| Initial AST (mean ± SD) | 780 ± 110 | 44 ± 54 | 360 ± 600 |
| Initial ALP (mean ± SD) | 190 ± 93 | 300 ± 25 | 220 ± 91 |
| R ratio (mean ± SD) | 12 ± 0.4 | 0.5 ± 0.1 | 6.4 ± 0.9 |
| Total bilirubin (mean ± SD) | 5.5 ± 0.4 | 4.3 ± 4.5 | 3.7 ± 1.5 |
| Platelet count/µL (mean ± SD) | 230000 ± 92000 | 230000 ± 130000 | 220000 ± 31000 |
| Drugs | Hepatocellular (n = 18) | Cholestatic (n = 5) | Mixed (n = 14) |
| Anti-infective agents | 3 | 1 | 4 |
| Analgesics | 7 | 0 | 0 |
| Psychotropic agents | 2 | 0 | 0 |
| Antineoplastic agents | 1 | 0 | 1 |
| Antilipedemic agents | 0 | 1 | 1 |
| Immunomodulatory agents | 2 | 0 | 0 |
| Others | 0 | Antithyroid: 1; Thrombolytic: 1; Total parenteral nutrition: 1 | Triptan: 1; Antiemetic: 1; Methyldrostanolone: 1; Anti-inflammatory: 1; Antiarrythmic: 2 |
| Herbal/Dietary supplements | 3 | 0 | 3 |
Biochemical and histopathological correlation was done for all the patients and an R ratio > 5 was identified in cases with a hepatitic histopathological pattern of injury, an R ratio < 2 corresponded to a cholestatic histopathological pattern and an R between 0.4 and 12.7 was observed in cases with mixed histopathological patterns listed in Table 3.
| Histopathological pattern | R ratio | Total, n = 38 (%) | Drugs/HDS (n) |
| Hepatitis | 12 ± 0.4 | 18 (47.4) | |
| Acute | 16 ± 0.1 | 5 (13.2) | Nitrofurantoin (1) |
| Black cohosh1 (1) | |||
| Sertraline (1) | |||
| Lamotrigine (1) | |||
| Green tea extract (1) | |||
| Acute with extensive necrosis | 11 ± 3.3 | 8 (21.1) | Diclofenac (1) |
| Acetaminophen (6) | |||
| Black cohosh1 (1) | |||
| Autoimmune-like | 6.2 ± 0.1 | 3 (7.9) | Infliximab (2) |
| Isoniazid (1) | |||
| Chronic | 8.8 | 1 (2.6) | Nitrofurantoin (1) |
| Non-necrotizing granulomatous hepatitis | 14 | 1 (2.6) | BCG (1) |
| Cholestasis | 0.5 ± 0.1 | 5 (13.2) | |
| Acute | 0.4 ± 0.5 | 4 (10.5) | Ceftriaxone (1) |
| Methimazole (1) | |||
| Total parenteral nutrition (1) | |||
| Rivaroxaban (1) | |||
| Chronic | 0.8 | 1 (2.6) | Triazolam and statin (1) |
| Cholestatic hepatitis | 9.0 ± 5.6 | 8 (21.1) | Ezetimibe (1) |
| Cefazolin (1) | |||
| Hydroxycut2 (1) | |||
| Rizatriptan5 (1) | |||
| Methyldrostanolone (1) | |||
| Trimethobenzamide Hydrochloride5 (1) | |||
| Mesalamine (1) | |||
| C4 extreme3 (1) | |||
| Macrovesicular steatohepatitis | 1.4 ± 1.2 | 5 (13.2) | Trimethoprim-sulfamethoxazole6 (1) |
| L-asparaginase (1) | |||
| Valproic acid (1) | |||
| Haart4 (1) | |||
| Amiodarone (1) | |||
| Mixed cholestatic hepatitis with steatosis and bile duct injury | 1.1 ± 0.8 | 2 (5.3) | Dronedarone6 (1) |
| Hydroxycut26 (1) |
In our cohort, 32 (84.2%) patients had DILI and 6 (15.8%) had HDS. The unique cases in our study included one patient with rivaroxaban related injury who presented with an ALT of 76 U/L, ALP of 214 U/L, R 0.9, total bilirubin of 6.8 mg/dL and a platelet count of 123000/µL. Another patient with rizatriptan related injury presented with an ALT of 147 U/L, ALP of 135 U/L, R 2.7, total bilirubin of 1.2 mg/dL and a platelet count of 132000/µL. Trimethobenzamide hydrochloride related DILI in our patient presented with an ALT of 630 U/L, ALP of 135 U/L, R 11.4, total bilirubin of 1.5 mg/dL and a platelet count of 350000/µL. One patient who used trimethoprim-sulfamethoxazole (TMP-SMX) presented with an ALT of 152 U/L, ALP of 198 U/L, R 1.9, total bilirubin of 10.9 mg/dL and a platelet count of 116000/µL. DILI caused by dronedarone in our patient presented with an ALT of 93 U/L, ALP of 477 U/L, R 0.5, total bilirubin of 3.3 mg/dL and a platelet count of 114000/µL. C4 extreme related injury presented with ALT of 3384 U/L, ALP of 175 U/L, R 49.5, total bilirubin of 22.5 mg/dL and a platelet count of 144000/µL. Cholestatic hepatitis caused by hydroxycut presented with ALT of 344 U/L, ALP of 500 U/L, R 1.7, total bilirubin of 0.7 mg/dL and a platelet count of 350000/µL. The features are listed in Table 4.
| Cases | ALT (U/L) | ALP (U/L) | R ratio | Total Bilirubin (mg/dL) | Platelet count (/µL) | Histopathologi-cal pattern | Outcome |
| Rivaroxaban | 76 | 214 | 0.9 | 6.8 | 123000 | Acute hepatocellular and canalicular cholestasis associated with bile duct damage, mild steatohepatitis with portal, periportal and pericentral fibrosis (stage 2 of 4), and nodular regenerative hyperplasia | LFTs downtrended after 15 d of drug removal |
| Rizatriptan | 147 | 135 | 2.7 | 1.2 | 132000 | Moderate to severe acute hepatitis with associated cholestasis and bile duct damage | LFTs downtrended after 45 d of drug removal |
| Trimethobenz-amide hydrochloride | 630 | 135 | 11.4 | 1.5 | 350000 | Cholestatic hepatitis with portal and lobular mononuclear inflammatory infiltrate, bile duct damage and bile ductular proliferation | LFTs downtrended after 60 d of drug removal |
| Trimethoprim-sulfamethoxazo-le | 152 | 198 | 1.9 | 10.9 | 116000 | Panacinar macrovesicular steatosis/mild steatohepatitis, cholestasis and mild portal tract fibrous expansion. (steatohepatitis necroinflammatory grade I; fibrosis stage 0-1) | LFTs downtrended after 90 d of drug removal |
| Dronedarone | 93 | 477 | 0.5 | 3.3 | 114000 | Pericentral hepatocyte and canalicular cholestasis, bile duct injury with bile ductular proliferation, mixed portal inflammatory infiltrates, mild interface hepatitis, lobular necroinflammatory activity with ballooning degeneration; mild macro and microvesicular steatosis | LFTs downtrended after 85 d of drug removal |
| C4 Extreme | 3384 | 175 | 49.5 | 22.5 | 144000 | Severe cholestatic hepatitis with sub massive hepatic necrosis involving approximately 70% of the liver parenchyma accompanied by severe centrilobular congestion, necrosis and extravasation of red blood cells | Underwent transplant; Alive and doing well |
| Hydroxycut | 344 | 500 | 1.7 | 0.7 | 350000 | Cholestatic hepatitis with bile duct injury and steatosis | LFTs downtrended after 45 d of drug removal |
The HDS agents identified in our study were: Black cohosh (2 cases), hydroxycut (2 cases), green tea extract (1 case) and C4 extreme (1 case). Black cohosh, green tea extract and C4 extreme displayed an R ratio of greater than 5 while hydroxycut displayed an R ratio of less than 2.
Histopathological patterns of injury and corresponding causative drugs/HDS are summarized in the Table 3. Hepatitic pattern was observed in 18 (47.4%). AH was the most common histopathological pattern of injury caused by nitrofurantoin, sertraline, lamotrigine, diclofenac and acetaminophen. AH with autoimmune-like features was caused by infliximab and isoniazid. CH was caused by nitrofurantoin. GH was the least common pattern of injury caused by Bacilli Calmette Guerin (BCG).
Cholestatic pattern of injury was found in 5 (13.2%) of the cases. AC was caused by ceftriaxone, methimazole, total parenteral nutrition, and rivaroxaban. CC was caused by triazolam and statins.
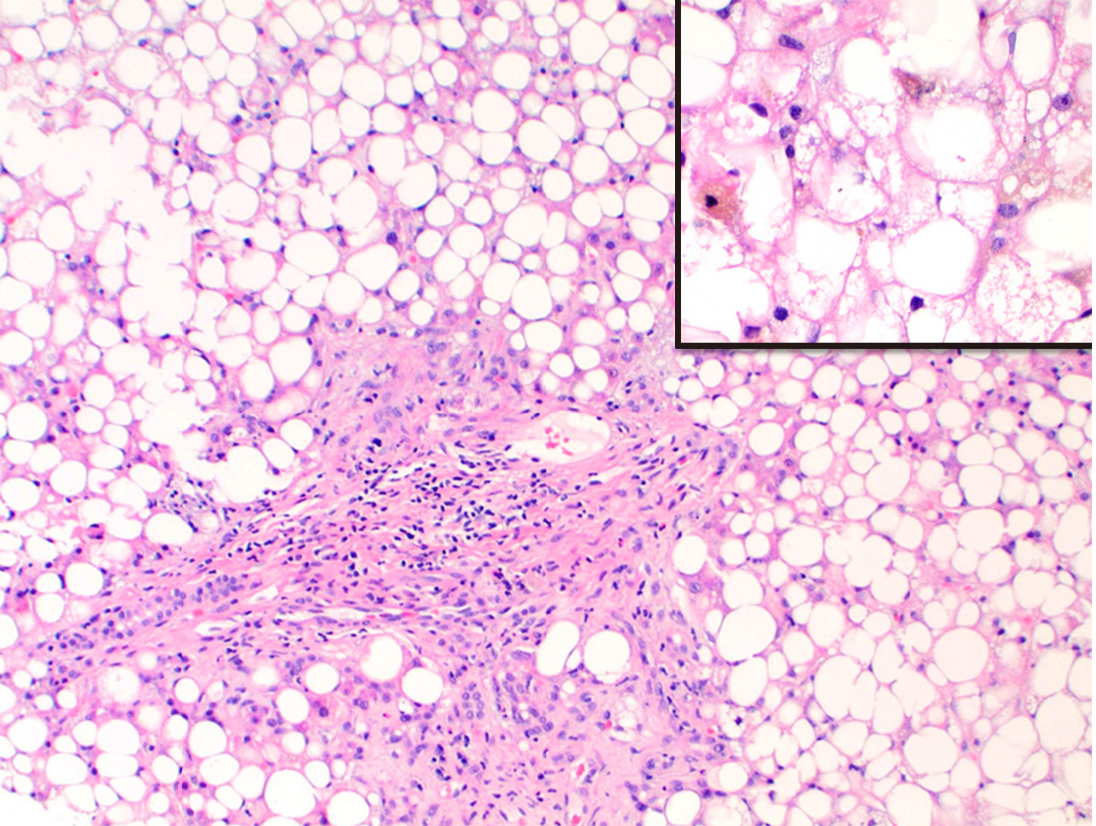
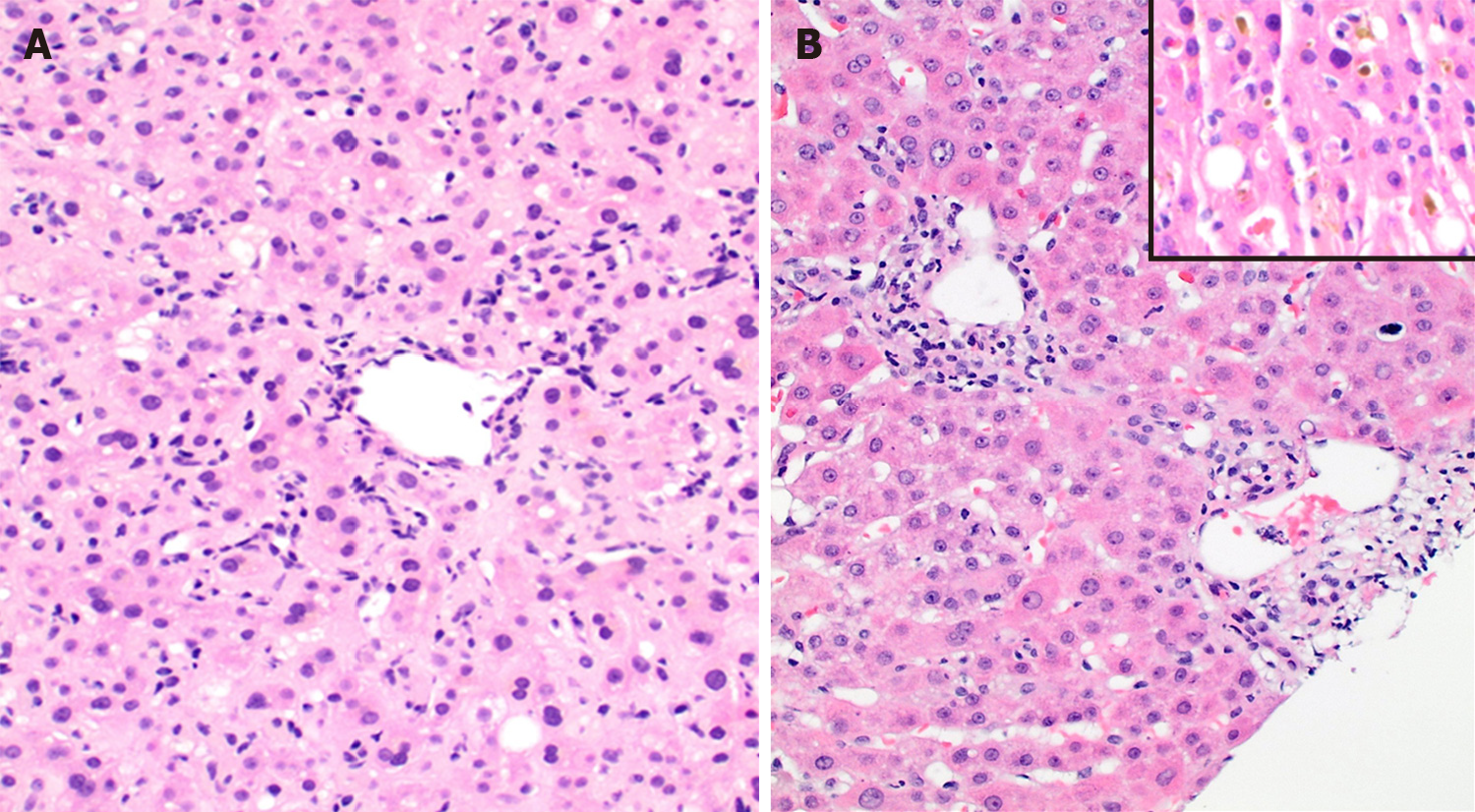
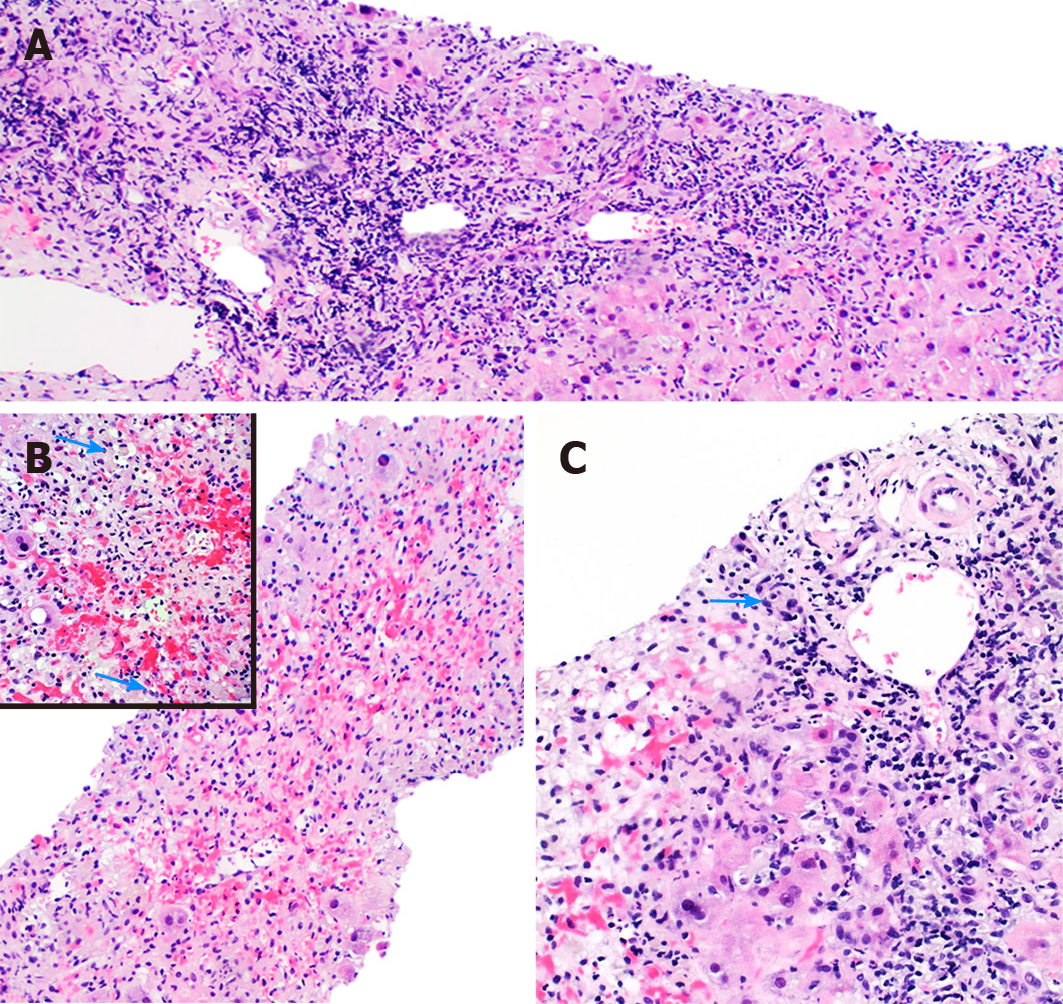
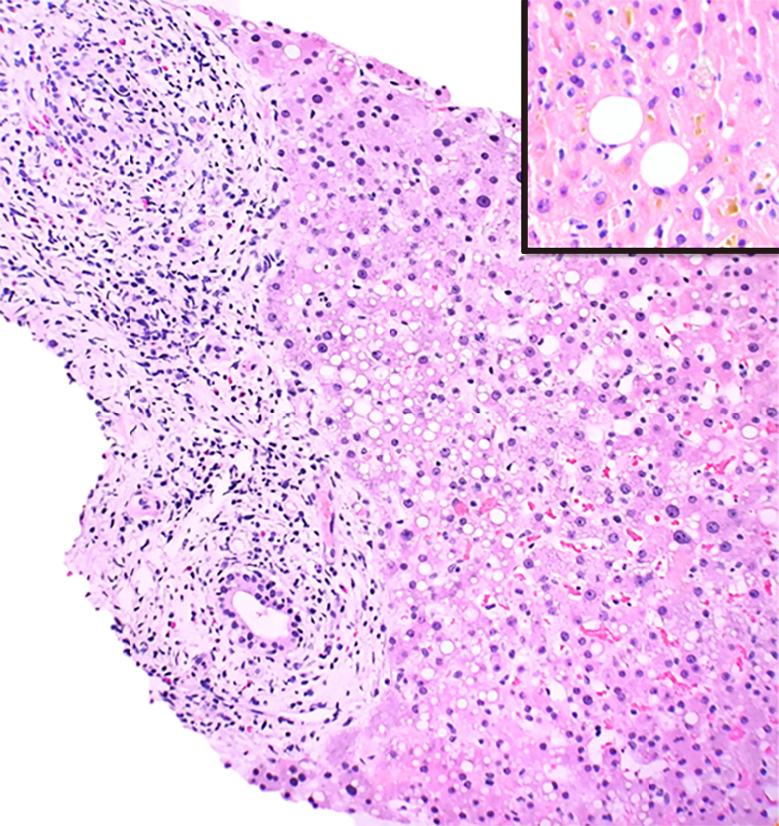
Mixed patterns 15 (39.5%) included macrovesicular steatohepatitis 5 (13.2%), cholestatic hepatitis 8 (21.1%) and MCH with steatosis and bile duct injury 2 (5.3%). The causal factors for steatohepatitis were TMP-SMX (Figure 2), L-asparaginase, valproic-acid, highly-active-antiretroviral-therapy and amiodarone. Drugs causing cholestatic hepatitis included ezetimibe, cefazolin, rizatriptan, methyldrostanolone, trimethobenzamide hydrochloride (Figure 3), C4 extreme (Figure 4) and mesalamine. MCH with steatosis and bile duct injury was caused by dronedarone.
In our cohort amongst HDS, black cohosh and green-tea extract caused AH while two cases of hydroxycut (Figure 5) displayed cholestatic hepatitis and an MCH with steatosis and bile duct injury (Table 4) respectively.
The clinical course and follow-up of all the patients with confirmed DILI/HDS was available. Thirty-one (81.5%) patients fared well with discontinuation of drug and/or treatment with N-acetyl cysteine/prednisone, while 7 (18.4%) underwent transplant due to severe acute liver injury. Patients with this AH with extensive necrosis were found to be mainly secondary to acetaminophen toxicity and less commonly black cohosh use; they had the worst outcome requiring transplant; of which 3 (42.9%) were deceased.
In this retrospective study we investigated the clinical and histopathological patterns of DILI/HDS at a single institution over a period of five years. The majority of the histopathological patterns of injury identified in our cohort were consistent with those previously reported in the literature[10] with 32 (84.2%) cases demonstrating DILI and 6 (15.8%) HDS. The histopathological spectrum of hepatic injury identified in our cohort was similar to those identified in other studies on DILI[10] and included: AH, CH, AC, CC and cholestatic hepatitis. Kleiner et al[10] reported that 83% of their DILI cases could be classified into one of the above patterns. In our analysis, AH (n = 16; 45.7%) was the most common histopathological pattern of injury while GH (n = 1; 2.6%) was the least common.
Analgesics were found to be the most common (n = 7; 38.9%) causative agents of hepatocellular injury while antimicrobial agents (n = 4; 26.7%) were the most common causal factors of mixed pattern of injury. Antimicrobials are the most frequent etiologic agents of DILI in the United States accounting for 45% of the cases[11] and analgesic induced DILI is a well-studied topic specifically those caused by acetaminophen[12]. Acetaminophen amongst analgesics is the most common single cause of acute liver failure in western nations with severe cases requiring liver transplantation[13]. In our study 15.8% of all DILI was caused by acetaminophen alone of which 5 (83.3%) patients underwent transplant. Additionally, acetaminophen-opiate or opioid combinations are declining in popularity but were responsible for at least 50% of serious acetaminophen related liver injury cases in the United States[13] compared to only 6 (15.8%) patients with acetaminophen related injury in our study. While most DILI cases are idiosyncratic, acetaminophen toxicity is dose related. In subtoxic cases, the toxic metabolite of acetaminophen (N-acetyl-p-benzoquinone imine) is rendered harmless by conjugation with glutathione, abundantly available within the hepatocytes. However, once the glutathione stores are depleted during higher levels of acetaminophen intake, fasting, malnutrition or chronic alcohol ingestion, N-acetyl-p-benzoquinone imine binds to cysteine residues in cellular proteins and causes cell necrosis, subsequently elevating aspartate aminotransferase and ALT levels in the blood[14]. The cell necrosis also releases acetaminophen adducts that can be measured by liquid chromatography[15] and recent immunoassays appear to be promising in detecting these adduct in acute liver failure[16,17].
Another interesting finding was GH secondary to BCG treatment for urothelial carcinoma. Disseminated BCG disease is a rare but life-threatening complication of BCG administration with symptoms similar to that of tuberculosis infection, including persistent fever, night sweats and weight loss[18]. The pathogenesis is thought to involve a combination of mycobacteraemia and local inflammatory hypersensitivity at various sites including the liver[18]. Our case of non-necrotizing GH secondary to BCG along with previously reported cases[18] emphasizes the significance of a complete history and clinicopathologic correlation of diagnosing DILI.
In addition, we identified some unusual patterns of hepatic injury including one case of cholestatic hepatitis with bile duct injury and steatosis due to dronedarone, and one case of steatohepatitis due to TMP-SMX. Dronedarone is a well-known antiarrhythmic agent and DILI related to it has been rarely reported. Most cases however, describe a hepatocellular pattern of injury similar to amiodarone unlike our case[19,20]. TMP-SMX is a fixed antibiotic combination widely used for mild to moderate bacterial infections and has been reported for cholestatic or mixed patterns of injury[21,22]. Our case was unique in that we identified steatohepatitis (Figure 2) similar to type-2 non-alcoholic steatohepatitis often identified in children. Since alcoholic liver disease and Non-alcoholic steatohepatitis were excluded in all our cases and the injury improved with removal of drug and symptomatic treatment, the diagnosis of DILI was confirmed.
Furthermore, we report two cases of cholestatic hepatitis, and one of AC that, to the best of our knowledge, have not been previously reported in the literature. The two cases of cholestatic hepatitis were secondary to the use of trimethobenzamide hydrochloride (Tigan) and rizatriptan (Maxalt). The patient with trimethobenzamide hydrochloride related DILI presented with altered liver function and a histopathological pattern displayed in Figure 3. The LFTs downtrended after 60 d of removal of drug. Trimethobenzamide hydrochloride was introduced in 1959 as an antinauseant-antiemetic. Chemically unrelated to the phenothiazines it is structurally closer to the dimethylaminoethoxy antihistamines. It was widely used prophylactically and therapeutically in vomiting induced by drugs, radiation therapy, infection, and motion sickness[23]. One case of hepatitis secondary to trimetho-benzamide hydrochloride was seen in 1967[24]. However, no recent data with detailed histopathological pattern of injury are available; in this report we provide detailed histopathological findings caused by this drug. Rizatriptan belongs to a group of serotonin receptor agonists that are useful in the therapy of vascular headaches and migraine. Cholestatic hepatitis secondary to triptans specifically rizatriptan has been unproven in the literature[25,26]. Our case presented with elevated liver enzymes and moderate to severe AH associated with cholestasis and bile duct damage on histology. LFTs downtrended after 45 days of drug removal. Lastly, the case of AC was caused by rivaroxaban (Xarelto) and displayed a histopathological pattern characterized by acute hepatocellular and canalicular cholestasis associated with mild steatohepatitis. LFTs downtrended after 15 d of drug removal. Rivaroxaban is a recently introduced oral anticoagulant and direct Factor Xa inhibitor. DILI secondary to rivaroxaban has been described as early as the preclinical licensure trials, but the details of patterns of injury and severity of disease are still lacking[27,28]. Hence it is important to report histopathology and outcome in such cases in order to become familiar with the newer patterns of injury.
One interesting case of C4 extreme presented with cholestatic hepatitis with sub massive necrosis. C4 extreme is an HDS and has the synephrine-containing Citrus aurantium extract. Synephrine is an analog of ephedra, which has previously been associated with liver toxicity[29]. However, DILI from C4 extreme in particular has just been reported in one series to date[30]. Our case resulted in a 24-year-old patient undergoing transplant (Table 4) displaying the significance of reporting such cases.
The diagnosis of DILI/HDS presents a daunting task for the clinician and pathologist alike. However, the collaboration of the clinician and pathologist in obtaining a complete medical and drug/alternative medication history along with the assessment of the histopathological findings and the review of previously reported cases in the literature (with the help of online resources such as “Livertox”) can help guide the way to a prompt and successful management of a DILI/HDS affected patient.
In conclusion, we present a five-year experience on the diagnosis of DILI/HDS in our institution. Despite the final inclusion being limited to 38 proven cases of DILI/HDS, we reported previously unreported, or rarely observed, drug-induced histopathological patterns of hepatic injury, reaffirming the pivotal role of the clinician and pathologist, in the diagnosis and care of patients with DILI/HDS.
Drug-induced liver injury (DILI) is a continuously evolving theme especially with the availability of over the counter medications and herbal/dietary supplements (HDS). The annual expenditure in the United States of hepatitis alone is 23 billion dollars and 11% of all cases of acute liver failure are related to DILI. These statistics alone highlight the importance of reporting the course of DILI cases and new confirmed cases with complete clinicopathological correlation. We believe these findings will be of interest to the readers of your journal.
The main idea behind this study is to continuously report previously unreported but proven cases of DILI/HDS and their patterns of injury for hepatologists and pathologists to consider while dealing with such cases.
The purpose of this study was to report our experience on the clinical-pathological findings including patterns of hepatic injury and its severity caused by drugs and HDS identified in our tertiary care center during the last five years.
A retrospective review of clinically proven cases of DILI/HDS who presented to our institution from January 1, 2013 to December 31, 2017 was performed. Slides were reviewed for histopathological patterns of injury and correlated with the causative agent. Out of 600 patients presenting with unexplained rise in liver enzymes undergoing biopsy, 107 were suspected to have DILI/HDS. Of these, 53 had a directly linked exposure to drug/herbal supplements. Fifteen patients were excluded for concurrent known liver disease.
Thirty-eight cases of DILI/HDS with a male:female of 1:1.5 and mean age of 51 ± 3 years were identified. DILI was identified in 84.2% cases while HDS injury in 15.8%. Acute hepatitis (42.1%) was the most common pattern of injury while granulomatous hepatitis (2.6%) was the least common. We found one case of acute-cholestasis due to rivaroxaban and two cases of cholestatic-hepatitis due to rizatriptan and trimethobenzamide-hydrochloride that, to the best of our knowledge, have not been previously reported. One case of steatohepatitis due to trimethoprim-sulfamethoxazole and three unusual cases of cholestatic-hepatitis with bile duct injury and steatosis due to dronedarone, C4-extreme and hydroxycut, were also seen. Of our cohort, 81.6% of the patients fared well with discontinuation of drug and 18.4% underwent transplant; of which 42.9% were deceased.
We report our experience on the hepatic injury caused by drugs and herbals at a tertiary care center including clinical findings, histopathological patterns of injury and clinical outcomes caused by these agents. In particular, we report on a few previously unreported/rarely observed clinical findings and histopathological patterns of hepatic injury caused by specific drugs and herbals. This implies that reporting of newer drugs/HDS and their related injury is important in this constantly changing era of medicine. Despite using a large cohort, we report only the proven cases of DILI/HDS increasing the impact of this study on clinical practice.
This study highlights a topic that has been addressed several times in the past but with the upcoming drugs/herbals it is important to continually work as a team of clinician and pathologist and report newer cases. Future prospective studies might reassure our findings further since these haven’t been reviewed in the past 10 years.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Ferreira CN, Yamamoto T S-Editor: Dou Y L-Editor: A E-Editor: Li X
| 1. | Kleiner DE. The histopathological evaluation of drug-induced liver injury. Histopathology. 2017;70:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 3. | Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 4. | Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934, 1934.e1-1934.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 612] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 5. | Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 6. | United States Food and Drug Administration. Drug Safety and Availability. Available from: https://www.fda.gov/Drugs/DrugSafety/default.htm. |
| 7. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (1)] |
| 8. | Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 411] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1071] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 10. | Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R, Talwalkar JA, Stolz A, Gu J, Barnhart H, Hoofnagle JH; Drug-Induced Liver Injury Network (DILIN). Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 11. | Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J; United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340-1352.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 12. | Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 13. | Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol. 2016;4:131-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Darr U, Sussman NL. Drug-Induced Liver Injury in the Setting of Analgesic Use. Clin Liver Dis. 2020;24:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Davern TJ 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM; Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Roberts DW, Lee WM, Hinson JA, Bai S, Swearingen CJ, Stravitz RT, Reuben A, Letzig L, Simpson PM, Rule J, Fontana RJ, Ganger D, Reddy KR, Liou I, Fix O, James LP. An Immunoassay to Rapidly Measure Acetaminophen Protein Adducts Accurately Identifies Patients With Acute Liver Injury or Failure. Clin Gastroenterol Hepatol. 2017;15:555-562.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Sussman NL, Remien CH. The Headache of Acetaminophen Overdose: Getting the NAC. Clin Gastroenterol Hepatol. 2017;15:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Moussa M, Abou Chakra M. Granulomatous hepatitis caused by Bacillus Calmette-Guerin (BCG) infection after BCG bladder instillation: A case report. Urol Case Rep. 2018;20:3-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. Inï¼Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: 648-652. |
| 20. | De Marzio DH, Navarro VJ. Antiarrhythmics. Hepatotoxicity of cardiovascular and antidiabetic drugs. In: Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: 519-540. [DOI] [Full Text] |
| 21. | Hanses F, Zierhut S, Schölmerich J, Salzberger B, Wrede CE. Severe and long lasting cholestasis after high-dose co-trimoxazole treatment for Pneumocystis pneumonia in HIV-infected patients--a report of two cases. Int J Infect Dis. 2009;13:e467-e469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Karnsakul W, Arkachaisri T, Atisook K, Wisuthsarewong W, Sattawatthamrong Y, Aanpreung P. Vanishing bile duct syndrome in a child with toxic epidermal necrolysis: an interplay of unbalanced immune regulatory mechanisms. Ann Hepatol. 2006;5:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Borda I, Jick H. Hepatitis following the administration of trimethobenzamide hydrochloride. Arch Intern Med. 1967;120:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Redondo Cerezo E, Espinosa Aguilar MD, Nogueras López F, Martín-Vivaldi Martínez R. Zolmitriptan-induced hepatotoxicity. Gastroenterol Hepatol. 2003;26:664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Deixler E, Helmke K. Extrahepatic cholestasia during therapy with Zolmitriptan (AscoTop). Z Gastroenterol. 2005;43:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1073] [Cited by in RCA: 1009] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 28. | Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 924] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 29. | Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int. 2011;31:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Peterson BR, DeRoche TC, Huber AR, Shields WW. Cholestatic liver injury associated with dietary supplements: a report of five cases in active duty service members. Mil Med. 2013;178:e1168-e1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |