Published online Nov 27, 2019. doi: 10.4254/wjh.v11.i11.725
Peer-review started: May 10, 2019
First decision: July 4, 2019
Revised: August 22, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 27, 2019
Processing time: 183 Days and 4.4 Hours
Gastrointestinal symptoms are prevalent in patients with cirrhosis. Cirrhotic patients have a known predilection to delayed gastric emptying compared to those without cirrhosis. However, the contributing factors have not been fully elucidated. Retained gastric food on esophagogastroduodenoscopy (EGD) has been used as a surrogate marker for delayed gastric emptying with reasonably high specificity. Therefore, we hypothesize that the frequency of retained gastric food contents at EGD will be higher in a cirrhotic population compared to a control population without liver disease. Additionally, we hypothesize that increased frequency of gastric food contents will be associated with increased severity of cirrhosis.
To determine the relative frequency of delayed gastric emptying among cirrhotics as compared to non-cirrhotics and to identify associated factors.
We performed a retrospective case-control study of cirrhotic subjects who underwent EGD at an academic medical center between 2000 and 2015. Three hundred sixty-four patients with confirmed cirrhosis, who underwent a total of 1044 EGDs for the indication of esophageal variceal screening or surveillance, were identified. During the same period, 519 control patients without liver disease, who underwent a total of 881 EGDs for the indication of anemia, were identified. The presence of retained food on EGD was used as a surrogate for delayed gastric emptying. The relative frequency of delayed gastric emptying among cirrhotics was compared to non-cirrhotics. Characteristics of patients with and without retained food on EGD were compared using univariable and multivariable logistic regression analysis to identify associated factors.
Overall, 40 (4.5%) patients had evidence of retained food on EGD. Cirrhotics were more likely to have retained food on EGD than non-cirrhotics (9.1% vs 1.4%, P < 0.001). Characteristics associated with retained food on univariable analysis included age less than 60 years (12.6% vs 5.2%, P = 0.015), opioid use (P = 0.004), Child-Pugh class C (24.1% Child-Pugh class C vs 6.4% Child-Pugh class A, P = 0.007), and lower platelet count (P = 0.027). On multivariate logistic regression analysis, in addition to the presence of cirrhosis (adjusted OR = 5.83; 95%CI: 2.32-14.7, P < 0.001), diabetes mellitus (types 1 and 2 combined) (OR = 2.34; 95%CI: 1.08-5.06, P = 0.031), opioid use (OR = 3.08; 95%CI: 1.29-7.34, P = 0.011), and Child-Pugh class C (OR = 4.29; 95%CI: 1.43-12.9, P = 0.01) were also associated with a higher likelihood of food retention on EGD.
Cirrhotics have a higher frequency of retained food at EGD than non-cirrhotics. Decompensated cirrhosis, defined by Child-Pugh class C, is associated with a higher likelihood of delayed gastric emptying.
Core tip: This is the first study to describe the frequency of retained gastric food contents on esophagogastroduodenoscopy (EGD) in a cirrhotic population. Our study reveals that cirrhotic patients are five times more likely to have retained food on EGD than controls. Additionally, this study investigates risk factors for gastric food retention in cirrhosis. Associated factors include age younger than 60, diabetes mellitus, opioid use, thrombocytopenia, and higher Child-Pugh class. A novel finding is the fact that gastric retention is associated with decompensated cirrhosis, as can be elucidated from the association with thrombocytopenia and higher Child-Pugh class.
- Citation: Snell DB, Cohen-Mekelburg S, Weg R, Ghosh G, Buckholz AP, Mehta A, Ma X, Christos PJ, Jesudian AB. Gastric food retention at endoscopy is associated with severity of liver cirrhosis. World J Hepatol 2019; 11(11): 725-734
- URL: https://www.wjgnet.com/1948-5182/full/v11/i11/725.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i11.725
Many patients with cirrhosis report gastrointestinal (GI) symptoms such as abdominal bloating, pain, and belching[1-6]. The prevalence of these symptoms has prompted investigation into abnormalities in GI function in cirrhosis. As suspected, cirrhotic patients have higher rates of gastrointestinal dysmotility, characterized by delayed gastric emptying and prolonged small bowel transit time, compared to those without cirrhosis[1,2,7-19]. While severity of cirrhosis has been associated with worsened small bowel motility, the relationship between gastric emptying and severity of liver disease has not been well established. Although some studies have correlated markers of portal hypertension with delayed gastric emptying[7,9,14,20], those examining the size of esophageal varices[11], variceal pressure[16], and hepatic venous pressure gradient[21], have failed to demonstrate an association with impaired gastric motility.
The presence of retained gastric food on esophagogastroduodenoscopy (EGD) can be used as a surrogate for delayed gastric emptying with a reasonably high specificity[22]. Furthermore, the risk of retained gastric food contents at EGD is higher among patients with severe emptying delays compared to mild or moderate delays[22]. Using this method of evaluating for retained food at EGD, the prevalence of delayed gastric emptying is less than 1% in the general population[23].
Since patients with cirrhosis frequently require EGD for surveillance and treatment of esophageal varices, evaluation for retained gastric food contents at EGD could provide important clinical information in this population. Therefore, we conducted this study to characterize the frequency of retained gastric food contents at EGD in a cirrhotic population compared to a control population without liver disease and to elucidate factors predictive of retained food.
We performed a retrospective case-control study of patients with cirrhosis who had an EGD for screening or surveillance of esophageal varices between 2000 and 2015. Cirrhotic patients who underwent EGD for an indication of screening or surveillance of varices were identified using the endoscopy electronic health record system, ProVation®, and the ICD-9-CM diagnosis codes 571.2, 571.5, or 571.6. A subsequent chart review confirmed a diagnosis of cirrhosis based on physician assessment. Patients younger than 18 years, those with intra-luminal tumor or mechanical bowel obstruction, those with a prior diagnosis of gastroparesis or prior esophageal, gastric or thoracic surgery, and those who had an EGD indication which could confound gastric emptying (food impaction, foreign body, active gastrointestinal bleed, abdominal pain, nausea, vomiting, dyspepsia, bloating, weight loss, early satiety, or post-prandial fullness) were excluded. Retained gastric food was defined as any EGD with retained food documented in the procedure note for a unique patient. A control group who underwent EGD for an indication of anemia was identified using ProVation® and the ICD-9-CM Diagnosis Codes 280.*, 281.*, or 285.9. Subsequent chart review excluded those with any known liver disease based on physician assessment. Anemia was chosen as the indication for EGD in the control group as it is unrelated to gastroparesis or its symptoms.
Demographic, clinical, laboratory, and endoscopic data were collected and managed using Research Electronic Data Capture tools hosted at Weill Cornell Medicine[24]. Demographic information included age, sex, ethnicity, and body mass index. Clinical data included documented symptoms of delayed gastric emptying in the six months preceding EGD (i.e., bloating, nausea/vomiting, early satiety/post-prandial fullness, upper abdominal pain, or weight loss); current or past history of diabetes mellitus type 1, diabetes mellitus type 2, human immunodeficiency virus, neurological disorders (such as parkinsonism, multiple sclerosis, stroke, primary dysautonomias), infiltrative diseases (such as scleroderma or amyloidosis); causes of drug-induced gastroparesis (α-2 adrenergic agonists, tricyclic antidepressants, calcium channel blockers, dopamine agonists, muscarinic cholinergic receptor antagonists, octreotide, glucagon-like peptide-1 agonists, phenothiazines, cyclosporine, and any opioid); and use of prokinetic medications (metoclopramide, domperidone, erythromycin, or cisapride). Cirrhosis-specific details included model for end-stage liver disease score, Child-Pugh score, transient elastography results, liver biopsy results, hepatic venous pressure gradient, history of spontaneous bacterial peritonitis, history of hepatic encephalopathy (along with highest grade noted), history of esophageal varices (along with highest grade noted), history of ascites, history or development of hepatocellular carcinoma, and liver transplantation. Routine blood testing within 3 months of EGD was also obtained, including hemoglobin, platelets, sodium, blood urea nitrogen, creatinine, prothrombin time/international normalized ratio, total bilirubin, albumin, total protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, hemoglobin A1C, thyroid stimulating hormone, and anti-nuclear antibodies. Endoscopic information included total number of endoscopies completed per patient over the study period, maximal point of EGD insertion, endoscopic findings, presence of pyloric stenosis or other evidence of gastric outlet obstruction, interventions performed, presence of solid gastric food contents, qualitative amount of retained gastric contents, visualization during EGD and whether lavage was required, and endoscopic findings on subsequent EGD within one year.
The primary outcome of the study was the frequency of retained gastric solid food contents, as documented in the procedural report by the endoscopist, in patients with cirrhosis undergoing EGD as compared to patients without liver disease undergoing EGD for anemia. Secondary outcomes included the relationship between retained gastric food contents with severity of cirrhosis by Child-Pugh score; and the association between retained gastric food contents with complications of decompensated cirrhosis as defined by presence of esophageal varices, ascites, or hepatic encephalopathy.
Categorical variables were described as frequencies (percentages) and continuous variables as (mean ± SD). Characteristics of patients with and without retained food on EGD were compared using the Kruskall-Wallis test for non-parametric continuous variables and χ2 or Fisher’s exact test for categorical variables, as appropriate. A multivariable logistic regression analysis was performed including co-variates statistically significant on univariable analysis. Statistical significance was defined by a two-tailed P value of less than 0.05. The statistical methods of this study were reviewed by biostatisticians in the Biostatistics, Epidemiology and Research Design Core within the Weill Cornell Clinical and Translational Science Center. Statistical analysis was performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC).
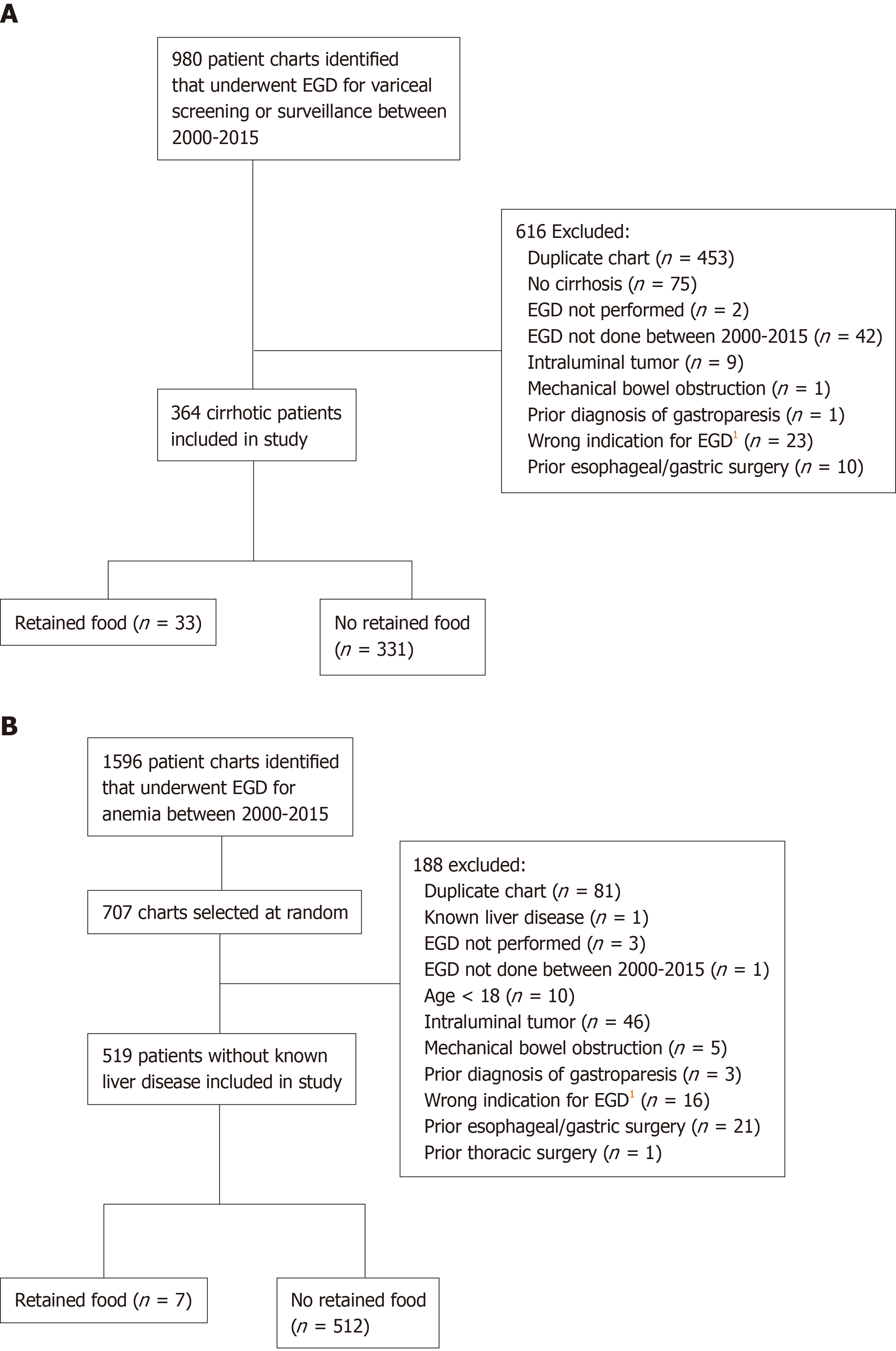
Between 2000 and 2015, 364 patients with confirmed cirrhosis, who underwent a total of 1044 EGDs for the indication of variceal screening or surveillance, were identified. During the same period, 519 control patients without liver disease, who underwent a total of 881 EGDs for the indication of anemia, were identified. Figure 1 shows the subject screening process and application of exclusion criteria.
Table 1 shows the baseline characteristics of these two groups. Cirrhotic patients had a mean age of 56 years as compared to 66 years in non-cirrhotic patients. Patients with cirrhosis were predominantly male (63%) compared to those without known liver disease who were predominantly female (55%). The vast majority of patients in both groups reported at least one upper gastrointestinal symptom within the six months prior to EGD. No patients had evidence of pyloric stenosis or other causes of gastric outlet obstruction on endoscopy. Well-established predisposing factors to gastroparesis, such as diabetes mellitus and opioid use, were similarly present in the two groups. Laboratory values demonstrated expected differences between the cirrhotic group and the non-cirrhotic, anemic group. Overall, 40 (4.5%) patients had evidence of retained food on EGD. Cirrhotics were more likely to have retained food on EGD than non-cirrhotics (9.1% vs 1.4%, adjusted OR = 5.83; 95%CI: 2.32-14.7, P < 0.001).
| Variables | Mean ± SD or % | P value1 | |
| Cirrhosis (n = 364) | No known liver disease (n = 519) | ||
| Age (yr) | 56 ± 11 | 66 ± 18 | < 0.001 |
| Sex | < 0.001 | ||
| Male | 227 (63) | 232 (45) | |
| Female | 133 (37) | 287 (55) | |
| Presence of an upper Gastrointestinal symptom | 357 (98) | 505 (97) | 0.457 |
| Diabetes mellitus type I | 2 (0.6) | 1 (0.2) | 0.572 |
| Diabetes mellitus type II | 112 (31) | 142 (27) | 0.271 |
| HIV | 22 (6) | 17 (3) | 0.049 |
| Neurological disorders | 7 (2) | 59 (11) | 0.001 |
| Infiltrative diseases (scleroderma or amyloidosis) | 7 (2) | 9 (2) | 0.836 |
| Opioid use | 46 (13) | 52 (10) | 0.223 |
| Calcium channel blocker use | 30 (8) | 110 (21) | 0.001 |
| Other gastric anti-kinetic medications | 19 (5) | 58 (11) | 0.002 |
| Prokinetic medications | 1 (0.3) | 3 (0.6) | 0.647 |
| Hemoglobin (g/L) | 129 ± 22 | 106 ± 22 | < 0.001 |
| Platelets (x 109/L) | 97 ± 50 | 230 ± 87 | < 0.001 |
| Creatinine (µmol/L) | 76 ± 21 | 83 ± 29 | < 0.001 |
| PT/INR | 1.2 ± 0.1 | 1.1 ± 0.1 | < 0.001 |
| Total Bilirubin (µmol/L) | 20.5 ± 13.7 | 10.3 ± 3.4 | < 0.001 |
| Albumin (g/L) | 34 ± 7 | 37 ± 6 | < 0.001 |
| AST (IU/L) | 58 ± 36 | 23 ± 7 | < 0.001 |
| ALT (IU/L) | 39 ± 31 | 18 ± 8 | < 0.001 |
| Hemoglobin A1C | 6.0 ± 1.3 | 6.2 ± 0.8 | 0.006 |
| TSH (mU/L) | 2.30 ± 0.40 | 3.47 ± 1.67 | 0.304 |
Table 2 demonstrates the results of univariate analysis of the relationship between patient characteristics and the presence or absence of gastric food retention. Age younger than 60 years was associated with retained food (12.6% vs 5.2%, P = 0.015). Diabetes mellitus types 1 and 2 showed a trend towards a significant association with retained food (P = 0.066). Opioid use was associated with retained food on EGD (P = 0.004). More severe thrombocytopenia, a marker of worse portal hypertension, was also associated with the presence of retained food (P = 0.027). Although no complications of decompensated cirrhosis were shown to be significantly associated, the presence of esophageal varices did show a trend towards significance (P = 0.084). On the other hand, severity of Child-Pugh class was associated with retained food on EGD (P = 0.007).
| Retained food (n = 33) | No retained food (n = 331) | P value1 | |
| Age group | 0.015 | ||
| < 60 yr | 12.6% | 87.4% | |
| ≥ 60 yr | 5.2% | 94.8% | |
| Sex | 0.942 | ||
| Male | 9.3% | 90.7% | |
| Female | 9.0% | 91.0% | |
| Diabetes Mellitus type I or II | 13.2% | 86.8% | 0.066 |
| Opioid use | 21.7% | 78.3% | 0.004 |
| Calcium channel blocker use | 16.7% | 83.3% | 0.173 |
| Child-Pugh class | 0.007 | ||
| A | 6.4% | 93.6% | |
| B | 10.3% | 89.7% | |
| C | 24.1% | 75.9% | |
| Alcoholic cirrhosis | 13.0% | 87.0% | 0.201 |
| Nonalcoholic steatohepatitis with cirrhosis | 4.3% | 95.7% | 0.406 |
| Lower platelet count (continuous) | 0.027 | ||
| Portal hypertensive gastropathy | 10.9% | 89.1% | 0.292 |
| Gastric varices | 5.6% | 94.4% | 0.758 |
| Esophageal varices | 11.0% | 89.0% | 0.084 |
| Hepatic encephalopathy | 13.6% | 86.4% | 0.118 |
| Ascites | 10.6% | 89.4% | 0.471 |
| SBP | 13.3% | 86.7% | 0.640 |
| Presence of an upper gastrointestinal symptom | 12.9% | 87.1% | 0.248 |
On multivariate logistic regression analysis, in addition to the presence of cirrhosis (adjusted OR = 5.83; 95%CI: 2.32-14.7, P < 0.001), diabetes mellitus (types 1 and 2 combined) (OR = 2.34; 95%CI: 1.08-5.06, P = 0.031), opioid use (OR = 3.08; 95%CI: 1.29-7.34, P = 0.011), and Child-Pugh class C (OR = 4.29; 95%CI: 1.43-12.9, P = 0.01) were also associated with a higher likelihood of food retention on EGD (Table 3).
| Adjusted odds ratio (95%CI) | P value1 | |
| Age | ||
| < 60 yr | ref | |
| ≥ 60 yr | 0.49 (0.21-1.14) | 0.098 |
| Diabetes | 2.34 (1.08-5.06) | 0.031 |
| Opioid use | 3.08 (1.29-7.34) | 0.011 |
| Child-Pugh class | ||
| A | ref | |
| B | 1.43 (0.62-3.28) | 0.403 |
| C | 4.29 (1.43-12.9) | 0.010 |
| Platelet count | 0.99 (0.99-1.00) | 0.117 |
This study is the first to describe the frequency of retained gastric food contents visualized on EGD in a cirrhotic population. Our study reveals that cirrhotic patients are five times more likely to have retained food on EGD than controls. In addition, more decompensated cirrhosis was associated with a higher likelihood of gastric food contents at EGD.
Cirrhosis has been associated with increased nitric oxide (NO) production, gut hormonal alterations, and autonomic neuropathy that can impact gastrointestinal motility[3]. Gut hormonal alterations related to insulin resistance, including hyperglycemia, hyperinsulinemia, and hypoghrelinemia can play a prominent role in the pathophysiology of delayed gastric emptying in patients with cirrhosis[17]. Portal hypertension has also been implicated as a potential mechanism given decreased postprandial portal blood flow resulting in congestion of the gastric wall as well as impaired antral compliance and motility[3]. Prolonged gastric emptying has been demonstrated in 24%-95% of patients with cirrhosis and upper gastrointestinal symptoms not attributable to other causes[1,2,4,5]. These often vague upper GI symptoms have been shown to contribute significant morbidity in the cirrhotic population through malnutrition[4], small intestinal bacterial overgrowth[4], psychological distress[6], and reduced health related quality of life measures[6].
The factors associated with gastric retention in the study population include age younger than 60, diabetes mellitus, opioid use, thrombocytopenia, and higher Child-Pugh class. Opioid use and diabetes mellitus are well described risk factors for gastroparesis. The association of diabetes with delayed gastric emptying lends further support to the role of insulin resistance in the pathogenesis of gastroparesis in cirrhotic patients, as previously described in Kalaitzakis et al[17]. Regarding the association of age and gastroparesis, it is unclear why gastric food retention was associated with younger age. Given that young age was associated with delayed gastric emptying on univariate analysis but not multivariate analysis, there are likely confounding factors at play. A novel finding is the fact that gastric retention is associated with decompensated cirrhosis as can be elucidated from the association with thrombocytopenia and higher Child-Pugh class. Additionally, there was a trend towards significance with the presence of esophageal varices that further supports an association between severity of cirrhosis, portal hypertension, and gastroparesis. Prior studies evaluating the association between severity of cirrhosis and gastroparesis have shown mixed results. The majority of studies have shown no association between severity of cirrhosis and gastroparesis[2,5,12,13,16,25]. However, the correlation between severity of cirrhosis and delayed gastric emptying seen in this study is similar to two previous studies[1,14]. Gumurdulu et al[1] demonstrated that Child-Pugh class correlated with delayed gastric emptying, as measured by scintigraphy, and Miyajima et al[14] concluded a similar association using measurements of autonomic function and portal blood flow via MRI. Despite the different methodologies used in those studies and the present study, the similar conclusions lend further credence to the results of the current study.
This study has several limitations. Given its retrospective non-interventional nature, no conclusions can be drawn regarding causality. Future studies should consider prospectively recruiting patients to confirm these results, though time constraints might make prospective recruitment and longitudinal follow-up difficult. Since this is a single center study at an academic center, the results may also lack generalizability. Additionally, the presence of retained food on EGD is not the gold standard method for diagnosing gastroparesis; but, there exists strong evidence for correlation[22].
In conclusion, we demonstrate that cirrhotic subjects have a higher likelihood of delayed gastric emptying than non-cirrhotics, particularly in those with decompensation of their liver disease. Providers who care for cirrhotic patients should have a high index of suspicion for symptoms related to delayed gastric emptying, a condition which is vastly underrecognized in this patient group. Ultimately, a prospectively validated prediction tool would be useful for the detection of impaired gastric motility in cirrhotic patients. Future studies should evaluate the effect of delayed gastric emptying on patient reported outcomes, quality of life and health care utilization.
Many patients with cirrhosis report gastrointestinal (GI) symptoms such as abdominal bloating, pain, and belching. Cirrhosis has been associated with increased nitric oxide (NO) production, gut hormonal alterations, and autonomic neuropathy that can impact gastrointestinal motility. Portal hypertension has also been implicated as a potential mechanism given decreased postprandial portal blood flow resulting in congestion of the gastric wall as well as impaired antral compliance and motility. Prolonged gastric emptying has been demonstrated in 24%-95% of patients with cirrhosis and upper gastrointestinal symptoms not attributable to other causes. These usual vague upper GI symptoms have been shown to contribute significant morbidity in the cirrhotic population through malnutrition, small intestinal bacterial overgrowth, psychological distress, and reduced health related quality of life measures.
The prevalence of GI symptoms has prompted investigation into abnormalities in GI function in cirrhosis. Cirrhotic patients have higher rates of gastrointestinal dysmotility, characterized by delayed gastric emptying and prolonged small bowel transit time, compared to those without cirrhosis. While severity of cirrhosis has been associated with worsened small bowel motility, the relationship between gastric emptying and severity of liver disease has not been well established. The mechanisms for gastrointestinal dysmotility in cirrhosis are also not fully understood. Although some studies have correlated markers of portal hypertension with delayed gastric emptying, those examining the size of esophageal varices, variceal pressure, and hepatic venous pressure gradient, have failed to demonstrate an association with impaired gastric motility. Examination of the risk factors for delayed gastric emptying in patients with cirrhosis could provide further insight into the underlying pathophysiology and could help identify patients who may benefit from therapeutic interventions aimed at improving gastric motility.
The presence of retained gastric food on esophagogastroduodenoscopy (EGD) can be used as a surrogate for delayed gastric emptying with a reasonably high specificity. Since patients with cirrhosis frequently require EGD for surveillance and treatment of esophageal varices, evaluation for retained gastric food contents at EGD could provide important clinical information in this population. Therefore, we conducted this study to characterize the frequency of retained gastric food contents at EGD in a cirrhotic population compared to a control population without liver disease and to elucidate factors predictive of retained food. Specifically, we examined the relationship between retained gastric food contents with severity of cirrhosis by Child-Pugh score; and the association between retained gastric food contents with complications of decompensated cirrhosis as defined by the presence of esophageal varices, ascites, or hepatic encephalopathy.
We performed a retrospective case-control study of patients with cirrhosis who had an EGD for screening or surveillance of esophageal varices between 2000 and 2015 at an academic medical center. Patients younger than 18 years, those with intra-luminal tumor or mechanical bowel obstruction, those with a prior diagnosis of gastroparesis or prior esophageal, gastric or thoracic surgery, and those who had an EGD indication which could confound gastric emptying (food impaction, foreign body, active gastrointestinal bleed, abdominal pain, nausea, vomiting, dyspepsia, bloating, weight loss, early satiety, or post-prandial fullness) were excluded. A control group who underwent EGD for an indication of anemia was identified as anemia is unrelated to gastroparesis or its symptoms. Three hundred sixty-four patients with confirmed cirrhosis, who underwent a total of 1044 EGDs for the indication of esophageal variceal screening or surveillance, were identified. During the same period, 519 control patients without liver disease, who underwent a total of 881 EGDs for the indication of anemia, were identified. The presence of retained food on EGD was used as a surrogate for delayed gastric emptying. The relative frequency of delayed gastric emptying among cirrhotics was compared to non-cirrhotics. Characteristics of patients with and without retained food on EGD were compared using the Kruskall-Wallis test for non-parametric continuous variables and χ2 or Fisher’s exact test for categorical variables, as appropriate. A multivariable logistic regression analysis was performed including co-variates statistically significant on univariable analysis. Statistical significance was defined by a two-tailed P value of less than 0.05.
Overall, 40 (4.5%) patients had evidence of retained food on EGD. Cirrhotics were more likely to have retained food on EGD than non-cirrhotics (9.1% vs 1.4%, OR = 5.83; 95%CI: 2.32-14.7, P < 0.001). Characteristics associated with retained food on univariable analysis included age less than 60 years (12.6% vs 5.2%, P = 0.015), opioid use (P = 0.004), Child-Pugh class C (24.1% Child-Pugh class C vs 6.4% Child-Pugh class A, P = 0.007), and lower platelet count (P = 0.027). Diabetes mellitus showed a trend towards a significant association with retained food (P = 0.066). Although no complications of decompensated cirrhosis were shown to be significantly associated, the presence of esophageal varices did show a trend towards significance (P = 0.084). On multivariate logistic regression analysis, in addition to the presence of cirrhosis, diabetes mellitus (types 1 and 2 combined) (OR = 2.34; 95%CI: 1.08-5.06, P = 0.031), opioid use (OR = 3.08; 95%CI: 1.29-7.34, P = 0.011), and Child-Pugh class C (OR = 4.29; 95%CI: 1.43-12.9, P = 0.01) were also associated with a higher likelihood of food retention on EGD.
This study is the first to describe the frequency of retained gastric food contents visualized on EGD in a cirrhotic population. Our study reveals that cirrhotic patients are five times more likely to have retained food on EGD than controls. In addition, more decompensated cirrhosis was associated with a higher likelihood of gastric food contents at EGD. The factors associated with gastric retention in the study population include age younger than 60, diabetes mellitus, opioid use, thrombocytopenia, and higher Child-Pugh class. Opioid use and diabetes mellitus are well described risk factors for gastroparesis. A novel finding is the fact that gastric retention is associated with decompensated cirrhosis as can be elucidated from the association with thrombocytopenia and higher Child-Pugh class. Additionally, there was a trend towards significance with the presence of esophageal varices that further supports an association between severity of cirrhosis, portal hypertension, and gastroparesis. Prior studies evaluating the association between severity of cirrhosis and gastroparesis have shown mixed results. However, the correlation between severity of cirrhosis and delayed gastric emptying seen in this study is similar to two previous studies. Gumurdulu et al demonstrated that Child-Pugh class correlated with delayed gastric emptying, as measured by scintigraphy, and Miyajima et al concluded a similar association using measurements of autonomic function and portal blood flow via MRI. Despite the different methodologies used in those studies and the present study, the similar conclusions lend further credence to the results of the current study. Clinicians should have a higher index of suspicion for upper GI symptoms related to dysmotility in those with more decompensated cirrhosis, so that these patients can undergo timely diagnosis and treatment.
We demonstrate that cirrhotic subjects have a higher likelihood of delayed gastric emptying than non-cirrhotics, particularly in those with decompensation of their liver disease. Future studies should consider prospectively recruiting patients in multiple centers to confirm these results, though time constraints might make prospective recruitment and longitudinal follow-up difficult. Additionally, since the presence of retained food on EGD is not the gold standard method for diagnosing gastroparesis, prospective studies could utilize gastric scintigraphy, which remains the gold standard for diagnosis. Providers who care for cirrhotic patients should have a high index of suspicion for symptoms related to delayed gastric emptying, a condition which is vastly underrecognized in this patient group. Ultimately, a prospectively validated prediction tool would be useful for the detection of impaired gastric motility in cirrhotic patients. Future studies should evaluate the effect of delayed gastric emptying on patient reported outcomes, quality of life and health care utilization.
The authors gratefully acknowledge the Clinical and Translational Science Center at Weill Cornell Medicine for their support in research design and biostatistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Campollo O, Coskun A, Saligram S, Vorobjova T S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
| 1. | Gumurdulu Y, Yapar Z, Canataroglu A, Serin E, Gumurdulu D, Kibar M, Colakoglu S. Gastric emptying time and the effect of cisapride in cirrhotic patients with autonomic neuropathy. J Clin Gastroenterol. 2003;36:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Verne GN, Soldevia-Pico C, Robinson ME, Spicer KM, Reuben A. Autonomic dysfunction and gastroparesis in cirrhosis. J Clin Gastroenterol. 2004;38:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Theocharidou E, Dhar A, Patch D. Gastrointestinal Motility Disorders and Their Clinical Implications in Cirrhosis. Gastroenterol Res Pract. 2017;2017:8270310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kalaitzakis E, Simrén M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Björnsson E. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 5. | Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Björnsson E. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Fritz E, Hammer J. Gastrointestinal symptoms in patients with liver cirrhosis are linked to impaired quality of life and psychological distress. Eur J Gastroenterol Hepatol. 2009;21:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Galati JS, Holdeman KP, Dalrymple GV, Harrison KA, Quigley EM. Delayed gastric emptying of both the liquid and solid components of a meal in chronic liver disease. Am J Gastroenterol. 1994;89:708-711. [PubMed] |
| 8. | Isobe H, Sakai H, Satoh M, Sakamoto S, Nawata H. Delayed gastric emptying in patients with liver cirrhosis. Dig Dis Sci. 1994;39:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Pimpo MT, Frieri G, Saltarelli P, Ciccocioppo R, Aggio A, Marchetti G, Taddei G, Carlei F, Lygidakis NJ, Onori L. Effects of cisapride on abnormally prolonged endogastric alkalinity time and delayed gastric emptying in cirrhotic patients. Hepatogastroenterology. 1996;43:1678-1684. [PubMed] |
| 10. | Tsai SC, Kao CH, Huang CK, Wang SJ, Chen GH. Abnormal gastric emptying in patients with liver cirrhosis. Kaohsiung J Med Sci. 1996;12:285-289. [PubMed] |
| 11. | Acalovschi M, Dumitraşcu DL, Csakany I. Gastric and gall bladder emptying of a mixed meal are not coordinated in liver cirrhosis--a simultaneous sonographic study. Gut. 1997;40:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Usami A, Mizukami Y, Onji M. Abnormal gastric motility in liver cirrhosis: roles of secretin. Dig Dis Sci. 1998;43:2392-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Chang CS, Kao CH, Yeh HZ, Lien HC, Chen GH, Wang SJ. Helicobacter pylori infection and gastric emptying in cirrhotic patients with symptoms of dyspepsia. Hepatogastroenterology. 1999;46:3166-3171. [PubMed] |
| 14. | Miyajima H, Nomura M, Muguruma N, Okahisa T, Shibata H, Okamura S, Honda H, Shimizu I, Harada M, Saito K, Nakaya Y, Ito S. Relationship among gastric motility, autonomic activity, and portal hemodynamics in patients with liver cirrhosis. J Gastroenterol Hepatol. 2001;16:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ishizu H, Shiomi S, Kawamura E, Iwata Y, Nishiguchi S, Kawabe J, Ochi H. Gastric emptying in patients with chronic liver diseases. Ann Nucl Med. 2002;16:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sadik R, Abrahamsson H, Björnsson E, Gunnarsdottir A, Stotzer PO. Etiology of portal hypertension may influence gastrointestinal transit. Scand J Gastroenterol. 2003;38:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Kalaitzakis E. Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol. 2014;20:14686-14695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Sato M, Chiba T, Kudara N, Takikawa Y, Suzuki K. Gastric motility and emptying in cirrhotic patients with portal hypersensitive gastropathy. Hepatogastroenterology. 2012;59:1464-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chander Roland B, Garcia-Tsao G, Ciarleglio MM, Deng Y, Sheth A. Decompensated cirrhotics have slower intestinal transit times as compared with compensated cirrhotics and healthy controls. J Clin Gastroenterol. 2013;47:888-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Günal O, Yeğen C, Aktan AO, Yalin R, Yeğen BC. Gastric functions in portal hypertension. Role of endothelin. Dig Dis Sci. 1996;41:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Madsen JL, Brinch K, Hansen EF, Fuglsang S. Gastrointestinal motor function in patients with portal hypertension. Scand J Gastroenterol. 2000;35:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Coleski R, Baker JR, Hasler WL. Endoscopic Gastric Food Retention in Relation to Scintigraphic Gastric Emptying Delays and Clinical Factors. Dig Dis Sci. 2016;61:2593-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Watanabe H, Adachi W, Koide N, Yazawa I. Food residue at endoscopy in patients who have previously undergone distal gastrectomy: risk factors and patient preparation. Endoscopy. 2003;35:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36737] [Article Influence: 2296.1] [Reference Citation Analysis (0)] |
| 25. | Schoonjans R, Van Vlem B, Vandamme W, Van Vlierberghe H, Van Heddeghem N, Van Biesen W, Mast A, Sas S, Vanholder R, Lameire N, De Vos M. Gastric emptying of solids in cirrhotic and peritoneal dialysis patients: influence of peritoneal volume load. Eur J Gastroenterol Hepatol. 2002;14:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |