Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1031
Peer-review started: June 12, 2020
First decision: July 30, 2020
Revised: August 15, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 27, 2020
Processing time: 165 Days and 3.8 Hours
MicroRNAs (miRNAs) have been suggested as biomarkers for malignant diseases including hepatocellular carcinoma (HCC). Specifically, hsa-miR-21-5p (miR-21) is among the most frequently deregulated miRNA in cancer. The diagnostic and prognostic value of miR-21 has been demonstrated in HCC tissue, mostly in the Asian population. Although the impact of various factors has been recently reported for circulating hsa-miR-122-5p (miR-122), at present only limited knowledge is available for miR-21.
To evaluate the value of miR-21 for the assessment of prognosis in HCC patients and to delineate the influence of clinical and preanalytical factors on miR-21 level in sera.
Patients with confirmed HCC from our European cohort with predominantly alcohol-associated liver damage were included in the study. All subjects were characterized according to their clinical and laboratory work-up and overall survival data were obtained. Quantitative real-time polymerase chain reaction was performed for miR-21 and spiked-in cel-miR-39-3p. The results were compared to previously reported miR-122 data.
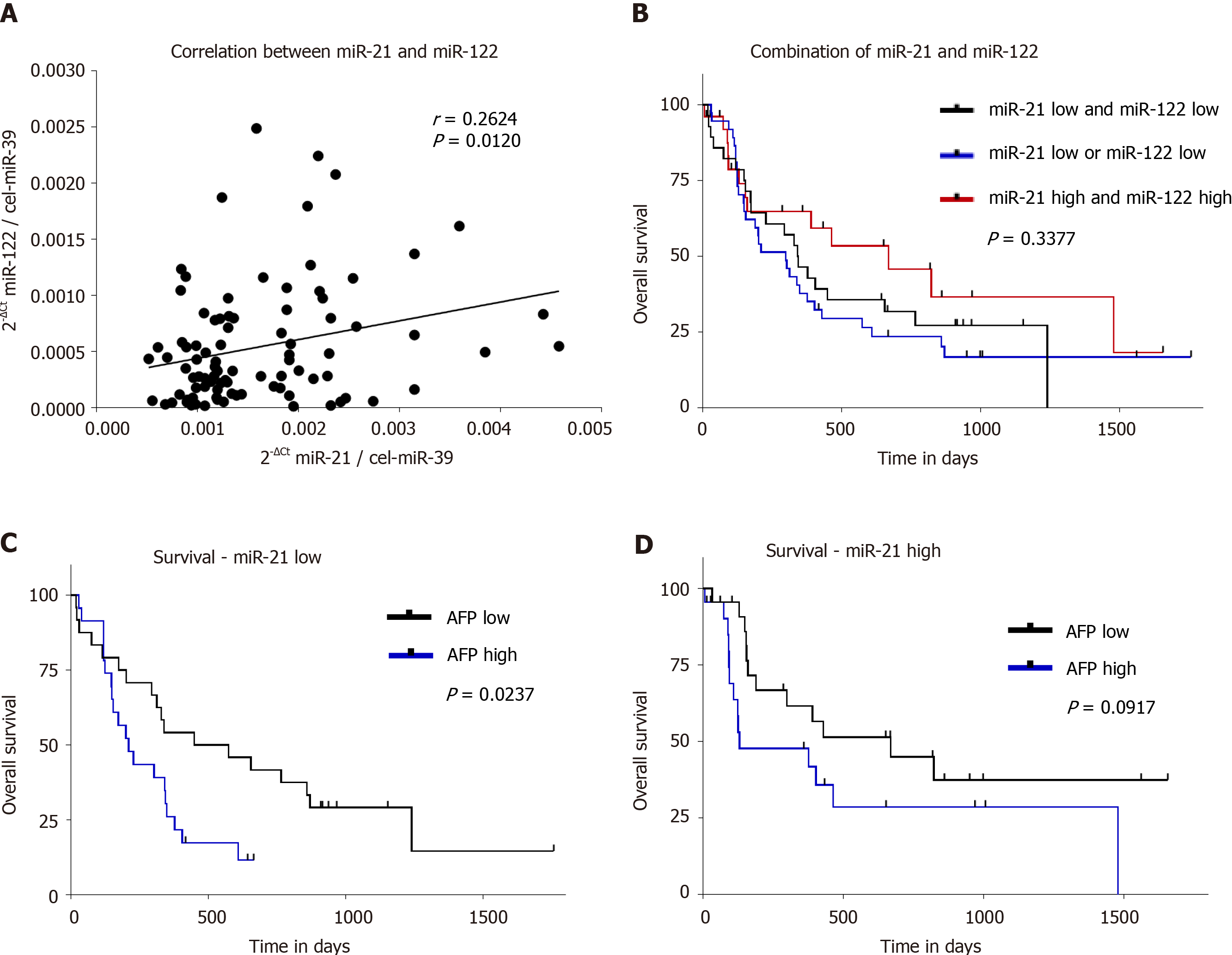
Survival of HCC patients was comparable between patients with low and high serum miR-21 concentration. No association was observed between miR-21 level in sera and Child-Pugh score, Barcelona Clinic Liver Cancer staging system, or etiology of HCC/liver disease. Age, gender, or pretreatment had no association with miR-21 level. A positive correlation was observed between miR-21 and aspartate aminotransferase (r = 0.2854, P = 0.0061), serum miR-122 (r = 0.2624, P = 0.0120), and the International Normalized Ratio (r = 0.2065, P = 0.0496). Negative correlation of miR-21 with serum creatinine (r = -0.2215, P = 0.0348) suggests renal function as a potential influencing factor in miR-21 biogenesis in blood.
The results from this work do not support clinically relevant prognostic value of circulating miR-21 in HCC patients in real-life settings. Following systematic evaluation, we identified renal function and aspartate aminotransferase as potential factors that may affect miR-21 concentration in blood. This knowledge should be considered in future miRNA-based biomarker studies not only for HCC but also for other diseases.
Core Tip: In our previous work, we identified renal function, hemoglobin, and liver injury as potential factors that may impact circulating microRNA expression. miR-21-5p is the most frequently deregulated miRNA in various types of cancer. Several reports have proposed miR-21-5p as a prognostic biomarker in hepatocellular carcinoma. In this study, serum miR-21-5p values were not associated with prognosis of hepatocellular carcinoma in a European cohort of patients with predominantly alcohol-related liver injury. In a similar fashion as previously reported for miR-122-5p, changes in circulating miR-21-5p level correlated with renal function and liver injury. This observation shows that caution should be taken in interpreting circulating miR-21-5p level and its biomarker potential.
- Citation: Franck M, Thon C, Schütte K, Malfertheiner P, Link A. Circulating miR-21-5p level has limited prognostic value in patients with hepatocellular carcinoma and is influenced by renal function. World J Hepatol 2020; 12(11): 1031-1045
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1031.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1031
There is a substantial effort to identify disease-specific biomarkers. Increasing evidence from intense research in the past several years strongly suggests that microRNAs (miRNAs) may become a valuable tool for the early identification and assessment of disease prognosis or treatment prediction. However, prior potential implementation of novel biomarkers including miRNAs in clinical settings, there is a great need for studies that deal with possible influencing factors[1]. At present, only limited knowledge is available regarding the translational utility of miRNAs as biomarkers in the real-life setting.
Hepatocellular carcinoma (HCC) is one of the most common cancers, even though the factors responsible for disease development are very heterogeneous[2,3]. Prognosis of patients with HCC is dependent on multiple factors including tumor biology, but is also strongly related to residual liver and kidney function. Therefore, HCC is probably the disease that may be best suited for evaluation of various influencing factors in biomarker research. There are several prognostic assessment models and staging systems for liver diseases and/or HCC available including Child-Pugh score[4,5], Model of End-Stage Liver Disease score[6], the Cancer of the Liver Italian Program score[7], Okuda staging system[8], and the Barcelona Clinic Liver Cancer (BCLC) staging system[9] and many of those include multiple variables related to liver and/or renal function.
There is a great need for novel diagnostic, prognostic, and predictive biomarkers for HCC patients that would help to refine the forecast and subsequently individualize treatment decisions. MiRNA analyses of different solid cancers suggest the extensive involvement of miRNAs in cancer pathogenesis[10]. Multiple miRNAs have been examined in HCC previously, but substantial heterogeneity of data has hampered its utility[11]. We recently re-evaluated the prognostic value of hsa-miR-122-5p (miR-122) in a European cohort of well-characterized patients[1]. According to our data, circulating miR-122 was associated with the overall survival (OS) of HCC patients only in a subgroup of patients. We and others have identified multiple co-existing factors that may influence circulating miR-122 levels such as liver injury, hemoglobin concentration, or kidney function, but knowledge of other miRNAs in HCC is limited.
Undoubtedly, hsa-miR-21-5p (miR-21) is among the most frequently deregulated miRNA in human cancers[10-12]. In-depth evidence gathered over the past several years clearly demonstrates the pro-oncogenic function of miR-21 in carcinogenesis[13,14]. For instance, an in vivo study used an miR-21-overexpression mouse model where miR-21 overexpression led to a pre-B malignant lymphoid-like phenotype, indicating that miR-21 is an oncogenic miRNA[15].
In HCC, miR-21 promotes lipid accumulation in the liver and carcinogenesis through the Hbp1-p53-Srebp1c pathway, and therefore represents a potential link between non-alcoholic fatty liver disease and HCC[16]. It may play a role in mediating sorafenib resistance of HCC[17]. In a mouse model, treatment with a miR-21 inhibitor led to inhibition of tumor growth implicating miR-21 as potential therapeutic target in HCC[18]. Overexpression of miR-21 in HCC tumors was uniformly described[19-21] and is associated with poor OS and disease-free survival of HCC patients[19,20].
Higher miR-21 blood level was described in patients with HCC compared to healthy donors[22-24], but the opposite observation has also been reported[25]. Several studies have reported discordant results on circulating miR-21, particularly in patients with chronic hepatitis B virus (HBV) infection and HCC[22,26,27]. Diagnostic value of miR-21 in HCC has mostly been evaluated in HBV-dominant cohorts[22,23,27]. However, a recently published meta-analysis suggested that high ubiquitous miR-21 expression may be a limiting factor in the diagnostic performance of circulating miR-21[28].
In this study, we evaluated the value of serum miR-21 as a prognostic marker in a European cohort with mainly alcohol-induced HCC. Furthermore, we identified and explored the potential clinical conditions and laboratory co-factors that may influence performance of miR-21 as a biomarker in HCC and potentially in other conditions.
In total, the sera of 91 patients with HCC were available for analysis. The samples were collected between January 2009 and April 2011 as previously described[1]. Diagnosis of HCC was confirmed either histologically or via non-invasive criteria based on typical imaging using computer tomography and magnetic resonance imaging. The study was performed according to the World Medical Association “Declaration of Helsinki–Ethical Principles for medical research involving human subjects,” and was approved by the local Institutional Review Board of Otto-von-Guericke University Magdeburg (No. 99/10). Written informed consent was obtained from patients prior inclusion in the primary study. All patients were well characterized according to laboratory parameters, concomitant liver disease, and HCC including Child-Pugh score and BCLC staging as previously reported[1].
Following centrifugation, all serum samples were stored at -80 °C prior to further use. We performed extraction of RNA (including miRNA) according to a previously described and established protocol using the miRNeasy Mini Kit (QIAGEN, Hilden, Germany)[29]. Briefly, 700 µL QIAzol Lysis Reagent were initially mixed with 100 µL serum. During this step, we added 5 μL of a 5 nM cel-miR-39 solution for internal normalization. The quality of RNA was examined using spectrophotometry. Following washing steps, RNA was finally eluted in 30 µL RNase-free water and stored at -80 °C until further use. Quantification of cel-miR-39-3p (assay ID: 000200) and hsa-miR-122-5p (assay ID: 002245) expression were assessed using the TaqMan miRNA assay and TaqMan Universal Master Mix II (Applied Biosystems, Foster City, CA, United States). Hsa-miR-21-5p was assessed with internally validated SYBR green method as previously described[30]. RNA (20 ng) was transcribed and used for the quantitative PCR (qPCR). The analyses were performed on the BioRad CFX Cycler System (BioRad, Hercules, CA, United States) in duplicate and the Ct or threshold cycle value was used for normalization and subsequent relative quantification.
For statistical analysis, we used GraphPad Prism Version 7.0 (GraphPad Software, San Diego, CA, United States). Two-sided P values ≤ 0.05 were defined as significant. Based on the normality of distribution of miRNA values (2-deltaCt [miR-21/cel-miR-39]), we used nonparametric tests such as Spearman’s rank correlation coefficient, Mann-Whitney test, Kruskal-Wallis test, and Dunn’s post-hoc test. Chi-square (χ2) test and Fisher’s exact test were used for contingency testing accordingly. Additionally, the unpaired t-test was used for analysis of laboratory parameters as appropriate. The data are presented as boxplots with whiskers for minimum and maximum. The survival time was defined as the time between inclusion in our study (date of blood withdrawal) and death or last documented contact. Kaplan-Meier survival curves and nonparametric log-rank test were used to evaluate survival differences. Median (50% percentile) was used to define the groups with high and low serum miR-21 expression, miR-122, alpha-fetoprotein (AFP) expression.
The analysis of miR-21 in serum showed high variability between samples (mean Ct value: 29.61 ± 0.78, [min: 27.38, max: 31.22, range: 3.84]). First, we divided the cohort in two groups with miR-21-high and miR-21-low serum levels based on the median as shown in Table 1. Most parameters showed no statistically significant difference between the groups including gender, age, etiology of HCC, BCLC stage, or treatment status.
| All patients | miR-21 low1 | miR-21 high2 | P value | ||
| Patient number: | 91 | 47 | 44 | ||
| Gender (n, %): | Women | 17 (18.7%) | 7 (14.9%) | 10 (22.7%) | 0.4231 |
| Men | 74 (81.3%) | 40 (85.1%) | 34 (77.3%) | ||
| Age in yr | mean ± SD | 67.91 ± 8.98 | 69.11 ± 8.84 | 66.64 ± 9.05 | 0.1912 |
| Etiology (n, %): | Alcohol abuse | 41 (45.1%) | 22 (46.8%) | 19 (43.2%) | 0.7560 |
| Viral hepatitis | 12 (13.2%) | 5 (10.6%) | 7 (15.9%) | ||
| NASH | 13 (14.3%) | 8 (17.0%) | 5 (11.4%) | ||
| Rare or other cause3 | 25 (27.5%) | 12 (25.5%) | 13 (29.5%) | ||
| BCLC stage (n, %): | A | 16 (17.6%) | 7 (14.9%) | 9 (20.5%) | 0.7787 |
| B | 37 (40.7%) | 20 (42.6%) | 17 (38.6%) | ||
| C + D | 38 (41.8%) | 20 (42.6%) | 18 (40.9%) | ||
| Child-Pugh score (n, %): | No liver cirrhosis | 16 (17.6%) | 8 (17.0%) | 8 (18.2%) | 0.7443 |
| A | 45 (49.5%) | 25 (53.2%) | 20 (45.5%) | ||
| B + C | 30 (33.0%) | 14 (29.8%) | 16 (36.4%) | ||
| Treatment (n, %)4: | Therapy naïve | 26 (28.6%) | 11 (23.4%) | 15 (34.1%) | 0.3535 |
| Pretreated | 65 (71.4%) | 36 (76.6%) | 29 (65.9%) | ||
| Ascites (n, %): | No ascites | 58 (63.7%) | 30 (63.8%) | 28 (63.6%) | 0.9999 |
| Ascites present | 33 (36.3%) | 17 (36.2%) | 16 (36.4%) | ||
| Bilirubin (µmol/L): | mean ± SD | 23.38 ± 31.73 | 24.27 ± 40.47 | 22.43 ± 18.78 | 0.7833 |
| AFP (ng/mL): | mean ± SD | 4593 ± 20162 | 540 ± 1533 | 8922 ± 28481 | 0.0469 |
| INR5: | mean ± SD | 1.076 ± 0.2127 | 1.034 ± 0.1450 | 1.122 ± 0.2611 | 0.0486 |
| Platelets (Gpt/L): | mean ± SD | 195.6 ± 118.7 | 182.7 ± 80.5 | 209.3 ± 149.0 | 0.2870 |
| ALAT (μmol/Ls): | mean ± SD | 0.7457 ± 0.4460 | 0.6955 ± 0.3425 | 0.7993 ± 0.5340 | 0.2697 |
| ASAT (μmol/Ls): | mean ± SD | 1.267 ± 1.041 | 1.040 ± 0.597 | 1.510 ± 1.330 | 0.0307 |
| Hemoglobin (mmol/L): | mean ± SD | 7.811 ± 1.254 | 7.655 ± 1.122 | 7.977 ± 1.375 | 0.2230 |
| Creatinine (µmol/L): | mean ± SD | 92.92 ± 67.95 | 102.3 ± 88.46 | 82.91 ± 33.08 | 0.1751 |
| Albumin (g/L): | mean ± SD | 36.29 ± 6.539 | 36.47 ± 5.668 | 36.10 ± 7.420 | 0.7938 |
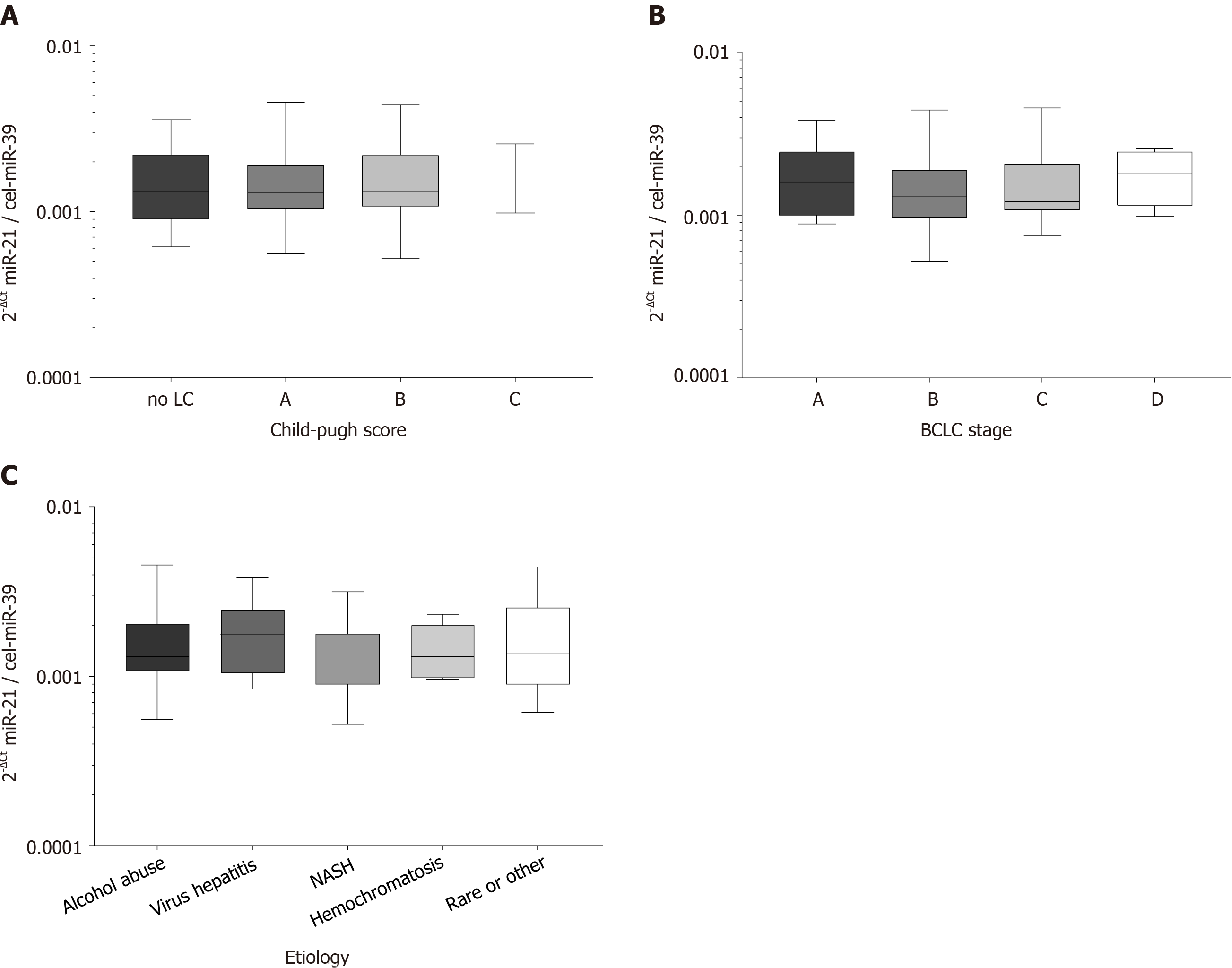
To evaluate the role of disease stage or etiology of the concomitant liver disease, we performed subgroup testing. Circulating miR-21 in patients with HCC were similar between patients with different Child-Pugh scores (P = 0.7991; Figure 1A), BCLC stages (P = 0.3947; Figure 1B) or was interdependent of etiologies of underlying liver disease (P = 0.6331; Figure 1C) suggesting that other factors than HCC or liver disease may contribute to miR-21 variation.
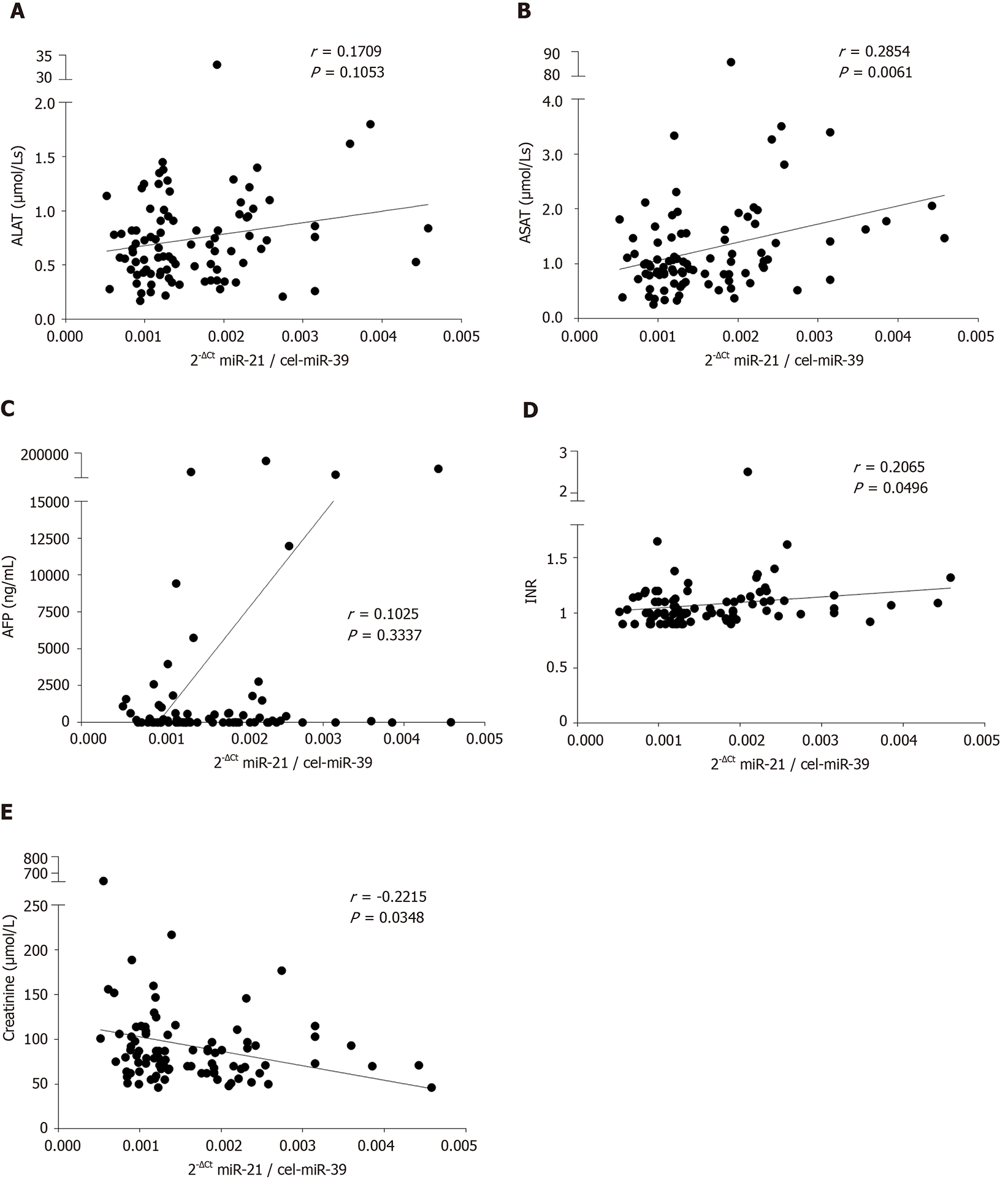
To evaluate if concomitant conditions including liver injury, liver function or kidney injury may affect miR-21 level, we performed comparison of various laboratory parameters and serum miR-21. As shown in Figure 2A, there was a non-significant trend for a positive correlation between miR-21 and alanine aminotransferase (ALAT) (r = 0.1709, P = 0.1053). In much stronger fashion, we observed a statistically significant positive correlation between miR-21 and aspartate aminotransferase (ASAT) (r = 0.2854, P = 0.0061; Figure 2B). Correspondingly, patients with higher miR-21 had also significantly higher ASAT levels (P = 0.0307; Table 1). Even though, miR-21 showed no correlation with AFP (r = 0.1025, P = 0.3337; Figure 2C), the miR-21-high group was associated with higher AFP levels (P = 0.0469; Table 1). MiR-21 levels were furthermore associated with the International Normalized Ratio (r = 0.2065, P = 0.0496; Figure 2D) and patients with high miR-21 had higher International Normalized Ratio accordingly (P = 0.0486; Table 1). Subsequent analysis of other parameters of the liver function such as bilirubin or albumin revealed not association with miR-21 (Tables 1 and 2). Most importantly from the clinical perspective, we observed a negative correlation between miR-21 level and creatinine level (r = -0.2215, P = 0.0348; Figure 2E), suggesting that renal function may impact miR-21 level in blood. Tables 1 and 2 provide a detailed overview on other factors that have been evaluated in the study with no significant difference.
| Parameter | 2-ΔCt miR-21 / cel-miR-39 | ||
| Spearman r | Confidence interval | P value | |
| Age in yr | -0.1147 | -0.3188 to 0.09956 | 0.2789 |
| Creatinine (µmol/L) | -0.2215 | -0.4139 to -0.01013 | 0.0348 |
| Bilirubin (µmol/L) | 0.1484 | -0.06547 to 0.3493 | 0.1603 |
| ALAT (µmol/Ls) | 0.1709 | -0.0425 to 0.3694 | 0.1053 |
| ASAT (µmol/Ls) | 0.2854 | 0.07828 to 0.4689 | 0.0061 |
| Albumin (g/L) | -0.01562 | -0.2267 to 0.1969 | 0.8832 |
| AFP (ng/mL) | 0.1025 | -0.1118 to 0.3077 | 0.3337 |
| Hemoglobin (mmol/L) | 0.1377 | -0.07638 to 0.3396 | 0.1931 |
| INR1 | 0.2065 | -0.005615 to 0.4008 | 0.0496 |
| Platelets (Gpt/L) | 0.09185 | -0.1224 to 0.2979 | 0.3865 |
| 2-ΔCt miR-122 / cel-miR-39 | 0.2624 | 0.05357 to 0.4493 | 0.0120 |
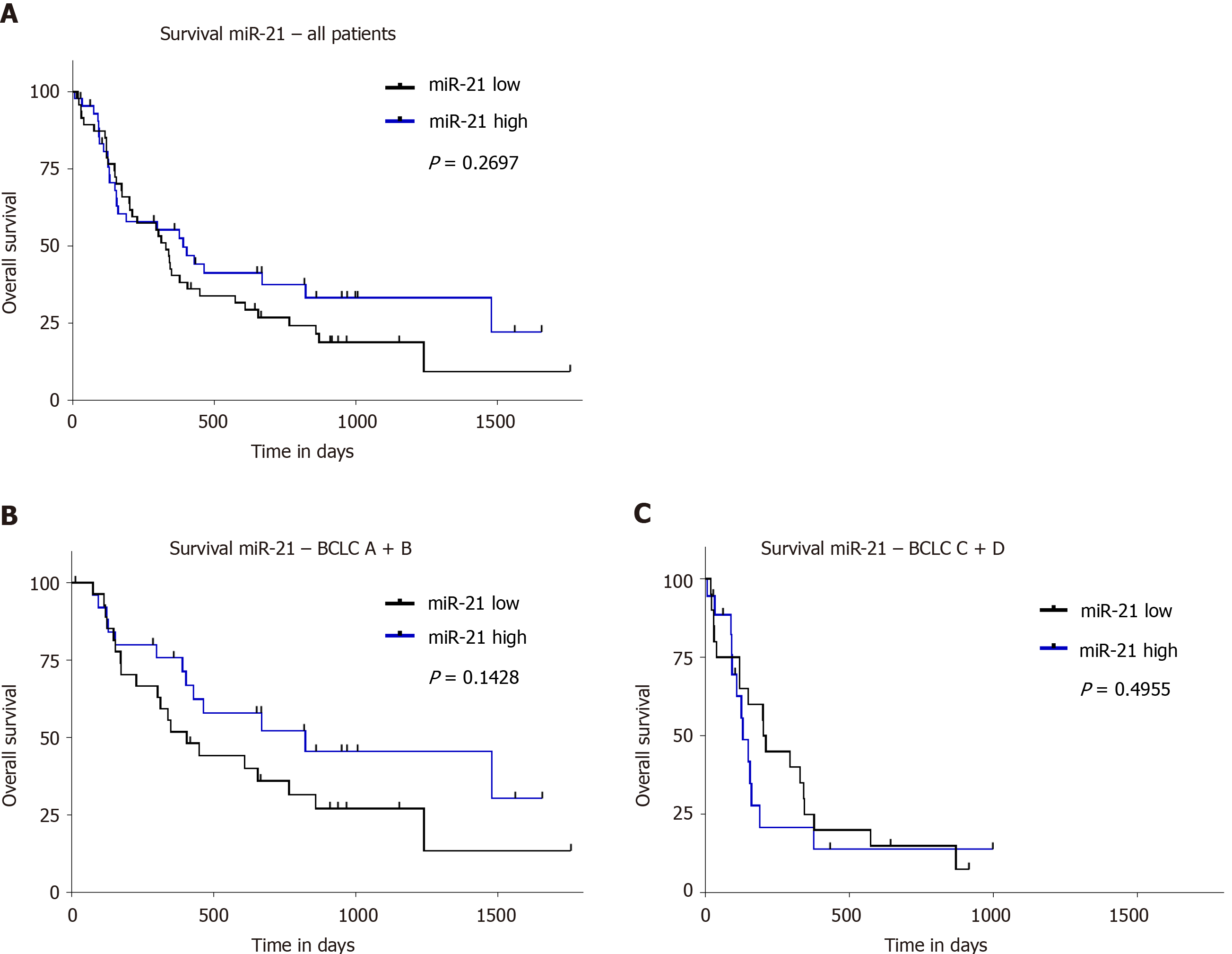
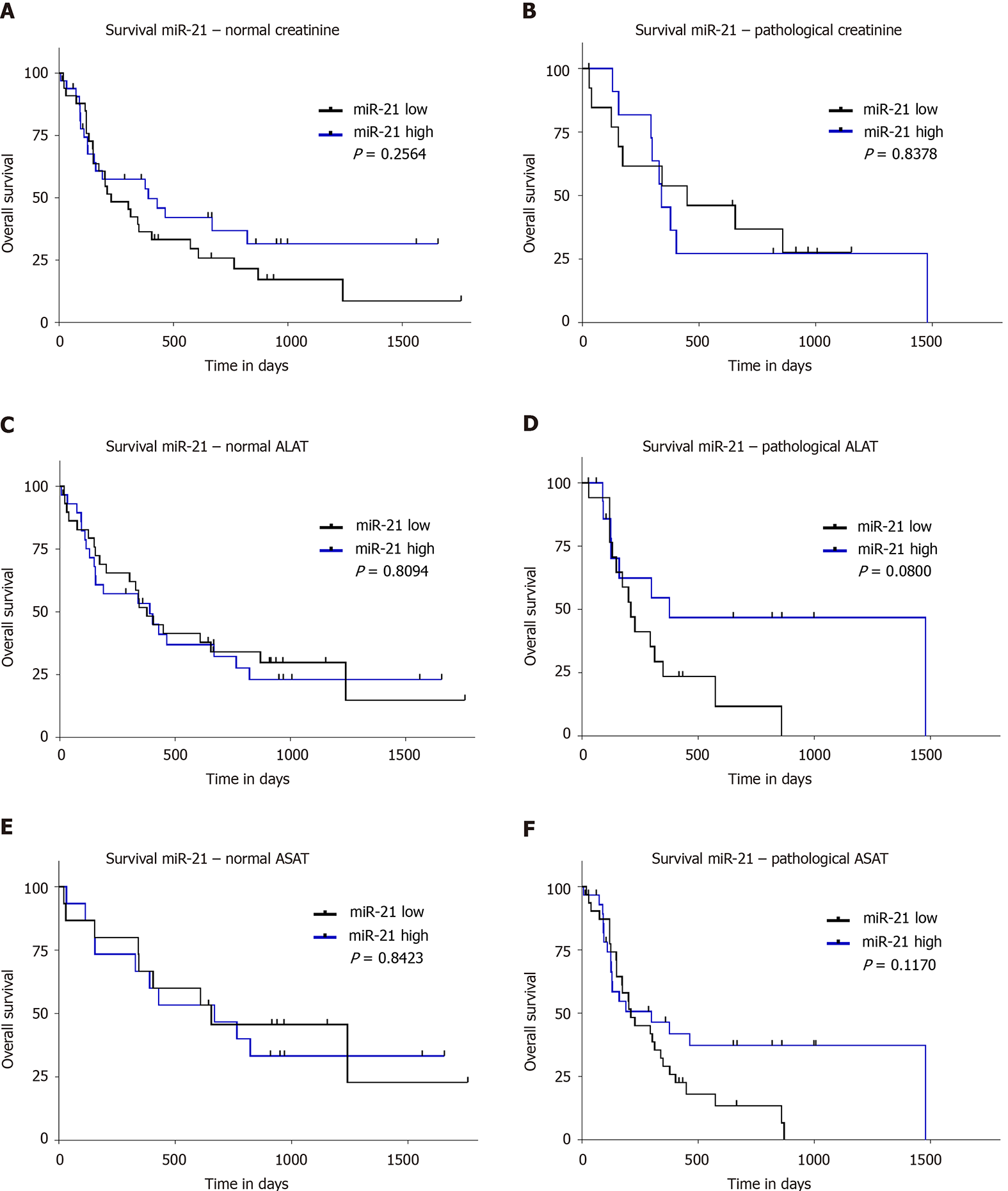
The survival data of our cohort have been previously validated and reported[1]. To evaluate the prognostic value of miR-21, we divided our study population based on the 50th percentile of miR-21 into patients with high and low level. We did not find any significant difference in survival between these groups (P = 0.2697; Figure 3A). Furthermore, no survival difference was found between the groups if the cohort was divided into three groups according to the 25th, 33th, and 75th percentile (data not shown). Taking into account the differences in tumor biology, we performed subgroup analysis for different BCLC stages. Neither separate analysis for combined BCLC A and B (P = 0.1428; Figure 3B) nor BCLC C and D (P = 0.4955; Figure 3C) were associated with survival differences. Having shown an association to creatinine, we further explored the prognostic value in subjects with normal and impaired renal function, but no prognostic difference was observed based on miR-21 level in patients with normal or pathological creatinine (P = 0.2564, Figure 4A and P = 0.8378, Figure 4B, respectively). Higher miR-21 levels were not associated with a worse prognosis in patients with normal ALAT (P = 0.8094, Figure 4C), while a slight trend for worse OS was observed in patients with pathological ALAT (P = 0.0800, Figure 4D). In a similar way, we found no significant difference in OS in patients with normal ASAT but a non-significant trend in patients with pathological ASAT (P = 0.8423, Figure 4E, P = 0.1170, Figure 4F, respectively).
Next, we evaluated the link between serum miR-21 and serum miR-122 levels. There was a positive correlation between miR-21 and miR-122 (r = 0.2624, P = 0.0120; Figure 5A), which suggests that circulating miRNAs may behave in a similar manner dependent on certain conditions like liver disease or renal function. We investigated if the combination of serum miR-21 and miR-122 may improve prognostic value in patients with HCC. After dividing our study group according to the median of miR-21 and miR-122 level, we formed three groups (patients with low miR-21 and low miR-122, patients with either low miR-21 or low miR-122, patients with high miR-21 and high miR-122); however, no significant difference in OS was observed (P = 0.3377; Figure 5B).
In a similar fashion, we investigated the association of serum miR-21 in correlation with AFP on OS. In HCC patients, higher AFP levels were associated with shorter OS in subjects with miR-21 Low (P = 0.0237; Figure 5C) but only with a trend for shorter OS in miR-21 high group (P = 0.0917; Figure 5D). In subgroup analysis, AFP was indeed associated with a worse prognosis, but the impact was independent of miR-21 level (data not shown).
Data on translational value of serum miR-21 in HCC in real-life settings are heterogeneous and only limited evidence is available for the European population. In this work, we systematically analyzed the prognostic value of serum miR-21 in our well-characterized European cohort of HCC patients, and evaluated the potential influence of various factors on its level in serum. The results of our study do not support the prognostic role of miR-21 in HCC. MiR-21 was neither associated with worse OS nor with etiology or tumor stage. Most interestingly, circulating miR-21 levels correlated significantly with ASAT, as surrogate for liver injury, and with creatinine, as a surrogate for renal function.
