Copyright
©The Author(s) 2024.
World J Hepatol. Mar 27, 2024; 16(3): 465-476
Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.465
Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.465
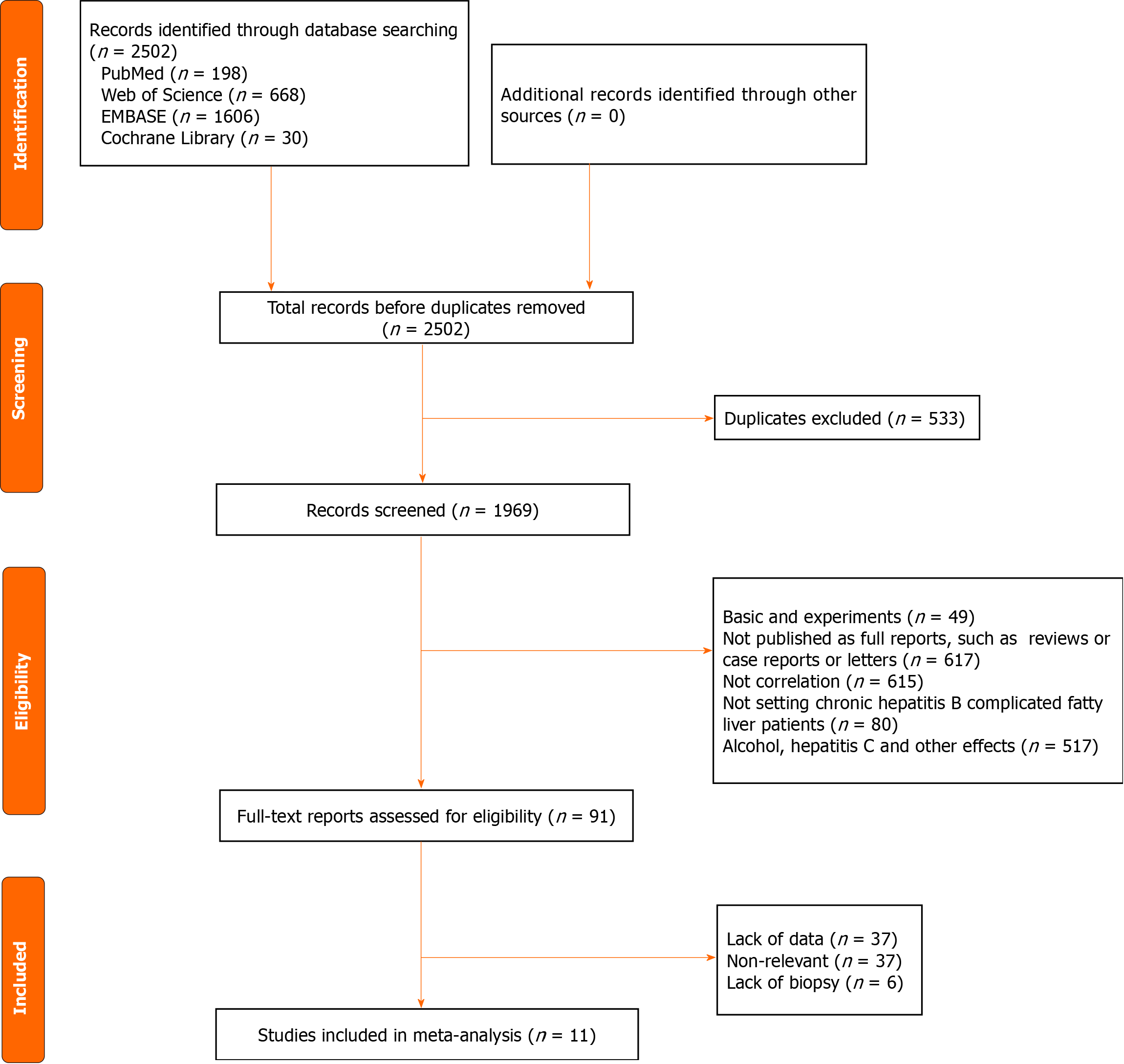
Figure 1 The flow diagram depicting the preferred reporting items for systematic reviews and meta-analyses in study selection.
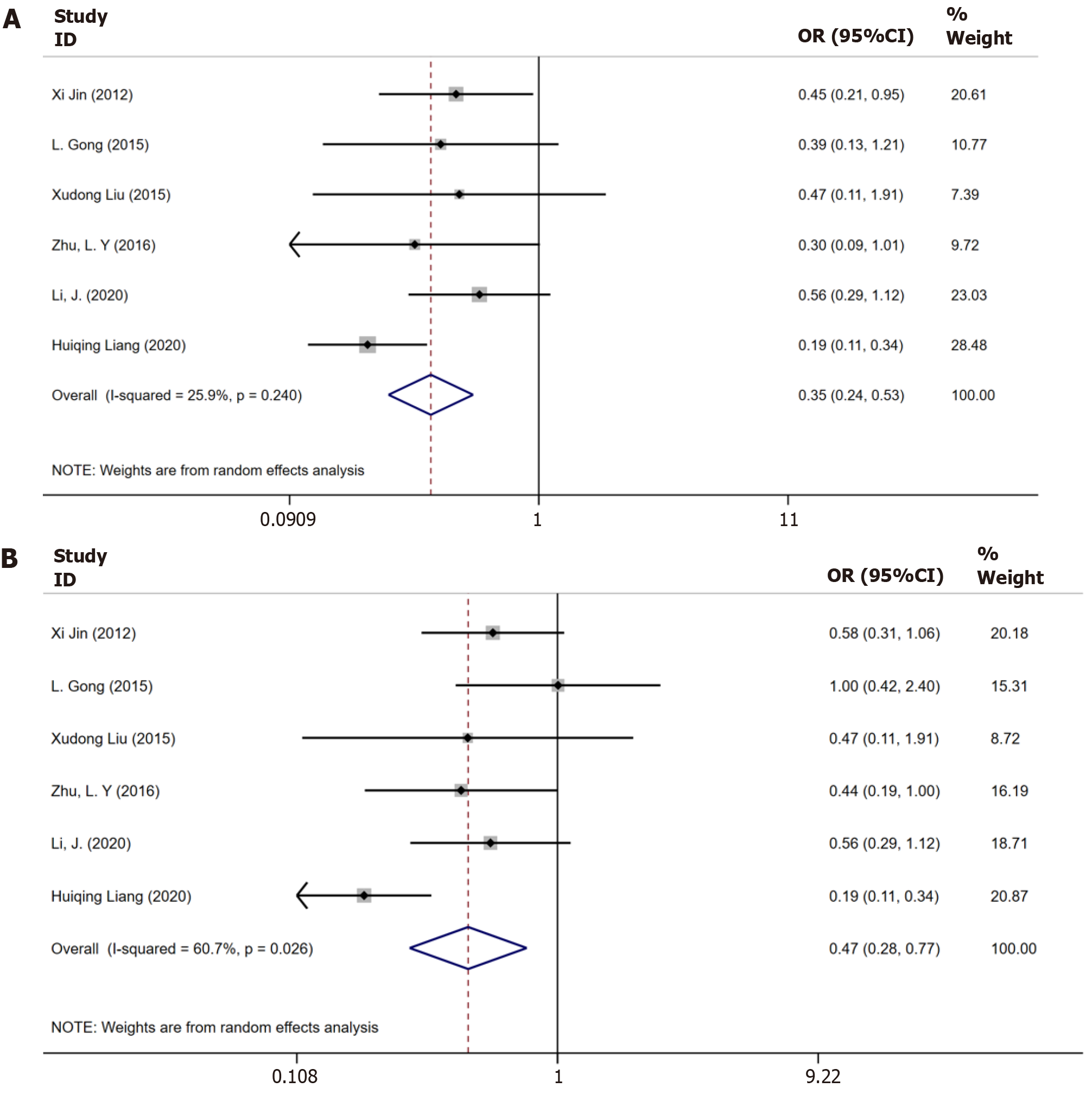
Figure 2 Meta-analysis of the biochemical responses in only chronic hepatitis B patients and in chronic hepatitis B with nonalcoholic fatty liver disease patients.
A: Biochemical response in only chronic hepatitis B (CHB) patients and in CHB with nonalcoholic fatty liver disease (NAFLD) patients until 48 wk; B: Biochemical response in only CHB patients and in CHB with NAFLD patients until 96 wk.
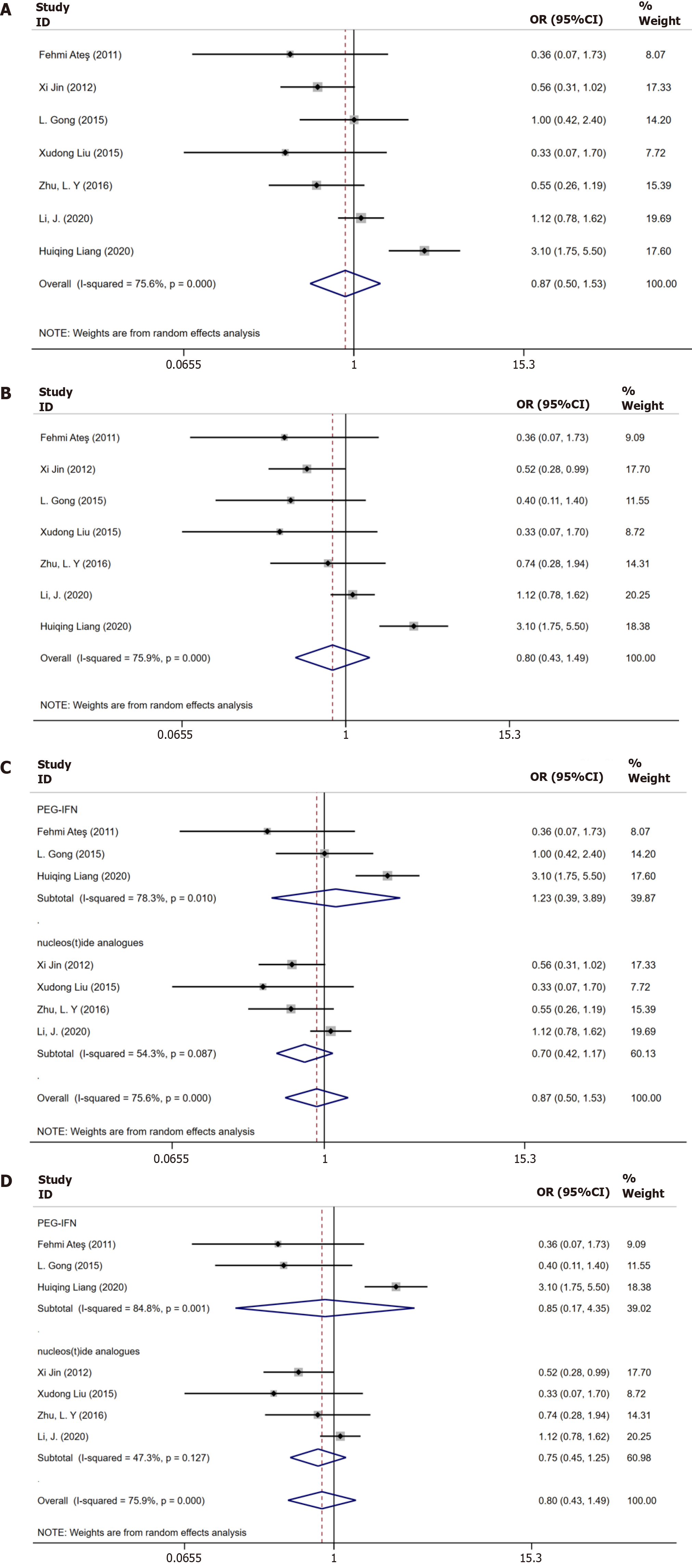
Figure 3 Meta-analysis of virological response in only chronic hepatitis B patients and in chronic hepatitis B with nonalcoholic fatty liver disease patients.
A: Virological response in only chronic hepatitis B (CHB) patients and in CHB with nonalcoholic fatty liver disease (NAFLD) patients until 48 wk; B: Virological response in only CHB patients and in CHB with NAFLD patients until 96 wk; C: Subgroup analysis according to the treatment regimens until 48 wk; D: Subgroup analysis according to the treatment regimens until 96 wk.
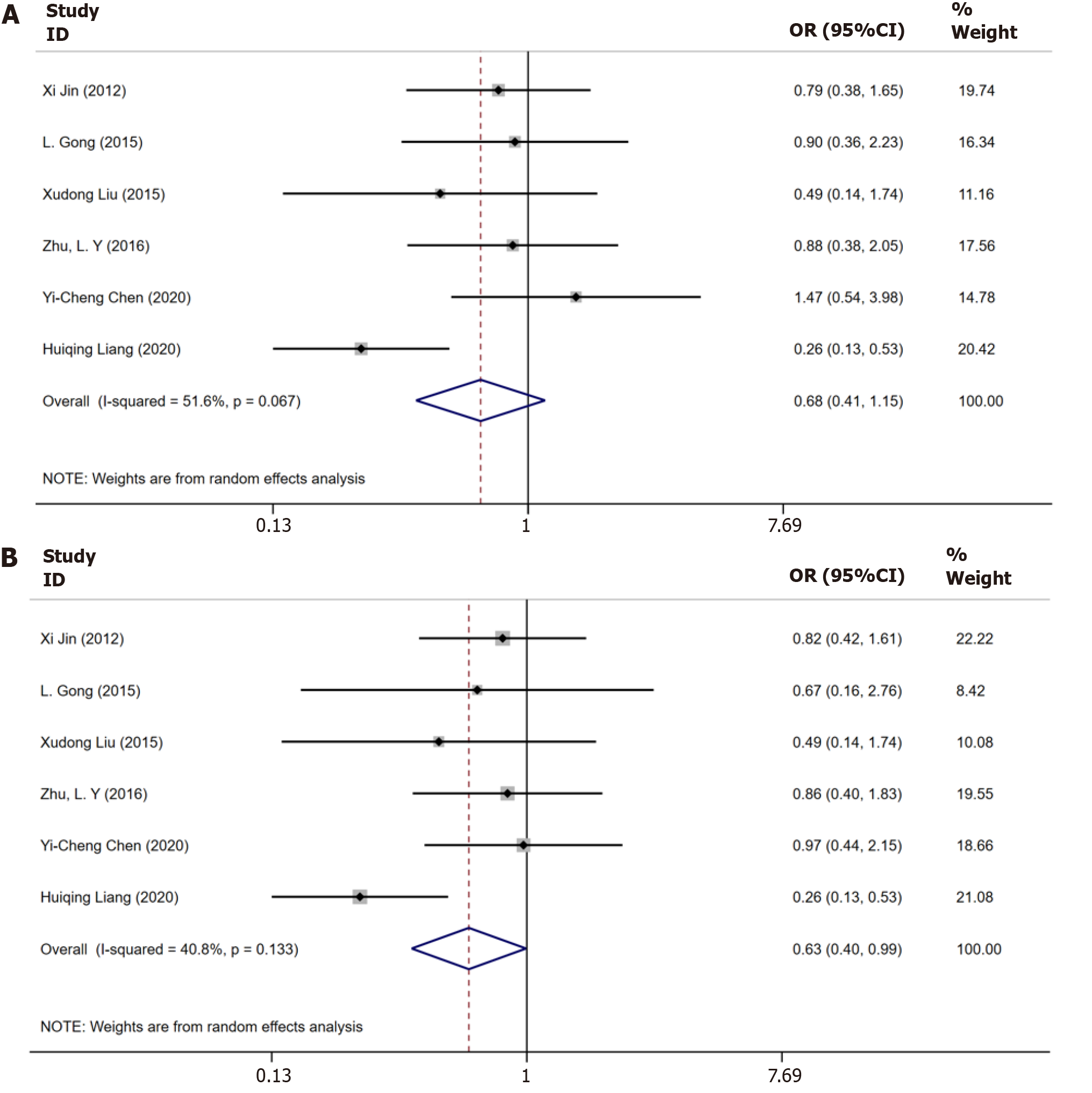
Figure 4 Meta-analysis of the serological responses in only chronic hepatitis B patients and in chronic hepatitis B with nonalcoholic fatty liver disease patients.
A: Serological response in only chronic hepatitis B (CHB) patients and in CHB with nonalcoholic fatty liver disease (NAFLD) patients until 48 wk; B: Serological response in only CHB patients and in CHB with NAFLD patients until 96 wk.
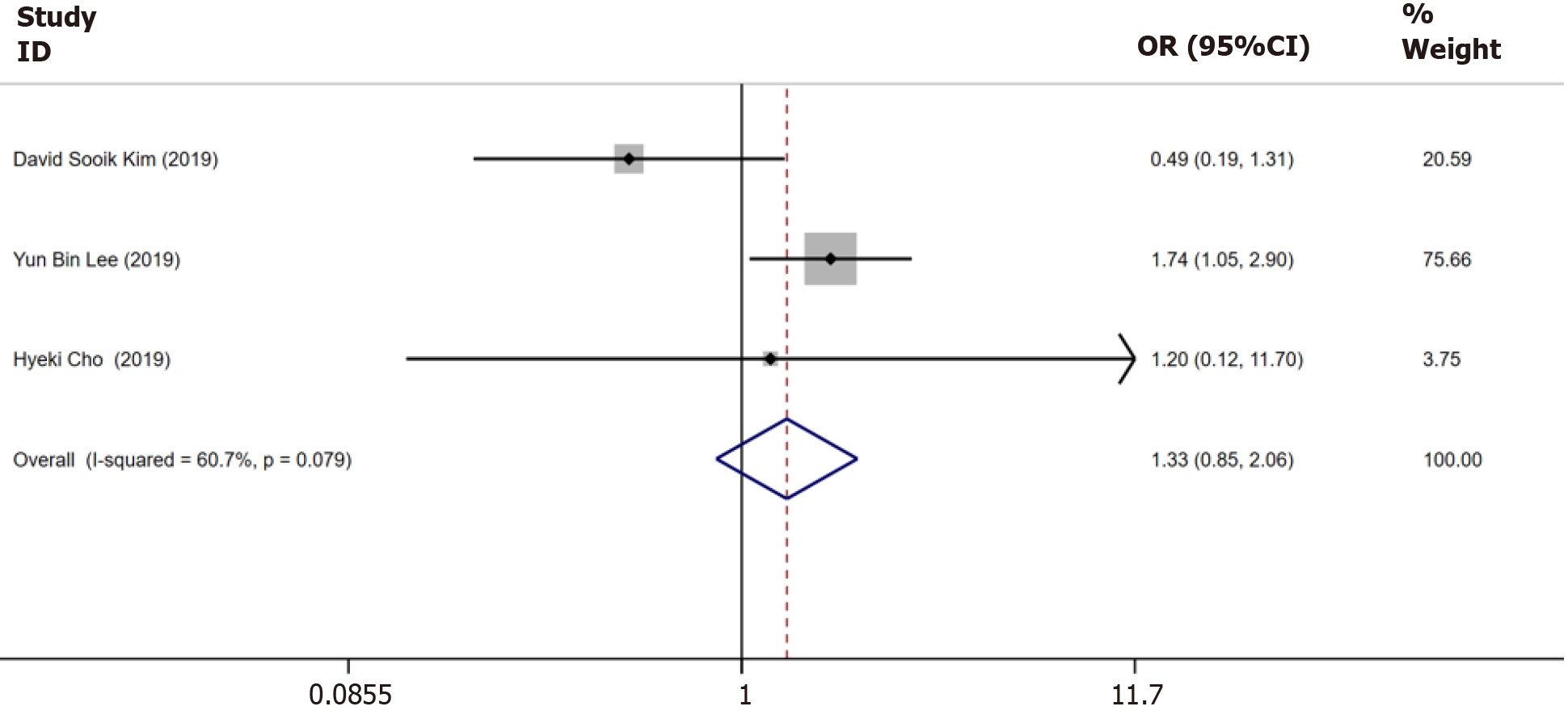
Figure 5 Meta-analysis of the incidence of hepatocellular carcinoma in only chronic hepatitis B patients and in chronic hepatitis B with nonalcoholic fatty liver disease patients.
- Citation: Liu SY, Wang D, Liu J, Yang LP, Chen GY. Influence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis. World J Hepatol 2024; 16(3): 465-476
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/465.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.465