Copyright
©The Author(s) 2021.
World J Hepatol. Sep 27, 2021; 13(9): 1079-1097
Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1079
Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1079
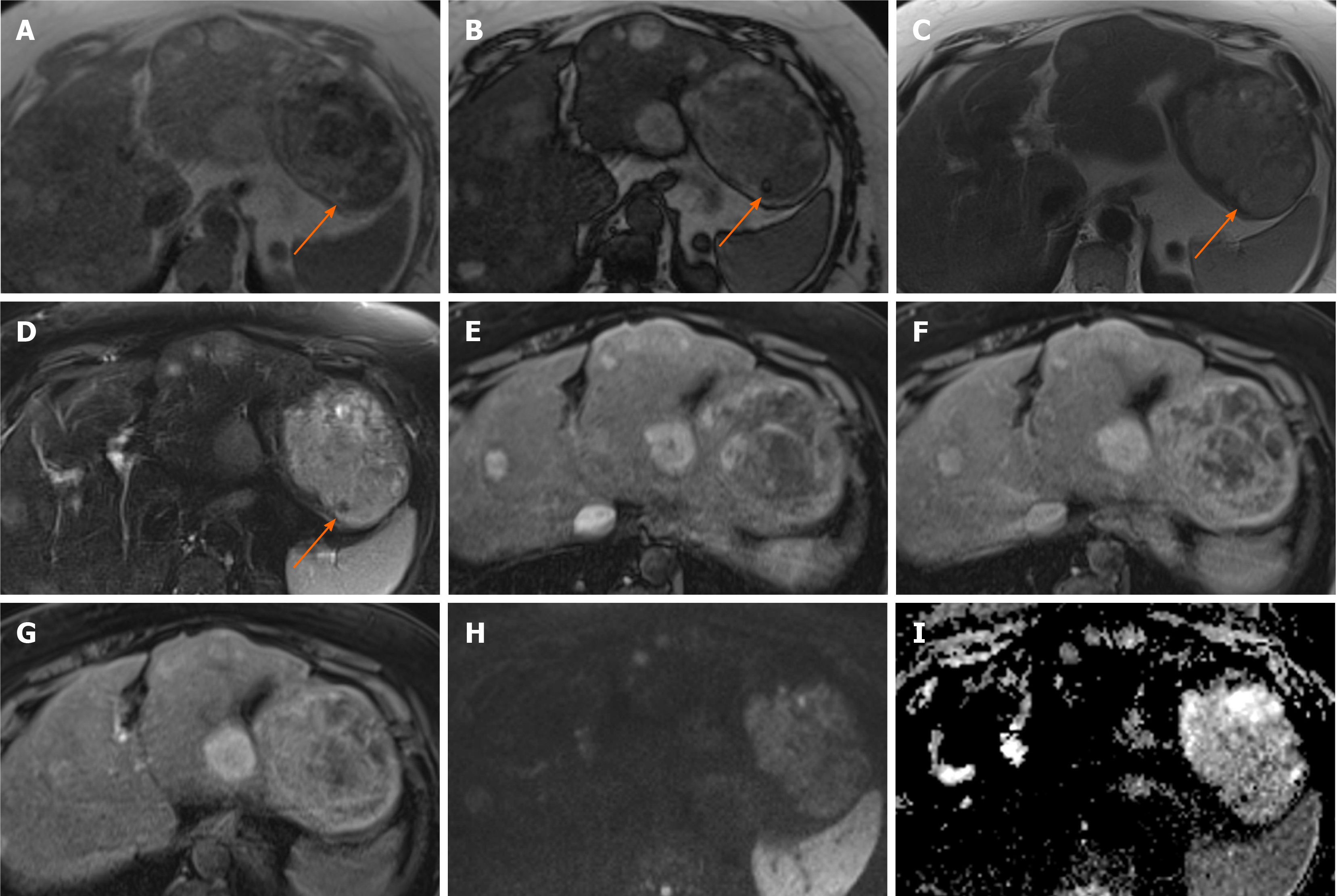
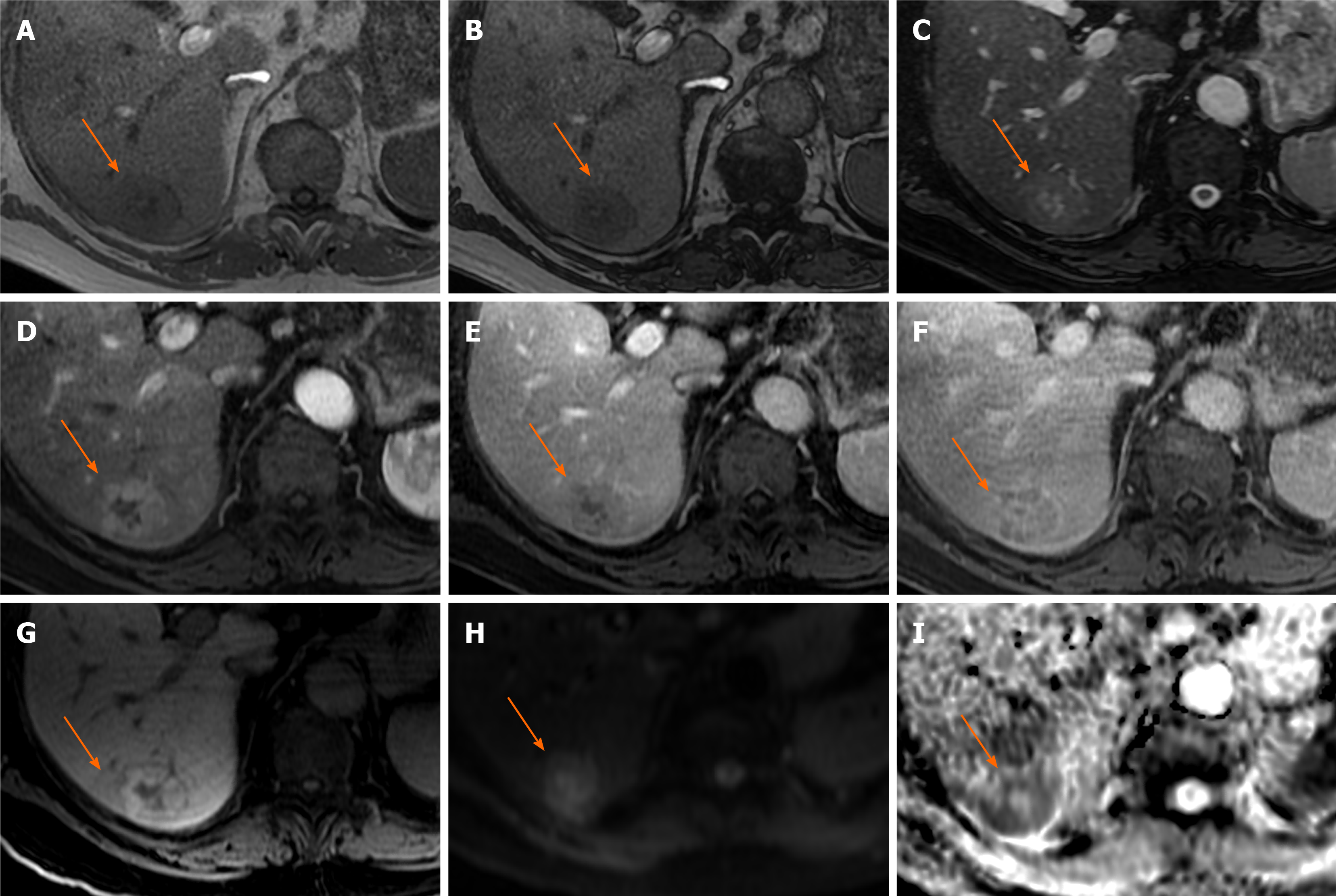
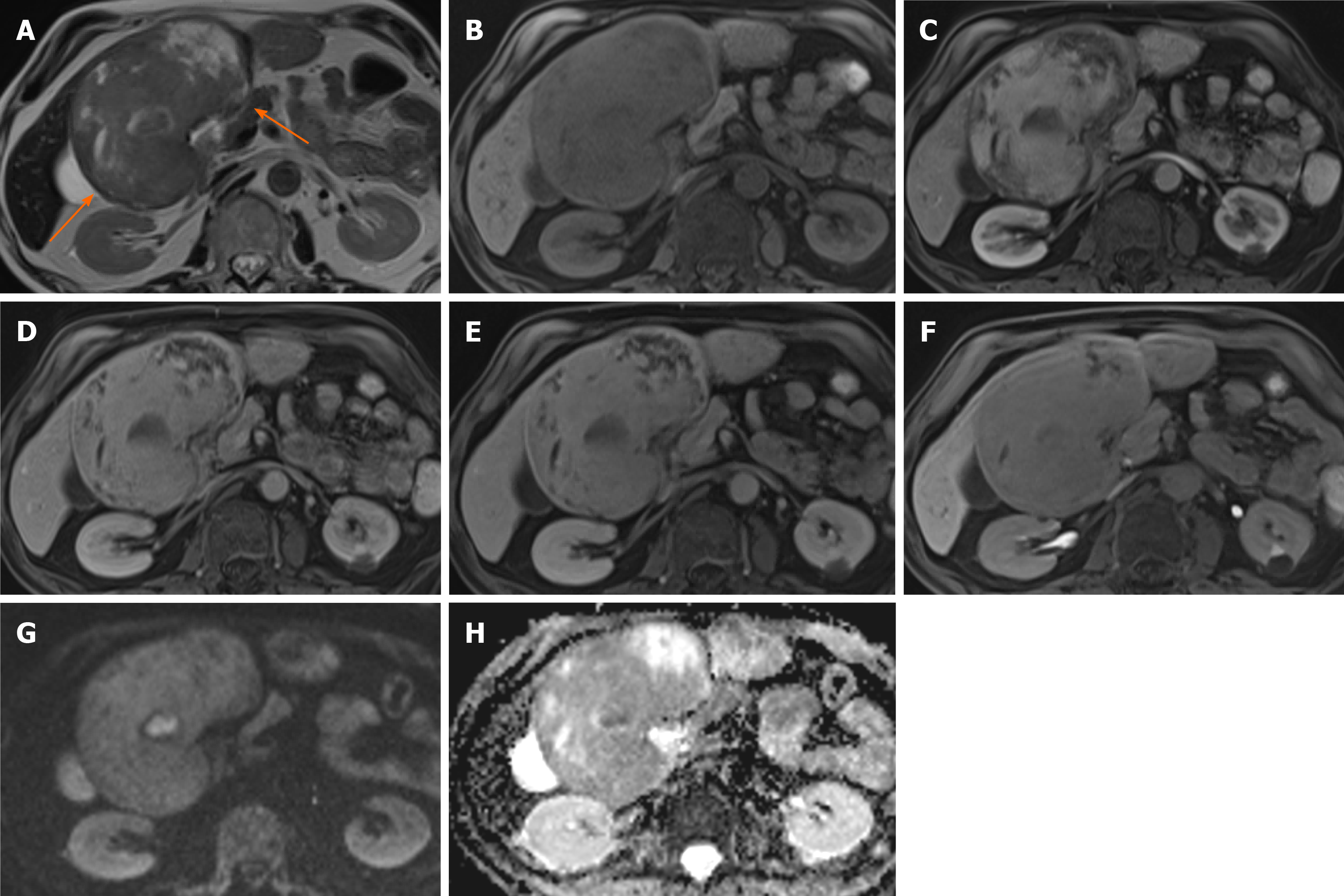
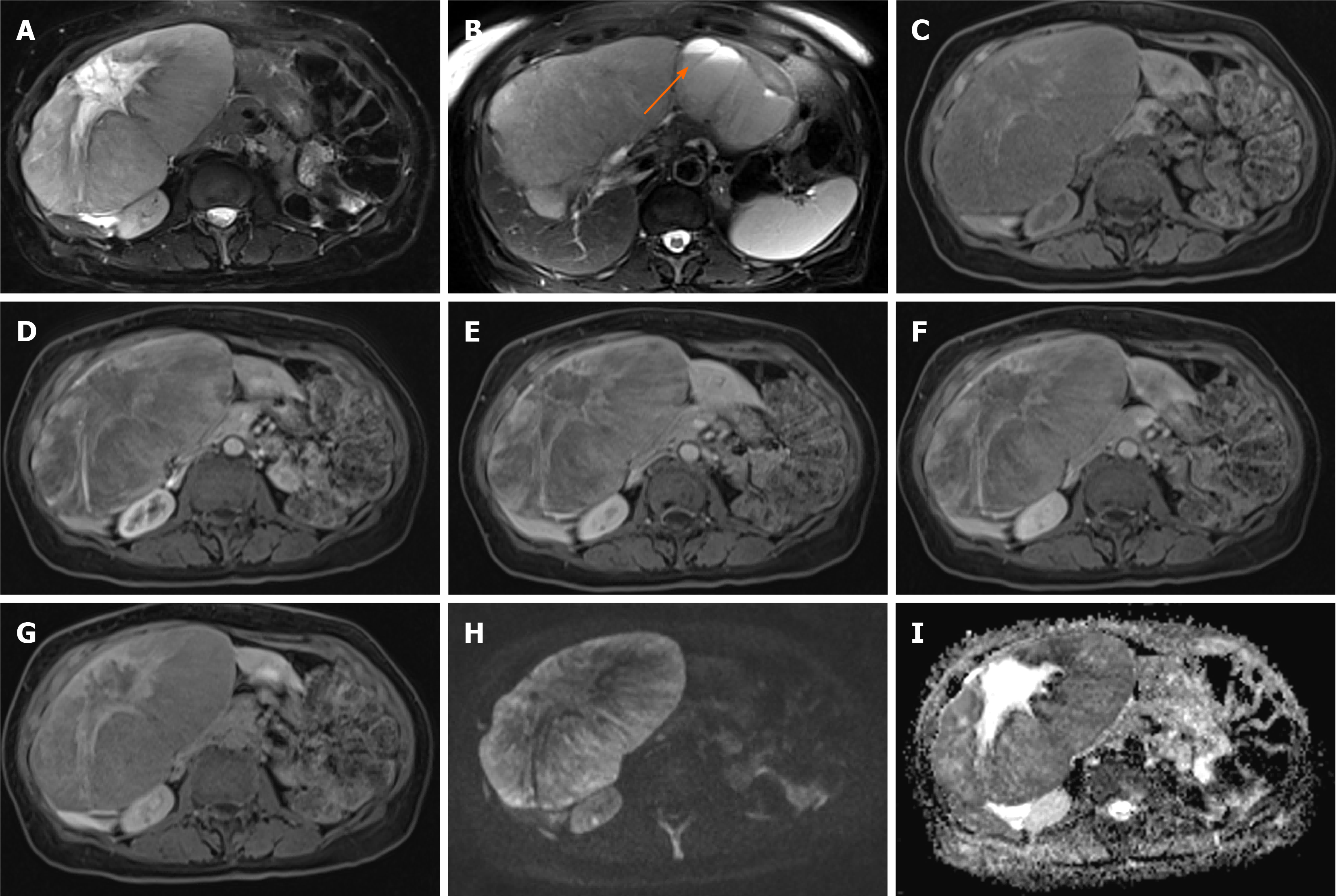
Figure 1 Hepatocellular adenoma.
A 42-year-old lady with congenital absence of portal vein and history of use of oral contraceptive medication presented with worsening jaundice. She underwent computed tomography that demonstrated multiple liver lesions that could not be characterised and subsequent magnetic resonance with gadoxetic acid was performed. This demonstrates multiple small lesions showing characteristics those of focal nodular hyperplasia. There is a further exophytic large lesion arising from the left liver lobe. The lesion is well-defined, T2 hyperintense and shows intratumoral fat (arrowed). A: In phase T1; B: Out-of-phase T1; C: T2-weighted imaging (T2-WI); D: Fat suppressed T2-WI; E-G: The arterial (E) and equilibrium (F) phase sequences demonstrates heterogenous enhancement with progressive filling in and there is contrast retention on hepatobiliary phase (G); H and I: Diffusion-weighted imaging (H) and apparent diffusion coefficient (I) sequences show no restricted diffusion. Due to atypical appearances this was resected and histology revealed this to be an adenoma with background steatotic liver.
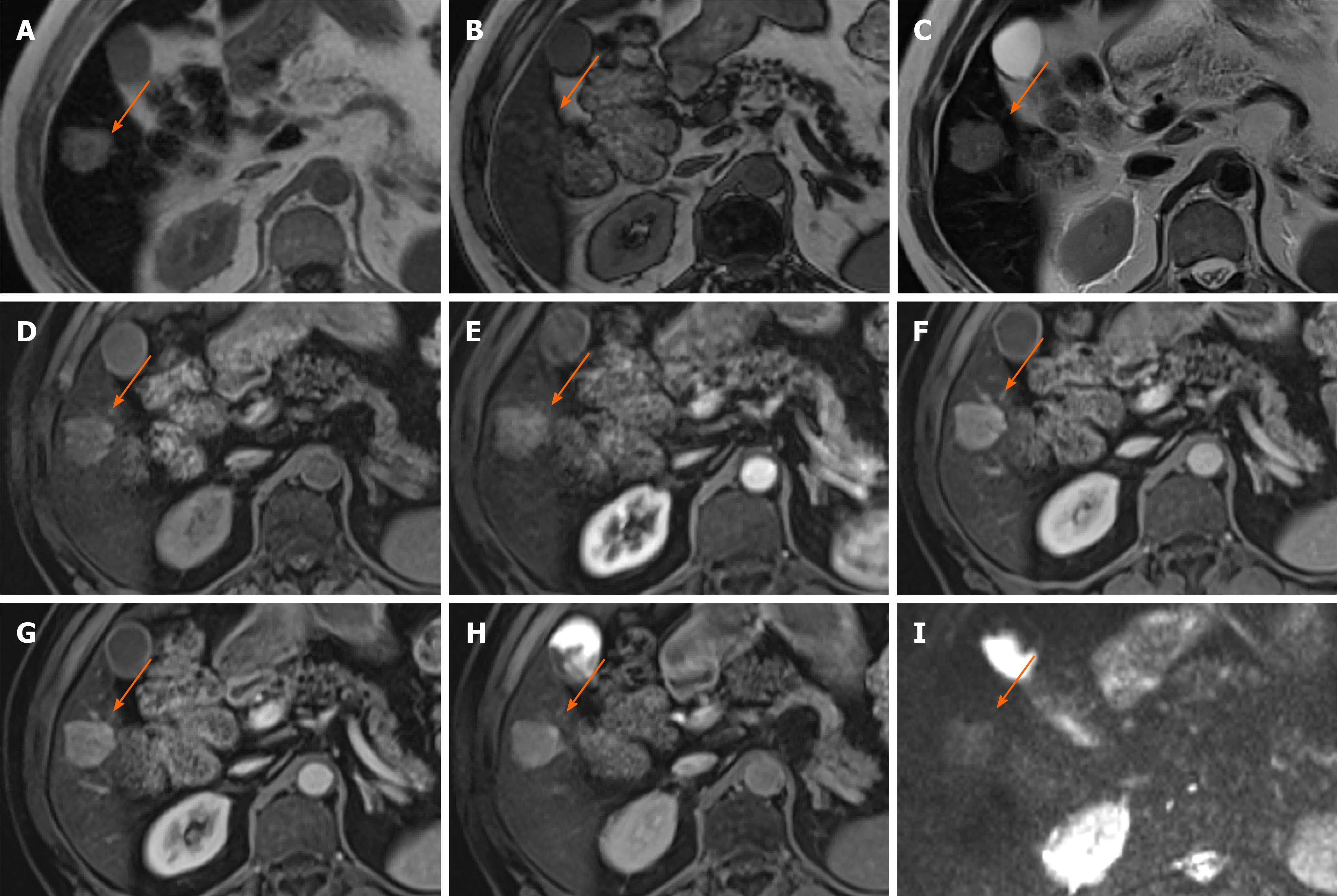
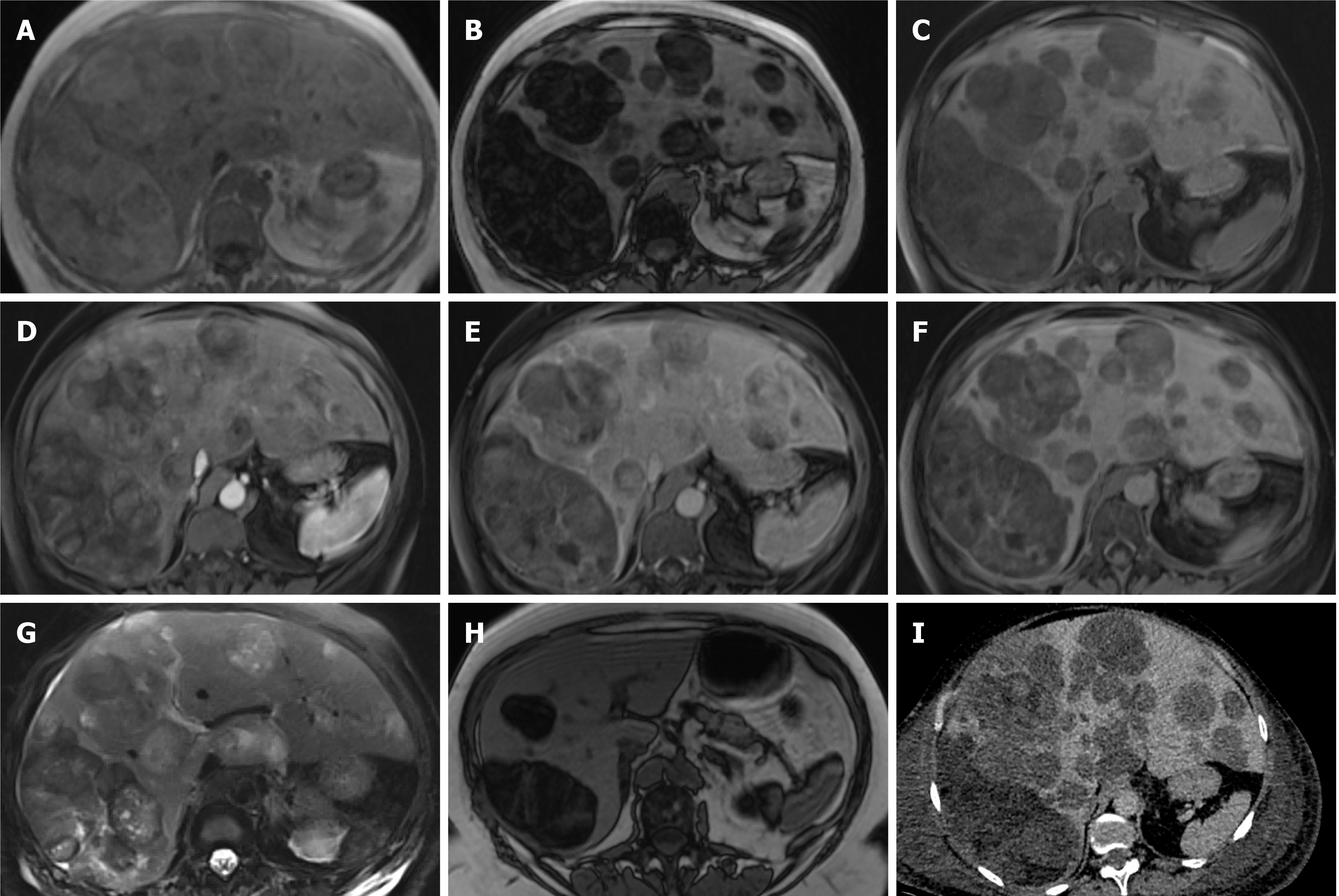
Figure 2 Hepatocellular adenoma.
A 27-year-old lady with background of glycogen storage type 1 disease. A and B: Segment IVA liver lesion demonstrating mild T2 hyperintensity with atoll sign (A) and cystic foci (B); C and F: No signal drop out on out-of-phase (F) when compared to in-phase (C) T1-weighted sequence; D, E and G: There is quite homogenous hyperenhancement on arterial phase (D) with no washout on portal venous (E) and delayed (G) phases; H: Hepatobiliary phase shows contrast retention within the lesion; I: Coronal T2-weighted shows hepatosplenomegaly as features of glycogen storage disease type I. The lesion has increased in size and therefore was resected, histology revealed an inflammatory subtype hepatocellular adenoma.
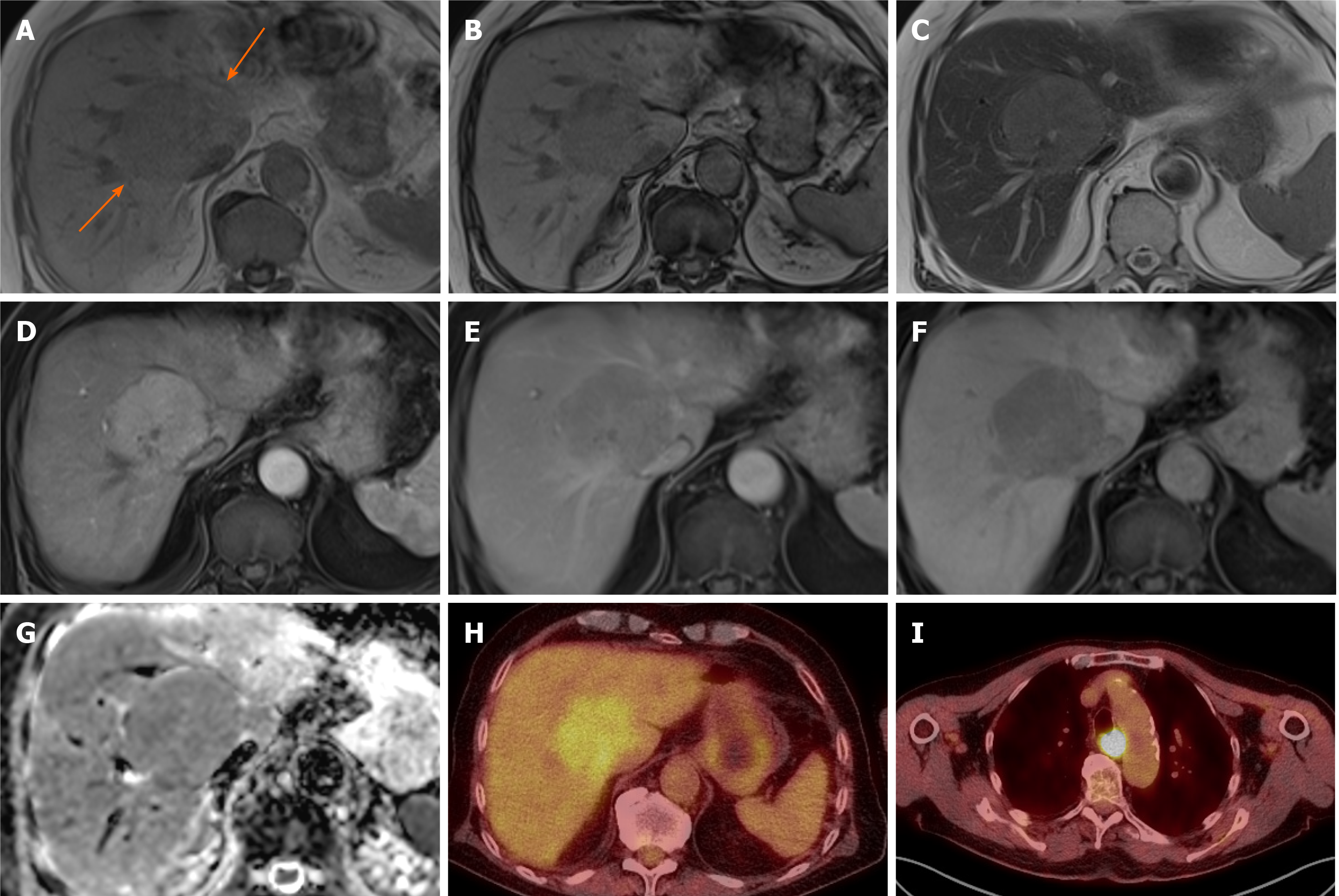
Figure 3 Focal nodular hyperplasia.
A 53-year-old woman with background of renal failure with renal transplant and history of autoimmune hepatitis since childhood. She underwent ultrasound (US) of the abdomen after an episode of pancreatitis which identified portal vein thrombosis. Subsequent unenhanced computed tomography (due to poor renal function) demonstrated a liver lesion in segment 5. Initially contrast US was attempted due to renal failure, which showed liver lesions to be multiple, but the lesions were indeterminate and subsequent magnetic resonance with gadoxetic acid was performed. Largest lesion in segment 5 selected as example. A and B: In-(A) and out-(B) of phase imaging shows some signal loss and mildly hypointense T1-weighted signal of the ill-defined right lobe lesion; C and G: T2-weighted without (C) and with fat suppression (G) show mildly hyperintense T2 signal; D and K: Diffusion-weighted imaging (D) and apparent diffusion coefficient (K) images show no diffusion restriction. E, F, and H: There is heterogenous enhancement on arterial phase (E) with no washout and slightly more homogenous contrast enhancement on portal venous (F) and delayed (H) phases; I and J: Heterogenous contrast uptake persists on hepatobiliary phase (I), which is mostly rim-like. Further similar lesion demonstrated on portal venous phase (J) in segment 7 (long arrow) and the known portal vein thrombus (short arrow). Initial radiological diagnosis favoured hepatocellular carcinoma. Liver function tests were normal. Initial non targeted liver biopsy was inconclusive for underlying cirrhosis. Second targeted lesion biopsy was performed. Both specimens were further reviewed in a national liver centre. Histology of the lesion was consistent with focal nodular hyperplasia and background liver demonstrated no cirrhosis, but signs consistent with nodular regenerative hyperplasia.
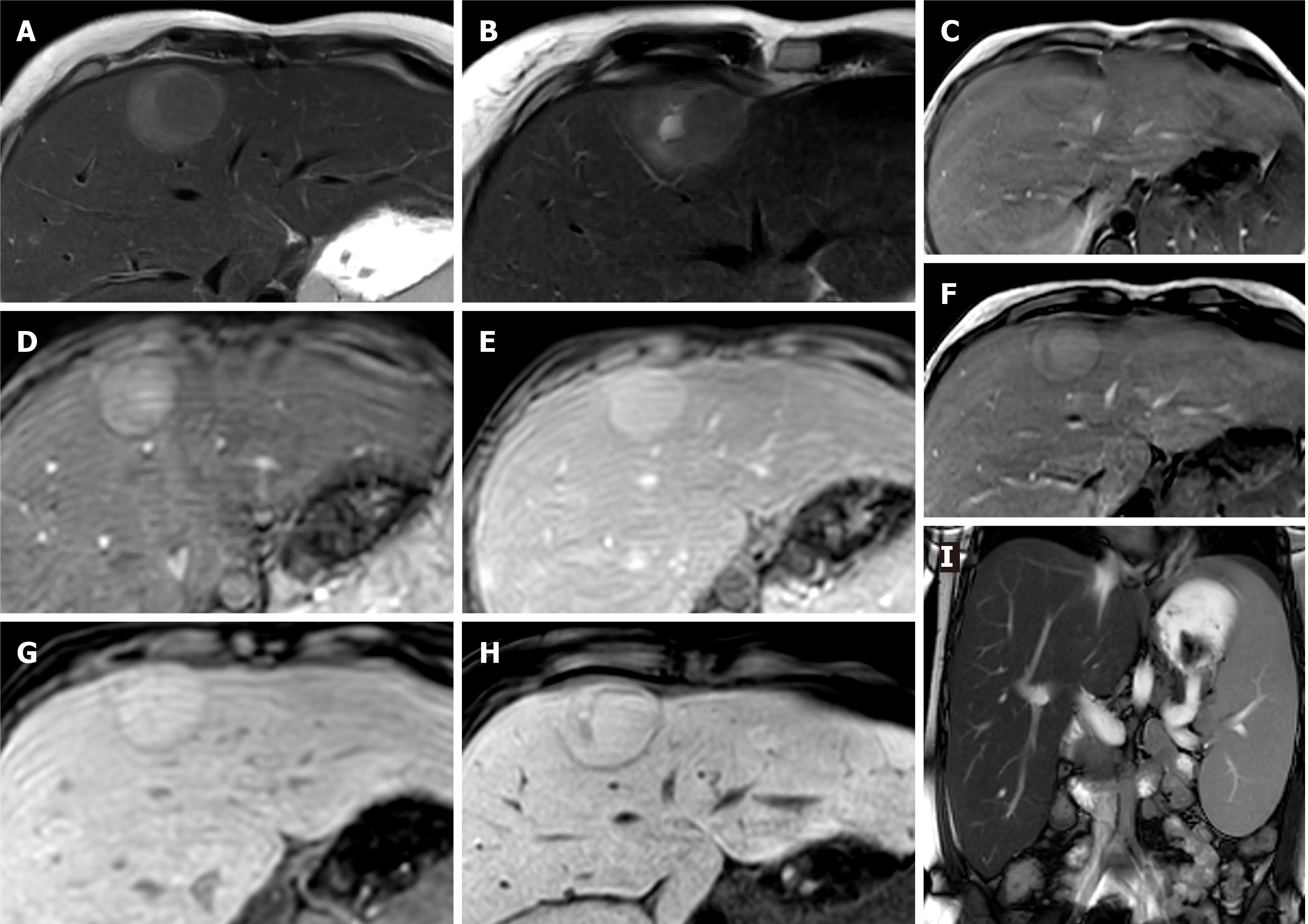
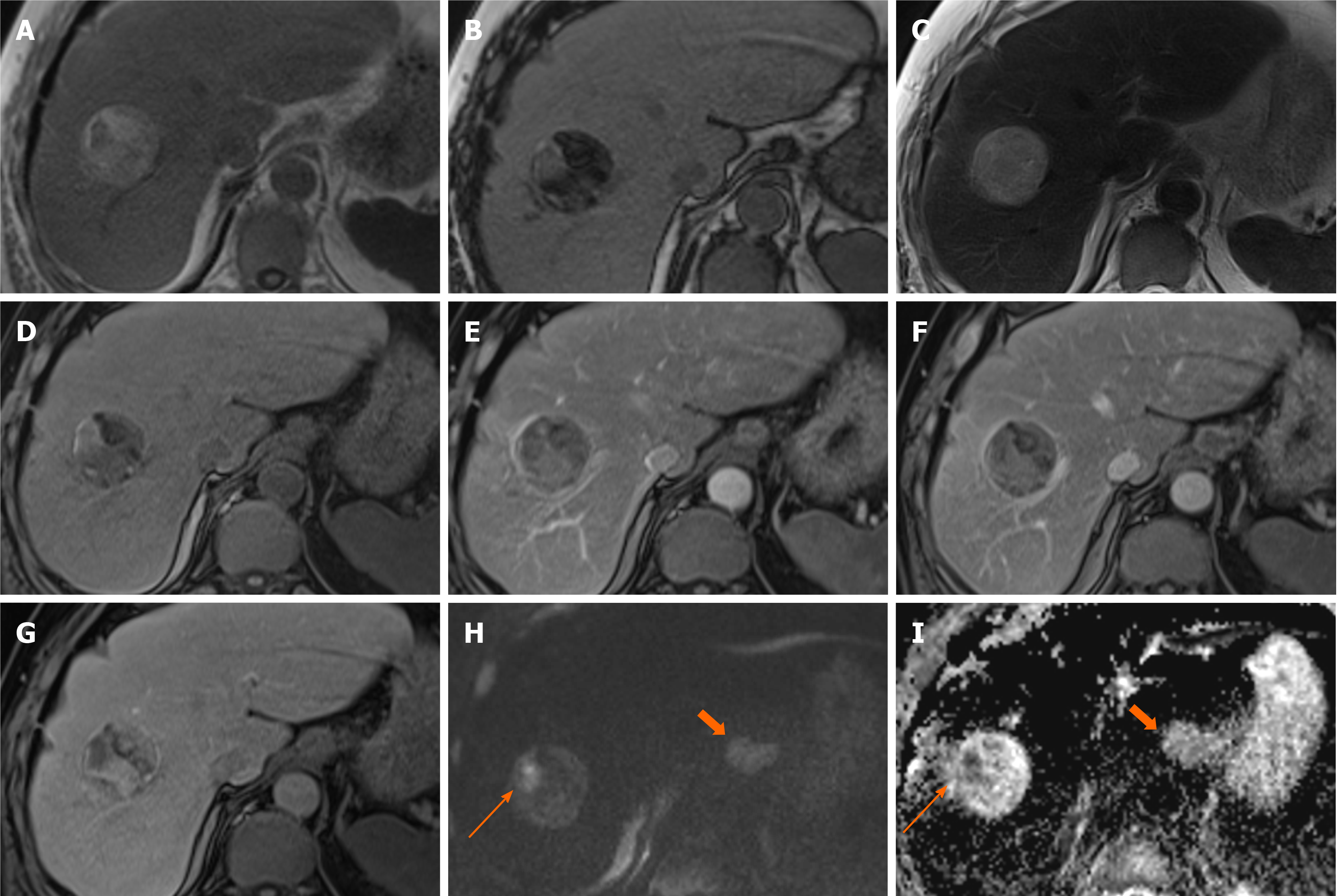
Figure 4 Hepatic angiomyolipoma.
A 21-year-old man referred by general practitioner for ultrasound of liver due to 6-mo history of intermittent abdominal pain and isolated raised bilirubin, treated as Gilbert’s syndrome. The patient had no prior medical history, no use of drugs or steroids and was not a heavy drinker. Incidental liver lesion was found and patient underwent subsequent magnetic resonance (MR) with gadoxetic acid to characterise this further. This was initially described as adenoma, but as the lesion increased in size on follow up imaging it was resected. Histology showed this to be an angiomyolipoma. A and B: MR demonstrates well-defined lesion with high signal foci on T1 in-phase (A) showing loss of signal on out-of-phase imaging (B); C and D: There are also hypointense foci on fat suppressed T2-weighted (C) when compared to T2-weighted imaging without fat suppression (D); E and F: The lesion shows enhancement on arterial phase (E) with no washout on equilibrium phase (F) and no pseudocapsule; G: There is no contrast uptake on hepatobiliary phase; H and I: No diffusion restriction as seen on diffusion-weighted imaging (H) and apparent diffusion coefficient (I) sequences.
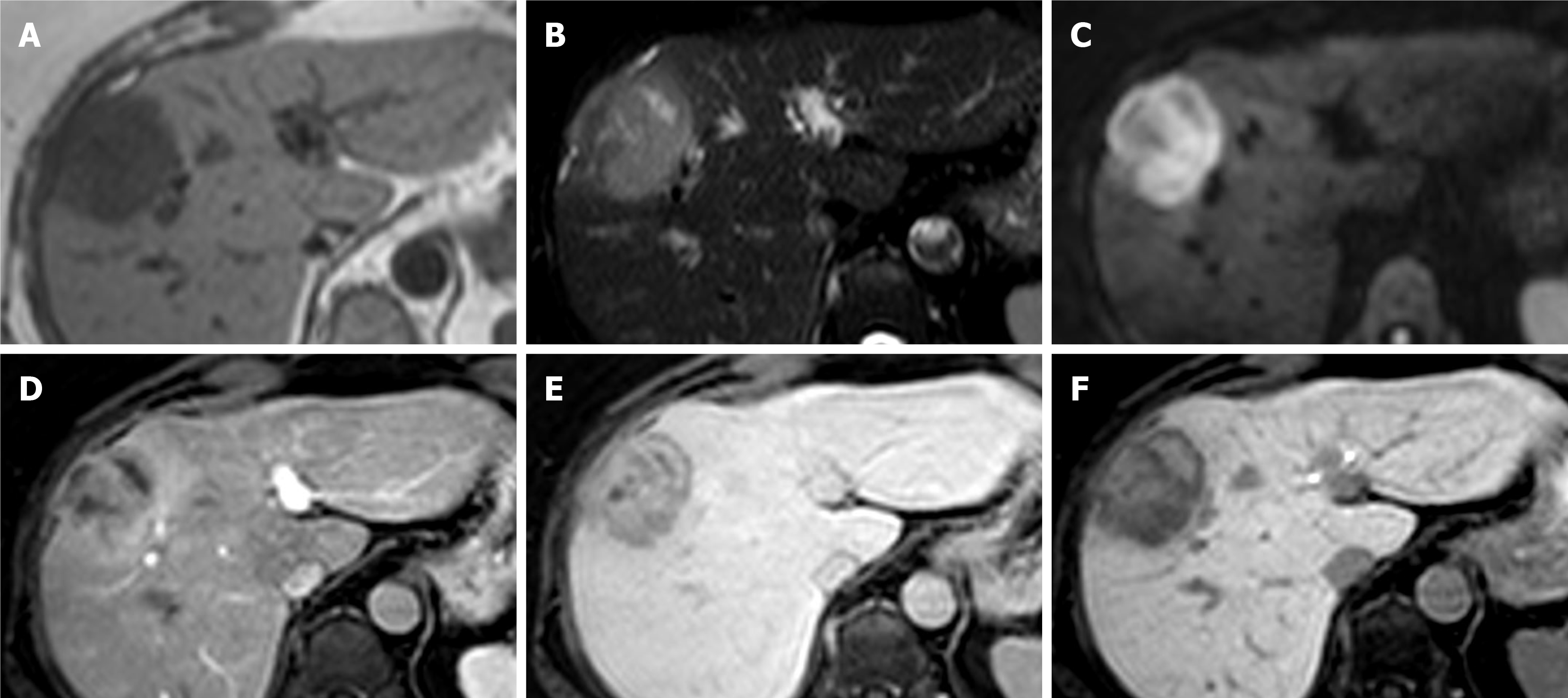
Figure 5 Hepatocellular carcinoma.
A 74-year-old man presented with incidental liver lesion found on routine computed tomography colonography. He had normal liver function and alpha-fetoprotein levels. The lesion had undergone further characterisation with magnetic resonance. A and B: There is no evidence of intralesional fat on T1-weighted in-phase (A) and out-of-phase (B) sequences; C: On T2-weighted images, the lesion is nearly isointense to the background liver and shows a hyperintense central scar, which can sometimes be seen in focal nodular hyperplasia; D-F: The lesion then demonstrates enhancement on the arterial phase (D) with evidence of washout as compared to background liver parenchyma on the portal venous (E) and delayed phases (F); there is also subtle peripheral enhancement on the delayed phase, likely representing a capsule, but the central scar remains largely unenhanced throughout; G: Hepatobiliary phase sequence demonstrates uptake of contrast in the majority of the lesion, with no uptake in the central scar and rim; H and I: diffusion-weighted imaging 500 (H) and low apparent diffusion coefficient (I) images suggest areas of diffusion restriction. Due to patient’s age, gender and indeterminate contrast characteristic, the lesion was resected. Histology showed the lesion was a well to moderately differentiated hepatocellular carcinoma. There was no background cirrhosis, but evidence of mild steatosis.
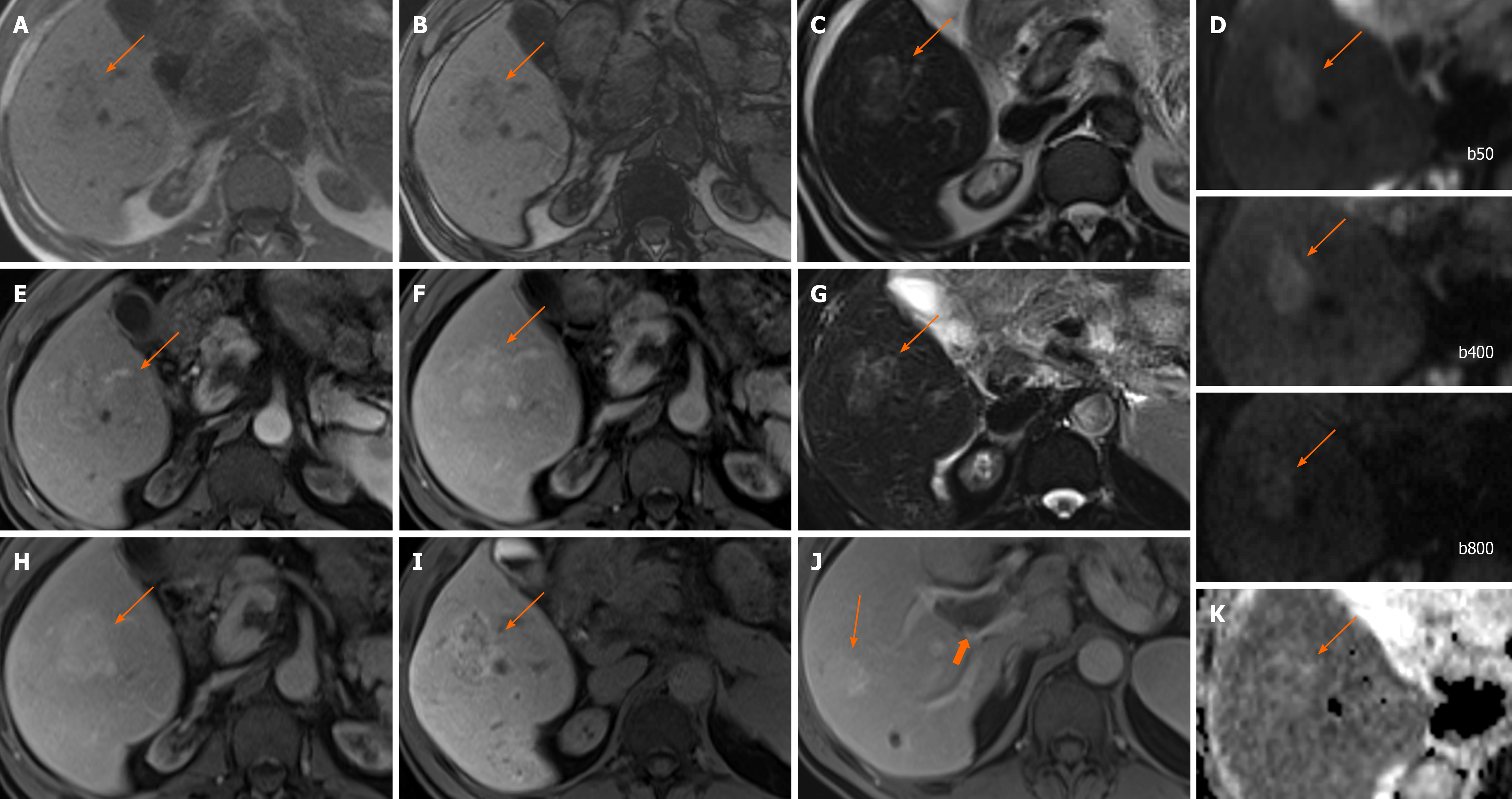
Figure 6 Hepatocellular carcinoma.
A 80-year-old man presented with haematuria and was found to have an incidental liver lesion on computed tomography. His liver function tests were normal. A and B: Magnetic resonance demonstrates signal loss throughout the liver, with paradoxical increase in signal on out-of-phase (B) imaging when compared to in-phase (A), suggestive of underlying iron overload; C: Segment 5 liver lesion shows signal loss on out-of-phase sequences suggesting fat contents and is of high T1 and T2 signal; D: Pre-contrast images; E-G: Subtraction sequences were not performed, but allowing for this, there is some enhancement on arterial phase (E), which persists into portal venous (F) and delayed phases (G); H and I: There is contrast retention on hepatobiliary phase (H) and no diffusion restriction (I–b400). Further tests performed confirmed genetic hemochromatosis. Portal venous pressure measurement also showed portal hypertension. Lesional biopsy confirmed this to be a moderately differentiated hepatocellular carcinoma in a background of cirrhosis, which was subsequently ablated.
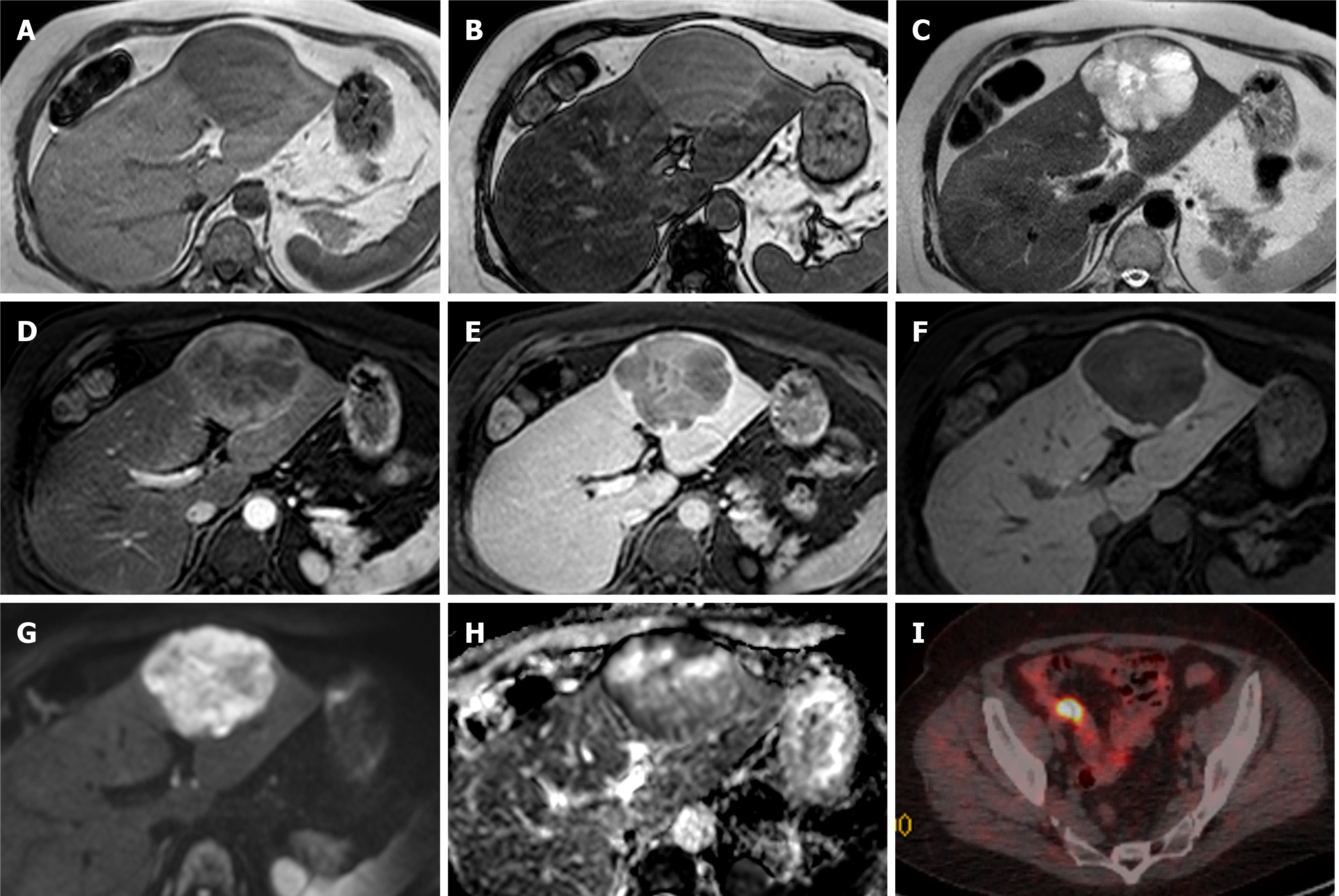
Figure 7 Hepatocellular carcinoma.
A 79-year-old with previous prostate cancer has undergone a magnetic resonance (MR) pelvis and was found to have prostatic cancer recurrence and a liver mass. He has undergone staging computed tomography which showed a further area of oesophageal thickening. Endoscopy revealed oesophageal tumour and biopsy confirmed this to be a squamous cell carcinoma. MR liver and positron emission tomography (PET) scan were performed to characterise these and determine whether liver lesion is a metastasis from oesophageal or prostate primary. Alpha-fetoprotein value was 10 at time of diagnosis. A and B: In- (A) and out-of-phase (B) sequences show low T1 signal liver mass with no intratumoral fat; C: It is of mildly high signal on T2 sequences; D and E: There is homogenous arterial enhancement (D) with washout on portal venous (E) phase; F and G: No contrast retention on hepatobiliary phase (F) and isointense to low signal on apparent diffusion coefficient (G); H and I: PET scan shows tracer uptake within the liver lesion (H), however this is of lower standardized uptake value than the oesophageal cancer (I). Targeted liver lesion biopsy confirmed this to be a hepatocellular carcinoma.
Figure 8 Hepatocellular carcinoma.
A 71-year-old underwent computed tomography chest, abdomen and pelvis for anaemia which identified ascending colon thickening and a liver lesion. Colonoscopy confirmed malignant lesion in the ascending colon and histology showed this to be an adenocarcinoma. Magnetic resonance of liver was performed to characterise the liver mass. A and B: This demonstrates a well-defined lesion with the majority of it showing fat component [signal loss on out-of-phase (B) compared to in-phase (A)] except for a small part laterally; C: It is of mildly high signal on T2 sequences; D: Unenhanced sequence; E-G: There are areas of patchy enhancement on arterial (E) and portal venous (F) phases with heterogenous contrast retention on hepatobiliary phase (G); H and I: This part also shows marked diffusion restriction (long arrow, H–diffusion-weighted imaging b800, I–apparent diffusion coefficient). Diffusion sequences also identified a lymph node showing restricted diffusion (short arrow). Subsequent endoscopy was organised which demonstrated an oesophageal lesion, and biopsies of this, and the adjacent lymph node proved it to be a squamous cell carcinoma. Even with two other primaries, the liver lesion was not considered typical for a metastasis radiologically and targeted biopsy was performed. Histology showed well to moderately differentiated hepatocellular carcinoma.
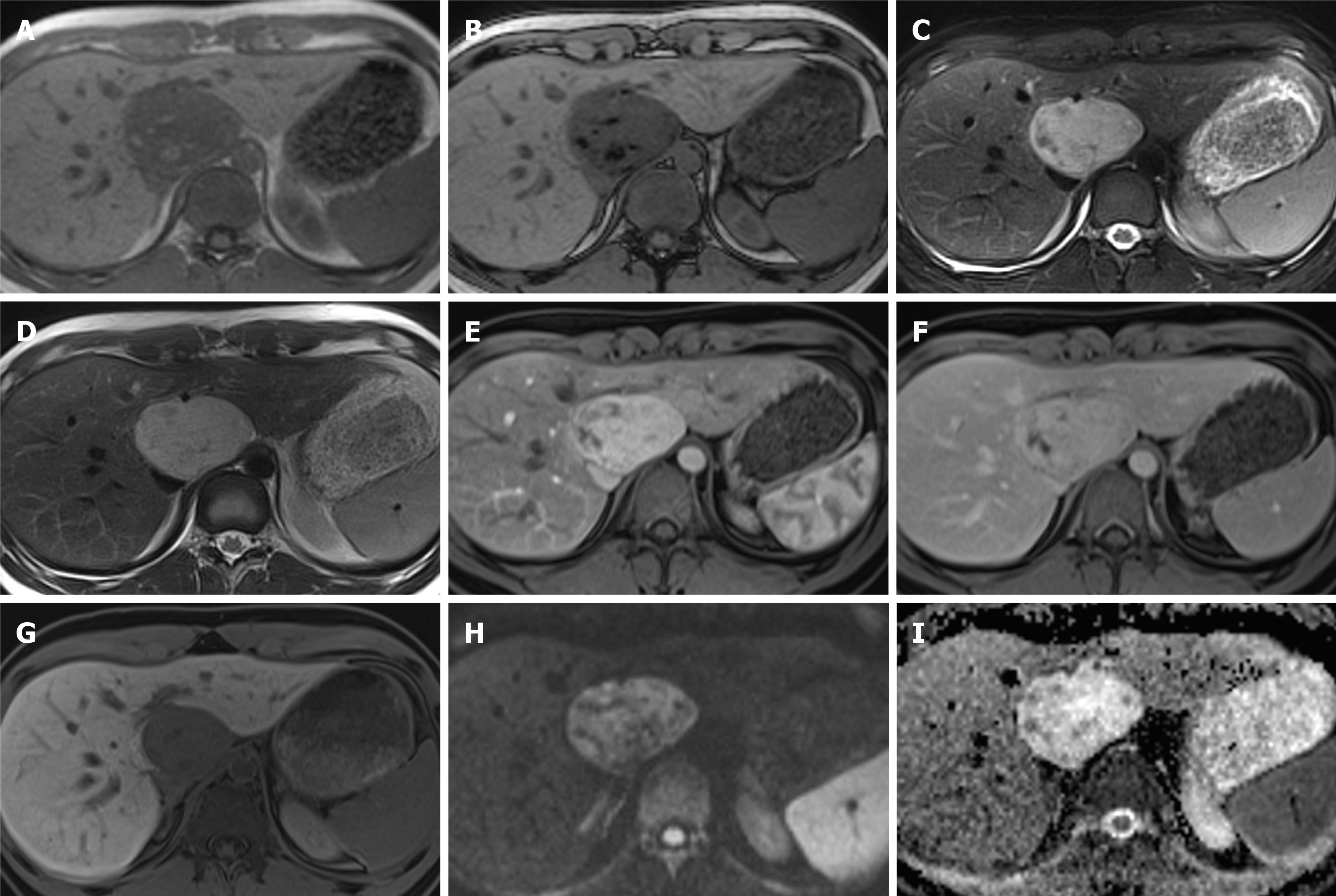
Figure 9 Hepatocellular carcinoma.
A 70-year-old man with a transient episode of frank haematuria as part of the investigations into this, was incidentally found to have a large liver mass arising from the left lobe of the liver. He had previous history of tongue cancer. Liver function tests were normal and alpha-fetoprotein was 2 throughout. A: The lesion (arrowed) is mostly hypointense on T2-weighted sequence with heterogenous areas of high signal; B and C: On T1-weighted sequence (B) it shows iso- to hypointense signal and there is heterogenous arterial enhancement (C); D and E: There is some further filling in on portal venous phase (D) where the lesion is now isointense to the liver parenchyma, similarly to delayed phase (E); F: On hepatobiliary phase the mass is hypointense to background liver; G and H: Diffusion-weighted imaging sequence (G) at b value of 800 shows a focal nodule within the lesion that is markedly hyperintense and on apparent diffusion coefficient (H) hypointense in keeping with diffusion restriction. The lesion was resected and histology confirmed moderately differentiated hepatocellular carcinoma.
Figure 10 Intrahepatic cholangiocarcinoma.
A 64-year-old female with background of hepatitis C cirrhosis was found to have a liver lesion on surveillance ultrasound. Initial magnetic resonance (MR) with extracellular contrast material was reported as likely hepatocellular carcinoma or metastasis. Biopsy confirmed cholangiocarcinoma and gadoxetic acid enhanced MR was organised to exclude satellite lesions and intrahepatic metastases. A-C: MR shows a right liver lobe lesion which is hypointense on T1-weighted imaging (A), hyperintense on T2-weighted imaging (B) and shows diffusion restriction on b800 diffusion-weighted imaging (C); D and E: On arterial phase (D) there is peripheral enhancement with progressive centripetal enhancement on delayed phases (E); F: Hepatobiliary phase shows a hypointense rim with a cloud-like inhomogeneous central enhancement. No further malignant liver lesions demonstrated.
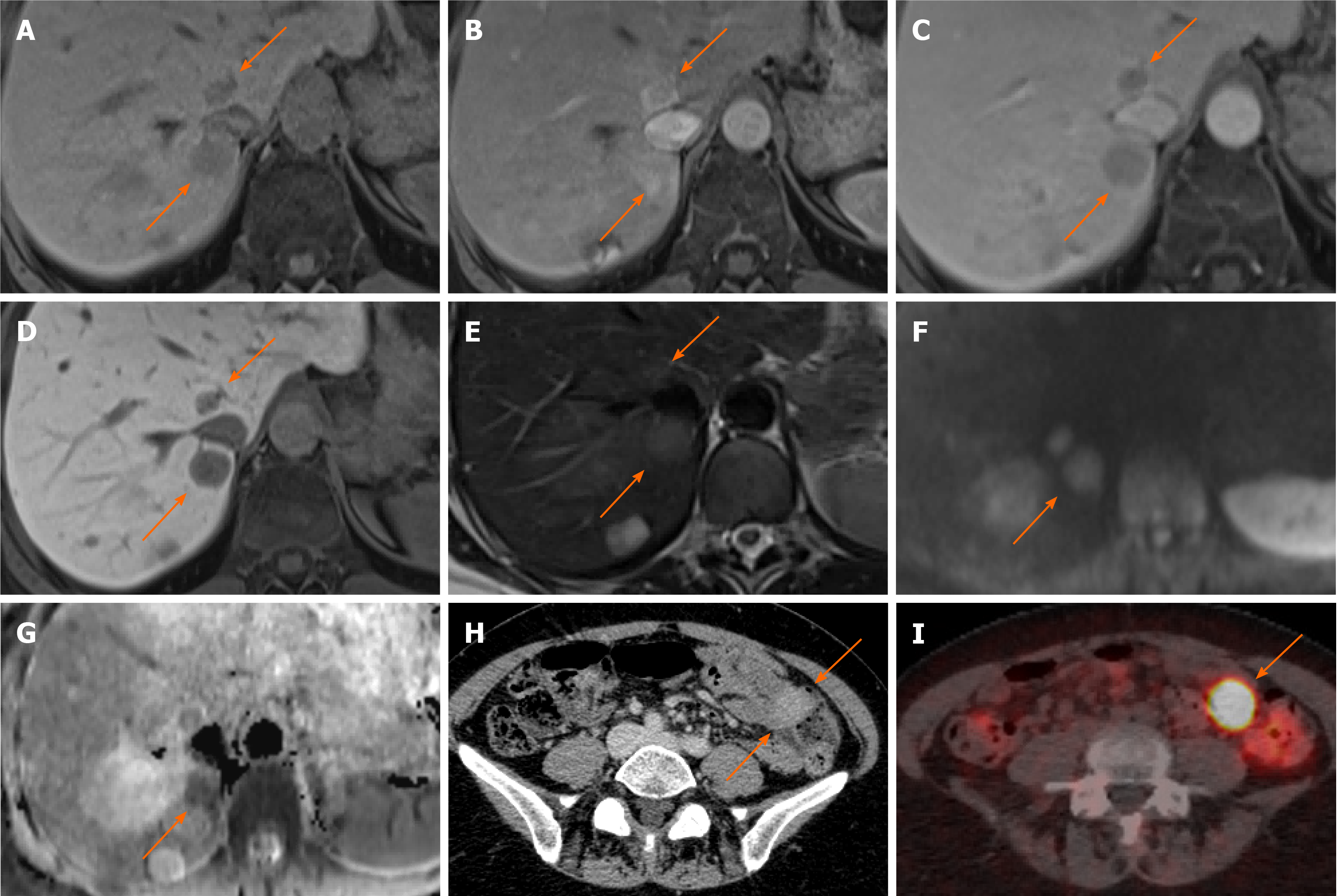
Figure 11 Neuroendocrine carcinoma metastases.
A 55-year-old female with anaemia underwent computed tomography (CT) which identified multiple liver lesions. Magnetic resonance liver was performed and confirmed multiple haemangiomas and few other lesions, two of which are shown here, showing atypical appearances. A: Pre contrast phase sequence shows two lesions of low signal on either side of the inferior vena cava; B and C: On arterial phase (B) there is enhancement followed by prompt washout on portal venous (C) phase; D: There is no contrast retention on hepatobiliary phase; E: Lesions are nearly isointense to liver on T2-weighted sequence; F and G: Diffusion weighted imaging (F) at b800 shows hyperintense signal followed by low signal on apparent diffusion coefficient (G) in keeping with diffusion restriction. The nature of these was not clear, but they were suspicious for hypervascular metastases. The patient underwent a number of investigations including oesophago-gastro-duodenoscopy, colonoscopy, CT chest, abdomen and pelvis and an ultrasound scan of pelvis. None of these investigations have identified a primary source of the liver lesions. Targeted liver biopsy was performed and histology revealed well differentiated neuroendocrine carcinoma (Ki-67 = 4%); H: In retrospect, there was an enhancing lesion within the small bowel also present on previous CT; I: Subsequent Ga68-Dotatoc positron emission tomography-CT was performed which confirmed uptake within the small bowel consistent with primary tumour.
Figure 12 Neuroendocrine carcinoma metastases.
A 59-year-old female was found to have a few liver lesions, the dominant lesion in the left lobe demonstrated here. A and B: In-phase (A) and out-of-phase (B) sequences show background hepatic steatosis, but no tumoral fat; C: The lesion shows heterogenous high T2 signal; D and E: There is mainly peripheral enhancement on the arterial phase (D) with washout on delayed phase (E). Delayed phase also shows an enhancing capsule; F: On hepatobiliary phase there is no contrast retention within the lesion except for the thin-rim of presumed capsule; G and H: There is high signal on diffusion weighted imaging b500 (G) with low signal seen on apparent diffusion coefficient (H), especially in the periphery. The other smaller lesions (not demonstrated here) showed similar signal characteristics. Initial staging computed tomography showed no primary tumour to suggest this is metastasis. The lesions were resected and histology confirmed low grade neuroendocrine tumour, with Ki-67 proliferation index of less than 1%; I: The patient underwent subsequent positron emission tomography scan that demonstrated the primary in the distal ileum.
Figure 13 Neuroendocrine carcinoma.
A 69-year-old female was found to have incidental large liver lesions in a non-cirrhotic liver while undergoing magnetic resonance (MR) pelvis for a uterine lesion, presumed to be fibroid. A: MR demonstrated large liver masses, the largest exophytic mass showing intermediate to high T2 signal with a high signal stellate scar; B: One of the lesions in the left liver lobe demonstrates a cystic component with fluid-fluid levels, which was presumed to represent previous haemorrhage; C: Majority of the lesions were of low T1 signal with a few hyperintense flecks surrounding the scar; D-F: There was heterogenous enhancement on arterial phase (D) with no washout demonstrated on portal venous (E) and delayed (F) phases; G: Hepatobiliary phase showed no contrast retention within the lesion except for the central scar; H and I: Diffusion weighted imaging at b800 (H) and apparent diffusion coefficient (I) show areas of diffusion restriction. These were biopsied and histology demonstrated well differentiated neuroendocrine carcinoma. The origin of this was not determinable from the immunohistochemical pattern. Overall, this was favoured to represent a primary neuroendocrine tumour of the liver as further imaging did not reveal another primary (although admittedly biopsy of the uterine lesion, radiologically presumed fibroid, was never performed). The patient represented a month later with haemorrhagic brain metastases.
Figure 14 Pleomorphic liposarcoma.
A 54-year-old underwent routine ultrasound for re-assessment of gallbladder polyps seen a year ago. Ultrasound revealed multiple liver lesions not present previously and magnetic resonance (MR) of the liver was organised. This showed multiple fat containing liver lesions favoured to represent adenomas. The patient was not on any steroid medication at the time and had no other risk factors for hepatocellular adenoma. A-G: She represented 3 mo later with right sided chest pain and computed tomography (CT) pulmonary angiogram demonstrated increase in the size and number of liver lesions, at which point a second MR liver with gadoxetic acid was performed and is shown here; A-C: MR shows multiple bilobar liver lesions of low T1 signal (C) and predominantly fat component as demonstrated by signal loss on out-of-phase sequence (B) when compared to in-phase (A); D and E: Arterial (D) and delayed phase (E) sequences show a few heterogenous areas of hyperenhancement some of which washout; F: Majority of the lesions did not retain contrast on hepatobiliary phase with only the larger lesions showing some areas of uptake, predominantly within septations; G: T2-weighted sequence (G) shows the lesions are heterogenous and of varied signal intensity; H: Image H demonstrated out-of-phase sequence on the MR performed 3 mo prior for comparison of lesion burden increase in the interim; I: demonstrates portal venous phase CT performed 1 mo since the second MR, again showing quick interval increase in size and number of the lesions. Targeted liver biopsy was performed which confirmed pleomorphic liposarcoma.
- Citation: Noreikaite J, Albasha D, Chidambaram V, Arora A, Katti A. Indeterminate liver lesions on gadoxetic acid-enhanced magnetic resonance imaging of the liver: Case-based radiologic-pathologic review. World J Hepatol 2021; 13(9): 1079-1097
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1079.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1079