The prognostic value of circulating miR-21 has been studied in several studies. The overview of the studies is provided in Table 3. Among those, four studies reported that high miR-21 was associated with a worse prognosis[18,24,31,32], while one study[25], in concordance with our data, did not support prognostic role of miR-21. Another study demonstrated significantly better liver transplantation-free survival in HCC patients with higher plasma miR-21 levels[33]. As mentioned above, the available data are mostly based on the evidence from Asian populations and mainly include HBV/hepatitis C virus-induced HCC, while our work is among the first to evaluate the survival in patients from European origin. One may speculate if etiology of HCC may be responsible for the differences in the study outcome; however, our subgroup analysis did not reveal any difference in this regards. From another point of view, differences in tumor stage may also influence miR-21 level. Our cohort involved a large proportion of patients with intermediate or advanced HCC (BCLC B and C). Nevertheless, the samples size in our work may be too low to precisely delineate the interaction and larger studies with possibility to perform multivariate adjustments for liver and renal injury in view of tumor stage and etiology of the disease will be needed.
| Characteristics | Qi et al[25] | Tomimaru et al[24] | Liu et al[32] | Wang et al[31] | Cho et al[33] | Zhang et al[18] | Current data |
| High miR-21 and prognosis | ↔ | Trend for worse prognosis | Worse prognosis | Worse prognosis | Better liver transplant-free survival | Worse prognosis | ↔ |
| Group allocation: Cut-off-value | NA | Cut-off based on ROC analyzes | 50th percentile | 50th percentile | 50th percentile | NA | 50th percentile |
| Origin of the cohort | Asia | Asia | Asia | Asia | Asia | Asia | Europe |
| Predominant etiology | HBV | HCV/HBV | HBV | HBV | HBV | NA | Mainly alcohol abuse |
| TNM reported | Yes | Yes | Yes | Yes | Yes | ||
| BCLC reported | Yes | Yes | Yes | ||||
| Child-Pugh score reported | Yes | Yes | Yes | Yes | Yes | ||
| Sampling | Before surgery | Before surgery | Before TACE | No exact information, surgical resection | Before surgery or RFA | Pre- and postoperative serum samples | Therapy naïve and pretreated |
| Patients | 70 | 126 | 136 | 97 | 120 | 46 | 91 |
| Specimen | Serum | Plasma | Serum | Serum | Plasma | Serum | Serum |
| qPCR method | TaqMan® | TaqMan® | SYBRGreen | SYBR Green | TaqMan® | Unknown | TaqMan® SYBRGreen |
| Normalization | miR-16 | miR-16 | Quanto EC1, Quanto EC2 | cel-miR-39 | MiR-16 | RNU6b | cel-miR-39 |
| Publishing year | 2011 | 2012 | 2014 | 2015 | 2017 | 2019 | 2020 |
AFP is among the most valuable prognostic markers in HCC. Wang et al[19] reported a positive correlation between miR-21 in tissues and serum AFP[19]. Tomimaru et al[24] and Liu et al[32] reported a similar correlation between circulating miR-21 in the blood and AFP[24,32]. In concordance with the data form Zhang et al[18], although our study demonstrated higher AFP values in patients with high serum miR-21 levels, it still did not reveal any correlation among the parameters.
An open question remains regarding the source of circulating miR-21 in HCC patients. Since miR-21 is present at a relatively high level in serum, it is hard to consider it a specific surrogate for the HCC tumor load. Nevertheless, several reports have suggested that circulating miR-21 may originate from tumor tissue. Studies have shown a reduction of miR-21 in blood after surgery[18,22,24,26]. Furthermore, concordant data have been reported for serum miR-21 and associated tissue levels[18,22,24], hypothesizing HCC tumor tissue as a potential source of miR-21 in HCC patients. At the same time, another hypothesis suggests that elevated circulating miR-21 may be triggered by non-specific liver damage. Based on our observation, miR-21 correlated with ASAT, which at least indirectly supports the hypothesis of liver injury. Additionally, miR-21 correlated significantly with serum miR-122 as highly abundant liver-specific miRNA[34,35]. Non-specific release of miR-122 from liver tissue has also been previously discussed[36,37]. Another study explored the influence of necroinflammation in patients with hepatitis C virus with and without HCC[38]. The authors demonstrated similar values of serum miR-21 between patients with and without HCC, suggesting that higher miR-21 level may be caused by non-specific liver injury rather than tumor-induced miR-21 release. Although our results do not link miR-21 with liver cirrhosis or liver function, Karakatsanis et al[20] showed that miR-21 was associated with cirrhosis[20], and Wang et al[39] correlated exosomal miR-21 with presence of liver cirrhosis[39].
Renal function seems to be a crucial confounding factor for miRNA analysis in blood not only for miR-122[1] as previously reported, but also for miR-21. On one hand, upregulation of miR-21 in kidney plays a role in acute kidney injury as a defense mechanism to reduce cell death. On the other hand, overexpression of miR-21, caused by massive cell death, leads to renal inflammation and fibrosis[40]. Renal injury in animal studies is associated with increased miR-21 expression in tissue and lower levels in blood[41]. In human, miR-21 was lower in subjects with cardiac surgery-associated acute kidney injury[42]. Our data strongly support the suggestion that renal function may affect circulating miRNAs as both miR-21 and miR-122 have shown a clear correlation with creatinine values. Taking into account the fact that miR-21 has been suggested as a potential biomarker for a large number of diseases including cancer[11,43] it will be necessary in the future to consider those variations in ongoing studies. In particular, miR-21 has been suggested as a diagnostic marker for malignant diseases including gastric, colorectal cancer, and HCC. Because of the miR-21 variation in our cohort, we speculate that the diagnostic value of miR-21 may be also affected by the renal and liver function. In-depth systematic data including miRNA-profiling are encouraged to address this topic in detail.
In summary, the results of this study do not support the role of circulating miR-21 as prognostic biomarker for patients with HCC in our European cohort. A positive correlation between miR-21 and ASAT and negative correlation with creatinine strongly emphasizes that non-specific liver injury and renal function may influence the sensitivity and specificity of miRNAs as biomarkers, and that those factors need to be carefully assessed in future studies.
MiR-21-5p (miR-21) is one of the most frequently deregulated microRNAs (miRNAs) in tissue and in body fluids in different diseases and most importantly in cancer. Overexpression and prognostic value of tissue miR-21 in hepatocellular carcinoma (HCC) has been previously suggested, but the data are heterogeneous. Greatest evidence in support of miR-21 as a biomarker originate from Asian population, highlighting the need for additional scientific effort and search for potential confounding factors including etiological background of HCC.
Data on prognostic value of serum miR-21 in patients with mainly alcohol abuse-induced HCC as well as the knowledge of potential influencing factors and underlying mechanism are still limited.
We evaluated the prognostic value of serum miR-21 in a European cohort of HCC patients with mainly alcohol-associated liver disease in real-life settings. We also explored the potential confounding clinical or laboratory influencing factors on the serum miR-21 levels.
Circulating miR-21 level were analyzed in 91 sera samples from well-characterized patients with HCC. Clinical and laboratory parameters (including alpha-fetoprotein), miR-122 as well as the overall survival (OS) were examined.
We observed no association between serum miR-21 and OS, Child-Pugh scores, or BCLC staging data. Significant correlation between serum miR-21 and aspartate aminotransferase, International Normalized Ratio, creatinine, and hsa-miR-122 suggested the potential influence of liver and renal function on circulating miR-21 levels.
Our data do not support prognostic role of miR-21 in HCC patients. An association of miR-21 with surrogate markers of liver injury and renal function strongly support the need for better understanding of influencing factors in miRNA biogenesis.
Systematic profiling studies that provide overall data assessment of miRNAs in view of potential influencing factors in blood are needed prior clinical implementation of miRNAs as clinical biomarkers.
The authors would like to thank Ursula Stolz (from the GI Research Laboratory of the Department of Gastroenterology, Hepatology and Infectious Diseases) for technical support.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues P S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Franck M, Schütte K, Malfertheiner P, Link A. Prognostic value of serum microRNA-122 in hepatocellular carcinoma is dependent on coexisting clinical and laboratory factors. World J Gastroenterol. 2020;26:86-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 4. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 5. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5738] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 6. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 7. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 8. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 10. | Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4471] [Cited by in RCA: 4523] [Article Influence: 238.1] [Reference Citation Analysis (0)] |
| 11. | Link A, Goel A. MicroRNA in gastrointestinal cancer: a step closer to reality. Adv Clin Chem. 2013;62:221-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029-6033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1865] [Cited by in RCA: 1897] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 13. | Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. 2011;30:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Link A, Kupcinskas J, Wex T, Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig Dis. 2012;30:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 16. | Wu H, Ng R, Chen X, Steer CJ, Song G. MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 2016;65:1850-1860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | He C, Dong X, Zhai B, Jiang X, Dong D, Li B, Jiang H, Xu S, Sun X. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6:28867-28881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Zhang N, Hu Z, Qiang Y, Zhu X. Circulating miR-130b- and miR-21-based diagnostic markers and therapeutic targets for hepatocellular carcinoma. Mol Genet Genomic Med. 2019;7:e1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Wang WY, Zhang HF, Wang L, Ma YP, Gao F, Zhang SJ, Wang LC. miR-21 expression predicts prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 21. | Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 22. | Guo X, Lv X, Lv X, Ma Y, Chen L, Chen Y. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget. 2017;8:44050-44058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, Wang Z, Qiu S, Wang X, Yang G, Sun H, Tang Z, Wu Y, Zhu H, Fan J. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 498] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 24. | Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K, Kanto T, Doki Y, Mori M. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Zhuang C, Jiang W, Huang D, Xu L, Yang Q, Zheng L, Wang X, Hu L. Serum miR-21, miR-26a and miR-101 as potential biomarkers of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, Lin D. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao H, Sun Q, Yan F, Yan C, Li H, Ren X. Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis. Gene. 2014;533:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Schönauen K, Le N, von Arnim U, Schulz C, Malfertheiner P, Link A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2018;24:1547-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 30. | Link A, Becker V, Goel A, Wex T, Malfertheiner P. Feasibility of fecal microRNAs as novel biomarkers for pancreatic cancer. PLoS One. 2012;7:e42933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Wang X, Zhang J, Zhou L, Lu P, Zheng ZG, Sun W, Wang JL, Yang XS, Li XL, Xia N, Zhang N, Dou KF. Significance of serum microRNA-21 in diagnosis of hepatocellular carcinoma (HCC): clinical analyses of patients and an HCC rat model. Int J Clin Exp Pathol. 2015;8:1466-1478. [PubMed] |
| 32. | Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H, Xu N, Xie Y. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS One. 2014;9:e109347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Cho HJ, Kim SS, Nam JS, Kim JK, Lee JH, Kim B, Wang HJ, Kim BW, Lee JD, Kang DY, Kim JH, Jae YM, Hwang JC, Shin SJ, Lee KM, Cho SW, Cheong JY. Low levels of circulating microRNA-26a/29a as poor prognostic markers in patients with hepatocellular carcinoma who underwent curative treatment. Clin Res Hepatol Gastroenterol. 2017;41:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2597] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 35. | Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, Tian W, Zhang Q, Wang C, Zhang Q, Zhuang SM, Zheng L, Liang A, Tao W, Cao X. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 588] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 36. | Roderburg C, Benz F, Vargas Cardenas D, Koch A, Janssen J, Vucur M, Gautheron J, Schneider AT, Koppe C, Kreggenwinkel K, Zimmermann HW, Luedde M, Trautwein C, Tacke F, Luedde T. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int. 2015;35:1172-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 38. | Bihrer V, Waidmann O, Friedrich-Rust M, Forestier N, Susser S, Haupenthal J, Welker M, Shi Y, Peveling-Oberhag J, Polta A, von Wagner M, Radeke HH, Sarrazin C, Trojan J, Zeuzem S, Kronenberger B, Piiper A. Serum microRNA-21 as marker for necroinflammation in hepatitis C patients with and without hepatocellular carcinoma. PLoS One. 2011;6:e26971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 40. | Li YF, Jing Y, Hao J, Frankfort NC, Zhou X, Shen B, Liu X, Wang L, Li R. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell. 2013;4:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Saikumar J, Hoffmann D, Kim TM, Gonzalez VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS, Vaidya VS. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci. 2012;129:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Arvin P, Samimagham HR, Montazerghaem H, Khayatian M, Mahboobi H, Ghadiri Soufi F. Early detection of cardiac surgery-associated acute kidney injury by microRNA-21. Bratisl Lek Listy. 2017;118:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:3313-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |