Published online Oct 27, 2018. doi: 10.4254/wjh.v10.i10.780
Peer-review started: May 18, 2018
First decision: June 6, 2018
Revised: June 19, 2018
Accepted: June 27, 2018
Article in press: June 28, 2018
Published online: October 27, 2018
Processing time: 164 Days and 4.7 Hours
To our best knowledge, no case of a tumor that was incidentally detected during living donor hepatectomy (LDH) has been reported in the English language medical literature. We present two cases in which grade I neuroendocrine tumors (NET) were incidentally detected during our twelve-year LDH experience. First Case: A 26-year-old male underwent LDH for his brother suffering from HBV-related chronic liver disease (CLD). After right lobe LDH, intestinal length was measured as part of a study concerning the relationship between small intestinal lengths and surgical procedure. At this stage, a mass lesion with a size of 10 mm × 10 mm was detected on the antimesenteric surface, approximately 90 cm proximal to the ileocecal valve. A wedge resection with primary intestinal anastomosis was performed. Second Case: A 29-year-old male underwent right lobe LDH for his father with hepatitis B virus (HBV)-related CLD. An abdominal exploration immediately prior to the closure of the incision revealed that the appendix vermiformis was edematous and had firmness with a size of 8-10 mm at its tip. An appendectomy was performed. The pathological examinations of the specimens of both patients revealed grade 1 NET. In conclusion, even if patients undergoing LDH are healthy individuals, whole abdominal cavity should be gently palpated and all findings recorded after completing laparotomy. Suspected masses or lesions should be confirmed by frozen section examination. Such an approach would avert potential medicolegal issues.
Core tip: To our best knowledge, no case of a tumor that was incidentally detected during living donor hepatectomy (LDH) has been reported in the English language medical literature. Herein, we present two cases in which grade I neuroendocrine tumors were incidentally detected during our twelve-year LDH experience. Even if patients undergoing LDH are healthy individuals, the whole abdominal cavity should be gently palpated and all findings be recorded after completing laparotomy. Suspected masses or lesions should be confirmed by frozen section examination. Such an approach would avert potential medicolegal issues.
- Citation: Akbulut S, Isik B, Cicek E, Samdanci E, Yilmaz S. Neuroendocrine tumor incidentally detected during living donor hepatectomy: A case report and review of literature. World J Hepatol 2018; 10(10): 780-784
- URL: https://www.wjgnet.com/1948-5182/full/v10/i10/780.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i10.780
While deceased donors are an important part of the liver donor pool in western countries, living donors constitute an important portion of the donor pool in many Asian countries including Turkey[1]. The most important problem with living donor liver transplantation is the mortality and morbidity risk faced by completely healthy donor candidates due to a major surgery like liver resection. In order to minimize those risks, all living liver donor (LLD) candidates undergo an examination according to an algorithm consisting of biochemical blood tests and advanced radiological instruments[2]. Incidental hemangioma, focal nodular hyperplasia, cystic lesions, median arcuate ligament, and ventricular septal defect have been rarely reported to be detected during examinations of LLD candidates[3-5]. On the other hand, no publication other than our study has ever reported unusual findings such as cancer detected incidentally in the liver or other intraabdominal organs during either preoperative investigations or in a living donor hepatectomy (LDH) procedure[6]. In this study we report two neuroendocrine tumor (NET) cases detected incidentally during our 12-year LDH experience.
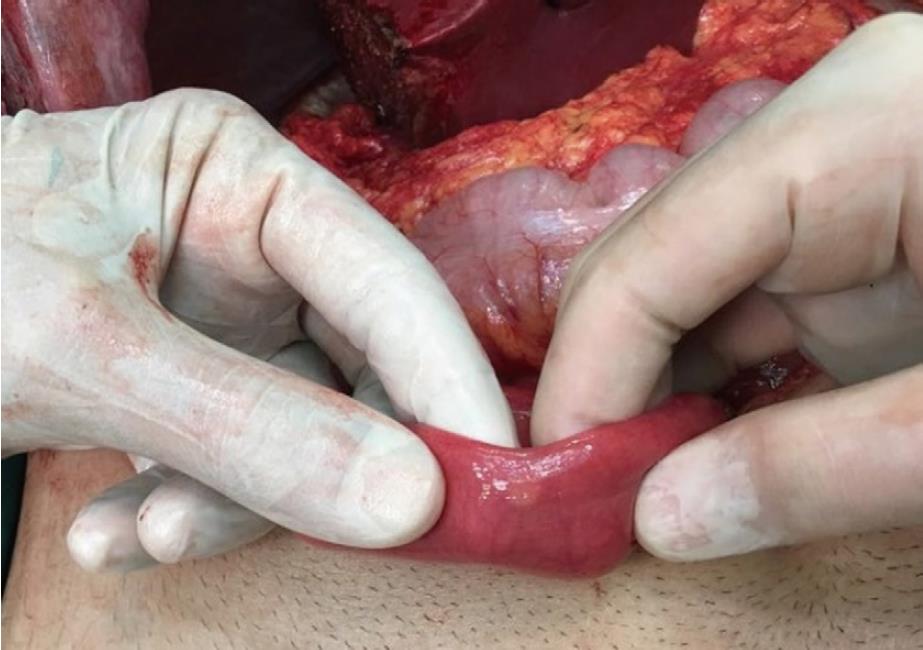
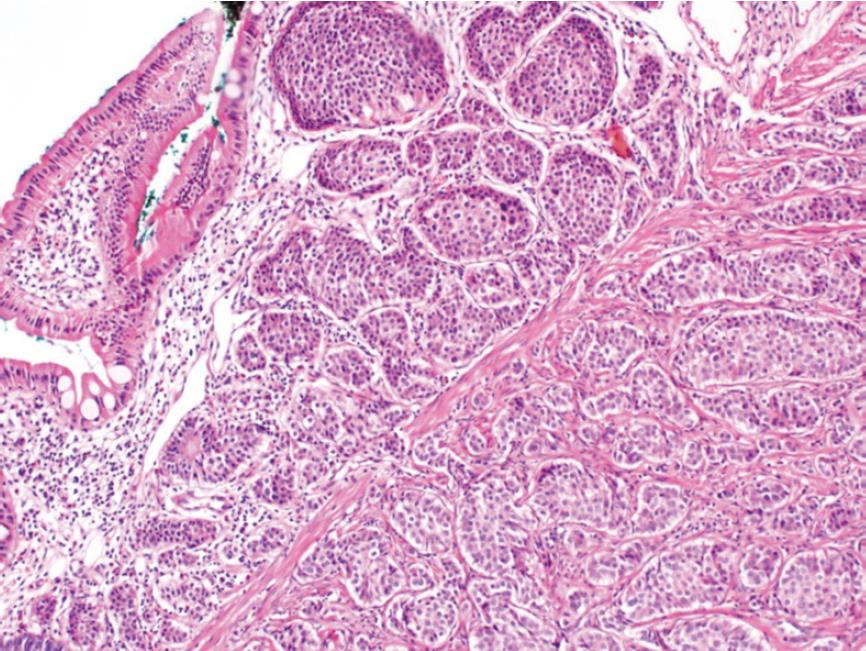
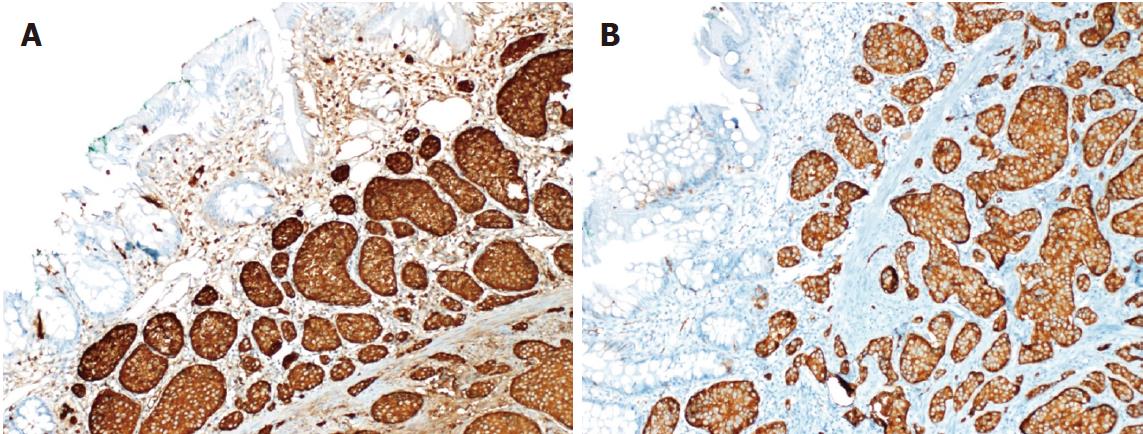
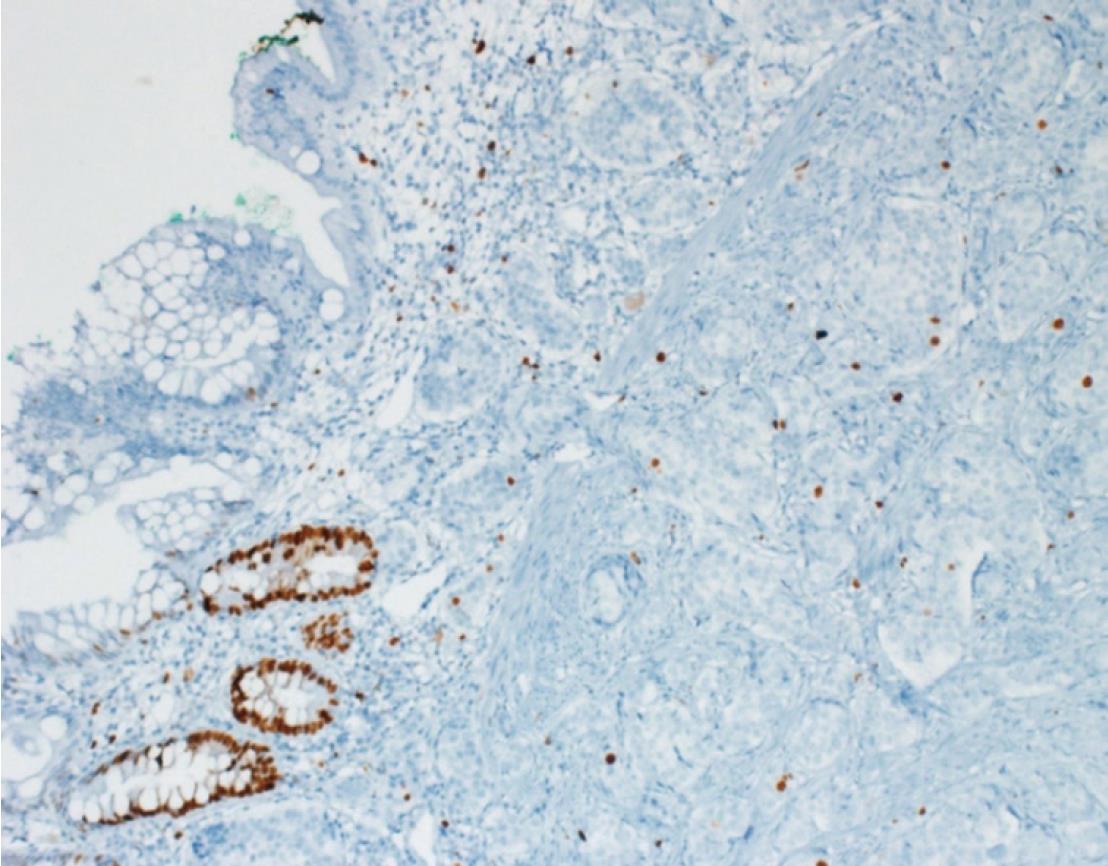
A 26-year-old healthy man applied to our transplant center for being a LLD to his 37-year-old brother with chronic liver disease (HBV). The donor candidate was taken into the operating room for LDH in May 2017. A laparotomy was performed through an incision starting from xiphoid to umblicus and extending laterally on the right side. As no macroscopic finding was detected in the liver, a right lobe LDH was performed. As part of a study conducted in our department investigating the relationship of mesenteric and antimesenteric lengths of small intestine with the surgical procedure, intestinal lengths of this patient were also measured. At this time, a mass lesion measuring approximately 10 mm was detected on the antimesenteric face of the intestine, approximately 90 cm proximal to the ileocecal valve (Figure 1). The mass was resected together with the mesentery of the adjacent intestinal segment. A wedge resection with primary intestinal anastomosis was performed. Intestinal mucosa and submucosa were closed with polyglactin 910 suture material while the seromuscular layer was closed with polypropylene. The patient experienced no complications during his postoperative follow-up and was discharged. A histopathological examination revealed a grade I neuroendocrine tumor (carcinoid tumor) with a size of 7 mm × 5 mm, which had intact surgical margins (Figure 2). An immunohistochemical analysis showed that it was NSE (+), Chromogranin (+), Synaptophysin (+), and Ki67 proliferation index (1%-2%) positive (Figures 3 and 4). The patient was put under follow-up by the medical oncology department, and a thoracoabdominal computerized tomography taken in the first controls revealed no additional lesions.
A 29-year-old healthy man applied to our transplant center for being a LLD candidate for his 51-year-old father who had chronic liver disease (HBV + HCC). His preoperative biochemical tests and radiological studies were normal. The patient was taken into the operating room for right donor hepatectomy in May 2008. A laparotomy was performed through an incision starting from xiphoid to umblicus and extending laterally on the right side. As no macroscopic finding was detected in the liver, right lobe LDH was performed. At the time when the surgeon suspended the anterior abdominal wall in order to place a drain into the surgical field, it was noticed that the appendix vermiformis was edematous and had prominent superficial arterioles. In addition, a firmness measuring 8-10 cm in size was palpated at the tip of the appendix. Therefore, an appendectomy was performed. As no complications developed at the postoperative follow-up, the patient was discharged to be seen at control visits. The pathology report of the appendix vermiformis showed a grade I neuroendocrine tumor (NET) with a diameter of 10 cm at the tip of the appendix vermiformis, which showed full-thickness invasion but did not spare the mesoappendix (Figure 2). In addition, the lesion was NSE (+), Chromogranin (+), Synaptophysin (+), and Ki67 (1%) positive upon immunohistochemical analysis (Figures 3 and 4). As the patient was living in another city, he is currently under follow-up of another center there. He has recently informed us that no problems have occurred in his final control visit.
The World Health Organization has classified NETs into two main categories: well differentiated (low grade, intermediate grade, high grade neuroendocrine tumor) and poorly differentiated (high grade neuroendocrine carcinoma). The basic criteria for this classification are the Ki67 proliferation index (< 3%, 3%-20%, > 20%) and number of mitosis (< 2 mitosis, 2-20 mitosis, > 20 mitosis).
NETs most commonly involve the small intestine. NETs are most commonly located in the first 60 cm segment before the ileocecal valve. Unlike appendix NETs, small intestinal NETs have the potential to make both nodal and distant metastasis independent of their size. Therefore, the most appropriate surgical approach is to perform a partial small intestinal resection to involve lymph nodes and mesentery, independent of the tumor size. As small intestinal NETs have the potential of being multiple, all intestinal loops should be carefully inspected during the operation[7-9].
Appendix vermiformis is the second most commonly involved organ by NETs in the gastrointestinal system[10]. The majority of appendix NETs are incidentally detected in patients taken into operating room for a presumed diagnosis of acute appendicitis. NETs are diagnosed at a rate of 0.3%-2.27% in patients diagnosed with acute appendicitis while they are detected in 1.8%-2.3% of patients undergoing incidental appendectomy[7-9]. The prognosis and metastasis potential of appendix NETs depend on their size. Ninety-five percent of appendix NETs are smaller than 2 cm at the time of diagnosis and have a low metastasis potential (4%). In tumors with a diameter ≥ 2 cm or patients with mesoappendix invasion, right hemicolectomy is the most appropriate approach. In tumors with a diameter ranging between 1.0 cm and 1.9 cm, adjunct hemicolectomy is the most appropriate approach when one or more of the following parameters exists: mesoappendix invasion, vascular invasion, high proliferation index (grade 2), suspicious/positive surgical margins, and mixed histology (goblet cell carcinoid, adenocarcinoid). Simple appendectomy is sufficient for patients without mesoappendix invasion despite a diameter of 1.0-1.9 cm and appendix NETs with a diameter < 1 cm. No adjunctive surgical procedure was undertaken since the patient presented here had a grade I NET.
The follow-up of patients with NET depends on tumor size and grade. One or a combination of several diagnostic modalities of biochemical (5-hydroxyindoleacetic acid, serum chromogranin), endoscopic (pan endoscopy, colonoscopy), radiological (computed tomography, magnetic resonance imaging, Indium-111 pentetreotide (OctreoScan), and functional PET imaging with 68-Ga DOTA-TATE can be used for diagnosis. Patients with an appendix NET having a diameter of ≥ 2 cm should be evaluated clinically and with biochemical/radiological studies whenever indicated at three and twelve months postoperation. Controls should be carried out after one year and advanced radiological tests should be performed as needed. In cases with a tumor size of less than 2 cm routine controls are not needed. The follow-up protocol of small intestinal NETs and appendix NETs should be in the form of close follow-up.
This case study brings into question whether it is necessary to palpate the whole abdominal cavity and especially gastrointestinal organs in healthy individuals undergoing laparotomy for LDH. Many studies so far have recommended not to touch the gastrointestinal tract unless indicated because even powder on surgical gloves may trigger the development of postoperative adhesions. In addition, handling, palpating, or removing the bowel loops may delay the return of intestinal motility during the postoperative period. Another question is what to do when a mass lesion is encountered in the gastrointestinal system. We suggest two methods: (1) when a tumoral lesion is detected in the gastrointestinal system before the LDH procedure, the lesion’s gross macroscopic features should be evaluated and the entire mass or part of it should be resected and sent for frozen examination. When the result of the latter is a benign pathology, the hepatectomy procedure should be resumed. Whenever the result of the frozen examination is a malignant condition, hepatectomy procedure should be aborted and a definitive surgery should be performed against the mass; and (2) when a tumoral lesion is encountered incidentally at the exploration after the completion of the LDH procedure, a frozen examination of the tumoral lesion should be performed. In these patients, too, the result of the frozen examination should be obtained and surgical treatment should be carried out on the basis of oncological principles. However, an important caveat is what would be the fate of the liver graft harvested from these patients. In our opinion, ultrasonography should be performed for the living liver graft on the back table, and it should be transplanted when no hepatic lesion is identified. We assume that preoperative hepatic MDCT and dynamic MRI examinations are already negative for hepatic lesions in such patients. Recipients of such a liver should still be closely followed.
Unfortunately, we did not perform frozen examination in both patients presented here. The lesion of the patient with the appendix NET was both very small and located at the tip of the appendix. Hence, the frozen report would not affect our surgical strategy. As for the intestinal NET case, the lesion was of completely benign appearance, as can be seen in Figure 1. Despite this, we resected the segment with the lesion on the basis of the oncological principles. The recipients of both donors were put under close follow-up, and neither developed any lesion suspected to be a tumor to the date of the writing of this article.
In patients undergoing LDH operation, the abdominal cavity may be explored very gently using powderless gloves. This approach both creates a chance for early detection of some unexpected lesions and avoids potential medicolegal problems.
Whenever a suspicious lesion is detected before starting the LDH procedure, a frozen examination should be done from that lesion. When the frozen report indicates a malignant lesion, the LDH procedure should be aborted and the lesion should be resected on the basis of oncological principles. However, the question whether donors with NETs having a diameter smaller than 2 cm that are located to the tip of the appendix should be used is open to discussion.
We aimed to present two cases in which grade I neuroendocrine tumors were incidentally detected during our twelve-year living donor hepatectomy experience.
Intraoperative appearance of the lesions of both patients was compatible with benign disease.
Differential diagnosis of the first case included calcified nodule, benign intestinal tumor, and early stage malignant tumor. Differential diagnosis of the second case includes acute appendicitis, mucocele, and carcinoid tumor
No abnormal findings were detected in preoperative biochemical blood tests in both living liver donor candidates.
No abnormal findings were detected in preoperative radiological examinations in both living liver donor candidates.
The immunohistochemical examinations of the specimens of both patients were reported as grade I neuroendocrine tumor.
While a wedge resection with primary intestinal anastomosis was performed in the first case, simple appendectomy was performed in the second case.
To our knowledge, no publication other than our study has ever reported unusual findings, such as cancer, detected incidentally during a living donor hepatectomy procedure.
Even if patients undergoing living donor hepatectomy are healthy individuals, the whole abdominal cavity should be gently palpated and all findings recorded after completing laparotomy. Suspected masses or lesions should be confirmed by frozen section examination.
CARE Checklist (2013) statement: Guidelines of the CARE Checklist (2013) have been adopted while writing this manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Maida M, Ozdemir F, Sazcı A, Sugimura H S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Akbulut S, Yilmaz S. Liver transplantation in Turkey: historical review and future perspectives. Transplant Rev (Orlando). 2015;29:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 2. | Sapisochin G, Goldaracena N, Laurence JM, Levy GA, Grant DR, Cattral MS. Right lobe living-donor hepatectomy-the Toronto approach, tips and tricks. Hepatobiliary Surg Nutr. 2016;5:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 3. | Salama IA, Dessouky BA, Korayem EM, Aal SA. Impact of multislice spiral computed tomography on donor selection and surgical planning in living-related liver transplant. Exp Clin Transplant. 2010;8:111-124. [PubMed] |
| 4. | Zirpe D, Muthukumaran CS, Vaidya A, Ramamurthy A. Incidental Ventricular Septal Defect (VSD) in the Donor of a Live Donor Liver Transplant: Tackle and Proceed. J Clin Diagn Res. 2016;10:PD06-PD07. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Akamatsu N, Sugawara Y, Tamura S, Kaneko J, Togashi J, Makuuchi M. Impact of celiac axis stenosis on living donor hepatectomy. Transplant Proc. 2006;38:2948-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Yilmaz M, Olmez A, Piskin T, Unal B, Ersan V, Sarici KB, Dirican A, Yilmaz S. Incidental appendectomy in donors undergoing hepatectomy for living-donor liver transplantation. Transplant Proc. 2012;44:1630-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Akbulut S, Tas M, Sogutcu N, Arikanoglu Z, Basbug M, Ulku A, Semur H, Yagmur Y. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol. 2011;17:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Tartaglia D, Bertolucci A, Galatioto C, Palmeri M, Di Franco G, Fantacci R, Furbetta N, Chiarugi M. Incidental appendectomy? Microscopy tells another story: A retrospective cohort study in patients presenting acute right lower quadrant abdominal pain. Int J Surg. 2016;28:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Lee JH, Choi JS, Jeon SW, Son CE, Bae JW, Hong JH, Lee KW, Lee YS. Laparoscopic incidental appendectomy during laparoscopic surgery for ovarian endometrioma. Am J Obstet Gynecol. 2011;204:28.e1-28.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol Oncol Res. 2011;17:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |