Published online Sep 27, 2018. doi: 10.4254/wjh.v10.i9.629
Peer-review started: May 3, 2018
First decision: May 24, 2018
Revised: July 20, 2018
Accepted: August 28, 2018
Article in press: August 28, 2018
Published online: September 27, 2018
Processing time: 152 Days and 11.4 Hours
To systematically review liver disease associated with hemophagocytic lymphohistiocytosis (HLH), propose reasonable contraindications for liver transplantation for liver failure in HLH, and report an illustrative case.
Systematic review according to PRISMA guidelines of hepatic manifestations of HLH using computerized literature search via PubMed of articles published since 1980 with keywords (“hemophagocytic lymphohistiocytosis” or “HLH”) AND (“liver” or “hepatic”). Two authors independently performed literature search and incorporated articles into this review by consensus. Illustrative case report presented based on review of medical chart, and expert re-review of endoscopic photographs, radiologic images, and pathologic slides.
A 47-year-old Caucasian male, was hospitalized with high-grade pyrexia, rash, total bilirubin = 45 g/dL, moderately elevated hepatic transaminases, ferritin of 3300 ng/dL, leukopenia, and profound neutropenia (absolute neutrophil count < 100 cells/mm³). Viral serologies for hepatitis A, B, and C were negative. Abdominal computed tomography scan and magnetic resonance imaging revealed no hepatic or biliary abnormalities. Pathologic analysis of liver biopsy revealed relatively well-preserved hepatic parenchyma without lymphocytic infiltrates or macrophage invasion, except for sparse, focal hepatocyte necrosis. Bone marrow biopsy and aspirate revealed foamy macrophages engulfing mature and precursor erythrocytes, consistent with HLH. Interleukin-2 receptor (CD25) was highly elevated, confirming diagnosis of HLH according to Histiocytic Society criteria. Patient initially improved after high-dose prednisone therapy. Patient was judged not to be a liver transplant candidate despite model for end stage liver disease (MELD) score = 33 because liver failure was secondary to severe systemic disease from HLH, including septic shock, focal centrilobular hepatocyte necrosis from hypotension, bone marrow failure, and explosive immune activation from HLH. The patient eventually succumbed to overwhelming sepsis, progressive liver failure, and disseminated intravascular coagulopathy. Systematic review reveals liver injury is very common in HLH, and liver failure can sometimes occur. Data on liver transplantation for patients with HLH are very limited, and so far the results have shown a generally much worse prognosis than for other liver transplant indications. Liver transplantation should not be guided solely by MELD score, but should include liver biopsy results and determination whether liver failure is from intrinsic liver injury vs multisystem (extrahepatic) organ failure from HLH.
This case report illustrates that liver transplantation may not be warranted when liver failure associated with HLH is primarily from multisystem failure from HLH. Liver biopsy may be very helpful in determining the severity and pathophysiology of the liver disease.
Core tip: This work systematically reviews liver disease in hemophagocytic lymphohistiocytosis (HLH), proposes contraindications for liver-transplantation in such patients, and reports an illustrative case. A 47-year-old-man was hospitalized with high-grade pyrexia, total-bilirubin = 45 g/dL, and profound neutropenia. Bone marrow biopsy/aspirate revealed hemophagocytic histiocytes. The patient had HLH, satisfying 6 Histiocytic Society criteria. Liver-biopsy histopathology revealed relatively well-preserved hepatic parenchyma, except for sparse hepatocytic necrosis. The patient was not a liver-transplant-candidate, despite model for end stage liver disease-score = 33, due to multi-organ failure from HLH. He expired from multi-organ failure despite receiving corticosteroids. This case and systematic review reveals patients with HLH may not be liver transplant candidates because of liver failure from multisystem failure.
- Citation: Cappell MS, Hader I, Amin M. Acute liver failure secondary to severe systemic disease from fatal hemophagocytic lymphohistiocytosis: Case report and systematic literature review. World J Hepatol 2018; 10(9): 629-636
- URL: https://www.wjgnet.com/1948-5182/full/v10/i9/629.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i9.629
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome of aggressive immune hyperactivation from hypercytokinemia that frequently causes liver injury and sometimes causes acute liver failure (ALF) which can contribute to the high syndromic mortality. Systematic literature review revealed hundreds of reported cases of HLH in adults, but provides insufficient data on indications and contraindications for liver transplantation for ALF associated with HLH[1]. Liver transplantation for ALF associated with HLH is currently controversial due to prominent systemic morbidities from HLH, the generally poor condition of patients suffering from both ALF and HLH, potential curability of ALF from HLH with HLH-specific therapy alone, and risk of recurrent HLH after liver transplantation[2,3]. A case is reported of fatal HLH in an adult presenting prominently with ALF, with the liver injury primarily due to severe systemic disease from HLH, as documented by liver biopsy and clinical evaluation, and liver transplantation was refused on this basis. This case report is important in illustrating potential pitfalls in liver transplantation for ALF associated with HLH.
This case was thoroughly reviewed based on medical chart, including re-review of original endoscopic photographs by an expert endoscopist, radiologic images by an expert radiologist, and pathologic slides by an expert pathologist. The IRB at William Beaumont Hospital, Royal Oak exempted/approved this case report on November 15, 2017.
Literature on hepatic manifestations of HLH was systematically reviewed searching PubMed for articles with the following medical subject headings/keywords (“hemophagocytic lymphohistiocytosis” or “HLH”) AND (“liver” or hepatic”), and by reviewing sections on HLH in standard pathology textbooks/monographs. Articles before 1980 were selectively excluded because clinical evaluations before 1980 often lacked currently required clinical tests. Large clinical trials, meta-analyses, systematic reviews, and controlled trials were assigned higher priority than review articles or small clinical series, and individual case reports were assigned the lowest priority. Two authors independently performed a literature search, and decided on which articles to incorporate in this review according to article priority based on consensus. Dr. Cappell has considerable experience in conducting systematic reviews, with 4 published systematic reviews in peer-reviewed journals indexed in PubMed during the last 2 years, and with a PhD in neurophysiology that involved 5 years of training and research in biomedical statistics.
A 47-year-old, non-alcoholic, non-obese, Caucasian man with type II diabetes mellitus treated with oral glipizide and metformin for 4 years, and no prior liver disease presented with a pruritic, maculopapular rash over his entire body associated with progressive fatigue, pyrexia, and profound jaundice for 7 d that began just after completing a 10-d course of oral trimethoprim-sulfamethoxazole therapy for an upper respiratory infection. Physical examination on admission revealed temperature = 39.5 °C, blood pressure = 89/48 mmHg, profound jaundice, no abdominal tenderness, no hepatosplenomegaly, no peripheral stigmata of chronic liver disease, and no peripheral lymphadenopathy. Laboratory values included: Leukocyte count = 700/µL (lab normal: 3500-10100/µL), and platelets = 283000/µL (normal: 150000-400000/µL). The sodium = 116 mmol/L (normal: 135-145 mmol/L), and creatinine = 1.67 mg/dL (normal: 0.60-1.40 mg/dL). Total bilirubin = 45.1 mg/dL (normal: 0.3-1.2 mg/dL), direct bilirubin = 26.4 mg/dL (normal: 0.0-0.3 mg/dL), alkaline phosphatase = 490 U/L (normal: 30-110 U/L), aspartate aminotransferase (AST) = 70 U/L (normal: 10-37 U/L), alanine aminoranferase (ALT) = 167 U/L (normal: 9-47 U/L), gamma glutamyl transferase = 730 U/L (normal: 13-60 U/L), albumin = 2.4 g/dL (normal: 3.5-5.1 g/dL), international normalized ratio (INR) = 1.9 (normal: 0.9-1.1), and lipase = 9 U/L (normal: 7-60 U/L). The cholesterol = 661 mg/dL (normal: 70-199 mg/dL), triglyceride = 184 mg/dL (normal: 30-149 mg/dL), and HDL cholesterol = 5 mg/dL (normal: 40-90 mg/dL). Serum IgG level was normal.
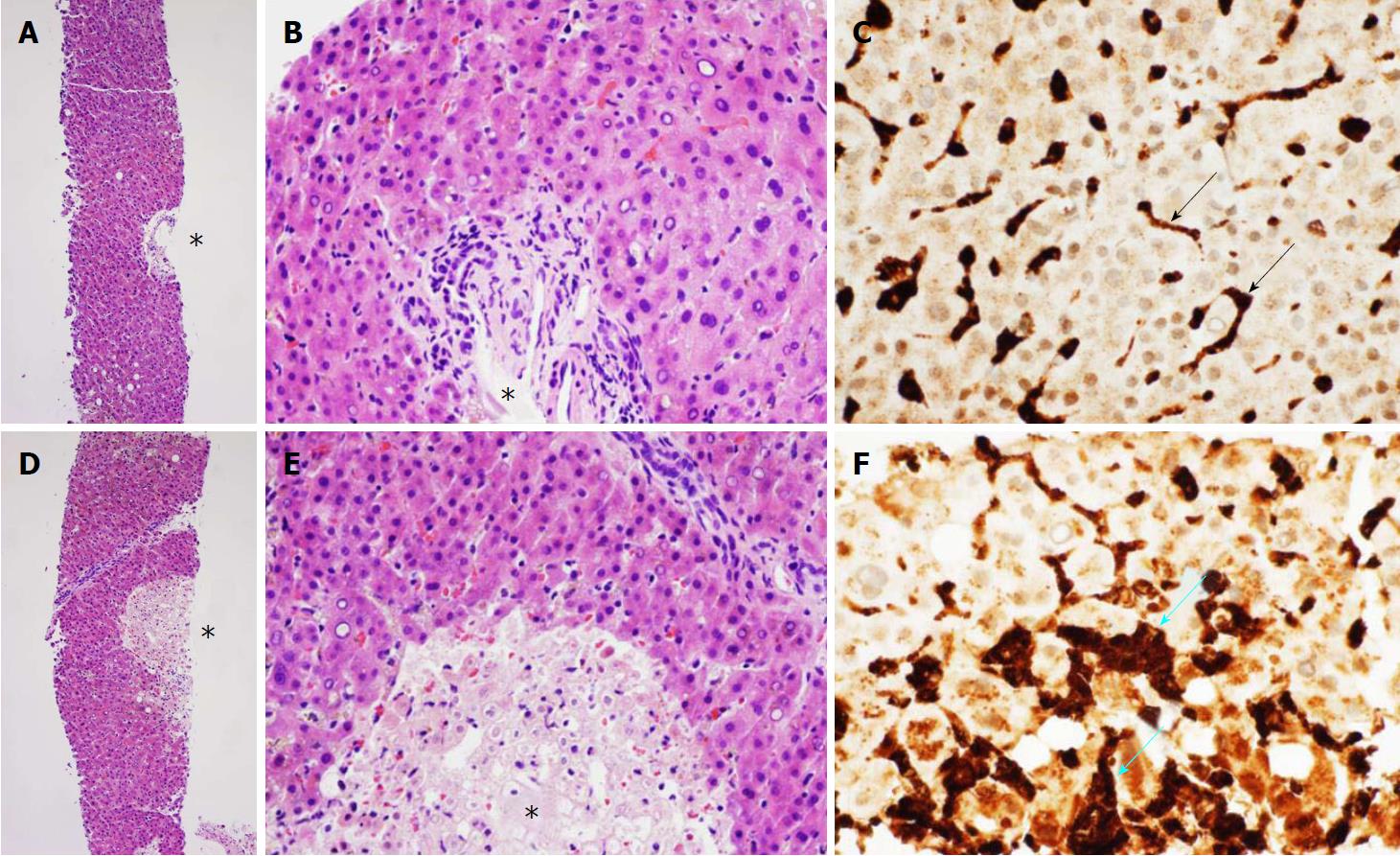
The acetaminophen level was 0. Acute viral hepatitis panel was negative for hepatitis A, B, C, and E. Anti-smooth muscle and anti-mitochondrial antibodies were absent in serum. Genotyping for hemochromatosis was negative for C282Y, and was heterozygous for H63D mutation. Abdominal ultrasound and computerized tomographic scans revealed normal hepatobiliary findings. Magnetic resonance cholangiopancreatography revealed a normal biliary tree, and mild, diffuse, hepatosplenic iron deposition. Histopathologic examination of a transjugular liver biopsy revealed relatively well-preserved hepatic parenchyma without lymphocytic infiltrates or macrophages in nearly all the cores (Figure 1A and B), except for sparsely distributed, focal, and small centrilobular hepatocellular necrosis (Figure 1D and E). Although M30 and M65 serum levels were not determined as assays for apoptosis, the patient only had aggregates of nonviable hepatocytes on liver biopsy examination which strongly favors the diagnosis of hepatocellular necrosis over that of apoptosis. Immunohistochemical staining for CD-68, a marker for Kupffer cells and tissue macrophages, revealed only normal-appearing CD-68-positive Kupffer cells (dark brown staining, black arrows) with characteristic slender, elongated morphology in non-ischemic areas (Figure 1C); but revealed an additional population of plump, CD-68 positive macrophages (blue arrows) within areas of focal hepatic necrosis (Figure 1F). CD-71, a marker for nucleated erythrocytes, showed no nucleated erythrocytes (no hemophagocytosis) within the Kupffer cells in the non-ischemic area or within macrophages in the ischemic areas (negative immunostains not shown). Due to his employment as a sewer inspector, leptospirosis was excluded by absence of leptospiral antibodies. He was treated with intravenous (IV) aztreonam and vancomycin after isolating Pseudomonas and group A streptococcus from blood cultures.
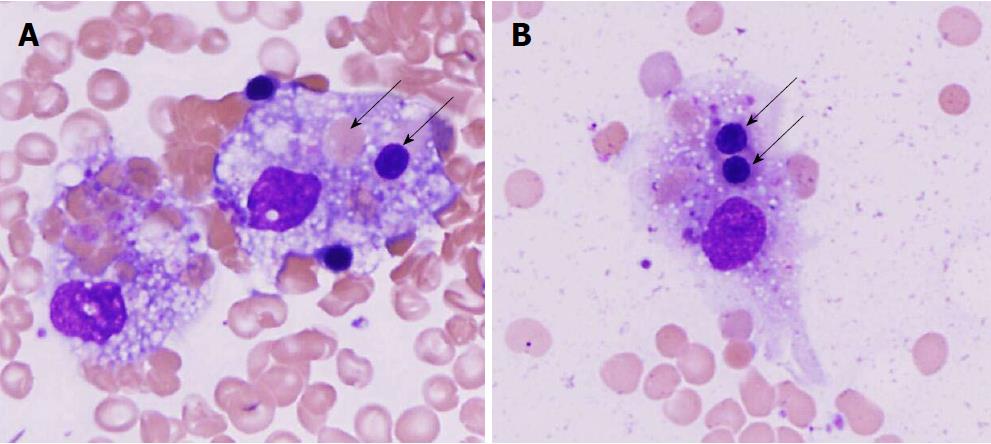
Histopathologic examination of a bone marrow biopsy and aspiration smears revealed hypocellular bone marrow due to marked granulocytic hypoplasia and prominent histiocytic hemophagocytosis (Figure 2). Plasma cells present in the marrow showed no light chain restriction, and appeared to be reactive. Concomitant flow cytometry showed no evidence of Hodgkin’s lymphoma or leukemia. The patient was diagnosed with HLH by satisfying the following 6 of 8 diagnostic criteria established by the Histiocyte Society (minimum 5 criteria required for diagnosis of HLH): (1) temperature = 39.5°C (qualifying-criterion: temperature > 38.5°C); (2) cytopenia of 2 blood cell lineages: hemoglobin = 8.6 g/dL (qualifying-criterion: hemoglobin < 9.0 g/dL, lab normal: 13.5-17.0 g/dL), and absolute neutrophil count < 100/µL (qualifying-criterion: < 1000/µL, lab-normal: 1600-7200 cells/µL); (3) fibrinogen = 135 mg/dL (qualifying criterion < 150 mg/dL; lab-normal: 175-375 mg/dL); (4) hemophagocytosis in bone marrow biopsy (qualifying-criterion: present); (5) ferritin = 3100 ng/mL (qualifying criterion: > 500 ng/mL, lab-normal: 14-338 ng/mL); and (6) elevated soluble interleukin 2 (IL-2)-receptor alpha = 9910 pg/mL (normal < 1033 pg/mL) (additionally, Histiocytic Society criterion #2-not satisfied, and criterion #6-not tested). Patient then received prednisone 100 mg orally daily. Liver transplant evaluation concluded that the patient was not a liver transplant candidate despite MELD score = 33 because of severe systemic illnesses. The patient and family refused experimental therapy with artificial hepatic assist devices, such as liver dialysis machines. Patient was not treated with porous CytoSorb polymer beads (CytoSorbents Corporation, Monmouth Junction, New Jersey, United States) to adsorb toxic molecules from septic shock because of no institutional experience with this treatment.
Patient then bled at the prior liver biopsy site because of disseminated intravascular coagulopathy. He became hypotensive, and was endotracheally intubated. Hemostasis was achieved by embolizing two major hepatic artery branches during two arteriogram sessions. He was transfused 13 units of packed erythrocytes, 6 units of fresh-frozen-plasma, 4 units of cryoprecipitate, and 4 units of platelets during this bleeding episode.
During the ensuing 10 d his ferritin increased > 33000 ng/mL, and the bilirubin and transaminase levels modestly declined. Despite temporary clinical stabilization after endotracheal intubation, numerous antibiotic therapeutic regimens, IV fluids, and IV vasopressors, the patient succumbed to overwhelming sepsis, disseminated intravascular coagulopathy, and liver failure 42-d after admission.
HLH has no racial or sexual predilection[4]. It most commonly affects infants < 18 mo old, but it can occur in older children and rarely occur in adults. It often presents with nonspecific symptoms and signs[4-6].
HLH has primary (genetic) etiologies, which usually manifest in infants with genetic immunologic mutations[7], and secondary (acquired) etiologies which typically manifest in older children or adults. Secondary etiologies include infection, especially with Epstein-Barr virus[8,9] or herpes simplex virus-1[10]; malignancy; or autoimmune diseases (called macrophage activation syndrome when HLH is associated with autoimmune diseases)[11]. The common denominator in all acquired etiologies is disrupted immune homeostasis.
Prompt diagnosis is critical for patient survival, but the diagnosis is often delayed or overlooked, which contributes to its high mortality. HLH occurs in about 1% of hematologic malignancies[12,13]; sometimes from chemotherapy for these malignancies[14]. Patients with malignancy have extremely high mortality, partly due to delayed diagnosis of HLH[15,16]. Suggested mechanisms of HLH associated with malignancy include: profound inflammation from immune activation, persistent antigen stimulation by cancer cells, and deranged immune response secondary to chemotherapy.
The Histiocyte Society criteria, which are the generally accepted diagnostic criteria, are very strict and can, therefore, miss some cases of HLH (false negatives). HLH should be clinically considered in patients satisfying 3 or 4 criteria[4]. Moreover, the Histiocyte Society incorporates two tests with limited clinical availability: Level of α chain of soluble interleukin-2 receptor (sIL-2r)[17], and 51-Cr release assay reflecting NK-cell activity; unavailability of these tests militate against early diagnosis and therapy. Ferritin levels provide a reliable prognostic indicator and monitor of treatment efficacy[18].
Treatment focuses on controlling the triggering event, such as underlying viral infection, when identified, and on 8-wk-induction-therapy with immunosuppresants, including corticosteroids, etoposide, and cyclosporine, to suppress exaggerated immune responses in HLH. The precipitating event in the current patient was likely the antecedent upper respiratory infection, but the specific (presumably viral) infectious agent was not identified despite extensive (viral) work-up. This patient was treated with high-dose prednisone, but not etoposide or cyclosporine because of the profound hyperbilirubinemia. Allogeneic hematopoietic stem cell transplantation (allo-HSCT), can sometimes achieve complete remission when the patient presents with profound HLH without life-threatening liver failure[19-21].
Patients with established HLH commonly demonstrate markedly elevated biochemical parameters of liver function. For example, among 30 adult patients with HLH, about 60% had AST > 2 times the upper limit of normal[22,23]. Likewise, about 60% of patients had lactate dehydrogenase (LDH) > 2 times the upper limit of normal[22,24]. About 80% of patients present with jaundice[23], which is often profound[25]. The hyperbilirubinemia is predominantly direct. In a study of 28 patients, 9 had moderately severe hypertriglyceridemia, with levels > 265 mg/dL, from cholestasis[26]. Hypoalbuminemia is common, reflecting decreased liver synthetic function[27]. Up to 90% of patients with advanced HLH have severe coagulopathies or disseminated intravascular coagulopathy[22,23,28]. Liver injury is associated with overproduction of cytokines[17]. Severely elevated ferritin levels, and iron overload, detected by hepatic magnetic resonance imaging (MRI), reflect severe hepatic injury.
Lymphocytes commonly infiltrate the liver from immune activation from HLH. For example, in a study of 27 autopsies of infants or children with congenital HLH, 22 (81%) had lymphocytic infiltrates, including 13 (48%) with dense lymphocytic infiltrates[27]. Lymphocytic infiltrates are more commonly periportal than centrilobular[28]. Treatment with corticosteroids or other immunosuppresants may decrease the density and distribution of intrahepatic lymphocytic infiltrates[27]. Macrophages are occasionally present in the liver, but hemophagocytic macrophages are detected in only about 10% of liver biopsies, whereas hemophagocytic macrophages are commonly detected in spleen or bone marrow biopsies[27].
ALF is currently reported secondary to HLH which was diagnosed based on satisfying 6 of 8 criteria for HLH. Despite biochemical evidence of severe hepatic injury, this patient had only focal hepatic necrosis, and relatively well-preserved hepatocytes in the rest of the biopsy sample, without intrahepatic lymphocytic infiltration or hemophagocytic macrophages. This patient had not received corticosteroids or other immunosuppresants prior to liver biopsy, which could have attenuated intrahepatic lymphocytic infiltration. These relatively bland liver biopsy findings in the setting of ALF strongly suggest that liver failure in this patient with HLH is not necessarily from intrinsic liver disease, say from viral infection or drug toxicity, but may be secondary to multi-organ, systemic, and extrahepatic injury from HLH.
Liver transplantation has been offered to patients who present predominantly with ALF, from severe hepatic inflammation and necrosis, in association with potentially reversible HLH[1-3]. In a literature review of 7 individual case reports, only 2 (28%) of 7 patients undergoing liver transplantation for ALF with HLH survived > 6 mo[2,3]. In a small clinical series, 9 pediatric patients underwent liver transplantation for life-threatening ALF associated with secondary HLH[3]. These patients typically had extremely abnormal liver function tests: ALT = 2512 ± 1158 U/L, (range: 1135-4113 U/L), AST = 3165 ± 2276 U/L (range: 779-8789 U/L), conjugated bilirubin = 257 ± 108 micromol/L (range: 141-448 micromol/L, normal 0-2 micromol/L), and INR = 7.7 ± 1.7 units (range: 3.9- > 9 units). Liver biopsy in these patients generally revealed severe-to-massive (25%-95%) hepatocyte necrosis, lymphocytic infiltrates, numerous macrophages, and intrahepatic erythrophagocytosis[3]. These patients had extremely frequent medical and surgical complications after transplantation, including: severe or opportunistic infections-6, acute liver rejection-5, recurrent HLH-5, bile duct strictures-3, post-transplant lymphoproliferative disease of liver-2, bowel obstruction-1, and wound dehiscence-1. Three patients died at 1.5, 8, and 14 mo after liver transplantation. The other 6 pediatric patients were alive and well at a median of 24 mo after liver transplantation[3].
In contradistinction, the currently reported patient had much less evident liver injury, but severe clinical manifestations of HLH. The current work may suggest that liver transplantation is not indicated for HLH when: (1)-ALF is not present or imminent (MELD score < 20-22); (2)-the patient has a poor prognosis due to the combined effects of ALF and HLH; (3)-the liver injury by itself does not underlie this poor patient prognosis; or (4)-the HLH is advanced and highly likely irreversible. This case illustrates three of these factors that militated against liver transplantation; in the current case the ALF was most likely from septic shock with focal centrilobular ischemia from hypotension, bone marrow failure, and explosive immune activation from HLH.
This prior clinical data suggest caution in liver transplantation for HLH. The data are limited. Reported outcomes for liver transplantation with HLH are much worse than that for liver transplantation without HLH[3]. The current work suggests that high mortality from advanced and likely irreversible HLH may limit the benefits of liver transplantation. Liver biopsy may be very helpful to assess the liver injury and to determine whether ALF associated with HLH is due primarily to intrinsic liver injury vs secondary to extrahepatic injury from HLH to help determine whether liver transplantation is warranted.
About 25% of patients had a rash associated with HLH in one large study[28]. The rash is most commonly generalized, maculopapular, and erythematous[29]. Patients can also have petechiae and purpura from thrombocytopenia. This patient’s rash started after administering trimethoprim-sulfamethoxazole and was most likely secondary to it.
HLH frequently involves the liver. It frequently causes AST levels > 2 times the upper limit of normal[22,23], frequently causes LDH levels > 2 times the upper limit of normal[22,24], and very frequently causes profound direct hyperbilirubinemia[23,25]. Patients may also commonly present with moderately severe hypertriglyceridemia from cholestasis[26], and hypoalbuminemia from decreased liver synthetic function[27]. The liver injury is associated with overproduction of cytokines[17], severe hepatic inflammation, and potentially hepatic necrosis.
When patients present with imminent or confirmed liver failure from predominantly liver involvement from HLH, liver transplant may be considered[1-3]. In such cases, the liver failure may be documented by a MELD score > 24-26, and the liver failure should contribute significantly to the poor patient prognosis. Liver biopsy should be performed to determine the extent of the liver injury and the relative roles of intrinsic liver injury vs systemic HLH in the patient’s ALF. However, the current case illustrates that patients may be poor candidates for liver transplantation from ALF secondary to HLH, if the HLH is likely irreversible, or if the patient has major comorbidities (systemic complications) from the HLH that are highly lethal. For example, the currently reported patient had highly lethal, comorbidities of overwhelming sepsis, shock, and disseminated intravascular coagulopathy, that precluded liver transplantation despite having a MELD score of 33 that would normally qualify the patient for liver transplantation. This work illustrates that decisions on liver transplantation in patients with HLH depend upon the presence of imminent or present ALF, the potential reversibility of the HLH, and the absence of severe comorbidities associated with the HLH that are most likely fatal. Patients with high MELD scores in the setting of HLH should be evaluated for liver transplantation before these highly lethal complications supervene.
Study limitations include: (1) this is a single retrospectively reported case report; (2) the etiology of HLH was undetermined, as occurs in about 25% of adult cases[4,30]; (3) the microbiology of the antecedent upper respiratory infection, that likely stimulated the HLH, is unknown; (4) the reported pathologic findings at liver biopsy are subject to sample bias; and (5) the possible, but unlikely, role of trimethoprim-sulfamethoxazole in the ALF[31]. Study strengths include the well-established diagnosis of HLH, and the academic evaluation of this patient at a tertiary-care-university hospital with a liver transplant center.
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome of aggressive immune hyperactivation from hypercytokinemia that frequently causes liver injury and sometimes causes acute liver failure (ALF) that can contribute to the high syndromic mortality. Systematic literature review revealed hundreds of reported cases of HLH in adults, but provided insufficient data on the patterns and pathophysiology of the liver injury. It is particularly important to clinically determine whether ALF associated with HLH is due primarily to intrinsic liver injury vs secondary to extrahepatic causes from the HLH, to help determine whether liver transplantation is potentially warranted. A case is reported of fatal HLH in an adult presenting prominently with ALF, with the liver injury secondary to severe systemic disease from HLH, as documented by liver biopsy and clinical evaluation, and liver transplantation refused on this basis.
It is clinically important to determine whether ALF associated with HLH is primarily from intrinsic liver injury vs secondarily from extrahepatic injury from HLH to help determine the potential efficacy of liver transplantation for ALF associated with HLH. ALF from direct liver injury from HLH might be treatable by liver transplantation, whereas liver injury secondary to extrahepatic damage from HLH would be unlikely to be successfully treated by liver transplantation.
To determine whether ALF associated with HLH is due primarily to intrinsic liver injury vs secondary to extrahepatic injury from HLH to help determine whether liver transplantation is potentially warranted. This study involves systematic review of the literature on liver injury associated with HLH. Additionally, a case report is presented to illustrate that a patient with ALF secondary to HLH may be an inappropriate candidate for liver transplantation, despite a high model for end stage liver disease (MELD) score, when the patient has other likely lethal complications of HLH, such as overwhelming sepsis, septic shock, or disseminated intravascular coagulation secondary to explosive immune activation from HLH.
Literature on hepatic manifestations of HLH, including liver injury or ALF secondary to HLH, was systematically reviewed by computerized search using PubMed for articles with the following medical subject headings/keywords (“hemophagocytic lymphohistiocytosis” or “HLH”) AND (“liver” or hepatic”), and by reviewing sections on HLH in standard pathology textbooks/monographs. Articles before 1980 were selectively excluded because clinical evaluations before 1980 often lacked currently required clinical tests of hepatic function, radiologic imaging of hepatic anatomy, and modern criteria for HLH. Large clinical trials, meta-analyses, systematic reviews, and controlled trials were assigned higher priority than review articles or small clinical series, and individual case reports were assigned the lowest priority. Two authors independently performed a literature search, and decided on which articles to incorporate into this review according to article priority based on consensus. Dr. Cappell has considerable experience in conducting systematic reviews, with 4 systematic reviews in peer-reviewed journals, indexed in PubMed, published during the last 2 years, and with a PhD in neurophysiology that involved 5 years of training and research in biomedical statistics. A case report is presented to illustrate that a patient with ALF secondary to HLH may be an inappropriate candidate for liver transplantation, despite a high MELD score, when the patient has other, likely lethal, complications of HLH. This case report was thoroughly analyzed based on the medical chart, including re-review of original endoscopic photographs by an expert endoscopist, radiologic images by an expert radiologist, and pathologic slides by an expert pathologist.
This systematic review shows that HLH frequently involves the liver. It frequently causes aspartate aminotransferase levels > 2 times the upper limit of normal, frequently causes LDH levels > 2 times the upper limit of normal, and very frequently causes profound direct hyperbilirubinemia. Likewise, patients commonly present with moderately severe hypertriglyceridemia from cholestasis, and hypoalbuminemia from decreased liver synthetic function. The liver injury is associated with overproduction of cytokines, severe hepatic inflammation, and potentially hepatic necrosis. When patients present with imminent or evident ALF predominantly from direct liver involvement from HLH, liver transplant may be potentially indicated. In such cases, the ALF may be documented by a MELD score > 24-26, and the ALF should contribute significantly to the poor prognosis. However, the currently reported case illustrates that patients may be poor candidates for liver transplantation from ALF secondary to HLH, when the HLH is likely irreversible, or if the patient has potentially lethal, major, comorbidities (systemic complications) from the HLH. For example, the currently reported patient had highly lethal, comorbidities of overwhelming sepsis, shock, and disseminated intravascular coagulopathy, that precluded liver transplantation despite having a MELD score of 33, which would normally have qualified the patient for liver transplantation. This work illustrates that decisions on liver transplantation in patients with HLH depend upon the presence of imminent or evident ALF, the potential reversibility of the HLH, and the absence of severe and most likely fatal comorbidities associated with HLH.
HLH frequently involves the liver and can directly cause severe liver injury or ALF from hepatic inflammation and hepatic necrosis from explosive immune hyperactivation related to hypercytokinemia. HLH can also indirectly cause livery injury from extrahepatic disease secondary to HLH, such as overwhelming sepsis, septic shock, hypoxemia, and disseminated intravascular coagulation. It is important to determine the relative contributions of these two alternative mechanisms of liver injury when contemplating liver transplantation for HLH. Intrinsic liver disease may respond to liver transplantation, whereas liver injury secondary to extrahepatic causes, such as septic shock, is unlikely to respond to liver transplantation if the extrahepatic causes are irreversible.
This work places in perspective that decisions on liver transplantation for ALF associated with HLH must consider not only the functional status of the liver, as indicated by the MELD score, but the etiology of the liver injury. When the liver injury is directly from the HLH (e.g., hepatic inflammation and necrosis from hypercytokinemia) liver transplantation may be considered, whereas when the liver injury is secondary to extrahepatic (other organ) failure (e.g., overwhelming sepsis, hyperimmune activation in other organs, disseminated intravascular coagulation, or respiratory failure) liver transplant is unlikely to be successful. This concept is illustrated by a case report wherein the ALF associated with HLH was secondary to extrahepatic causes and not amenable to liver transplantation, and by systematic review of liver injury from HLH. This work may prove useful to clinicians in decisions on whether to perform liver transplantation for ALF associated with HLH.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Inoue K, Sonzogn A, Tahiri MJH S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Lin S, Li Y, Long J, Liu Q, Yang F, He Y. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults: A case report and review of the literature. Medicine (Baltimore). 2016;95:e5431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Wright G, Wilmore S, Makanyanga J, McKerrell T, Watkins J, Patch D, Burroughs AK. Liver transplant for adult hemophagocytic lymphohistiocytosis: case report and literature review. Exp Clin Transplant. 2012;10:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Amir AZ, Ling SC, Naqvi A, Weitzman S, Fecteau A, Grant D, Ghanekar A, Cattral M, Nalli N, Cutz E. Liver transplantation for children with acute liver failure associated with secondary hemophagocytic lymphohistiocytosis. Liver Transpl. 2016;22:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Li J, Wang Q, Zheng W, Ma J, Zhang W, Wang W, Tian X. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Rice L, Pacheco J. Adult Hemophagocytic Lymphohistiocytosis: More Data; Even More Questions. Crit Care Med. 2016;44:2119-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cetica V, Sieni E, Pende D, Danesino C, De Fusco C, Locatelli F, Micalizzi C, Putti MC, Biondi A, Fagioli F. Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. J Allergy Clin Immunol. 2016;137:188-196.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Pinto-Patarroyo GP, Rytting ME, Vierling JM, Suarez-Almazor ME. Haemophagocytic lymphohistiocytosis presenting as liver failure following Epstein-Barr and prior hepatitis A infections. BMJ Case Rep. 2013;2013:pii: bcr2013008979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kitazawa Y, Saito F, Nomura S, Ishii K, Kadota E. A case of hemophagocytic lymphohistiocytosis after the primary Epstein-Barr virus infection. Clin Appl Thromb Hemost. 2007;13:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Drori A, Ribak Y, van Heerden PV, Meir K, Wolf D, Safadi R. Hemophagocytic lymphohistiocytosis due to acute primary herpes simplex virus 1 infection. J Clin Virol. 2015;68:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kelkar AH, Shah AA, Yong SL, Ahmed Z. An unusual association between hemophagocytic lymphohistiocytosis, mixed connective tissue disease, and autoimmune hemolytic anemia: A case report. Medicine (Baltimore). 2017;96:e7488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C, Blechacz B, Wang S, Minkov M, Jordan MB. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. 2017;123:3229-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, Allen CE, Pierce SA, Cortes JE, Ravandi F. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: Relation to hemophagocytosis, characteristics, and outcomes. Cancer. 2016;122:2857-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Delavigne K, Bérard E, Bertoli S, Corre J, Duchayne E, Demur C, Mansat-De Mas V, Borel C, Picard M, Alvarez M. Hemophagocytic syndrome in patients with acute myeloid leukemia undergoing intensive chemotherapy. Haematologica. 2014;99:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Patel R, Patel H, Mulvoy W, Kapoor S. Diffuse Large B-Cell Lymphoma with Secondary Hemophagocytic Lymphohistiocytosis Presenting as Acute Liver Failure. ACG Case Rep J. 2017;4:e68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Bains A, Mamone L, Aneja A, Bromberg M. Lymphoid malignancy-associated hemophagocytic lymphohistiocytosis: Search for the hidden source. Ann Diagn Pathol. 2017;28:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lin M, Park S, Hayden A, Giustini D, Trinkaus M, Pudek M, Mattman A, Schneider M, Chen LYC. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. 2017;96:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Grangé S, Buchonnet G, Besnier E, Artaud-Macari E, Beduneau G, Carpentier D, Dehay J, Girault C, Marchalot A, Guerrot D. The Use of Ferritin to Identify Critically Ill Patients With Secondary Hemophagocytic Lymphohistiocytosis. Crit Care Med. 2016;44:e1045-e1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Ayala E, LaFave D, Nishihori T, Kharfan-Dabaja MA. Haploidentical transplantation as a promising therapy for relapsed hemophagocytic lymphohistiocytosis in an older adult patient. Hematol Oncol Stem Cell Ther. 2018;11:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Fu L, Wang J, Wei N, Wu L, Wang Y, Huang W, Zhang J, Liu J, Wang Z. Allogeneic hematopoietic stem-cell transplantation for adult and adolescent hemophagocytic lymphohistiocytosis: a single center analysis. Int J Hematol. 2016;104:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Kuriyama T, Kawano N, Yamashita K, Kikuchi I. Cord Blood Transplantation Following Reduced-Intensity Conditioning for Epstein-Barr Virus-Associated Hemophagocytic Lymphohistiocytosis during Systemic Lupus Erythematosus Treatment. J Clin Exp Hematop. 2016;56:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Fukaya S, Yasuda S, Hashimoto T, Oku K, Kataoka H, Horita T, Atsumi T, Koike T. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: analysis of 30 cases. Rheumatology (Oxford). 2008;47:1686-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Palazzi DL, McClain KL, Kaplan SL. Hemophagocytic syndrome in children: an important diagnostic consideration in fever of unknown origin. Clin Infect Dis. 2003;36:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Shao X, Xu Y, Xu X, Xu Y, Chen H, Hong M, Liu L. Epstein-Barr Virus-associated Hemophagocytic Lymphohistiocytosis in Adults: A Retrospective Analysis of 23 Patients in China. Isr Med Assoc J. 2018;20:80-85. [PubMed] |
| 25. | Jagtap N, Sharma M, Rajesh G, Rao PN, Anuradha S, Tandan M, Ramchandani M, Reddy DN. Hemophagocytic Lymphohistiocytosis Masquerading as Acute Liver Failure: A Single Center Experience. J Clin Exp Hepatol. 2017;7:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Okamoto M, Yamaguchi H, Isobe Y, Yokose N, Mizuki T, Tajika K, Gomi S, Hamaguchi H, Inokuchi K, Oshimi K. Analysis of triglyceride value in the diagnosis and treatment response of secondary hemophagocytic syndrome. Intern Med. 2009;48:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Ost A, Nilsson-Ardnor S, Henter JI. Autopsy findings in 27 children with haemophagocytic lymphohistiocytosis. Histopathology. 1998;32:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 960] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 29. | Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 796] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 30. | Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 31. | Hayashi M, Abe K, Imaizumi H, Okai K, Kanno Y, Takahashi A, Ohira H. Drug-induced liver injury with autoimmune features complicated with hemophagocytic syndrome. Clin J Gastroenterol. 2016;9:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |