Copyright
©The Author(s) 2018.
World J Hepatol. Nov 27, 2018; 10(11): 822-836
Published online Nov 27, 2018. doi: 10.4254/wjh.v10.i11.822
Published online Nov 27, 2018. doi: 10.4254/wjh.v10.i11.822
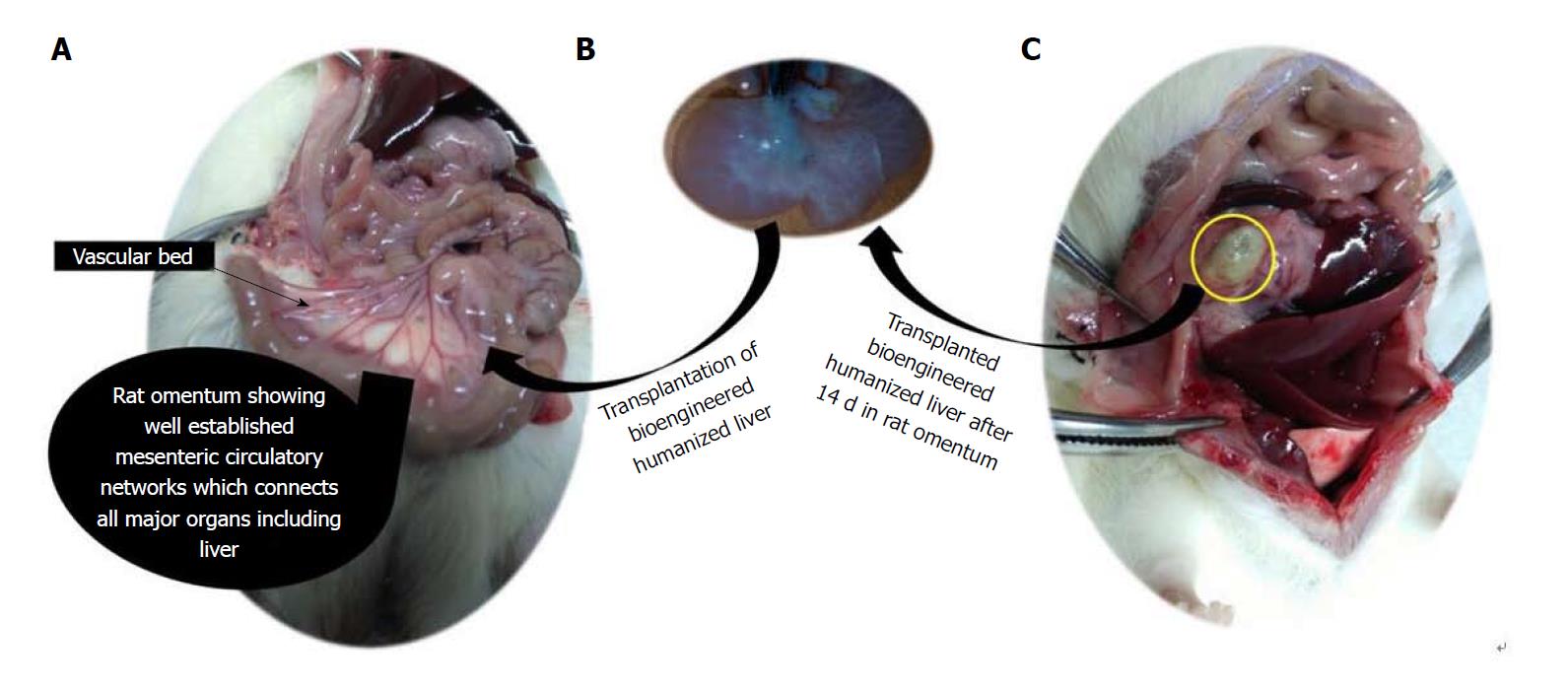
Figure 1 Intra-omental transplantation of bioengineered humanized livers showing development of secondary liver after 14 d.
A: Anatomy of rat omentum showing well-established web of circulatory networks which connected with major organs; B: Developed bioengineered humanized liver in our lab ex vivo; C: Intra-omentally transplanted bioengineered humanized liver showing well engraftment with the surrounding tissue.
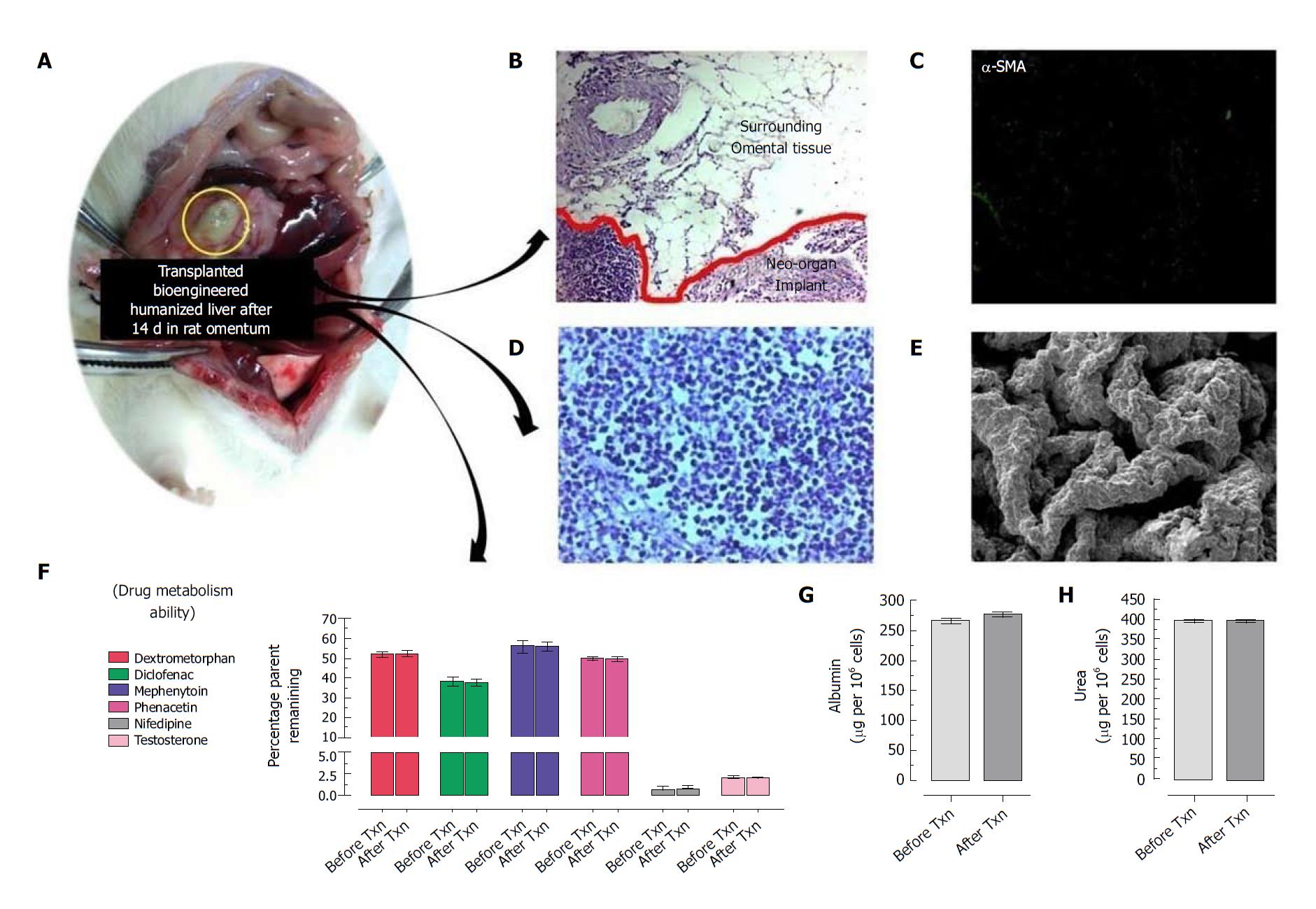
Figure 2 Intra-omental transplantation of bioengineered humanized livers showing no sign of fibrosis or immunological response at transplantation site.
A: Optical image of transplanted implant at intra-omental site; B: Hematoxyeleine and eosin (HE) staining of the transplanted implant along with surrounding tissues showing no sign of immunological cells infiltration or tissue damage. Moreover, neo-vascularization was seen into nearby surrounding tissues which connects with the implant; C: Immunocytochemical staining using α-SMA showed no sign of fibrotic reactions to implant; D: HE staining showed well organized distribution and proliferation of hepatic cells into the implant post-transplantation; E: Scanning electron microscopy (SEM) image of retrieved graft at day 15 post-transplantation showing almost similar anatomy of bioengineered livers with natural liver; F: Retrieved livers at day 15 post intra-omental transplantation showed almost similar metabolic activity to before transplantation (P > 0.05). The other two important liver cell functions such as G: Albumin synthesis and H: Ammonia detoxification (i.e. urea production) is almost similar to the bioengineered humanized livers prior to transplantation (P > 0.05).
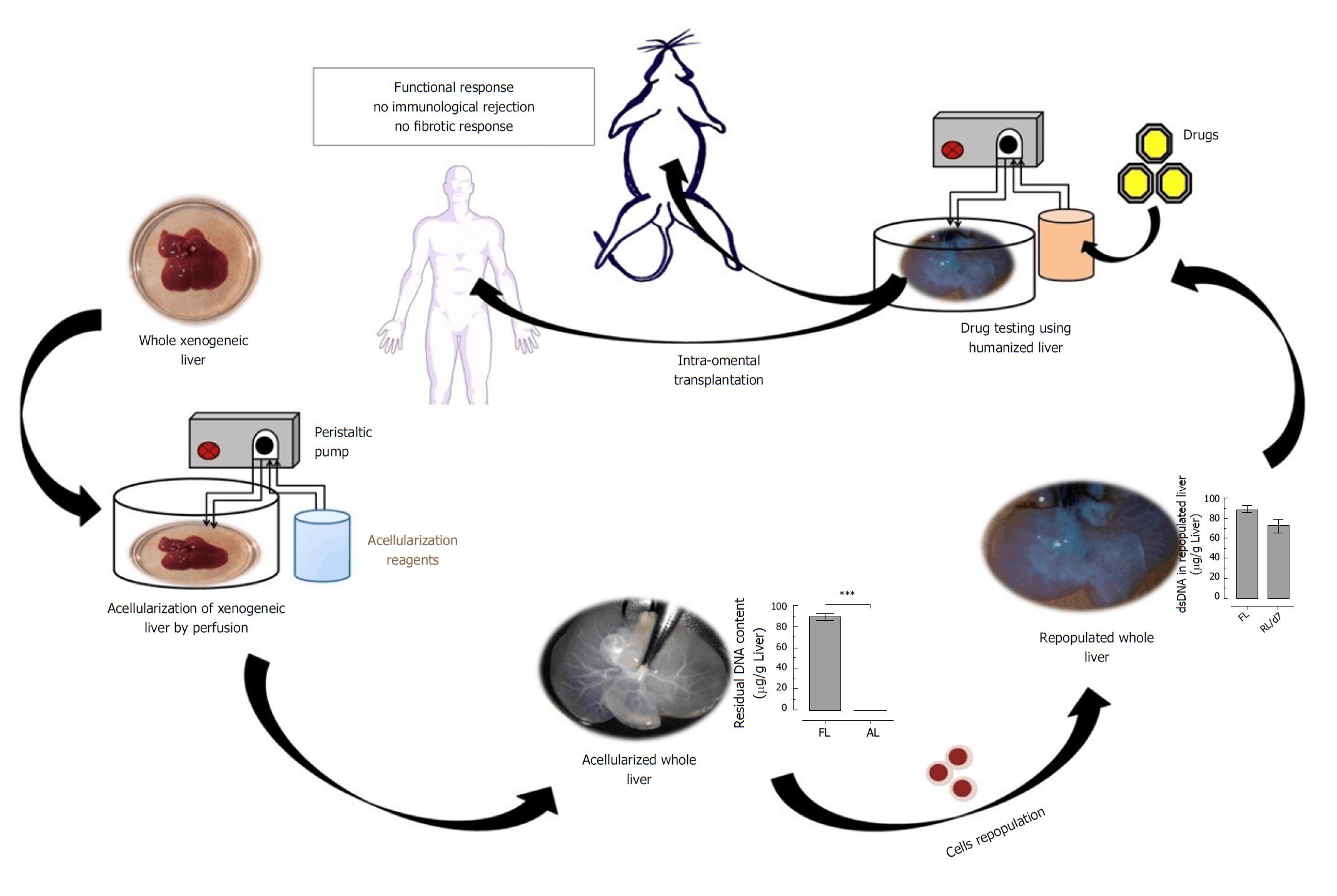
Figure 3 Brief overview of strategy for the development of immune-competent bioengineered humanized liver using acellularization and repopulation technology for future biomedical applications.
- Citation: Vishwakarma SK, Lakkireddy C, Bardia A, Paspala SAB, Tripura C, Habeeb MA, Khan AA. Bioengineered functional humanized livers: An emerging supportive modality to bridge the gap of organ transplantation for management of end-stage liver diseases. World J Hepatol 2018; 10(11): 822-836
- URL: https://www.wjgnet.com/1948-5182/full/v10/i11/822.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i11.822