Published online Mar 27, 2019. doi: 10.4254/wjh.v11.i3.305
Peer-review started: October 26, 2018
First decision: December 19, 2018
Revised: February 22, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: March 27, 2019
Processing time: 153 Days and 8.6 Hours
Preoperative supplementation with immunonutrients, including arginine and n-3 fatty acids, has been shown in a number of systematic reviews to reduce infectious complications in patients who have undergone gastrointestinal surgery. Limited information, however, is available on the benefits of nutritional supplementation enriched with arginine and n-3 fatty acids in patients undergoing liver resection.
To evaluate the effects of preoperative nutritional supplementation enriched with arginine and n-3 fatty acids on inflammatory and immunologic markers and clinical outcome in patients undergoing liver resection.
Thirty-four patients undergoing liver resection were randomized to either five days of preoperative Impact® [1020 kcal/d, immunonutrition (IMN) group], or standard care [no supplementation, standard care (STD) group]. Nutritional status was measured at study entry by subjective global assessment (SGA). Functional assessments (grip strength, fatigue and performance status) were carried out at study entry, on the day prior to surgery, and on postoperative day (POD) 7 and 30. Inflammatory and immune markers were measured at study entry, on the day prior to surgery, and POD 1, 3, 5, 7, 10 and 30. Postoperative complications were recorded prospectively until POD30.
A total of 32 patients (17 IMN and 15 STD) were analysed. All except four patients were SGA class A. The plasma ratio of (eicosapentaenoic acid plus docosahexaenoic acid) to arachidonic acid was higher in IMN patients on the day prior to surgery and POD 1, 3, 5 and 7 (P < 0.05). Plasma interleukin (IL)-6 concentrations were elevated in the IMN group (P = 0.017 for POD7). No treatment effect was detected for functional measures, immune response (white cell count and total lymphocytes) or markers of inflammation (C-reactive protein, tumour necrosis factor-α, IL-8, IL-10). There were 10 patients with infectious complications in the IMN group and 4 in the STD group (P = 0.087). Median hospital stay was 9 (range 4–49) d in the IMN group and 8 (3-34) d in the STD group (P = 0.476).
In well-nourished patients undergoing elective liver resection, this study failed to show any benefit of preoperative immunonutrition.
Core tip: Whether immunonutritional supplementation provided preoperatively to patients undergoing liver resection can reduce postoperative inflammation and improve clinical outcome compared to standard care remains unclear. We conducted a prospective randomized trial to clarify this. We found no significant suppression of postoperative inflammation or reduction in infectious complications or length of hospital stay post-surgery through providing preoperative immunonutrition.
- Citation: Russell K, Zhang HG, Gillanders LK, Bartlett AS, Fisk HL, Calder PC, Swan PJ, Plank LD. Preoperative immunonutrition in patients undergoing liver resection: A prospective randomized trial. World J Hepatol 2019; 11(3): 305-317
- URL: https://www.wjgnet.com/1948-5182/full/v11/i3/305.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i3.305
Immunonutrition, the provision of specific nutrients in supra-physiological doses, is suggested to provide vital substrates that act to modulate immune and metabolic responses and improve clinical outcome[1]. Nutrients that have been identified to offer immunological benefit include n-3 polyunsaturated fatty acids, arginine, glutamine and nucleotides. A number of systematic reviews have shown that immunonutrition provided as a preoperative supplement to patients undergoing elective gastrointestinal surgery leads to significant reductions in postoperative infectious complications[2-5]. Benefit has been demonstrated in both malnourished[6] and normally nourished patients[7] and is thought to be due to the down regulation of the inflammatory responses to surgery and amelioration of the postoperative immune depression.
Despite the reported benefits of this therapy, we are aware of only two published randomized, prospective studies investigating the effect of preoperative immunonutrition on postoperative inflammation and clinical outcome in liver resection patients[8,9]. Mikagi et al[8] randomized 41 patients but only 26 were analysed. Some evidence for reduction of inflammation [interleukin-6 (IL-6)] on postoperative day (POD) 1 was seen and only one infectious complication was reported. Uno et al[9] randomised 40 patients, 83% of whom had surgery for (presumably perihilar) bile duct carcinoma, a relatively rare condition. Significant reductions in IL-6 on POD1, infectious complications and length of hospital stay were reported.
The primary objective of the present study was to investigate postoperative inflammatory and immunologic responses in patients undergoing liver resection who were randomized to either preoperative treatment with an immunonutritional supplement (IMN) or standard care (STD). Secondary objectives included clinical outcomes and physiological function.
Patients over 16 years of age scheduled for non-laparoscopic elective hepatic resection for primary or secondary liver cancer between December 2012 and April 2014 were recruited from the hepaticopancreaticobiliary outpatient clinic at Auckland City Hospital. Exclusion criteria included immunosuppression, cirrhosis (biopsy proven or fibroscan result), chemotherapy within 3 wk prior to study entry, taking fish oil supplements, and pregnancy. Ethics approval was granted by the Northern A Ethics Committee. Each patient provided written informed consent.
Hepatic resection was performed by an open technique through a right subcostal incision. All patients received intra-thecal opiate and general anaesthesia. Parenchymal resection was performed without inflow occlusion by anatomical dissection. Drains were routinely placed at completion and removed post-operatively depending upon volume and character of effluent. All patients received antibiotic prophylaxis (cefoxitin 1 g) given intravenously at induction of anaesthesia.
This was a prospective, randomized, assessor-blinded, clinical trial. At recruitment, patients were allocated to IMN or STD groups in a 1:1 ratio using opaque sealed envelopes prepared according to a computer-derived random sequence with variable block sizes. In addition to their usual intake, IMN patients were prescribed for each of the 5 consecutive days preceding surgery 3 x 237 mL tetra packs of IMPACT Advanced Recovery® (Nestle) providing 1020 kcal energy, 54 g protein, 12.6 g arginine, 1.3 g nucleotides, and 3.3 g eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) per day. Patients were telephoned to remind them to commence taking the supplement and asked to keep a daily record of the timing and volume of Impact consumed, as well as all other oral intake over this period. Those randomized to the STD group were advised to continue with their usual oral intake. Patients in this group assessed as having malnutrition were provided with a standard nutritional supplement (Fortisip®, Nutricia) twice daily (providing 600 kcal energy, 24 g protein), in addition to their usual intake, for the period preceding and including 5 days prior to surgery. At recruitment, blood samples were taken for inflammatory and immune status markers and measurement of plasma fatty acids. These measurements were repeated on the day prior to surgery (D-1) and on POD 1, 3, 5 and 7. An additional C-reactive protein (CRP) measurement was taken on POD30. CRP and full blood count were determined by the hospital accredited laboratory. Remaining blood was centrifuged at 4 °C and plasma separated and frozen at –80 °C until analysis. Nutritional status was assessed at study entry and functional status at study entry and on D-1, POD7 and POD30.
Using methods described in detail previously[10], total lipids were extracted, phosphatidylcholine (PC), the major phospholipid in plasma, was isolated, and gas chromatography was performed to determine the fatty acid composition of PC.
An immunoturbidimetric method (Roche Diagnostics) was used for high-sensitivity CRP assay. Simultaneous quantification of plasma tumour necrosis factor (TNF)-α, IL-6, IL-8 and IL-10 was carried out using a high- sensitivity multiplex immunoassay kit (Milliplex®, Millipore Corp, Billerica, MA, United States) and a micro-beads system following the manufacturer’s instructions (Luminex Corp., Austin, TX, United States).
Nutritional intake was assessed using the 24 hour diet recall technique[11]. Body weight to the nearest 0.1 kg was measured using electronic scales and an estimated clothing weight was subtracted. Height was measured using a stadiometer. Subjective global assessment (SGA) of nutritional status was performed as described by Detsky et al[12]. The Christensen Fatigue Scale was used to quantify subjective feelings of fatigue[13]. The Karnofsky Performance Scale was used to quantify general well-being and ability to complete activities of daily living[14]. Physiological function was measured by maximum voluntary grip strength in the dominant hand as the best of three attempts using a spring loaded analogue dynamometer (model 78010, Lafayette Instrument Co., Lafayette, IN, United States).
Patients were assessed daily while in hospital for infectious and other complications. Clinical notes were reviewed post-discharge to ensure no complication was missed. Patients were monitored for 30 days for complications. Postoperative complications were assessed and graded according to the Clavien-Dindo system[15]. A bacterial infection was defined by administration of antibiotics when signs and symptoms of sepsis were evident, indicating possible chest, urinary, line or wound infection. Infectious complications were categorised according to the Centers for Disease Control Classification System[16]. The surgical team in charge of each patient had sole discretion over adjudication of complications and determination of date of hospital discharge and were blinded to group allocation.
Sample size calculations demonstrated that 15 patients per group would provide > 90% power for detecting a significant difference in plasma IL-6 concentrations on POD1, based on the results of Braga et al[17] in gastrointestinal surgery patients.
Repeated measures data were analysed by the general linear mixed model. Inflammatory markers were log-transformed prior to analysis. Between-group comparisons used Student’s t test or Mann-Whitney U test for normally distributed and non-normally distributed data respectively. Fisher’s exact test was used for categorical data. Time-to-event data were compared between groups using the log-rank test. All analysis was performed on an intention-to-treat basis. Data are presented as mean ± SE or median (range). P values of less than 0.05 were considered to indicate statistical significance. Statistical analyses were performed using SAS release 9.4 (SAS Institute, Cary, NC, United States).
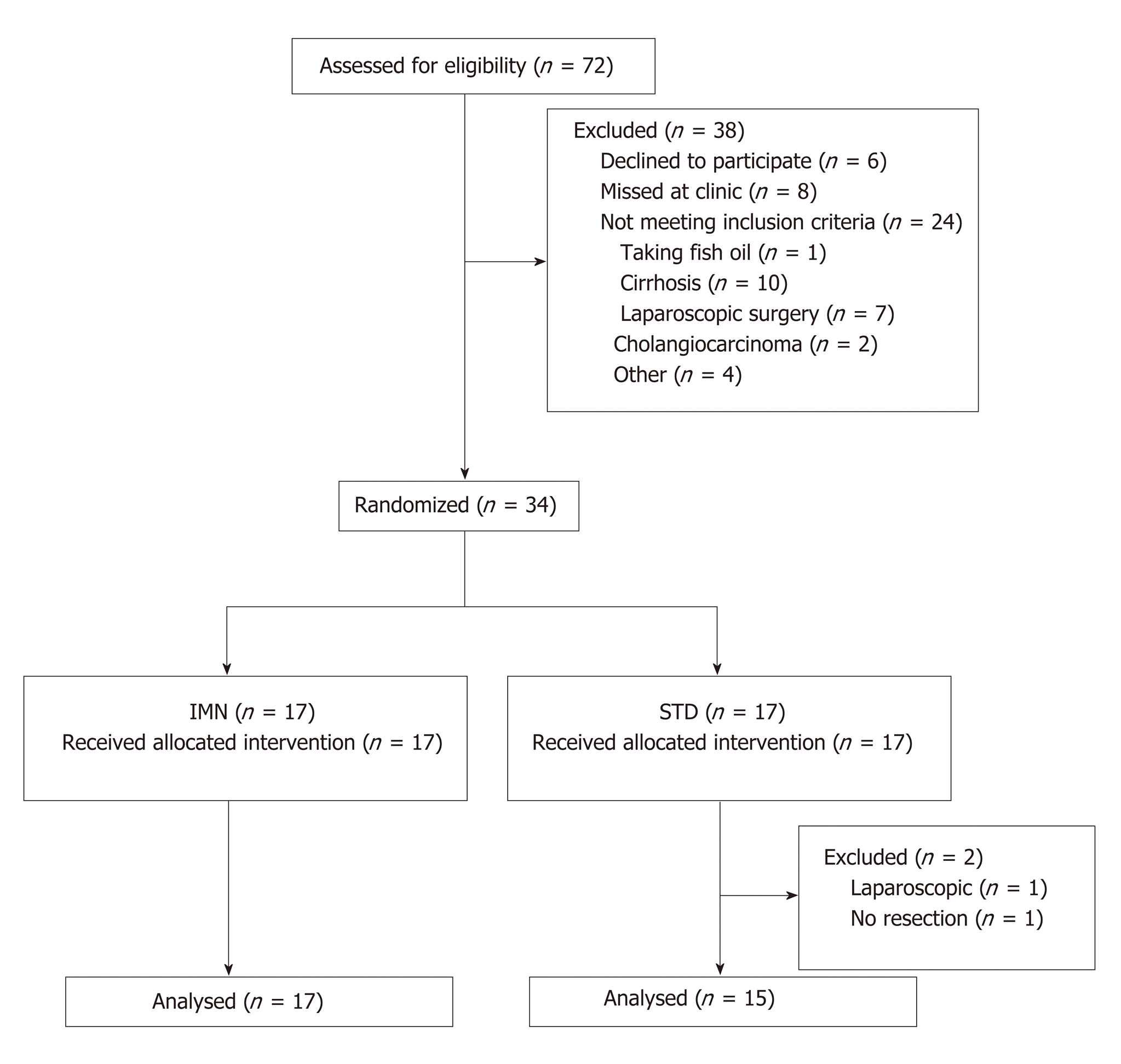
Thirty-four patients were randomized to IMN or STD groups between November 2012 and April 2014 (Figure 1). Two STD patients were withdrawn after randomization, one undergoing laparoscopic instead of open surgery and the other did not proceed to resection because of unexpected disease at laparotomy. Baseline characteristics for the remaining 32 patients are summarised in Table 1.
| Immunonutrition | Standard care | |
| n | 17 | 15 |
| Male/Female | 11/6 | 10/5 |
| Age (yr) | 61 (28 – 76) | 63 (31–79) |
| SGA grade (A/B/C) | 15/1/1 | 13/2/0 |
| Indication for surgery | ||
| Metastatic disease | 15 | 13 |
| Hepatocellular carcinoma | 2 | 1 |
| Granulomatous liver disease | 0 | 1 |
| Hepatectomy | ||
| Major resection ( ≥ 3 segments) | 14 | 10 |
| Minor resection ( < 3 segments) | 3 | 5 |
| ASA grade (I/II/III) | 1/9/6 | 0/8/6 |
| Tissue removed (g) | 815 ± 123 | 610 ± 94 |
| Duration of surgery (min) | 173 (104-337) | 155 (128-246) |
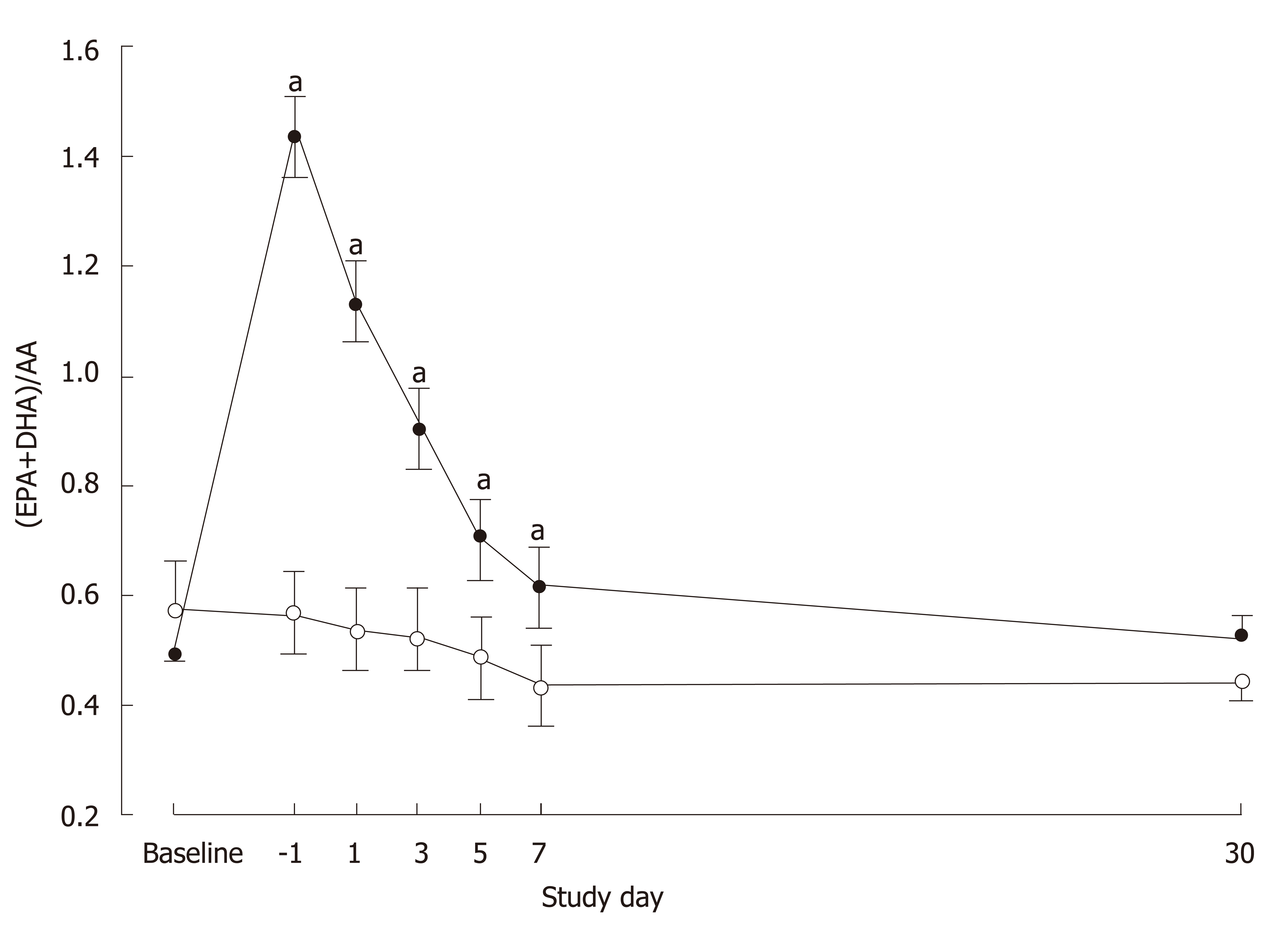
The ratio of EPA + DHA to arachidonic acid differed significantly between the groups over time (P < 0.0001; Figure 2). No difference was seen at baseline (P = 0.36) but the ratio was higher in the IMN group on D-1 (P < 0.0001) and on POD1 (P < 0.0001), POD3 (P < 0.0001), POD5 (P = 0.003) and POD7 (P = 0.014). In the IMN group, compared with the preoperative period, plasma PC EPA+DHA (as percent of total fatty acids) increased more than two-fold.
Baseline dietary assessment showed no significant differences between the groups for consumption of energy (P = 0.27), carbohydrate (P = 0.11), protein (P = 0.37), total fat (P = 0.93), PUFAs (P = 0.47), MUFAs (P = 0.88), or fibre (P = 0.12). Compliance with the full preoperative course of immunonutrition was 100% in 16 out of 17 patients with one patient consuming one less tetra pack than prescribed. As shown in Table 2, changes over time did not differ between the groups for fatigue score (P = 0.342), performance status (P = 0.810) or grip strength (P = 0.849). Compared to D-1, patients were more fatigued on POD7 (P < 0.0001) and performance status (P < 0.0001) and grip strength (P = 0.009) deteriorated. Over the subsequent 3 weeks fatigue and performance improved (P < 0.0001) but no change in grip strength was seen (P = 0.802).
| Study entry | Day -1 | POD 7 | POD 30 | P value1 | |||
| Group | Time | Group×Time | |||||
| Fatigue score | |||||||
| STD group | 4.2 ± 0.8 | 4.0 ± 0.6 | 7.1 ± 0.5 | 5.4 ± 0.7 | 0.121 | < 0.0001 | 0.342 |
| IMN group | 4.3 ± 0.6 | 2.7 ± 0.4 | 6.7 ± 0.5 | 3.5 ± 0.8 | |||
| Performance status | |||||||
| STD group | 84.0 ± 3.9 | 85.7 ± 2.8 | 59.3 ± 4.2 | 73.5 ± 3.9 | 0.867 | < 0.0001 | 0.810 |
| IMN group | 81.9 ± 2.8 | 86.8 ± 3.1 | 57.7 ± 4.8 | 75.0 ± 6.9 | |||
| Grip strength (kg) | |||||||
| STD group | - | 37.8 ± 2.7 | 35.4 ± 2.6 | 33.8 ± 2.3 | 0.825 | 0.019 | 0.849 |
| IMN group | - | 38.9 ± 3.4 | 36.5 ± 3.3 | 33.2 ± 2.4 |
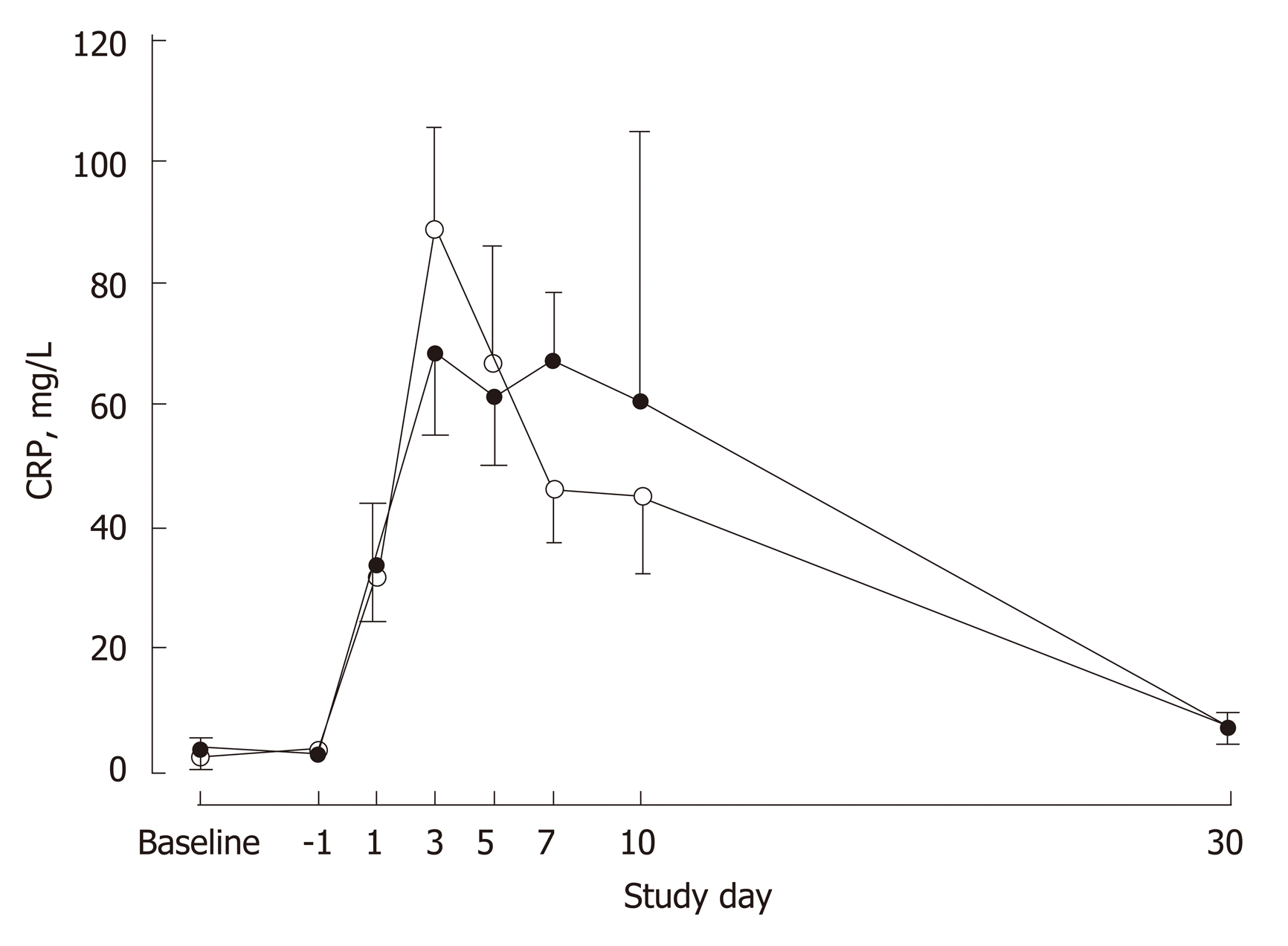
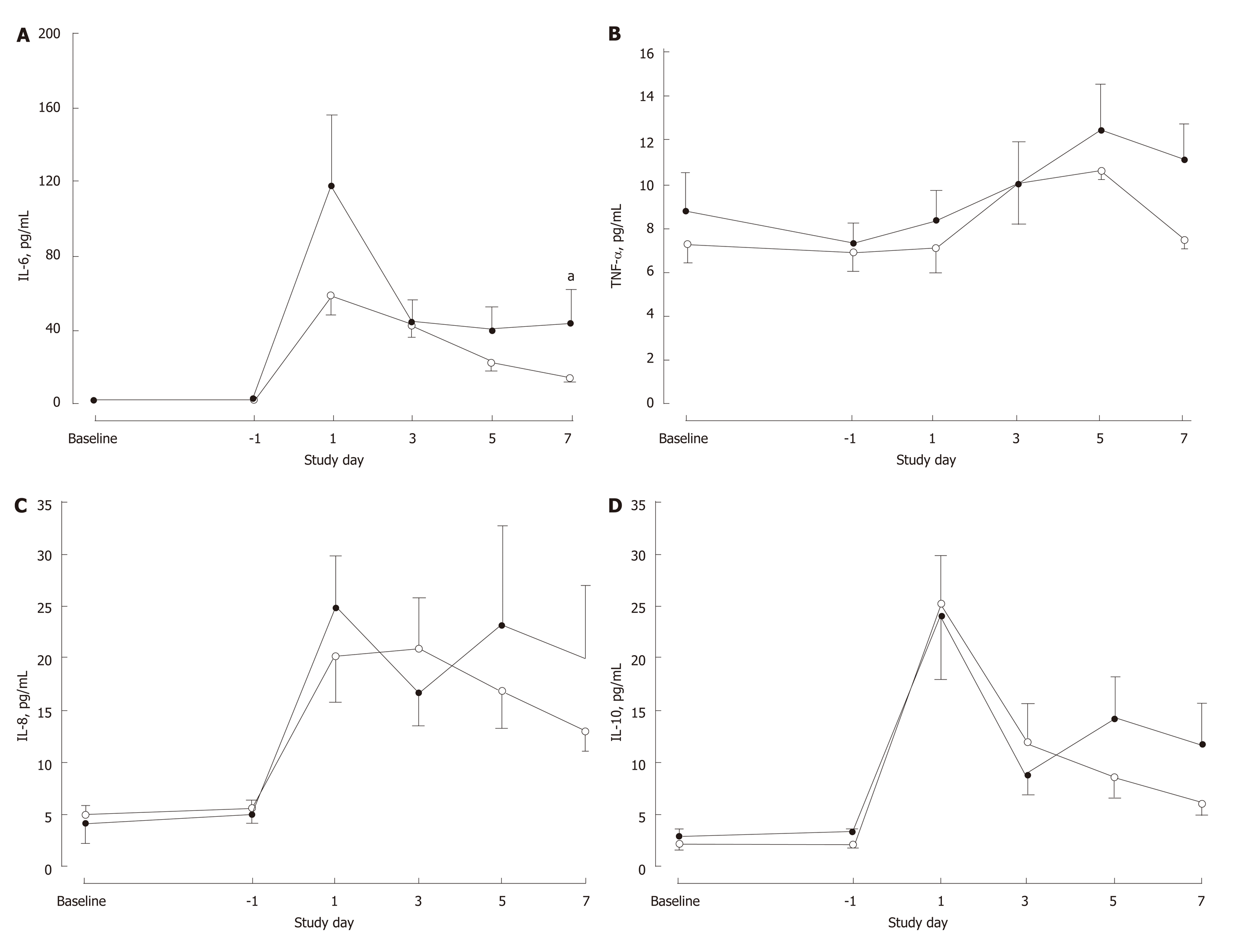
There were no differences in white cell count (P = 0.201) or total lymphocytes (P = 0.575) between the groups over the study period (Table 3). White cell count was elevated and lymphocyte count was depressed over the first 10 postoperative days before returning to preoperative levels by POD30. Plasma concentrations of CRP, TNF-α, IL-6, IL-8 and IL-10 to POD7 are shown in Figures 3 and 4 for the two groups. Except for IL-6 (P = 0.034), there were no significant differences between the groups for the profiles over time for these markers. Circulating IL-6 concentrations were higher in the IMN group on POD7 (P = 0.017) and tended to be higher on POD1 (P = 0.087) and POD5 (P = 0.088). In both groups on POD7, IL-6 concentrations were elevated (P < 0.0001) compared to baseline.
| Preoperative | Postoperative | P value1 | |||||||||
| Study entry | Day-1 | POD 1 | POD 3 | POD 5 | POD 10 | POD 30 | Group | Time | Group×time | ||
| Total lymphocytes (x109/L) | |||||||||||
| IMN group | 1.41 ± 0.13 | 1.54 ± 0.12 | 0.93 ± 0.09 | 0.96 ± 0.10 | 0.95 ± 0.09 | 1.19 ± 0.09 | 1.47 ± 0.13 | 0.091 | < 0.001 | 0.575 | |
| STD group | 1.66 ± 0.16 | 1.78 ± 0.13 | 1.12 ± 0.15 | 1.21 ± 0.09 | 1.07 ± 0.13 | 1.38 ± 0.12 | 1.47 ± 0.10 | ||||
| White cell count (x109/L) | |||||||||||
| IMN group | 5.80 ± 0.60 | 7.05 ± 0.78 | 12.78 ± 1.10 | 9.81 ± 1.29 | 7.40 ± 0.91 | 12.28 ± 2.27 | 6.80 ± 0.86 | 0.416 | < 0.001 | 0.201 | |
| STD group | 6.86 ± 0.83 | 6.75 ± 0.54 | 12.79 ± 1.32 | 8.99 ± 0.77 | 9.65 ± 0.98 | 13.53 ± 1.63 | 7.40 ± 0.68 | ||||
Postoperative complications are summarized in Table 4. Postoperative complications occurred in 12 patients in the IMN group and 11 patients in the STD group (P = 0.598). Ten patients in the IMN group and 4 in the STD group developed infectious complications (P = 0.087). The median length of hospital stay (LOS) was 9 (range 4– 49) d in the IMN group and 8 (3–34) d in the STD group (P = 0.476). Seven patients in the IMN group developed a major postoperative complication (Clavien-Dindo grade ≥III) as did one patient in the STD group (P = 0.047). However, no association was seen between the severity of postoperative complications and whether the surgery was major or minor (Table 5).
| IMN | STD | P value 1 | |
| Infectious complications | |||
| Urinary tract | 3 | 0 | |
| Surgical site | 2 | 2 | |
| Blood stream | 5 | 0 | |
| Gastrointestinal | 2 | 1 | |
| Lower respiratory tract | 3 | 3 | |
| Non Infectious complications | |||
| AF/Bradycardia/Tachycardia | 3 | 2 | |
| Acute kidney injury | 3 | 0 | |
| Aspiration pneumonia | 1 | 0 | |
| Acute respiratory distress syndrome | 1 | 0 | |
| Atelectasis | 7 | 6 | |
| Bowel obstruction | 1 | 3 | |
| Diarrhoea | 1 | 0 | |
| Electrolyte derangement | 4 | 3 | |
| Encephalopathy | 1 | 0 | |
| Hypotension | 2 | 0 | |
| Ileus | 4 | 2 | |
| Ischaemic optic neuropathy | 1 | 0 | |
| Leak | 1 | 0 | |
| Nausea and vomiting | 1 | 2 | |
| Pleural effusion | 5 | 3 | |
| Pain requiring epidural | 2 | 0 | |
| Pneumothorax | 1 | 0 | |
| Non infected collection | 1 | 0 | |
| Wound dehiscence | 1 | 0 | |
| Total infectious complications | 15 | 6 | |
| Total non-infectious complications | 40 | 21 | |
| Total complications | 55 | 27 | |
| Patients with an infectious complication | 10 | 4 | 0.087 |
| Patients with a non-infectious complication | 11 | 11 | 0.445 |
| Patients with any complication | 12 | 11 | 0.598 |
| Severity of complication | 0.047 | ||
| Patients with major complication (Clavien-Dindo Grade ≥ III) | 7 | 1 | |
| Patients with minor complication (Clavien-Dindo Grade < III) | 5 | 10 | |
| Patients without any complication | 5 | 4 |
In this study, preoperative nutritional supplementation enriched in n-3 fatty acids and arginine did not result in suppression of postoperative inflammation compared to standard care. The primary measure of inflammatory response was IL-6 and this marker was persistently elevated in the IMN group, significantly so on POD7, in comparison to the STD group. The pattern of changes for other markers of inflammation did not differ between the groups but also tended to be elevated in the IMN group on POD7. Similarly, there were no differences in immune markers between the groups. While the study was not powered for clinical outcome, the results for infectious complications and length of stay were consistent with a failure of preoperative immunonutrition to dampen the postoperative inflammatory response. The incidence of infectious complications was 59% and 27% in the IMN and STD groups, respectively. Notably, there was a higher incidence of major complications in the IMN group.
These results contrast with those in colorectal, pancreatic and gastric surgery where reductions of around 50% in infectious complication rates and 2-3 d in length of hospital stay were seen in meta-analyses of studies where supplementation with arginine and n-3 fatty acid based formulas were provided only preoperatively[3-5]. Studies included in these meta-analyses that have examined inflammatory and immune responses after preoperative feeding with IMN have reported reduced IL-6 concentrations on POD1[17] and increased total lymphocyte numbers postoperatively[18]. Limited published work is available in patients undergoing liver resection with only two published randomized trials of immunonutrition. Mikagi et al[8] administered IMPACT at 750 kcal/d for 5 d preoperatively in the immunonutrition group. A drop-out rate of 37% was reported with no significant reductions in infectious complications, non-infectious complications or length of hospital stay in the 26 patients analysed. Uno et al[9] carried out an intention to treat analysis on 40 patients and reported a significant reduction in infectious complications, no difference in non-infectious complications and a significant reduction in length of stay. However, case-mix differed markedly from the present study. Patients largely underwent surgery for bile duct carcinoma compared to metastatic colorectal cancer in the current study and the differing pathologies may have contributed to the contrasting results, as infectious complications in their control group were much higher (75%) than in the current study (27%).
Liver resections were included in recently published randomized trials from Hubner et al[19] (28 of 145 patients) and Giger-Pabst et al[20] (11 of 105 patients) but subgroup analyses were not conducted. In a non-randomized, propensity score matched case-control study[21] of 49 patients receiving immunonutrition and 49 controls, most of whom were well nourished, IMPACT was provided preoperatively in the same dose as the current study (3 x 237 mL, 1020 kcal) for 7 d. Definition and grading of severity of complications were identical to the current study and the authors reported no significant difference in infectious complications (38.7% immunonutrition vs. 28.5% control) or median length of stay (10 d in both groups). This evident lack of benefit also extends to liver transplantation where perioperative administration of IMPACT did not result in improved clinical outcome[22].
In contrast to the studies of Mikagi et al[8] and Zacharias et al[21], major hepatectomy was performed in 75% of our patients compared to 8% and 21% in the respective earlier studies. This may account, at least in part, for the higher incidence of major complications in our study (25%) compared to the Zacharias study (11%). The markedly higher number of non-infectious complications in the immunonutrition group in the current study may have been a chance effect rather than a result of the treatment. A larger study is required to confirm this. This finding however may have contributed to the greater and more sustained inflammatory response postoperatively in the immunonutrition group, predisposing those patients to infection. One patient in the IMN group contributed 3 of the 15 infectious and 10 of the 40 non-infectious complications observed in that group having suffered aspiration pneumonia and acute respiratory distress syndrome.
The majority of patients in the current study were well-nourished with two patients in each group assessed as malnourished preoperatively (SGA B + C). Malnourished patients experience significantly longer LOS and more major postoperative complications[23], which are attributed to malnutrition associated immune depression[24]. Consequently, it has been hypothesized that by providing immunonutrition containing key nutrients with the ability to minimise the early inflammatory response to surgery a more substantial benefit may be seen in malnourished patients[25]. If this is the case it might explain, at least in part, the lack of any indication of improved clinical outcomes in the present study. Uno et al[9] did not report nutritional status of their patients and a high prevalence of malnutrition in their cholangiocarcinoma patients may help explain the significant benefits seen with immunonutrition. To date, there are no published meta-analyses evaluating the impact of nutritional status on the treatment effect of immunonutrition. Published meta-analyses have pooled data from randomized trials that include both well-nourished and malnourished patients, with a number of studies not reporting on baseline nutritional status.
Dose and timing of preoperative immunonutrient supplementation are not well-defined. Our protocol conformed to the 500-1000 kcal/d recommended by Waitzberg et al[2] and the consensus guidelines from ASPEN[26] and ESPEN[27]. Giger-Pabst et al[20] investigated the effect of immunonutrition administered for only 3 days preoperatively based on the findings of a prior study suggesting that the anti-inflammatory effect of immunonutrition starts after only two days[28]. However, 3 days of IMPACT supplementation preoperatively was insufficient to provide any benefit in terms of infectious complications or LOS. The authors concluded based on their own findings and review of the literature that at least five days of preoperative supplementation are required to achieve benefit.
Limitations of the current study include its small size, given it was focused on inflammatory and immune parameters rather than clinical outcome. It was also not placebo-controlled and double-blinded, the latter being less important for endpoints based on blood assays. However, there was potential for bias in the reporting of complications, most especially for those occurring after hospital discharge. In-hospital complications were assessed by clinicians who were blinded to the group allocation. After discharge, patients were contacted or assessed at clinic visits to monitor complications over the first postoperative month. However, not all complications may have been captured. Up to 25% of postoperative infections, which are largely surgical wound infections, may occur after discharge[17]. We did not perform dietary assessments during or at the end of the period of nutritional supplementation so cannot comment on any difference in caloric load between the groups prior to surgery and impact on outcome.
Strengths of the study include: measurement of EPA and DHA plasma concentrations which support likely cell membrane incorporation[29] and resultant biological effects[30]; the near-perfect compliance with the immunonutrition product, verified by the EPA+DHA levels in plasma; hospital discharge determination by staff independent of the study; operations performed by the same surgeon using the same surgical technique in all except one patient; and assessment of inflammatory and immune markers in the very early postoperative period (from POD1).
In conclusion, this study failed to show any evidence for suppression of postoperative inflammation or improvement in clinical outcome through providing immunonutrition to well-nourished patients undergoing liver resection. There remains no large scale double-blind trial in liver resection on which to base more definitive conclusions and such a trial, particularly in patients having major resections, is warranted.
Immunonutrients provided pre- and perioperatively to patients undergoing major gastrointestinal surgery have been shown in a number of studies to reduce postoperative morbidity. Nutritional supplementation enriched in n-3 long-chain fatty acids and arginine has been used in the majority of these studies and these nutrients are thought to modulate the inflammatory and immune responses to surgery leading to improved clinical outcome.
We were motivated to design and implement a randomized trial of immunonutrition in patients undergoing liver resection given that only one such prospective trial had previously been reported. That trial had a high dropout rate and we considered further work was needed.
The main objective of this study was to evaluate the effect of preoperative supplemental immunonutrition, enriched in n-3 fatty acids and arginine, on postoperative inflammatory and immune markers. A secondary objective was to examine effects on clinical outcome.
Patients scheduled for non-laparoscopic elective hepatic resection for primary or secondary liver cancer were randomized in an assessor-blinded prospective trial to preoperative immunonutrition (IMPACT Advanced Recovery®, 1020 kcal/d) for 5 consecutive days or to standard care. Blood samples were obtained at recruitment, on the day prior to surgery and on postoperative day (POD) 1, 3, 5 and 7 for measurement of plasma fatty acids and markers of inflammation and immune status. Patients were monitored for 30 POD for infectious and other complications.
Immune markers did not differ between the groups. Postoperative inflammatory response, as assessed by interleukin-6 concentrations, was more pronounced in the immunonutrition group. Ten patients in the immunonutrition group and 4 in the standard care group developed infectious complications. Major postoperative complications were more common in the immunonutrition group.
In this study, provision of a preoperative immunonutritional supplement was not associated with post-surgery suppression of inflammation nor with improved clinical outcomes. The higher incidence of major complications in the immunonutrition group may have contributed to these findings.
Since completion of this study a similar preoperative immunonutrition regime was reported in a randomized trial demonstrating reduced postoperative inflammatory response and improved clinical outcome with immunonutrition. The majority of patients in that study had a relatively rare indication for liver resection. Future efforts should be directed at double-blind trials of immunonutritional formulae, in patients undergoing major liver resections for commonly seen indications, that are adequately powered to assess postoperative infectious complications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abdel-Salam OME, Aoki H, Frena A S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Calder PC. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;98 Suppl 1:S133-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30:1592-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Cerantola Y, Hübner M, Grass F, Demartines N, Schäfer M. Immunonutrition in gastrointestinal surgery. Br J Surg. 2011;98:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012;255:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Gu Y, Guo T, Li Y, Cai H. Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trials. Surg Oncol. 2012;21:e87-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Mikagi K, Kawahara R, Kinoshita H, Aoyagi S. Effect of preoperative immunonutrition in patients undergoing hepatectomy; a randomized controlled trial. Kurume Med J. 2011;58:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Uno H, Furukawa K, Suzuki D, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery. 2016;160:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Burdge GC, Wright P, Jones AE, Wootton SA. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br J Nutr. 2000;84:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Gibson RS, Ferguson EL. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries. HarvestPlus Technical Monograph 8. Washington D.C. : HarvestPlus 2008; . |
| 12. | Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 1951] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 13. | Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg. 1982;69:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Karnofsky DA, Albelman WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634-656. [DOI] [Full Text] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24744] [Article Influence: 1178.3] [Reference Citation Analysis (0)] |
| 16. | Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4717] [Article Influence: 277.5] [Reference Citation Analysis (0)] |
| 17. | Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33:1815-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Hübner M, Cerantola Y, Grass F, Bertrand PC, Schäfer M, Demartines N. Preoperative immunonutrition in patients at nutritional risk: results of a double-blinded randomized clinical trial. Eur J Clin Nutr. 2012;66:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Giger-Pabst U, Lange J, Maurer C, Bucher C, Schreiber V, Schlumpf R, Kocher T, Schweizer W, Krähenbühl S, Krähenbühl L. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition. 2013;29:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Zacharias T, Ferreira N, Carin AJ. Preoperative immunonutrition in liver resection-a propensity score matched case-control analysis. Eur J Clin Nutr. 2014;68:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Plank LD, Mathur S, Gane EJ, Peng SL, Gillanders LK, McIlroy K, Chavez CP, Calder PC, McCall JL. Perioperative immunonutrition in patients undergoing liver transplantation: a randomized double-blind trial. Hepatology. 2015;61:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Krenitsky J. Nutrition and the immune system. AACN Clin Issues. 1996;7:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Barker LA, Gray C, Wilson L, Thomson BN, Shedda S, Crowe TC. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr. 2013;67:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Consensus recommendations from the US summitt on immune-enhancing enteral therapy. JPEN J Parenter Enteral Nutr. 2001;25:S61-S63. [PubMed] |
| 27. | Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P; DGEM (German Society for Nutritional Medicine), Jauch KW, Kemen M, Hiesmayr JM, Horbach T, Kuse ER, Vestweber KH; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 659] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 28. | Giger U, Büchler M, Farhadi J, Berger D, Hüsler J, Schneider H, Krähenbühl S, Krähenbühl L. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery-a randomized controlled pilot study. Ann Surg Oncol. 2007;14:2798-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S-599S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 594] [Article Influence: 45.7] [Reference Citation Analysis (0)] |