Copyright
©The Author(s) 2024.
World J Hepatol. Mar 27, 2024; 16(3): 405-417
Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.405
Published online Mar 27, 2024. doi: 10.4254/wjh.v16.i3.405
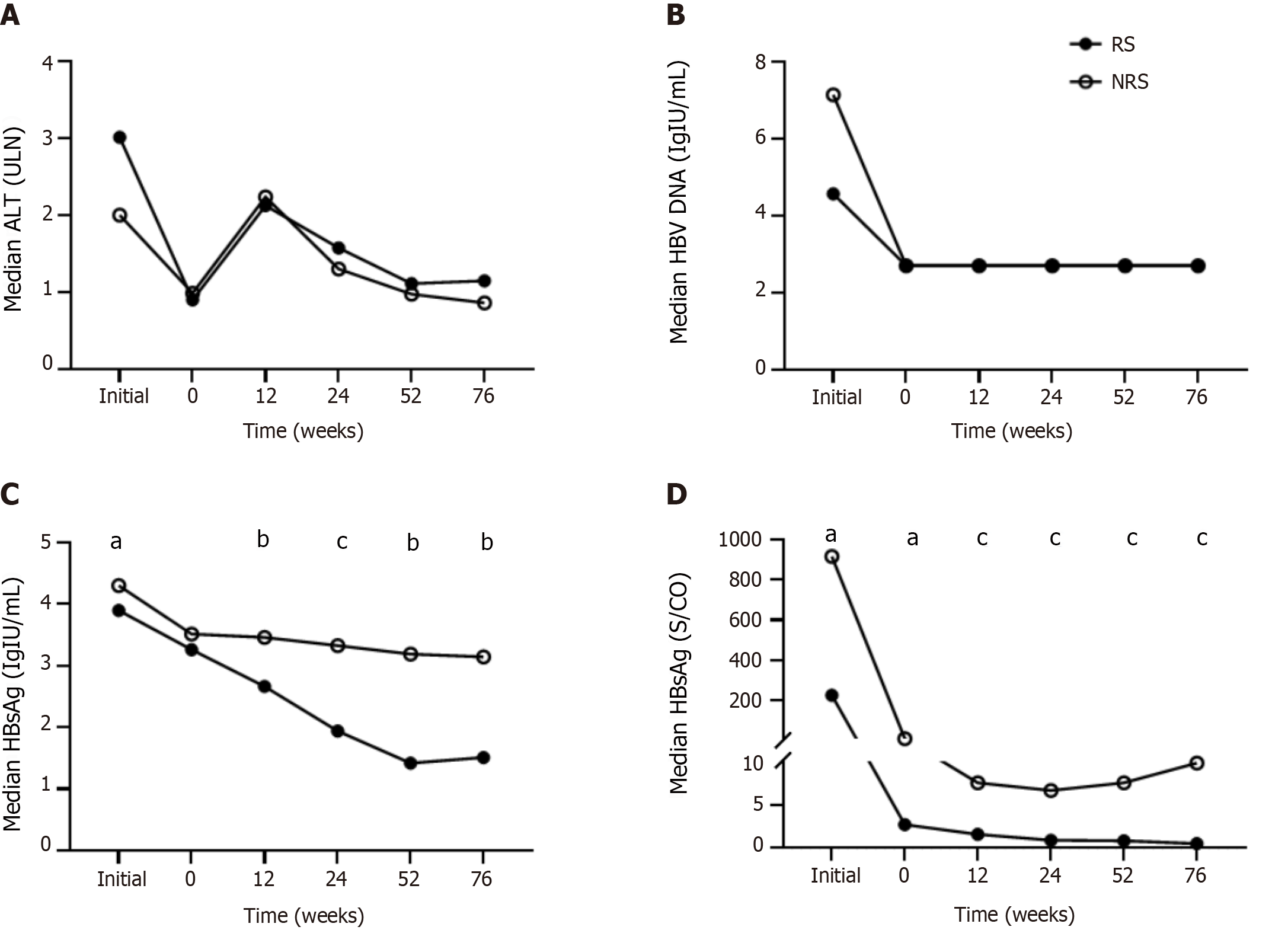
Figure 1 Kinetics of serum markers in patients with chronic hepatitis B during peginterferon alpha treatment and follow-up between responders and non-responders.
A: Alanine aminotransferase; B: Hepatitis B virus DNA; C: Hepatitis B surface antigen; D: Hepatitis B e antigen. aP < 0.05, bP < 0.01, cP < 0.001. RS: Responders; NRS: Non-responders; ALT: Alanine aminotransferase; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen.
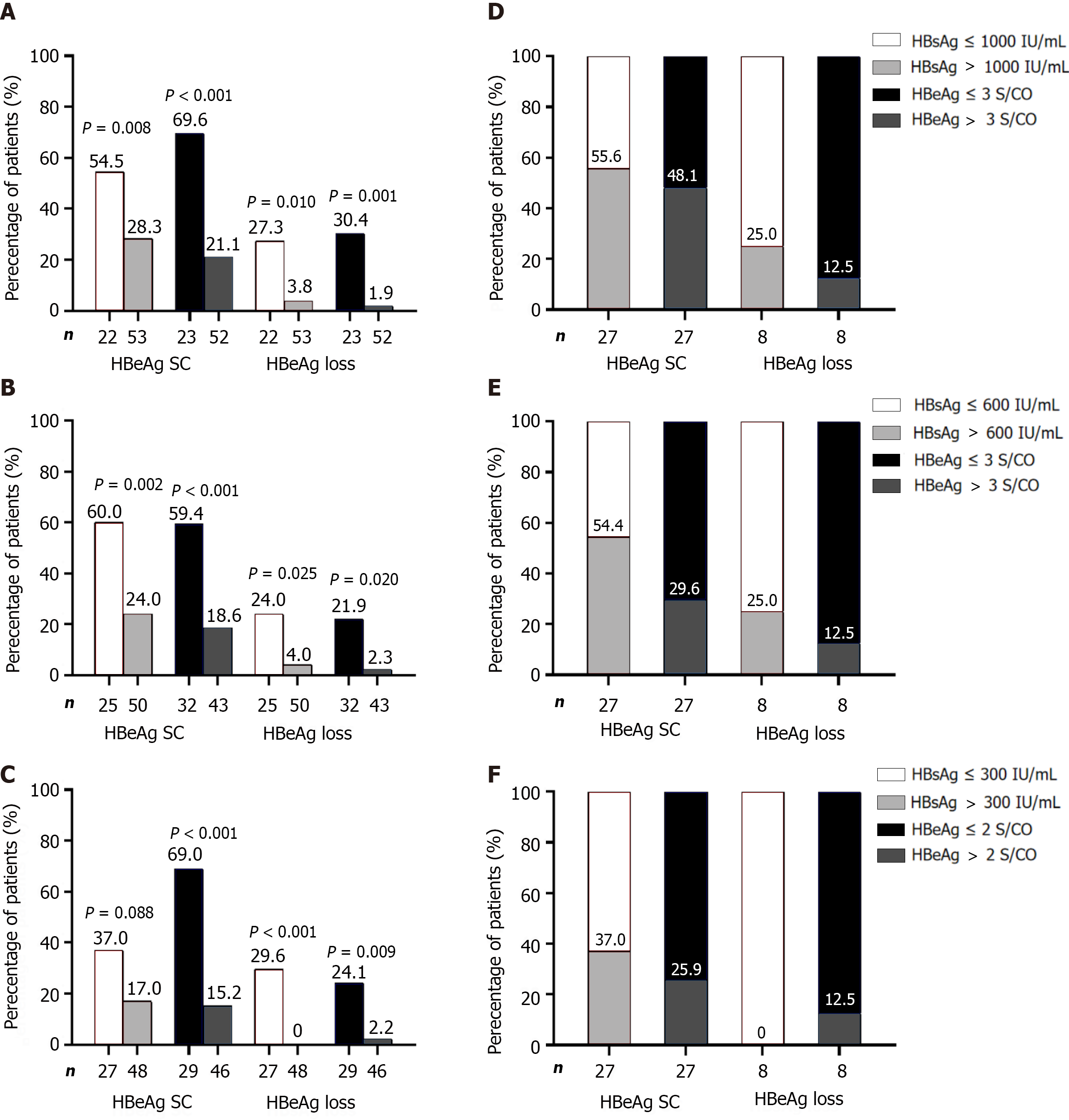
Figure 2 Response and hepatitis B surface antigen loss rates at 24 wk post-treatment based on patients who met single hepatitis B surface antigen or hepatitis B e antigen cutoffs at each time point.
A-F: Moreover, the proportion of patients who met the single hepatitis B surface antigen (HBsAg) or hepatitis B e antigen (HBeAg) cutoffs at each time point was determined based on patients that achieved response and HBsAg loss at 24 wk post-treatment. Baseline (A and D), week 12 (B and E), week 24 (C and F). HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen.
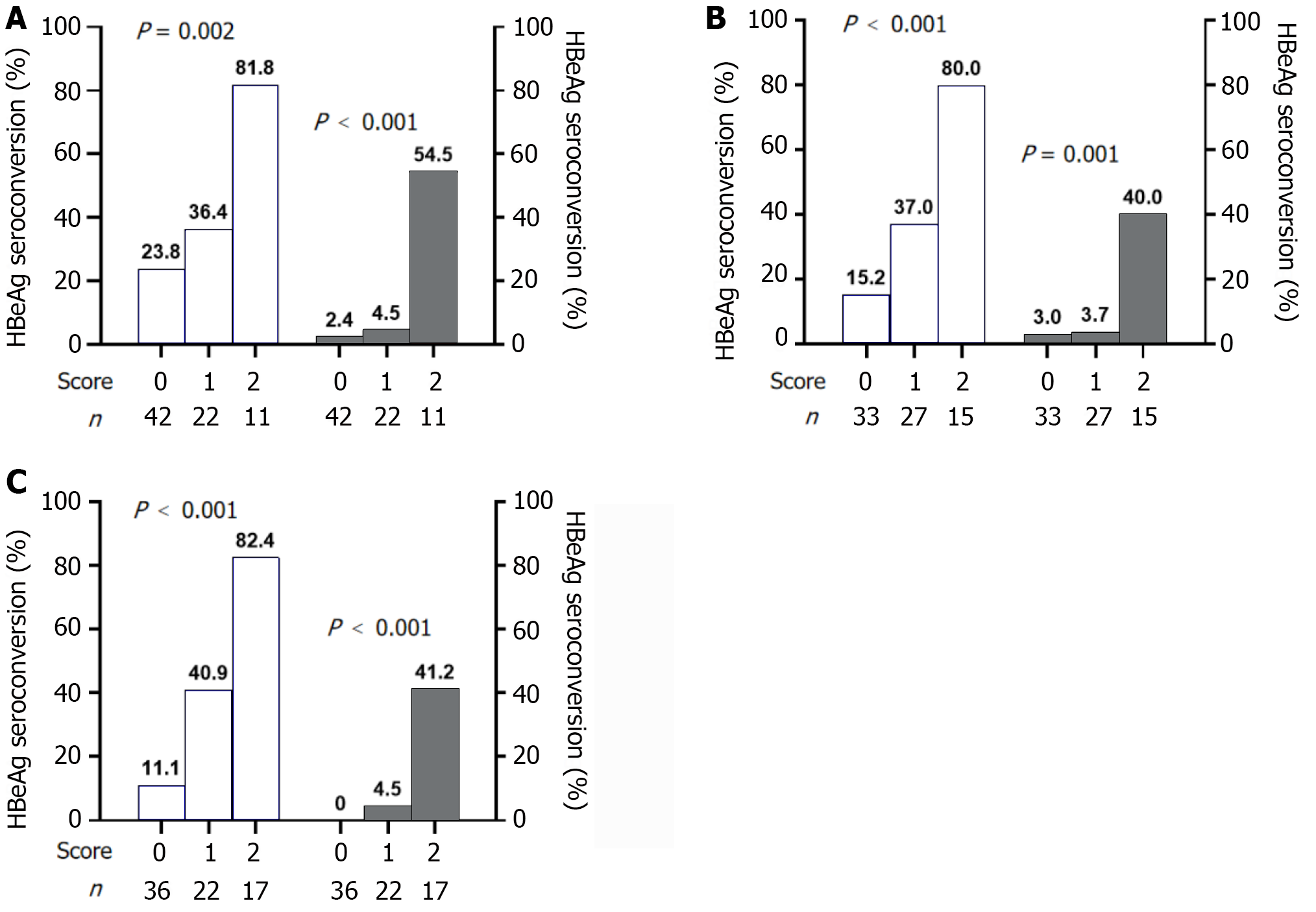
Figure 3 Performance of predictive models at baseline, week 12, and week 24 for evaluating hepatitis B e antigen seroconversion (blue bars) and hepatitis B surface antigen loss (pink bars) at 24 wk post-treatment in patients with chronic hepatitis B treated with peginterferon alpha.
A: Baseline; B: Week 12; C: Week 24. HBeAg: Hepatitis B e antigen.
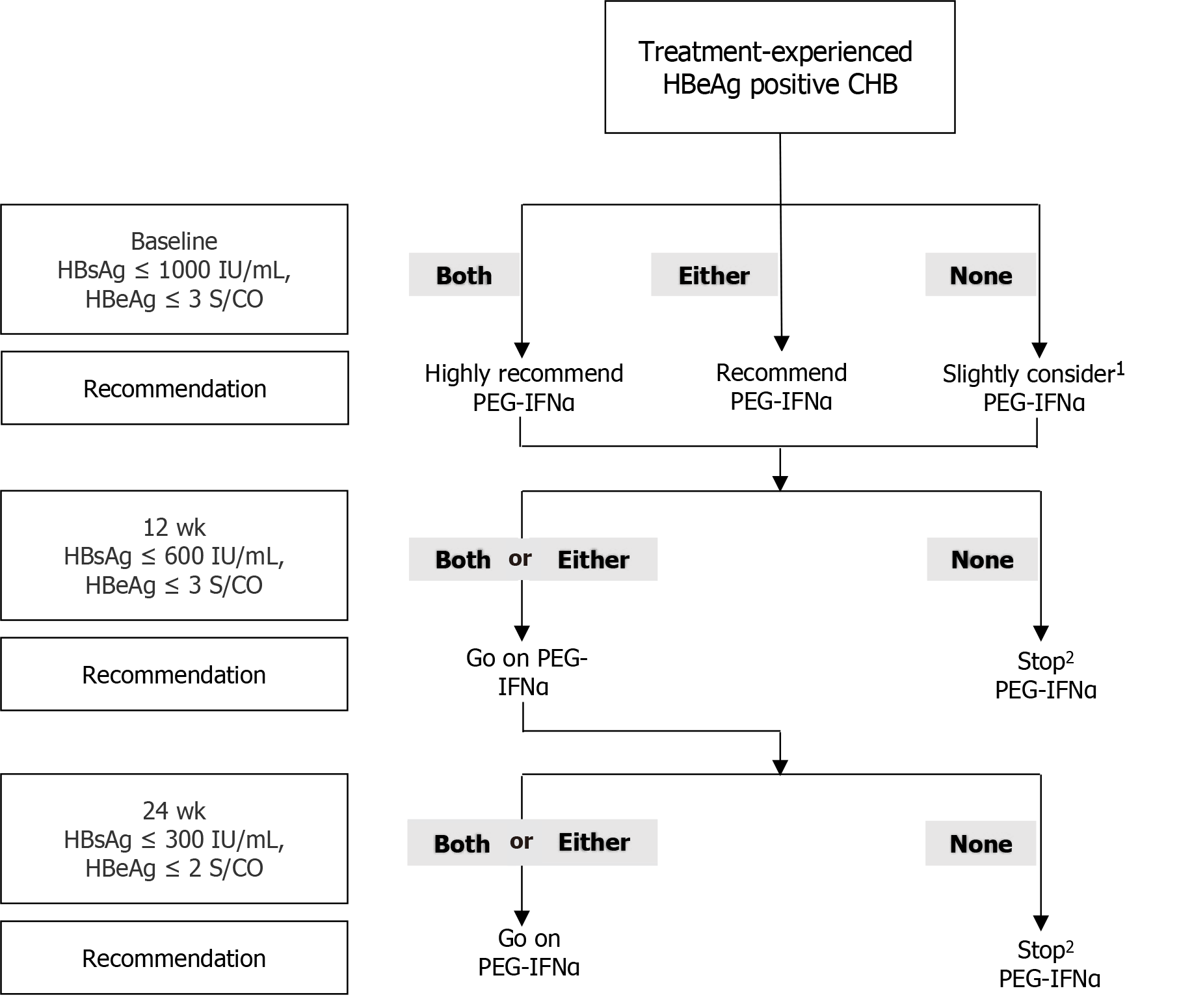
Figure 4 Response-guided therapy strategy for predicting response at 24 wk post-treatment based on hepatitis B surface antigen and hepatitis B e antigen levels at baseline, week 12, and week 24 in the management of previously treated patients with hepatitis B e antigen-positive chronic hepatitis B.
1According to the prediction models, patients who didn’t meet the two cutoffs at baseline had a response of 23.8% at end of follow-up. So we gave a recommendation of slightly considering peginterferon-alfa. 2For patients who didn’t meet corresponding two cutoffs at week 12 or week 24 but met cutoffs at baseline, peginterferon-alfa could be considered because there was a possibility of attaining a response at end of follow-up. CHB: Chronic hepatitis; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; PEG-IFNα: Peginterferon-alfa.
- Citation: Zhang PX, Zheng XW, Zhang YF, Ye J, Li W, Tang QQ, Zhu J, Zou GZ, Zhang ZH. Prediction model for hepatitis B e antigen seroconversion in chronic hepatitis B with peginterferon-alfa treated based on a response-guided therapy strategy. World J Hepatol 2024; 16(3): 405-417
- URL: https://www.wjgnet.com/1948-5182/full/v16/i3/405.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i3.405